Abstract
Melon, Cucumis melo L. is an important vegetable crop worldwide. At present, there are phenomena of homonyms and synonyms present in the melon seed markets of China, which could cause variety authenticity issues influencing the process of melon breeding, production, marketing and other aspects. Molecular markers, especially microsatellites or simple sequence repeats (SSRs) are playing increasingly important roles for cultivar identification. The aim of this study was to construct a DNA fingerprinting database of major melon cultivars, which could provide a possibility for the establishment of a technical standard system for purity and authenticity identification of melon seeds. In this study, to develop the core set SSR markers, 470 polymorphic SSRs were selected as the candidate markers from 1219 SSRs using 20 representative melon varieties (lines). Eighteen SSR markers, evenly distributed across the genome and with the highest contents of polymorphism information (PIC) were identified as the core marker set for melon DNA fingerprinting analysis. Fingerprint codes for 471 melon varieties (lines) were established. There were 51 materials which were classified into17 groups based on sharing the same fingerprint code, while field traits survey results showed that these plants in the same group were synonyms because of the same or similar field characters. Furthermore, DNA fingerprinting quick response (QR) codes of 471 melon varieties (lines) were constructed. Due to its fast readability and large storage capacity, QR coding melon DNA fingerprinting is in favor of read convenience and commercial applications.
Introduction
Melon (C. melo L., 2n = 24), which belongs to Cucumis, Cucurbitaceae, is an important vegetable crop worldwide. Among Cucurbitaceae, C. melo is one of the most important cultivated cucurbits, due to its economic and nutraceutical importance [1].
Global melon yield in 2009 was over 25.5 million tons, and in the past 30 years has increased 1.7 fold with an average annual growth rate of 3.6%. China is the biggest melon producer in the world, accounting for about half of global production in recent years. The total yield in China in 2009 was over 12.22 million tons (FAO Statistics 2009, http://faostat3.fao.org/home/index.html#VISUALIZE_BY_DOMAIN).
In China, a large number of melon varieties are being released and commercialized every year. While some of these commercial varieties have the same parents, many different trade names (synonym) are used, and on the other hand, some of the commercial varieties have the same names but their parents are different (homonym). This situation in China is widespread, which confuses melon breeders, seed producers, as well as melon producers, and damages their economic interests, including also negative influence on exporting of seeds and fruit produce to other countries due to the confusion and inconvenience it has caused. Another situation is that some seed companies’ lax quality control results in problems of seed purity and affects the interests of farmers seriously. On the other hand, assessment of genetic variation is important for melon breeding: efficient management, protection and application of germplasm resources [2]. Detection and utilization of the genetic variation and cultivar identification are thus some important tasks for melon breeders.
Traditionally, evaluation of seed quality and germplasm management has been based on morphological descriptors. However, many modern varieties and hybrids are phenotypically less distinct than traditional varieties, and morphological evaluation is more difficult. Furthermore, identification based on morphological descriptors is time consuming and often influenced by environmental conditions. All of these factors increase the difficulties of monitoring the melon seed market. Therefore, new, reliable, and timesaving methods are needed for accurately assessing cultivar identification and genetic diversity [3].
Molecular marker technologies offer alternatives for the identification of cultivars and genetic diversity. Techniques have included, for melon, the utilization of isozymes, random amplified polymorphic DNA, amplified fragment length polymorphisms, single nucleotide polymorphisms, and simple sequence repeats (SSRs) [4]–[7]. Among all the marker systems, single nucleotide polymorphisms are the best for marker-based cultivar identification and genetic diversity studies [6]. However, the high cost and the difficulties associated with single nucleotide polymorphism genotyping prevent it from being used in most melon breeding programs [8], and especially by monitoring laboratories supported by local governments and small breeding laboratories in developing countries such as China.
SSRs are a viable alternative for these laboratories, because the co-dominant and multi-allelic nature and high reproducibility of SSR markers are sufficient for identification of germplasm and genetic variation [9]–[11]. More importantly, the experimental conditions necessary to perform SSR genotyping are realizable for small laboratories. During the past decade, much data have been generated using SSR markers for the evaluation of the genetic diversity of crops such as barley, wheat, sorghum, potato, rice, and maize [12]–[21]. An overview of these results indicates that the SSR marker system is a powerful and economical tool for analyzing crop genetic diversity.
In melon, SSR markers have been used broadly to define genetic associations among botanical groups and commercial market classes [22]–[25], and assessments of the major primary and secondary geographic origins of melon diversity. However, there has been very few polymorphism analysis of Chinese marketable melons. Therefore, it is necessary to establish an SSR-based DNA fingerprint database for melon which could provide support for evaluations of varietal purity and authenticity.
In the present study we developed and validated a core set of SSR markers to establish a DNA fingerprint database for melon. We detected polymorphism of 1219 SSRs in 20 representative melon varieties (lines), with 470 polymorphic SSRs selected. Eighteen SSR markers with rich contents of polymorphism information (PIC) were selected as the core SSRs to establish the DNA fingerprint database. The utility of this core set of SSRs was further demonstrated and validated in 471 melon accessions including commercial cultivars and elite lines.
Materials and Methods
Plant Materials
Four hundred and seventy-one melon varieties (lines) were employed in this study (Table S1). Plant materials were obtained mainly from the major melon producing areas of China, covering 20 provinces, municipalities, and autonomous regions. A small number of melon varieties (lines) originated from Japan, the United States, India, and some other countries which further broadened the representativeness of target populations. These 471 melon materials comprised two categories: thin rind melon (294) and thick rind melon (177). According to botanical classification, subspecies included C. melo subsp. agrestis (Naud) Greb (2 materials), C. melo subsp. dudaim (L.) Greb (one), C. melo subsp. flexuosus (L.) Greb (7), C. melo subsp. conomon (Thunb.) Greb (284) and C. melo subsp. melo Pang (177).
Three hundred and forty-five commercial cultivars were obtained directly from seed market vendors. A small number of commercial cultivars were from researchers who commissioned us to determine if plants with the same identifier were from different genetic lines. Of the remaining 126 breeding elite lines, outside breeders and researchers provided some, and others were our own stock kept in the laboratory. All the tested melon varieties were grown in the greenhouse at 25/18°C day/night.
