Abstract
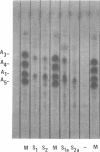
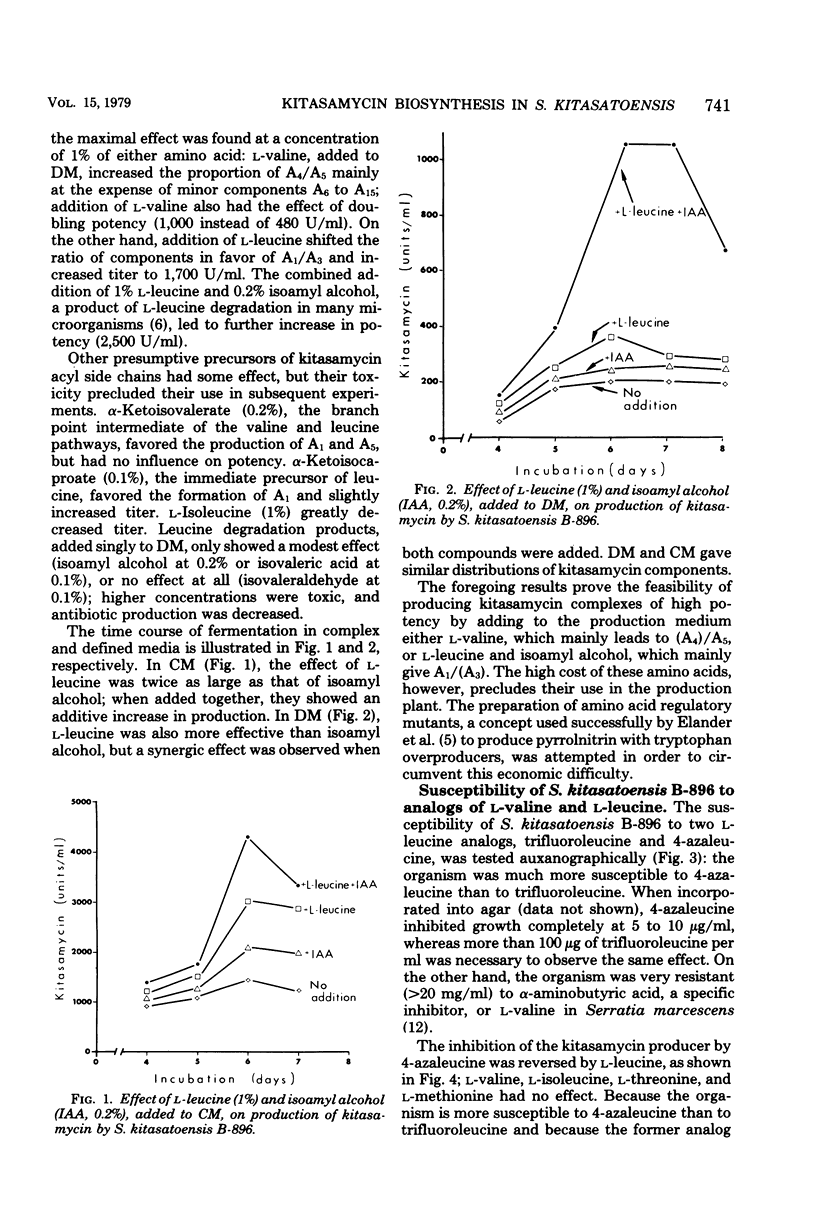
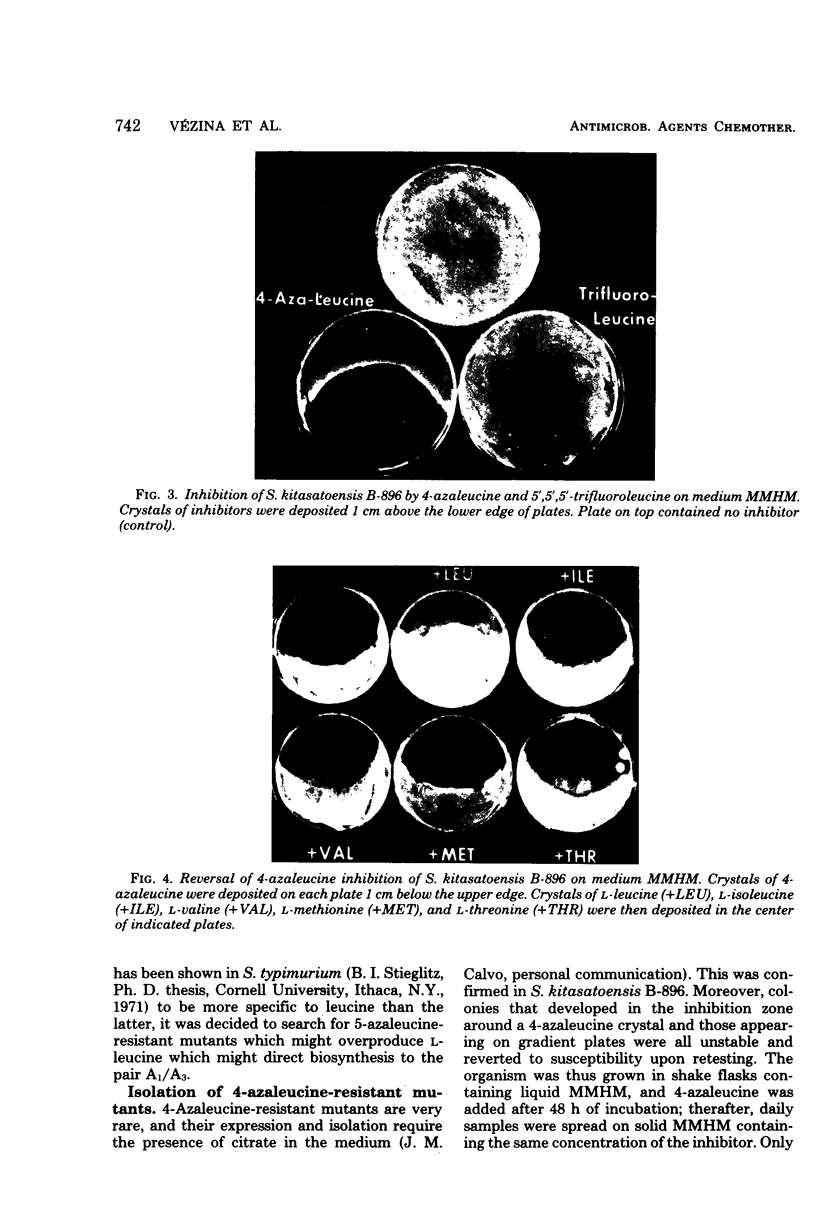
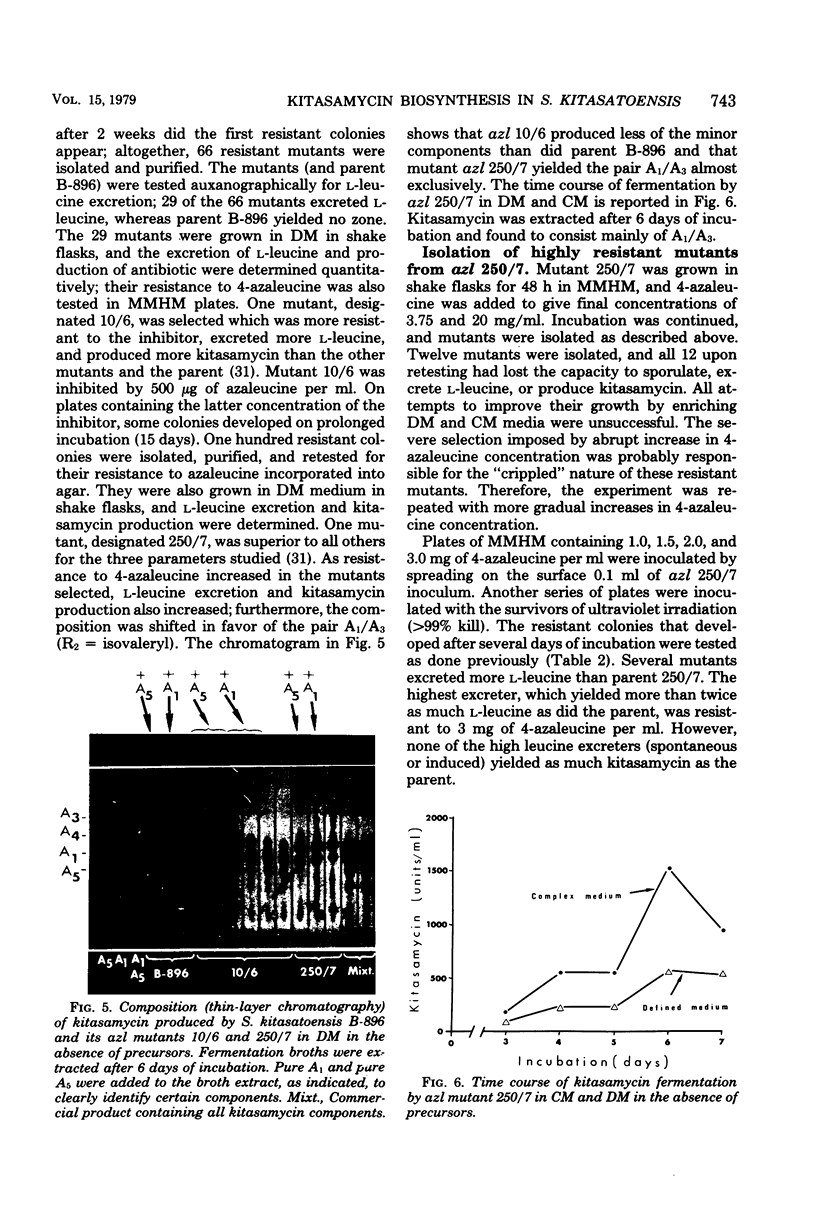
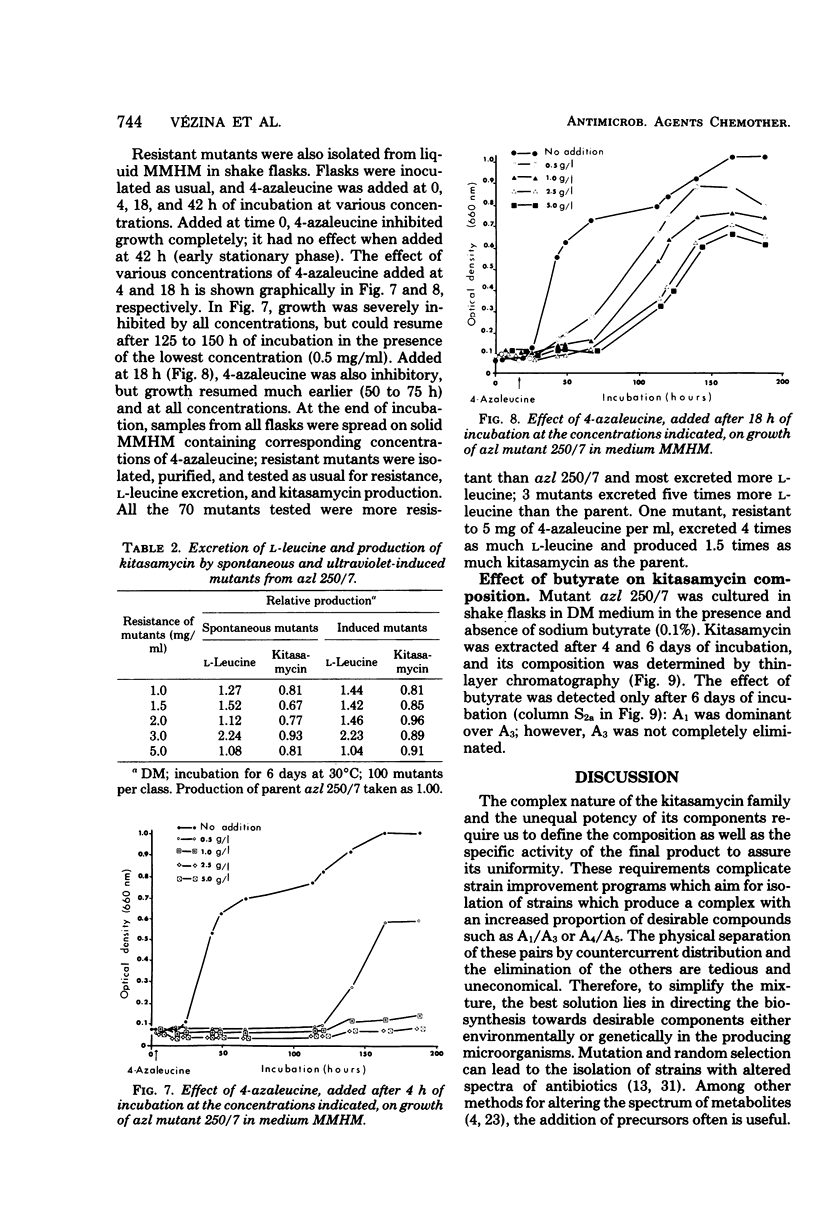
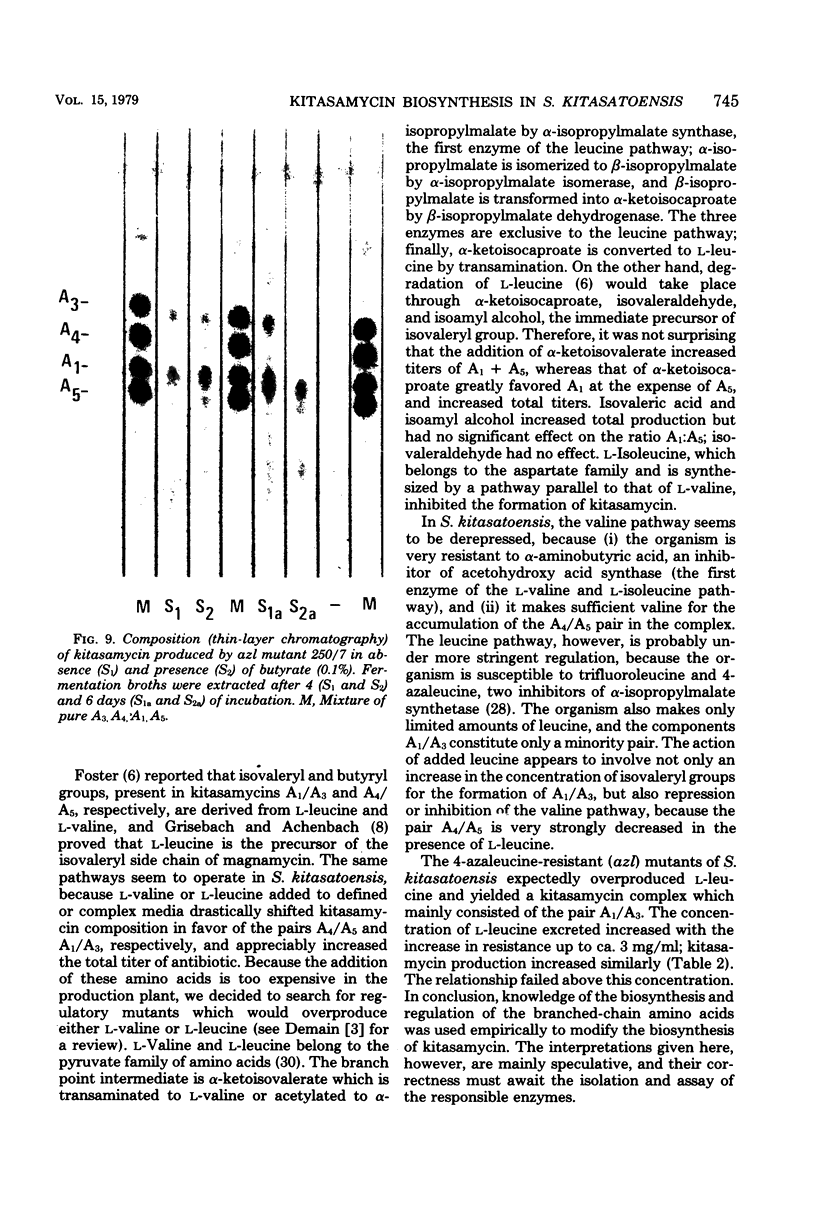
The biosynthesis of kitasamycin in Streptomyces kitasatoensis B-896 was profoundly influenced by the addition of precursors to complex and defined media: l-valine and l-leucine directed biosynthesis towards the pairs A4/A5 (R2 = butyryl) and A1/A3 (R2 = isovaleryl), respectively, and total kitasamycin titers were doubled and quadrupled, respectively. S. kitasatoensis B-896 was very resistant (>20 mg/ml) to α-aminobutyric acid, an analog of l-valine, but very susceptible to l-leucine analogs 5′, 5′, 5′-trifluoroleucine and 4-azaleucine (5 to 10 μg/ml). The inhibition by 4-azaleucine could be reversed by l-leucine, but by none of the other amino acids of the pyruvate family or the amino acids of the aspartate pathway. 