Abstract
Delta-like 4 (Dll4) is a ligand of the Notch pathway family which has been widely studied in the context of tumor angiogenesis, its blockade shown to result in non-productive angiogenesis and halted tumor growth. As Dll4 inhibitors enter the clinic, there is an emerging need to understand their side effects, namely the systemic consequences of Dll4:Notch blockade in tissues other than tumors. The present study focused on the effects of systemic anti-Dll4 targeting in the bone marrow (BM) microenvironment. Here we show that Dll4 blockade with monoclonal antibodies perturbs the BM vascular niche of sub-lethally irradiated mice, resulting in increased CD31+, VE-Cadherin+ and c-kit+ vessel density, and also increased megakaryocytes, whereas CD105+, VEGFR3+, SMA+ and lectin+ vessel density remained unaltered. We investigated also the expression of angiocrine genes upon Dll4 treatment in vivo, and demonstrate that IGFbp2, IGFbp3, Angpt2, Dll4, DHH and VEGF-A are upregulated, while FGF1 and CSF2 are reduced. In vitro treatment of endothelial cells with anti-Dll4 reduced Akt phosphorylation while maintaining similar levels of Erk 1/2 phosphorylation. Besides its effects in the BM vascular niche, anti-Dll4 treatment perturbed hematopoiesis, as evidenced by increased myeloid (CD11b+), decreased B (B220+) and T (CD3+) lymphoid BM content of treated mice, with a corresponding increase in myeloid circulating cells. Moreover, anti-Dll4 treatment also increased the number of CFU-M and -G colonies in methylcellulose assays, independently of Notch1. Finally, anti-Dll4 treatment of donor BM improved the hematopoietic recovery of lethally irradiated recipients in a transplant setting. Together, our data reveals the hematopoietic (BM) effects of systemic anti-Dll4 treatment result from qualitative vascular changes and also direct hematopoietic cell modulation, which may be favorable in a transplant setting.
Introduction
Hematopoiesis is the process by which new blood cells are generated and occurs mainly in the adult bone marrow (BM). The importance of the BM microenvironment in regulating hematopoiesis has been amply demonstrated by studying the so-called “stem cell niches”, in which the endosteal and vascular niches were shown to support hematopoietic stem cells (HSCs) self-renewal, proliferation, and differentiation [1]–[4]. However, recent findings have proven this interpretation of the BM stem cell niches may to be too simplistic [5], [6]. Interestingly, the vascular niche is not only critical for HSC maintenance[7]–[9] and differentiation [10], but also for hematopoietic reconstitution and recovery [11]–[15]. Mechanistically, the BM endothelial cells were shown to express different “angiocrine genes”, whose production is dependent on the activation of Akt or MAP kinase signaling pathways [29], and whose function is to restore hematopoiesis following insults such as irradiation. Therefore, targeting the BM vascular niche and angiocrine genes production to modulate hematopoietic recovery and function may be of clinical relevance. We found Delta-like 4 (Dll4, a ligand of the Notch signaling pathway expressed by BM endothelial cells) targeting to potentially fulfill this aim.
Blockade of Dll4-mediated Notch signaling has been described as a modulator of tumor angiogenesis. Indeed, its inhibition, by promoting non-productive angiogenesis, was shown to be an effective treatment strategy in pre-clinical solid tumor models [16]–[19], and is already being tested in clinical trials [20], [21].
We have explored the effects of Dll4 blockade in the BM vascular niche using two strategies, first by using different endothelial cell markers, to assess qualitative changes in BM vasculature, and secondly by exploring the modulation of “angiocrine genes” in vivo and EC-specific activation of signaling pathways in vitro. To characterize the phenotypic response of the BM vascular niche to anti-Dll4 antibody treatment, we used different EC markers (CD31, CD105, VE-Cadherin, vascular endothelial growth factor receptor 3 (VEGFR3) and Lycopersicon esculentum lectin [22]–[24]), SMA (smooth muscle actin, a pericyte marker) [25], and by counting megakaryocyte numbers (which are part of the BM vascular niche, and are CD41+ [26]–[28]). Additionally, we assessed the effect of Dll4 blockade in modulating the expression of angiocrine genes [29] and activation of signaling pathways on BM endothelial cells in vitro.