Strategies for Developing a Core Set of SSR Markers in Melon
Twenty melon genotypes with diverse genetic backgrounds and horticultural traits were screened to develop a core set of SSR markers (Table 1). These were chosen as representatives of the melon collection for maximum diversity. The starting marker set contained 1219 SSRs, for developing the core set of SSRs. Our objective was to eliminate sequentially the SSR markers with low power of discrimination to achieve a final combination of markers that could be used for DNA fingerprinting and genetic diversity analysis. The 1219 SSRs were obtained from the literature [26]–[32], online databases (NCBI Expressed Sequence Tags database, http://www.ncbi.nlm.nih.gov/dbEST/; the Curcurbit Genomics Database, http:/www.icugi.org/) and the Chinese Academy of Agricultural Sciences.
Table 1. Characteristics of 20 melon varieties used for developing the melon DNA fingerprinting core SSRs.
| No. | Name | Systematics | Fruit shape | Rind features | Flesh color | Fruit weight (g) | Soluble solid content (Brix) |
| 1 | Little Melon (wild) | C. melo ssp. agrestis | Round | Green | White | 15.63 | 5 |
| 2 | Play Melon | C. melo ssp. dudaim | Round | Yellow | Yellow | 43.11 | 12.5 |
| 3 | Qingpi Caigua No.1 | C. melo ssp. flexuosus | Elongated | Green | White | 360.09 | 5.4 |
| 4 | Huapi Sushaogua | C. melo ssp. conomon | Elongated | Green, dark green stripes | Green | 176.62 | 8.2 |
| 5 | Heipi Sugua | C. melo ssp. conomon | Elongated | Dark green | Green | 730.49 | 3.3 |
| 6 | No.20 | C. melo ssp. conomon | Oblate | Green | White | 153.7 | 6.2 |
| 7 | 3-2-2 | C. melo ssp. conomon | Oblong | Green, white stripes | White | 261.24 | 12.3 |
| 8 | 7-1-1-2 | C. melo ssp. conomon | Oblong | Green, dark green stripes | Green | 215.32 | 7.7 |
| 9 | 13-4-6-1 | C. melo ssp. conomon | Oblong | Yellow, white stripes | White | 334.82 | 8.6 |
| 10 | 16-8-1-1 | C. melo ssp. conomon | Oblong | Yellow, white stripes | Orange | 307.45 | 9.5 |
| 11 | Tianshuai (parent) | C. melo ssp. conomon | Oblong | White | White | 198.83 | 9.2 |
| 12 | Bailangua | C. melo ssp. melo | Round | White | Green | 536.54 | 11.5 |
| 13 | Tiedanzi | C. melo ssp. melo | Round | Orange | Green | 388.75 | 11.3 |
| 14 | Hongxincui | C. melo ssp. melo | Oblong | Grey-white | Red orange | 803.21 | 12.8 |
| 15 | Kalakesai | C. melo ssp. melo | Round | Green, dark green stripes | Red orange | 613.05 | 12.1 |
| 16 | PMR45 | C. melo ssp. melo | Round | Densely netted | Red orange | 353.92 | 12.3 |
| 17 | TopMark | C. melo ssp. melo | Round | Densely netted | Orange | 388.04 | 6.2 |
| 18 | WI998 | C. melo ssp. melo | Oblate | Orange, netted | Red orange | 374.26 | 7.3 |
| 19 | Elizabeth Male Parent | C. melo ssp. melo | Round | Yellow | Green | 188.88 | 12.3 |
| 20 | Yourangsika | C. melo ssp. melo | Round | Orange, crazing | White | 222.76 | 10.6 |
The 20 (maximum diversity) melon genotypes were screened for the 1219 SSRs. A final core set of SSRs was determined based on the following criteria: 1) a PIC value ≥0.55, 2) SSRs were evenly distributed across the melon genome with ≥one marker from each linkage group, 3) easy to use in a PCR assay (i.e., a clear PCR product under standard reaction conditions), and 4) each SSR detected one and only one locus.
SSR Marker Analysis
For each melon variety, young tender leaves were collected from five 13-day-old seedlings, and stored at –80°C for DNA isolation as recommended by Luan et al. [33]. PCR reactions were performed in accordance with the procedures described by Luan et al. [34], with modifications. Briefly, each 15-µL PCR reaction mixture contained 15 ng template DNA, 0.4 µM each of the left and right primers, 2 mM MgCl2, 0.2 mM each of dNTPs, and 1 U Taq DNA polymerase in 1× PCR buffer (Takara, China). The PCR reaction began with 94°C for 5 min; then 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 7 min. The PCR products were analyzed via 6% polyacrylamide gel electrophoresis in 1× TBE buffer. The gel was silver-stained using the Silver Sequence DNA Sequencing System (Promega, Madison, WI, USA).
Data Analysis
Polymorphic SSR markers were scored for the presence or absence of corresponding bands among the tested accessions. Stutter and background bands were excluded. The scores ‘1’ and ‘0’ indicated the presence and absence of the bands, respectively. The PIC of each marker was calculated in accordance with Kirst et al. [35] as:
where fi is the band frequency of the i th allele.
Genetic similarities between melon varieties were calculated using Nei and Li’s coefficients index [36] with Free-Tree software [37]. A dendrogram was constructed with MEGA5 [38] software using the unweighted pair-group method with arithmetic mean (UPGMA).
Representation of DNA Fingerprinting
The coding of fingerprints was expressed in accordance with Wang et al. [39] and Ma et al. [40]. Core SSRs were sorted in a fixed order, and expressed in capital letters. Each pair of SSR primers amplified polymorphic bands, and the amplicon sizes of variable alleles were recorded (in base pairs [bp]), and if the amplicon of a core SSR was polymorphic in one material, the polymorphic amplicon sizes were sorted in descending order and connected by a dash (i.e., “-”). For example, the fingerprinting code A100B300-200C0 means the variety has an amplicon of 100 bp at SSR locus A, amplicons of 300 bp and 200 bp at SSR locus B, and no amplicon at SSR locus C.
Results
Development of the Core Set of SSR Markers for DNA Fingerprinting
In total, 1219 SSRs were screened from 20 melon genotypes of diverse genetic backgrounds and horticultural traits, and 470 polymorphic SSRs were screened out. Taking into account PIC values, genomic distribution uniformity, PCR amplification efficiency, and other factors relevant to SSRs, finally 18 polymorphic SSRs were selected as the core set of SSRs (Table 2). Each pair of these selected SSR primers could detect varying numbers of polymorphic bands (range, 4 to 14; mean, 9). The PIC value ranged between 0.55 and 0.82, with an average of 0.68. Their amplified bands were legible, easy to count, and distinguishable from one another. These markers were, for the most part, evenly distributed across the melon genome.