4-Azaleucine-resistant mutants were isolated which in the absence of any precursors overproduced l-leucine and a kitasamycin complex mainly consisting of the pair A1/A3. These 4-azaleucine-resistant mutants are presumed to be regulatory mutants in which α-isopropylmalate synthase, the first enzyme of the l-leucine pathway, has become either derepressed or desensitized to leucine feedback inhibition. l-Leucine-regulatory mutants have economic value: in the absence of expensive precursors, they produce a kitasamycin complex in which the most potent pair A1/A3 is dominant and the least active components are absent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L. Mutation and the production of secondary metabolites. Adv Appl Microbiol. 1973;16:177–202. doi: 10.1016/s0065-2164(08)70027-3. [DOI] [PubMed] [Google Scholar]
- Elander R. P., Mabe J. A., Hamill R. L., Gorman M. Biosynthesis of pyrrolnitrins by analogue-resistant mutants of Pseudomonas fluorescens. Folia Microbiol (Praha) 1971;16(3):156–165. doi: 10.1007/BF02884206. [DOI] [PubMed] [Google Scholar]
- HATA T., SANO Y., OHKI N., YOKOYAMA Y., MATSUMAE A., ITO S. Leucomycin, a new antibiotic. J Antibiot (Tokyo) 1953 Apr;6(2):87–89. [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Valine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. J Bacteriol. 1971 May;106(2):493–499. doi: 10.1128/jb.106.2.493-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluepfel D., Sehgal S. N., Vézina C. Antimycin A components. I. Isolation and biological activity. J Antibiot (Tokyo) 1970 Feb;23(2):75–80. [PubMed] [Google Scholar]
- Omura S., Hironaka Y., Hata T. Chemistry of leucomycin. IX. Identification of leucomycin A3 with josamycin. J Antibiot (Tokyo) 1970 Oct;23(10):511–513. [PubMed] [Google Scholar]
- Omura S., Miyazawa J., Takeshima H., Kitao C., Aizawa M. Induction of the bioconversion of leucomycins by glucose in a producing strain. J Antibiot (Tokyo) 1977 Feb;30(2):192–193. doi: 10.7164/antibiotics.30.192. [DOI] [PubMed] [Google Scholar]