We also determined how Dll4 systemic blockade interfered with hematopoiesis by directly affecting hematopoietic cells. Dll4 has been shown to be involved in HSCs self-renewal and proliferation [30]–[32], megakaryocytic differentiation [33], [34] and lymphoid modulation [33], [35]–[37]. However, the hematopoietic effects of Dll4 blockade, namely in the setting of perturbed BM function, had not been previously shown.
We performed in vivo phenotypic characterization of the main BM hematopoietic lineages following anti-Dll4 treatment, in vitro functional assays to identify hematopoietic cell-specific modulation of anti-Dll4, and an in vivo BM transplant (BMT) following lethal irradiation. For the in vivo characterization of the main BM hematopoietic lineages we quantified myeloid (CD11b+) and lymphoid (B, B220+ and T, CD3+) BM content [38]–[41]. Additionally, we measured hematopoietic stem/progenitor cells (HSPCs; stem cell antigen (Sca)-1+ and fetal liver kinase (Flk)-1−) [42], [43] and endothelial progenitor cells (EPC; Sca1+Flk1+ [44]–[46], in BM and peripheral blood (PB). The effects of anti-Dll4 treatment in HSPCs commitment and differentiation was assessed in vitro by performing colony-forming units (CFU) assays in methylcellulose [47], [48].
We show that systemic Dll4 blockade affects the BM vascular niche and hematopoietic cell differentiation, while having limited effects on the expression of “angiocrine genes” or on EC activation. Interestingly, in a BMT setting, anti-Dll4 treatment of donor mice results in faster lymphoid and erythroid recovery of recipient mice.
Together, we show that anti-Dll4 treatment perturbs BM recovery following irradiation, which can be clinically relevant in a BMT setting.
Methods
Animals and Experimental Design
The following animal experiments were performed following approval of the Instituto Gulbenkian de Ciência Animal Care Committee and Review Board.
Balb/c mice (6–8 weeks old) were sub-lethally irradiated (300rad), and subjected to treatment with neutralizing anti-mouse Dll4 antibody (HMD4-2) [19], [49], [50], 12.5 g/kg, intraperitoneally (IP), every 2 days or every 3 days, for 15 days, starting 1 day after irradiation. In parallel, control mice were injected with phosphate-buffered saline (PBS). All experiments refer to 15–20 days counting from the day of irradiation. Each irradiated group consisted of 3 control and 3 anti-Dll4 treated animals, and the experiments were performed 3 times.
The Dll4 knockout mice experiments were performed with the approval of the Faculty of Veterinary Medicine of Lisbon Ethics and Animal Welfare Committee. Dll4 conditional knockout mice (Dll4lox/lox) were generated as follows: conditional KO Dll4 vector with 2 loxP sequences flanking the 3 first gene exons was inserted in EE cells by electroporation. The neomycin resistant clones were selected, injected in blastocysts and transferred to pseudo-pregnant females. The offspring were crossed with h-ActB-flp mice to remove neoR, and the resulting littermates were crossed to obtain Flp−/−. These mice were then crossed with VECadCreERT2 mice, a gift from Dr. Ralph Adams, to produce a tamoxifen-inducible endothelial-specific Dll4 loss-of-function line (VECadCreERT2Dll4lox/lox). Tamoxifen induction was performed for 5 days, 50mg/kg/day. All experiments refer to 31–34 days counting from the first day of induction. Each group consisted of 12 Dll4lox/lox and 11 VECadCreERT2Dll4lox/lox animals.
Sample Collection
Peripheral blood was collected from the heart in EDTA-coated tubes (Multivette 600, Sarstedt, Nümbrecht, Germany) and centrifuged at 1200 rpm for 5 minutes.