Table 2. The 18 core SSRs for DNA fingerprinting.
| No. | Name | Primer sequences (5'-3', forward/reserve) | PIC | Size (bp) | Bands | Linkage group | Code name of SSRs | |||
| A 1 | B 2 | C 3 | D 4 | |||||||
| 1 | SSR12833 | TCCCGACCTCTTCACGTAAC/GGAAGGCTCATACAGTGGGA | 0.82 | 180–350 | 12 | V | I | – | – | A |
| 2 | CMBR088 | CCACTAAAGTTTCCTTATGTTTTGG/TGGTTGAGGAAGACTACCATCC | 0.82 | 255–460 | 14 | – | – | VII | X | B |
| 3 | CMBR052 | CAGCGATGATCAACAGAAACA/GGCTGACACTCCCTGTACCT | 0.82 | 100–215 | 14 | – | – | VII | VII | C |
| 4 | GCM548 | AACAGGTAGAGGAAAGCATG/TGACCCACTAGTACATCTCTC | 0.78 | 200–330 | 12 | – | XVI | II | – | D |
| 5 | SSR12083 | GAATTGGCCCATCCTTCATT/GCCATTCCAAAAACTTTTCAAC | 0.74 | 195–400 | 10 | – | VI | – | XI | E |
| 6 | CMBR097 | CGACAATCACGGGAGAGTTT/CATATTAGACCCATATTTGTTGCAT | 0.72 | 340–400 | 7 | – | XII | XII | XII | F |
| 7 | MU4104-1 | TTTCCCGCATTGATTTTCTC/GAGAAACGCTTCCCACAAAC | 0.71 | 275–440 | 8 | IV | VII | – | I | G |
| 8 | NR38 | TAAAACACTCTCGTGACTCC/GATCTGAGGTTGAAGCAAAG | 0.69 | 225–360 | 8 | XIX | XI | – | – | H |
| 9 | ECM147 | GAAAGGTAGGAAGAAAGTGAAGA/ACTCTTGAAGCTGACCGATG | 0.67 | 230–275 | 5 | – | – | XI | XI | I |
| 10 | TJ138 | AAAATGAAAACTCTTCGGCAAG/AAAACCCTTCTTGCCCTTGT | 0.67 | 140–250 | 8 | XVII | IV | – | I | J |
| 11 | CMBR002 | TGCAAATATTGTGAAGGCGA/AATCCCCACTTGTTGGTTTG | 0.65 | 165–295 | 9 | – | – | VI | – | K |
| 12 | CM26 | CCCTCGAGAAACCAGCAGTA/CACCTCCGTTTTTCATCACC | 0.63 | 300–360 | 8 | – | II | – | VII | L |
| 13 | MU9175-1 | CAATTTCCAATCCATCTGCTC/ATCGAAATTCCTCCCTCGTT | 0.63 | 295–345 | 8 | – | VIII | – | II | M |
| 14 | MU5554-1 | CCTTCATGATCCTCTACTAAACCC/TCTTCCATGCTTTTCTCGCT | 0.60 | 230–290 | 8 | – | XI | – | VI | N |
| 15 | SSR00398 | ATTCAAACCCCGTTTAACCC/AGTGAAAATGGCGGAAACTG | 0.60 | 185–340 | 11 | – | XIII | – | – | O |
| 16 | ECM150 | ACACACCTAATCTCCCTACCTTC/CTCAAACAACGTCAGCTGGT | 0.59 | 170–205 | 8 | – | – | IX | IX | P |
| 17 | TJ10 | ACGAGGAAAACGCAAAATCA/TGAACGTGGACGACATTTTT | 0.57 | 170–195 | 4 | IX | V | III | – | Q |
| 18 | CMBR154 | GATTCTTCCTCCTTCTAAAGGATA/AATGTGGGTGAGAGGACATT | 0.55 | 100–180 | 8 | – | XVIII | IV | – | R |
Zhu et al. [41].
Gao et al. [42].
Diaz et al. [43] (ICUGI, http://www.icugi.org/).
Localization on melon genome [44] using SSR primer sequences by BLAST.
–, not on the linkage group.
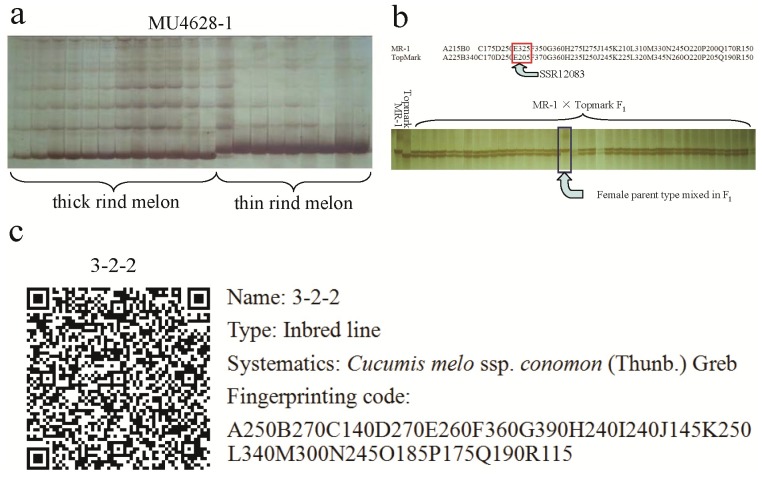
Of the 1219 SSRs, there were 52 SSRs which could differentiate thin rind from thick rind melon, as exemplified by the SSR primer MU4628-1 (Figure 1a). Although some of them matched well according to the screening criteria of the core set of SSRs, they were not selected based on visible phenotypic traits.
Figure 1. Application of the core set of SSR primers and the expression of melon DNA fingerprinting QR Code.
a. SSR primer MU4628-1 could distinguish the thin rind melon and the thick rind melon. In this study, the total number of SSR primer pairs which could distinguish thin rind melon and thick rind melon clearly was 52. b. Each pair of the core SSR primers could be used in seed purity tests. In this example, fingerprinting codes of MR-1 and Topmark were analyzed. We found that SSR E (SSR12083, labeled by a red frame) had the bigger amplicon length difference in the two parents, so we chose SSR12083 to identify F1 seed purity. Most of the F1 were parent hybrid types, while few of them were female parent type (labeled by a blue frame). c. The DNA fingerprinting QR code of melon line 3-2-2 is at the left. This picture could be scanned by computers or a mobile device, such as a smartphone, PDA and so on. The right side shows the translation result of the left picture after scanning.