- Omura S., Miyazawa J., Takeshima H., Kitao C., Atsumi K. Bioconversion of leucomycins and its regulation by butyrate in a producing strain. J Antibiot (Tokyo) 1976 Oct;29(10):1131–1133. doi: 10.7164/antibiotics.29.1131. [DOI] [PubMed] [Google Scholar]
- Omura S., Nakagawa A. Chemical and biological studies on 16-membered macrolide antibiotics. J Antibiot (Tokyo) 1975 Jun;28(6):401–433. doi: 10.7164/antibiotics.28.401. [DOI] [PubMed] [Google Scholar]
- Omura S., Nakagawa A., Takeshima H., Atusmi K., Miyazawa J., Piriou F., Lukacs G. Letter: Biosynthetic studies using 13C enriched precursors on the 16-membered macrolide antibiotic leucomycin A3. J Am Chem Soc. 1975 Oct 29;97(22):6600–6602. doi: 10.1021/ja00855a065. [DOI] [PubMed] [Google Scholar]
- Omura S., Suzuki Y., Nakagawa A., Hata T. Letter: Fast liquid chromatography of macrolide antibiotics. J Antibiot (Tokyo) 1973 Dec;26(12):794–796. doi: 10.7164/antibiotics.26.794. [DOI] [PubMed] [Google Scholar]
- Omura S., Takeshima H., Nakagawa A., Miyazawa J., Piriou F., Lukacs G. Studies on the biosynthesis of 16-membered macrolide antibiotics using carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1977 Jun 28;16(13):2860–2866. doi: 10.1021/bi00632a009. [DOI] [PubMed] [Google Scholar]
- Queener S. W., Sebek O. K., Vézina C. Mutants blocked in antibiotic synthesis. Annu Rev Microbiol. 1978;32:593–636. doi: 10.1146/annurev.mi.32.100178.003113. [DOI] [PubMed] [Google Scholar]
- Rakhit S., Singh K. Structure activity relationship in sixteen membered macrolide antibiotics. J Antibiot (Tokyo) 1974 Mar;27(3):221–224. doi: 10.7164/antibiotics.27.221. [DOI] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz B. I., Calvo J. M. Distribution of the isopropylmalate pathway to leucine among diverse bacteria. J Bacteriol. 1974 Jun;118(3):935–941. doi: 10.1128/jb.118.3.935-941.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]