BM was flushed from the long bones with PBS 0.5% BSA and centrifuged at 800 rpm for 15 minutes. PB and BM cells were collected for FACS analysis.
Femur BM was flushed with PBS and immediately centrifuged at 800 rpm for 15 minutes. Plasma was then collected for enzyme-linked immunosorbent assay (ELISA) analysis.
Bone Marrow Transplants
Balb/c mice (6 weeks old) were lethally irradiated (800rad), and subjected to BMT 24 hours later. Cells for BMT were collected from the femur of previously treated or control animals (two recipients per donor animal), on day 15 of treatment. Viable nucleated cells were counted in a Countless Automated Cell Counter (Invitrogen, Carlsbad, CA). 2.5×106 total BM cells were injected intravenously. BM for BMT was collected from 3 control and 3 anti-Dll4 treated animals. Recipient animals were treated with enrofloxacin 10 mg/kg every day for 7 days post-irradiation.
Complete blood counts (CBC) of tail vein PB was performed at weeks 1 and 2 post-transplantation.
Cell Culture
Human umbilical cord vein ECs (HUVECs) (Clonetics, Lonza, Switzerland) were cultured in EBM-2 supplemented with EGM-2 SingleQuots, 2 mg/mL BBE (Lonza, Walkersville, MD) and 10% heat-inactivated fetal bovine serum (FBS) (Gibco Invitrogen, Carlsbad, CA).
Murine bone marrow-derived stromal cell line S17 was cultured in complete medium – Roswell Park Memorial Institute (RPMI) 1640 medium, 2 mM L-Glutamine, antibiotic-antimycotic (all from Gibco Invitrogen, Carlsbad, CA) and 50 µM β-mercaptoethanol (Sigma-Aldrich, Germany) – plus 10% FBS.
In vitro Colony Forming Assays
BM mononuclear Lin−Sca1+ cells (104), collected from anti-Dll4 treated and control animals and sorted in FacsAria (Becton Dickinson, Franklin Lakes, NJ), were plated onto cytokine-supplemented methylcellulose medium (MethoCult GF M3434, Stem Cell Technologies, Vancouver, BC, Canada). Resulting colonies are single-cell derived and represent the original cell’s identity [47], [48]. Colonies were scored after 1 and 2 weeks of culture, according to the manufacturer’s instructions.
Human cord blood mononuclear cells were lineage depleted using lineage cell depletion kit, as shown in Table S1. 104 Lin− cells were plated onto cytokine-supplemented methylcellulose medium (MethoCult GF H4434, Stem Cell Technologies, Vancouver, BC, Canada). Treatment with neutralizing anti-human Dll4 antibody (MHD4-46) [51], [52], 50 µg/mL, and/or anti-human Notch1 antibody (MHN1-128) [53], 10 µg/mL, started the day after the establishment of the culture and was performed every 2 days. Colonies were scored after 1 week of culture, according to the manufacturer’s instructions.
Flow Cytometry
BM and PB mononuclear cells were stained for T, B, myeloid and progenitor cell markers, using the antibodies indicated on Table S1, 1 h at 4°C. BM cells were stained for megakaryocytes, following the same protocol. Flow cytometry was performed on FACSCalibur and analyzed with Cell Quest Software (Becton Dickinson, Franklin Lakes, NJ).
Histological and Immunohistochemical Analysis
Livers were formalin-fixed and processed for routine histopathology and immunohistochemistry. Bones were formalin-fixed, EDTA-decalcified and processed for routine histopathology. Immunohistochemistry for the antigens indicated on Table S1 was performed in the humerus, on 3 µm slices, at 3 distinct levels for each bone/mouse (40 µm distance). Sections were incubated with primary antibody at room temperature for 1 h, immunostaining proceeded according to the visualization system manufacturer’s instructions and counterstained with Mayer’s hematoxylin.
Immunofluorescence for the antigens indicated on Table S1 was performed in the humerus, on 3 µm slices. Primary antibodies were incubated at room temperature for 1 hour, secondary antibodies were incubated at room temperature for 2 hours. Slides were mounted with Vectashield mounting medium with DAPI (VectorLaboratories, Burlingame, CA).