Each pair of the core set of SSR primers could be used to identify the purity of F1 seeds (Figure 1b). For example (described in Figure 1b), we analyzed the fingerprinting codes of MR-1 and Topmark. SSR E (SSR12083, labeled with a red frame) had the bigger amplicon length difference in two parents, so SSR12083 was chosen to identify the purity of F1. Most of the F1 were parent hybrid types, and few of them were the female parent type (labeled with a blue frame). This feature could be used in purity tests of hybrid seeds.
DNA Fingerprinting and Genetic Diversity Analysis of 471 Melon Materials
All of the 471 melon materials were subjected to polymorphism and genotyping screening using the core set of melon DNA fingerprinting SSR primers. In the present study, we used the SSR label plus the PCR amplicon length to represent the fingerprint code of each germplasm, and every fingerprint code corresponded to a long string.
The original purpose for melon germplasm DNA fingerprinting was to quickly gain genetic information for each melon line, input it into a computer database, and then be able to realize the fast alignment of genetic information of varieties of melon materials. Text information is difficult to read quickly from a computer, so the string fingerprinting code was transposed into graphical coding, the Quick Response (QR) barcode, which can be quickly scanned into the computer. Figure 1c is an example of the melon DNA fingerprinting QR code, which contains DNA fingerprinting, variety name, variety type and other information. The DNA fingerprinting QR code can be quickly scanned by computer or mobile operating systems. The QR code could thus be printed onto the packages of different varieties to quickly identify the contents for buyers and accreditation bodies, or be pasted onto seed bags or labels in experimental fields to enable the rapid identification of the germplasm, to support breeding material management and germplasm secrecy for breeders.
The fingerprint codes of these melon materials are listed in Table S2. The number of the amplified bands in these 471 melon materials ranged between 17 and 31, with an average of 21.46.
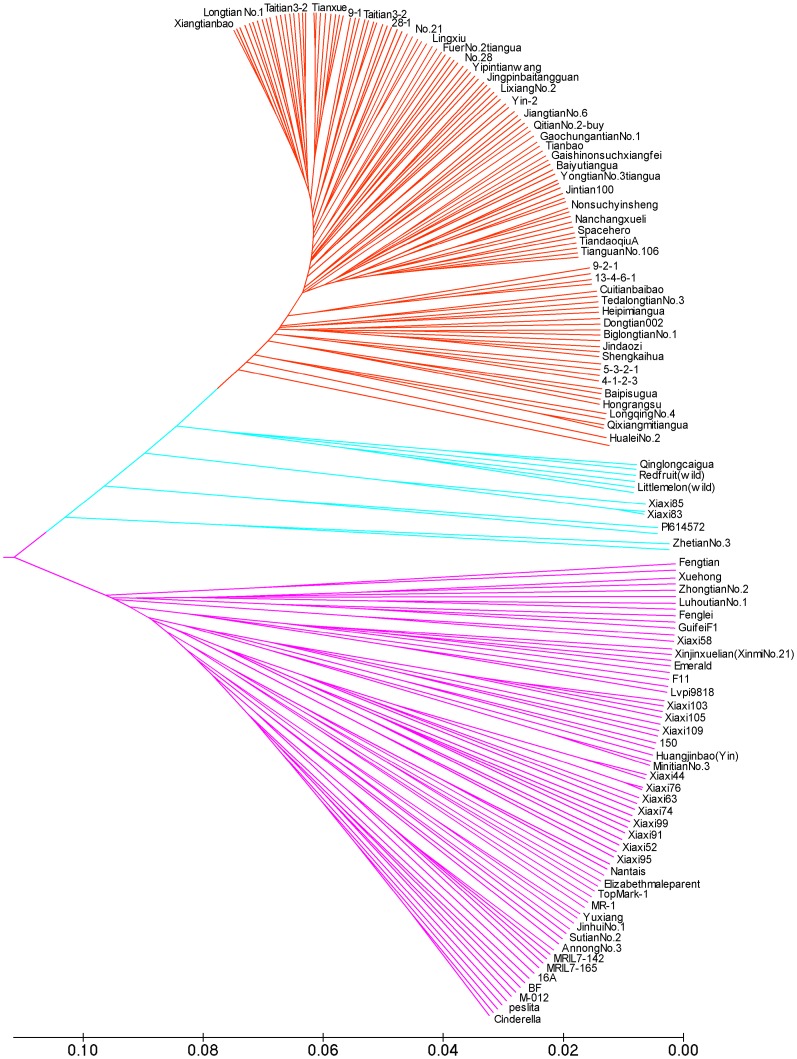
The dendrogram of the 471 melon entries included in this study was based on UPGMA analysis, using a similarity matrix generated by the Nei and Li coefficient after amplification with 18 pairs of microsatellite primers (Figure 2). The resulting dendrogram had 3 distinct clusters. The first cluster (shown in red) consists only of C. melo subsp. conomon (Thunb.) Greb (284 varieties/lines). The second (blue) comprises C. melo subsp. flexuosus (L.) Greb (7 varieties/lines), C. melo subsp. dudaim (L.) Greb (1 variety), C. melo subsp. agrestis (Naud) Greb (2 varieties), and C. melo subsp. melo Pang (3 varieties). The third cluster (pink) contains only C. melo subsp. melo Pang (174 varieties/lines).
Figure 2. UPGMA dendrogram of 471 melon accessions based on the 18 core set of SSR markers.
Materials with the same fingerprinting codes had been merged. The end of each branch represents a melon variety, and many names of varieties had been omitted due to space considerations. For more detailed classification information, see Figure S1. The 471 test accessions were in 3 distinct clusters: color codes red, blue, and pink.
Interestingly, based on the screening by the core set of melon DNA fingerprinting SSR primers, there were 51 materials used in this study that could be separated into 17 groups and each group has the same fingerprinting codes, with the names and fingerprinting codes of these materials listed in Table 3. Further field traits survey results (data not shown) indicated that the materials with the same fingerprinting codes (each of these 17 groups) have also same or similar field characters. These materials could be considered as synonym materials.
Table 3. Materials with the same fingerprinting codes.
| Group No. | Material Name | Fingerprinting code |
| 1 | No.22, 12-2-2-1, 14-1-3-1 | A215B270C115D200E265F340G390H240I240J220K225L310M295N245O185P175Q195R115 |
| 2 | 28-1, 26-Yellow, 3-1-3, Wang-1-2, 03-1-3, Jingxuan Tiebaqing | A250B270C215D260E260F350G390H240I240J190K250L310M295N245O185P175Q195R115 |
| 3 | Xiangtiancui, Zhentianmei, Lvzhou Tianbao, Gagatian | A250B270C215-115D260E260F350G390H240I240J220-195K250L310M295N245O185P175Q195-190R115 |
| 4 | 9-1, Taitian1-5-5, Taitian2-1-1, Taitian2-2-1, Taitian2-2-2, Taitian2-3-1, Taitian2-3-2, Taitian2-5-1 | A250B270C140D305E265F350G390H235I240J190K250L310M295N245O185P175Q190R115 |
| 5 | Qi-2-3-4, Taitian2-5-5 | A250B270C215D330E265F350G380H240I240J190K250L310M295N245O185P175Q190R115 |
| 6 | Taitian1-3-5, Taitian1-4-3 | A215B270C140D200E260F340G390H240I240J190K250L310M295N245O185P175Q190R115 |
| 7 | Zhen Xiangtian, Gaotang Prince, Mengtianbaibao, Super Xiangmiguawang, Jiangtian, Jingxuan Disease-resistant Hefeng No.5 | A250B270C215D270E260F350-340G390H240I240J190K250L310M295N245O260-185P175Q195-190R115 |
| 8 | Xiangtian No.1, Super Tianmi | A250B270C215D325E260F350G390H240-235I240J190K250L310M295N245-235O185P175Q195R115 |
| 9 | Longbai No.1, Longtianwang | A320B270C115D270E265F390G390H240I240J195K250L310M295N245O185P175Q195R115 |
| 10 | Tiangua, Koukoutian | A320-250B270C215-115D260E260F350G390H240I240J190K250L310M295N245O185P175Q195R115 |
| 11 | Xiangpiaopiao, Jinshuai | A250-210B270C215-140D260E260F380-350G390H240I240J190K250L310M295N245O185P175Q195R115 |
| 12 | Jingmei 009, Gaochun Rainbow No.7 F1 | A320-250B270C140D260-200E260F350G390H240I240J190K250L310M295N245O185P175Q195R115 |
| 13 | Jilinnongda No.8, Northeast Guawang | A320-215B270C145D200E250F350-340G390H240I240J190K290-225L310M295N245O185P175Q195R115 |
| 14 | Xinfu No.19, Yate | A250B270C215-140D260E260F350G390H240I240J190K250L340-310M315-295N245O185P175Q195-190R115 |
| 15 | Jinmi, Jinrui | A210-180B340C185-140D270-255E205F370-360G440-430H265I250J245-220K295-225L310M345-325N260O260P180Q170R180-115 |
| 16 | Jingpin Xuemeiren, Balengcui | A320B270C140D200E265F350G395H235I240J195K250L310M295N245O185P175Q195R115 |
| 17 | Big Longtian No.1, Big Hongchengcui | A320B270C115D260E265F350G395H235I240J195K250L310M295N245O185P175Q195R115 |
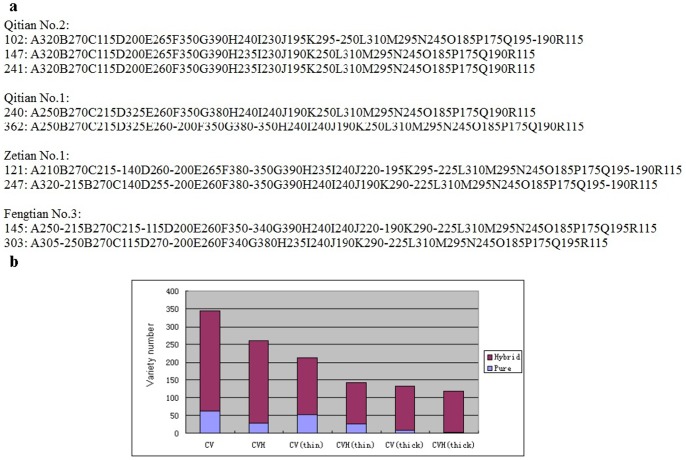
Screening with the core set of SSR primers also identified four group of homonym commercial varieties: Qitian No.1, Qitian No.2, Zetian No.1, and Fengtian No.3 (Figure 3a). These are all well-known thin rind melon commercial varieties in China. On the other hand, some homozygous melon lines were detected in commercial melon varieties (Figure 3b), where the total number of commercial melon varieties was 345, and the number of identified pure materials was 62, or 18% of the total number of varieties. Even in the commercial cultivars labeled “hybrid” or “F1”, there were still 11% that were actually pure materials. This confusion was more common in thin rind melons than in thick rind–about 25% of thin rind melon varieties were identified as pure materials, while only about 7% of thick rind melon varieties were identified as pure. In the thick rind melon cultivars labeled “hybrid” or “F1”, all were hybrid cultivars. This could indicate that the thick rind melon seed market is more mature than the thin rind melon seed market.
Figure 3. Alignment of homonym melon varieties and genetic purity detection of commercial melon varieties. a.
Alignment of 4 groups of homonym melon varieties. Qitian No.1, Qitian No.2, Zetian No.1 and Fengtian No.3 represent four commercial varieties in which homonym phenomena were found. Numbers to the left of colons are the numbers of varieties in Table S2; fingerprinting codes are listed to the right of the colons. b. Genetic purity detection results of commercial melon varieties. CV: commercial cultivar; CVH: commercial cultivar labeled “hybrid” or “F1”; CV (thin): commercial thin rind melon cultivar; CVH (thin): commercial thin rind melon cultivar labeled “hybrid” or “F1”; CV (thick): commercial thick rind melon cultivar; CVH (thick): commercial thick rind melon cultivar labeled “hybrid” or “F1”.
Discussion
Selection and Application of the Core Set of SSR Markers in Melon
In this study, 18 SSRs were selected from an initial 1219 as the core set of SSRs. The selected SSR markers have high PIC values, and are evenly distributed across the melon genome with at least one marker from each linkage group. They are easy to use in PCR assays under standard reaction conditions, and each SSR detects a single locus. The utility of this core set of SSRs was validated when used to analyze 471 melon accessions, including commercial cultivars and elite lines, and could sufficiently distinguish between cultivars and lines. Furthermore, results of the field trait survey indicated that materials with identical fingerprint codes (Table 3) based on these SSRs also shared the same or similar field characters, further validating the reliability of these SSRs. Importantly, each pair of the core set of SSR primers could establish the purity of F1 seeds (Figure 1b), and thus could be used for purity tests of hybrid seeds. This study is the first attempt to develop a core set of SSR markers to establish a melon DNA fingerprint database, and we will continue to monitor its application and validation and to improve it whenever necessary.