Vascular Perfusion
Fluorescein isothiocyanate (FITC) Lycopersicon esculentum lectin (VectorLaboratories, Burlingame, CA) was injected in the tail vein (100 µg, from a 500 µg/mL solution). Mice were euthanized 5 minutes later, and perfused with 4% paraformaldehyde (PFA) in PBS. Femur BM was then flushed off and further fixed in 4% PFA overnight, dehydrated in a sucrose gradient for one day, and cryopreserved in Tissue-Tek Optimum Cutting Temperature (Sakura, Torrance, CA).
Cryosections (15 µm) were stained with ToPro-3 (Table S1) plus 100 µg/mL ribonuclease A (Sigma-Aldrich, Germany) at 4°C overnight, to visualize nuclei, and mounted in Mowiol 4–88 (pH 8.5 in Tris-HCl and glycerol; Calbiochem Merk Millipore, Darmstadt, Germany).
Western Blotting
Third passage HUVEC at 70% confluence were cultured in EBM-2 plus 1% FBS for 17 hours, left untreated or treated with neutralizing anti-human Dll4 antibody (MHD4-46) [51], [52], 50 µg/mL, or PBS, for 2 hours. Cells were then lysed with RIPA buffer (20 mM Tris pH 7.5, 150 mM NaCl, 5 mM KCl, 5 mM MgCl, 1% Triton X-100, protease inhibitor cocktail and 1 mM sodium orthovanadate), and equal amounts of proteins were subjected to SDS–polyacrylamide gel electrophoresis with 12% Mini-Protean TGX precast gel (BioRad, US). Proteins were transferred onto nitrocellulose membrane (Hybond-C Extra, GE Healthcare Life Sciences, Roosendaal, Netherlands) and subjected to standard immunoblotting with the antibodies indicated on Table S1.
Reverse Transcriptase PCR (RT–PCR)
For in vivo assessments, total BM from control or anti-Dll4 treated mice was flushed off in PBS, centrifuged 1200 rpm 5 min, and collected to TRIzol Reagent (Invitrogen, Carlsbad, CA). For in vitro assessments, third passage HUVEC at 70% confluence were starved with EBM-2 plus 1% FBS overnight, and treated with neutralizing anti-human Dll4 antibody (MHD4-46) [51], [52], 50 µg/mL, or PBS, for 16 hours, then collected to TRIzol Reagent (Invitrogen, Carlsbad, CA).
RNA was extracted according to the manufacturer’s instructions. cDNA was produced with SuperScript II (Invitrogen, Carlsbad, CA) by using random-sequence hexamer primers (Roche Applied Science, Indianapolis, IN). Real-time PCR was performed with Power SYBR Green PCR Master Mix in 7900HT Fast Real-Time PCR System (both from Applied Biosystems, Foster City, CA). Amplification of 18S rRNA, hypoxanthine guanine phosphoribosyl transferase (HPRT) and β2-microglobulin (β2MG) were used for sample normalization; data were analyzed using all these endogenous controls and plotted using HPRT only. Primer sequences are as described on Table S2.
RT-PCR data were analyzed by DataAssist software (Applied Biosystems Foster City, CA) using 18S, β2MG and HPRT as endogenous controls, and plotted using HPRT as endogenous control.
Statistical Analysis
Results are expressed as mean ± standard error. Data were analyzed using unpaired two-tailed student's t test. P values of <0.05 were considered statistically significant.