Chinese Melon Diversity Analysis
In this study, we constructed a UPGMA dendrogram, which indicated that Chinese marketable melons could be divided into three distinct hierarchical clusters (Figure 2). Such a classification accords well with Chinese traditional melon classification, which divides melons into two groups by rind: thin rind (red, in the figure) and thick rind (pink). Wild melon, vegetable melon, and some kinds of thin-thick rind hybrids are in an intermediate position (blue) between the two groups above.
The thin rind melons had an average amplification band number of 20.61, less than that of the thick rind melons with an average number of 22.88. Furthermore, genetic similarities among the thin rind melons were 0.79–0.99, with an average of 0.94, which were higher than that of the thick rind melons (range 0.70–0.99, average 0.85). These results showed that the two groups are genetically distinct from each other, which might be because they have been developed independently for many years. Variations within genomic regions might be due to differences in selection pressure on target genes and traits between the two groups. The large difference between the average genetic diversity of the thin rind and thick rind melons could be because the thick rind melons in this study were collected from diverse regions, including southern, northern, northeastern, and Yangtze River regions of China, while most of the thin rind melons were collected from only northern and northeastern China. In addition, the genetic base of Chinese thin rind melon is relatively narrow. Therefore, detection and utilization of genetic variations and cultivar identification, and the expansion of genetic diversity are important tasks for Chinese thin rind melon breeders.
Problems in Chinese Melon Breeding and the Seed Market
In this study, we found three main problems in the Chinese melon seed market. First, some varieties with the same parentage have different trade names. Second, there are varieties that have different parentage but carry the same name. Finally, some pure melon lines are sold as commercial varieties; there are even some commercial varieties that are labeled “hybrid” or “F1” but in fact are pure lines. Based on the screening by the core set of melon DNA fingerprinting SSR primers, 51 materials were reclassified into 17 groups and each group has the same fingerprinting codes, while these materials in each group also share the same or similar field characters. Thus these materials could be considered as synonym materials, and the majority of the synonym commercial melon cultivars are thin rind melon. On the other hand, we found 4 groups of homonym commercial varieties, which were all well-known thin rind melon commercial varieties in China: Qitian No.1, Qitian No.2, Zetian No.1 and Fengtian No.3 (See Figure 3a). These discrepancies, discovered through DNA fingerprinting, indicate that some seed producers or dealers have mislabeled produce in favor of the more popular sold, which infringes on the rights of melon breeders and seed production enterprises.
Another notable phenomenon was that not all varieties in Chinese melon seed markets were F1 generation hybrids. In a mature seed market, varieties sold should be hybrids. However, through the DNA fingerprinting, we found some pure melon lines were detected in commercial melon varieties(Figure 3b), and this phenomenon was more common in thin rind melon than in thick rind melon, indicating that the thick rind melon seed market could be more mature than thin rind melon seed market. The above results indicated that the melon market in China was still immature, especially for thin rind melon. One of the important reasons could be due to the narrow genetic resources of Chinese thin rind melon, which made the breeding relatively difficult. To solve this problem, efforts should be made to create new germplasm resources, while strengthening seed market management is also necessary.
Supporting Information
Detailed UPGMA dendrogram of 471 melon accessions based on the 18 core set of SSR markers.
(PDF)
Varieties (lines) employed in this study. Name: *materials used to screen SSRs; Type: L inbred or elite line, CV commercial cultivar, CV (H) commercial cultivar labeled with “hybrid” or “F1”; Systematics: A Cucumis melo ssp. conomon (Thunb.) Greb, B Cucumis melo ssp. melo Pang, C Cucumis melo ssp. dudaim (L.) Greb, D Cucumis melo ssp. agrestis (Naud) Greb, E Cucumis melo ssp. flexuosus (L.) Greb; Obtain way: K kept in our laboratory, B bought in the market, 1 Daqing Branches of Heilongjiang Academy of Agricultural Sciences, 2 Zhengzhou Fruit Research Institute of Chinese Academy of Agriculture Sciences, 3 Vegetable Research Institute of Shandong Academy of Agricultural Sciences, 4 Horticultural Research Institute of Guangxi Academy of Agricultural Sciences, 5 Ningbo Agricultural Scientific Research Institute, 6 Horticultural Research Institute of Anhui Academy of Agricultural Sciences, 7 Vegetable Research Institute of Gansu Academy of Agricultural Sciences, 8 Shanghai Jiading District Agro-Technology Extension Service Center, 9 Vegetable Research Institute of Jiangsu Academy of Agricultural Sciences, 10 Center of Hami Melon of Xinjiang Academy of Agricultural Sciences, 11 Horticultural Research Institute of Henan Academy of Agricultural Sciences, 12 The Institute of Vegetables and Flowers of Chinese Academy of Agricultural Sciences, 13 Institute of Germplasm Resources of Ningxia Academy of Agriculture and Forestry Sciences, O other ways.
(DOC)
The fingerprint codes of varieties (lines) employed in this study.
(DOC)
Acknowledgments
We thank Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (Northeast Region), Ministry of Agriculture, P. R. China for kindly providing experimental instruments and technical support.
We thank Daqing Branches of Heilongjiang Academy of Agricultural Sciences, Zhengzhou Fruit Research Institute of Chinese Academy of Agriculture Sciences, Vegetable Research Institute of Shandong Academy of Agricultural Sciences, Horticultural Research Institute of Guangxi Academy of Agricultural Sciences, Ningbo Agricultural Scientific Research Institute, Horticultural Research Institute of Anhui Academy of Agricultural Sciences, Vegetable Research Institute of Gansu Academy of Agricultural Sciences, Shanghai Jiading District Agro-Technology Extension Service Center, Vegetable Research Institute of Jiangsu Academy of Agricultural Sciences, Center of Hami Melon of Xinjiang Academy of Agricultural Sciences, Horticultural Research Institute of Henan Academy of Agricultural Sciences, The Institute of Vegetables and Flowers of Chinese Academy of Agricultural Sciences and Institute of Germplasm Resources of Ningxia Academy of Agriculture and Forestry Sciences for providing melon germplasm resources.
Funding Statement
This work was funded by China Agriculture Research System (CARS-26-02), Ministry of Agriculture, People's Republic of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hector G, Nunez-Palenius, Miguel Gomez-Lim, Neftali Ochoa-Alejo, Rebecca Grumet, et al. (2008) Melon Fruits: Genetic Diversity, Physiology, and Biotechnology Features. Critical Reviews in Biotechnology 28(1): 13–55. [DOI] [PubMed] [Google Scholar]
- 2. Krishna GK, Zhang J, Burow M, Pittman R, Delikostadinov S, et al. (2004) Genetic diversity analysis in Valencia peanut (Arachis hypogaea L.) using microsatellite markers. Cellular and Molecular Biology Letters 9: 685–697. [PubMed] [Google Scholar]
- 3. Naito Y, Suzuki S, Iwata Y, Kuboyama T (2008) Genetic diversity and relationship analysis of peanut germplasm using SSR markers. Breeding Science 58: 293–300. [Google Scholar]
- 4. Staub JE (2001) Inheritance of RAPD markers in melon (Cucumis melo L.). Cucurbit Genetics Cooperative Report 24: 29–32. [Google Scholar]
- 5. Garcia-Mas J, Oliver M, Gómez H, de Vicente MC (2000) Comparing AFLP, RAPD and RFLP markers to measure genetic diversity in melon. Theoretical and Applied Genetics 101: 860–864. [Google Scholar]
- 6. Deleu W, Esteras C, Roig C, González-To M, Fernández-Silva I, et al. (2009) A set of EST-SNPs for map saturation and cultivar identification in melon. BMC Plant Biology 9: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monforte AJ, Garcia-Mas J, Arús P (2003) Genetic variability in melon based on microsatellite variation. Plant Breeding 122: 153–157. [Google Scholar]
- 8. Ding C, Jin S (2009) High-throughput methods for SNP genotyping. Methods in Molecular Biology 578: 245–254. [DOI] [PubMed] [Google Scholar]
- 9. Arif IA, Bakir MA, Khan HA, Farhan AH, Homaidan AA, et al. (2010) A brief review of molecular techniques to assess plant diversity. International Journal of Molecular Sciences 11: 2079–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mujaju C, Sehic J, Werlemark G, Garkava-Gustavsson L, Fatih M, et al. (2010) Genetic diversity in watermelon (Citrullus lanatus) landraces from Zimbabwe revealed by RAPD and SSR markers. Hereditas 147(4): 142–153. [DOI] [PubMed] [Google Scholar]
- 11. Danin-Poleg Y, Reis N, Baudracco-Arnas S, Pitrat M, Staub JE, et al. (2000) Simple sequence repeats in Cucumis mapping and map merging. Genome 43(6): 963–974. [PubMed] [Google Scholar]
- 12. Matus IA, Hayes PM (2002) Genetic diversity in three groups of barley germplasm assessed by simple sequence repeats. Genome 45: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 13. Maestri E, Malcevschi A, Massari A, Marmiloni N (2002) Genomic analysis of cultivated barley (Hordeum vulgare) using sequence tags molecular markers. Estimates of divergence based on RFLP and PCR markers derived from stress-responsive genes, and simple sequence repeats (SSRs). Molecular Genetics and Genomics 267: 186–201. [DOI] [PubMed] [Google Scholar]
- 14. Baek HJ, Beharav A, Nevo E (2003) Ecological-genomic diversity of microsatellites in wild barley, Hordeum spontaneum, population in Jordan. Theoretical and Applied Genetics 106: 397–410. [DOI] [PubMed] [Google Scholar]
- 15. Achtar S, Moualla MY, Kalhout A, Röder MS, MirAli N (2010) Assessment of genetic diversity among Syrian durum (Triticum turgidum subsp. durum) and bread wheat (Triticum aestivum L.) using SSR markers. Genetika 46(11): 1500–1506. [PubMed] [Google Scholar]
- 16. Ng'uni D, Geleta M, Bryngelsson T (2011) Genetic diversity in sorghum (Sorghum bicolor (L.) Moench) accessions of Zambia as revealed by simple sequence repeats (SSR). Hereditas 148(2): 52–62. [DOI] [PubMed] [Google Scholar]
- 17. Bornet B, Goraguer F, Joly G, Branchard M (2002) Genetic diversity in european and Argentinian cultivated potatoes (Solanum tuberosum subsp. tuberosum) detected by inter-simple sequence repeats (ISSRs). Genome 45(3): 481–484. [DOI] [PubMed] [Google Scholar]
- 18. Zhang P, Li J, Li X, Liu X, Zhao X, et al. (2011) Population structure and genetic diversity in a rice core collection (Oryza sativa L.) investigated with SSR markers. PLoS One 6(12): e27565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashfaq M, Khan AS (2012) Genetic diversity in basmati rice (Oryza sativa L.) germplasm as revealed by microsatellite (SSR) markers. Genetika 48(1): 62–71. [PubMed] [Google Scholar]
- 20. Reif JC, Warburton ML, Xia XC, Hoisington DA, Crossa J, et al. (2006) Grouping of accessions of Mexican races of maize revisited with SSR markers. Theoretical and Applied Genetics 113(2): 177–185. [DOI] [PubMed] [Google Scholar]
- 21. Yu RH, Wang YL, Sun Y, Liu B (2012) Analysis of genetic distance by SSR in waxy maize. Genetics and Molecular Research 11(1): 254–260. [DOI] [PubMed] [Google Scholar]
- 22. Katzir N, Danin-Poleg T, Tzuri G, Karchi Z, Lavi U, et al. (1996) Length polymorphism and homologies of microsatellites in several Cucurbitaceae species. Theoretical and Applied Genetics 93: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 23. Stepansky A, Kovalski I, Perl-Treves R (1999) Intraspecific classification of melon (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Systematics and Evolution 217: 313–332. [Google Scholar]
- 24. Staub JE, Danin-Poleg Y, Fazio G, Horejsi T, Reis N, et al. (2000) Comparison analysis of cultivated melon groups (Cucumis melo L.) using random amplified polymorphic DNA and simple sequence repeat markers. Euphytica 115: 225–241. [Google Scholar]
- 25. Monforte AJ, Garcia-Mas J, Arus P (2003) Genetic variability in melon based on microsatellite variation. Plant Breeding 122: 153–157. [Google Scholar]