Results
Systemic Anti-Dll4 Treatment Interferes with the BM Vascular Niche
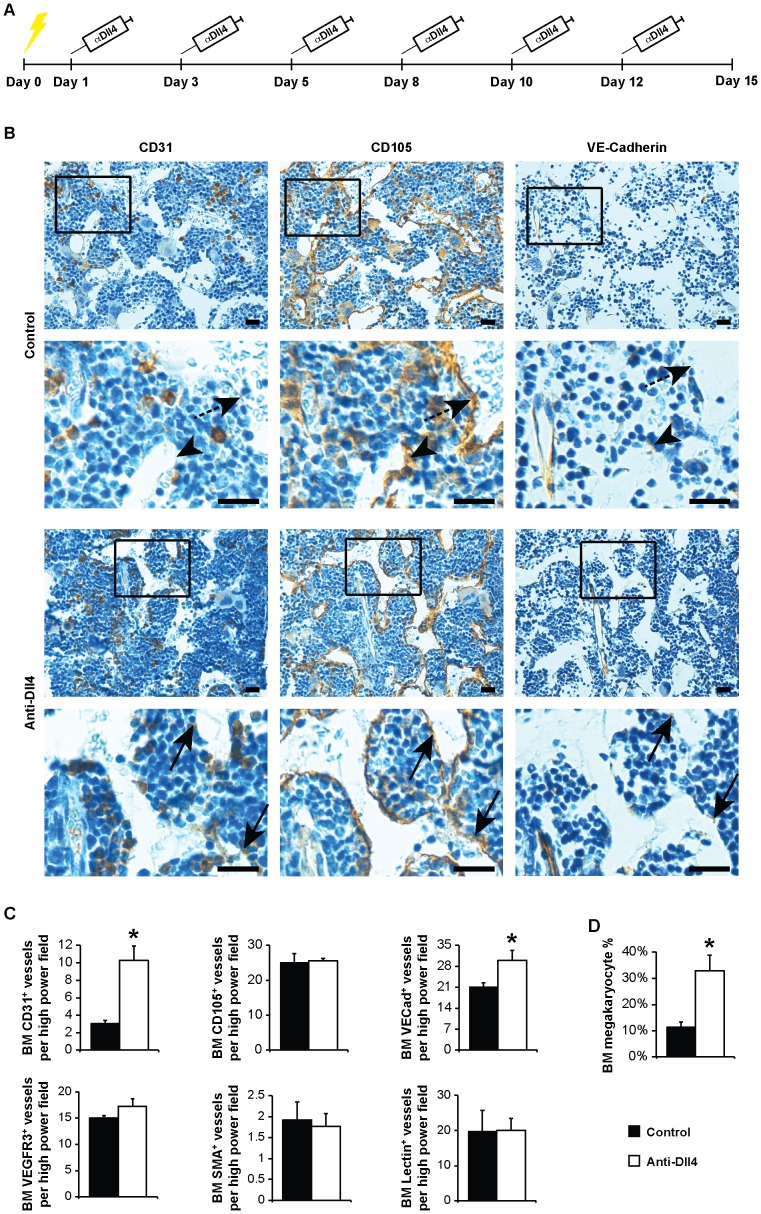
We asked whether a therapeutic (systemic) approach of Dll4 blockade would affect the BM vascular niche. For that, we sub-lethally irradiated mice (300rad), therefore inducing myeloablation and BM turnover, and systemically treated them with a neutralizing anti-Dll4 antibody, HMD4-2 (Figure 1A).
Figure 1. Therapeutic anti-Dll4 blockade interferes with the BM vascular niche.
(A) Schematic representation of the clinical assessment of anti-Dll4 treatment. Yellow lightening bolt, sub-lethal irradiation. (B) Immunohistochemistry for CD31, CD105 and VE-Cadherin counterstained with Mayer’s haemalum (Leica DMD 108). Sequential sections represent the same blood vessels. Arrowhead, CD31-CD105+Ve-Cadherin+ blood vessel; dashed arrow, CD31−CD105+VE-Cadherin− blood vessel; arrow, CD31+CD105+VE-Cadherin+ blood vessel. Bar = 20 µm. (C) CD31, CD105, VE-Cadherin, VEGFR3, SMA and Lectin-positive vessel count, per high power field (400x, Leica DMD 108), reveal an increase of CD31 and VE-Cadherin-positive BM vessels in anti-Dll4 treated mice. (D) Flow cytometric analysis of the percentage of megakaryocytes (CD41+ cells) in the BM shows an increase of BM megakaryocyte cell percentage in anti-Dll4 treated mice. Data are means ± s.e.m. *, p<0.05; data represents one of three experiments in which n = 3.