- 26. Danin-Poleg Y, Reis N, Tzuri G, Katzir N (2001) Development and characterization of microsatellite in Cucumis . Theoretical and Applied Genetics 102: 61–72. [Google Scholar]
- 27. Fazio G, Staub J E, Chung SM (2002) Development and characterization of PCR markers in cucumber. American Society for Horticultural Science 127 (4): 545–557. [Google Scholar]
- 28. Silberstein L, Kovalski I, Brotman Y, Perin C, Dogimont C, et al. (2003) Linkage map of Cucumis melo including phenotypic traits and sequence-characterized genes. Genome 46 (5): 761–773. [DOI] [PubMed] [Google Scholar]
- 29. Gonzalo MJ, Oliver M, Garcia-Mas J, Monfort A, Dolcet-Sanjuan R, et al. (2005) Simple-sequence repeat markers used in merging linkage maps of melon (Cucumis melo L.). Theoretical and Applied Genetics 110 (5): 802–811. [DOI] [PubMed] [Google Scholar]
- 30. Joobeur T, Gusmini G, Zhang X, Levi A, Xu Y, et al. (2006) Construction of a watermelon BAC library and identification of SSRs anchored to melon or Arabidopsis genomes. Theoretical and Applied Genetics 112: 1553–1562. [DOI] [PubMed] [Google Scholar]
- 31. Zalapa JE, Staub JE, McCreight JD, Chung SM, Cuevas H (2007) Detection of QTL for yield-related traits using recombinant inbred lines derived from exotic and elite US western shipping melon germplasm. Theoretical and Applied Genetics 114 (7): 1185–1201. [DOI] [PubMed] [Google Scholar]
- 32. Fernandez-Silva I, Eduardo I, Blanca J, Esteras C, Picó B, et al. (2008) Bin mapping of genomic and EST-derived SSRs in melon (Cucumis melo L.). Theoretical and Applied Genetics 118: 139–150. [DOI] [PubMed] [Google Scholar]
- 33. Luan F, Delannay I, Staub JE (2008) Melon (Cucumis melo L.) diversity analyses provide strategies for genetic improvement and evidentiary support of domestication patterns. Euphytica 164: 445–461. [Google Scholar]
- 34. Luan F, Sheng Y, Wang Y, Staub JE (2010) Performance of melon hybrids derived from parents of diverse geographic origins. Euphytica 137(1): 1–16. [Google Scholar]
- 35. Kirst M, Cordeiro GD, Rezende SP, Grattapaglia D (2005) Power of microsatellite markers for fingerprinting and parentage analysis in Eucalyptus grandis breeding populations. Journal of Heredity 96: 1–6. [DOI] [PubMed] [Google Scholar]
- 36. Nei M (1977) F-statistics and analysis of gene diversity in subdivided populations. Annals of Human Genetics 41: 225–233. [DOI] [PubMed] [Google Scholar]
- 37. Pavlicek A, Hrda S, Flegr J (1999) FreeTree-freeware program for construction of phylogenetic trees on the basis of distance data and bootstrap/jackknife analysis of the tree robustness. Application in the RAPD analysis of the genus Frenkelia . Folia Biologica (Praha) 45: 97–99. [PubMed] [Google Scholar]
- 38. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang JY, Chen YY, Huang BZ, Yu F, Wu YT (2009) Establishment of fingerprinting for bananas (Musa nana) by SSR marker. Journal of Fruit Science 26 (5): 733–738 (in Chinese). [Google Scholar]
- 40. Ma HB, Xu XM, Wei XY, Yang WX, Zou WG (2010) DNA fingerpringing and genetic analysis based on SSR markers for rice cultivars in Fujian. Fujian Journal of Agricultural Science 25(1): 33–38 (in Chinese). [Google Scholar]
- 41. Zhu Z, Gao M, Gao P, Luan F (2011) QTL Analysis of the First Fertile Flower Node of Cucumis melo L. Acta Horticulturae Sinica. 38(9): 1753–1760. [Google Scholar]
- 42. Gao M, Zhu Z, Gao P, Luan F (2011) A Microsatellite-based Genetic Map of Melon and Localization of Gene for Gynoecious Sex Expression Using Recombinant Inbred Lines. Acta Horticulturae Sinica 38(7): 1308–1316. [Google Scholar]
- 43. Diaz A, Fergany M, Ziarsolo P, Blanca J, Fei Z, et al. (2011) A consensus linkage map for molecular markers and Quantitative Trait Loci associated with economically important traits in melon (Cucumis melo L.). BMC Plant Biology 11: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garcia-Mas J, Benjak A, Sanseverino W, Bourgeois M, Mir G, et al. (2012) The genome of melon (Cucumis melo L.). Proceedings of the National Academy of Sciences USA 109(29): 11872–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed UPGMA dendrogram of 471 melon accessions based on the 18 core set of SSR markers.
(PDF)
Varieties (lines) employed in this study. Name: *materials used to screen SSRs; Type: L inbred or elite line, CV commercial cultivar, CV (H) commercial cultivar labeled with “hybrid” or “F1”; Systematics: A Cucumis melo ssp. conomon (Thunb.) Greb, B Cucumis melo ssp. melo Pang, C Cucumis melo ssp. dudaim (L.) Greb, D Cucumis melo ssp. agrestis (Naud) Greb, E Cucumis melo ssp. flexuosus (L.) Greb; Obtain way: K kept in our laboratory, B bought in the market, 1 Daqing Branches of Heilongjiang Academy of Agricultural Sciences, 2 Zhengzhou Fruit Research Institute of Chinese Academy of Agriculture Sciences, 3 Vegetable Research Institute of Shandong Academy of Agricultural Sciences, 4 Horticultural Research Institute of Guangxi Academy of Agricultural Sciences, 5 Ningbo Agricultural Scientific Research Institute, 6 Horticultural Research Institute of Anhui Academy of Agricultural Sciences, 7 Vegetable Research Institute of Gansu Academy of Agricultural Sciences, 8 Shanghai Jiading District Agro-Technology Extension Service Center, 9 Vegetable Research Institute of Jiangsu Academy of Agricultural Sciences, 10 Center of Hami Melon of Xinjiang Academy of Agricultural Sciences, 11 Horticultural Research Institute of Henan Academy of Agricultural Sciences, 12 The Institute of Vegetables and Flowers of Chinese Academy of Agricultural Sciences, 13 Institute of Germplasm Resources of Ningxia Academy of Agriculture and Forestry Sciences, O other ways.
(DOC)
The fingerprint codes of varieties (lines) employed in this study.
(DOC)