We used six different vascular markers to characterize the effects of anti-Dll4 in the BM vascular niche: CD31, CD105 and VE-Cadherin antibodies, widely used to identify BM endothelial cells [9], [13]; VEGFR3 antibody, described as a specific marker of BM sinusoids [13]; SMA antibody, which labels pericytes in arteries and capillaries [22]-[24]; and Lycopersicon esculentum lectin, used as a pan-endothelial marker that stains perfused vessels [18], [54].
By day 15 post-irradiation, increased number of CD31+ and VE-Cadherin+ vessels were scored in the BM of anti-Dll4 treated mice, with no significant changes in CD105+, VEGFR3+, SMA+, and lectin+ vessels (Figure 1B, C, S1A). VE-Cadherin mRNA expression was also increased in vivo and in vitro following anti-Dll4 treatment (Figure S1B, C).
Furthermore, anti-Dll4 treatment following myeloablation also increased BM megakaryocyte content (Figure 1D). BM VE-Cadherin (endothelial) expression had been previously associated with an increase in megakaryocyte numbers [55]. Moreover, it has been reported that Dll4 impairs the final stages of megakaryocytic differentiation, without affecting its early stages, also concordant with our data [34]. Therefore, the increase in megakaryocyte numbers herein described might be due to the increase in VE-Cadherin expression, to a direct effect of anti-Dll4 treatment on megakaryocytes, or both.
These results were surprising, as previous work has shown quantitative vascular changes in tumors upon anti-Dll4 treatment [17], [18]. However, in our study, we observed a qualitative modulation of the BM vascular niche (as suggested by the use of the different vascular markers). Therefore, we further characterized the type of blood vessels in the BM microenvironment, following anti-Dll4 treatment. As previously described, we found VEGFR3 to be a specific sinusoidal marker [13], lectin to stain all types of blood vessels in the BM [54] (Figure S1A), and SMA to stain for pericyte-covered (stable) blood vessels, such as arteries and capillaries [23], [24] (Figure S1A, S2a,c). CD31, CD105 and VE-Cadherin have been extensively used as BM endothelial cells markers [5], [6], [9], [13], but the CD31 and VE-Cadherin specific modulation led us to further characterize these vessels. As shown in Figure S2, BM stable vessels are CD105high/low, VE-Cadherinhigh and CD31+, whereas BM sinusoids are CD105+, VE-Cadherin+/−, and CD31+/− in sub-lethally irradiated mice.
Next, we asked whether these BM vascular niche-specific changes were a direct effect of Dll4 blockade on the endothelial cells. For that, we used inducible, conditional knockout (VECad-Cre-ERT2Dll4lox/lox) mice and assessed the number of CD31, CD105 and VE-Cadherin vessels, as well as the percentage of megakaryocytes in the BM. Consistent with the effects reported earlier (seen after systemic anti-Dll4 treatment), we observed a similar phenotype in this genetic targeting of Dll4, with an increase in CD31+ and VE-Cadherin+ vessels without modulation of CD105+ vessels, and an increase in the percentage of CD41+ megakaryocytes (Figure S3). These data suggest the effects of anti-Dll4 blockade in the BM vascular niche are exerted predominantly on VE-Cadherin-expressing BM endothelial cells.
The BM vascular modifications herein described were accompanied by systemic defects in the vascular compartment of the liver (Figure S4), as previously reported by others [56].
Together, these data suggest systemic Dll4 blockade perturbs the BM vascular niche, favoring CD31+ and VE-Cadherin+ endothelial cells expansion and increasing BM megakaryocyte content.
Specific Effects of Anti-Dll4 Treatment on Endothelial Cells
Next, we investigated the mechanisms by which anti-Dll4 could affect endothelial cells function.
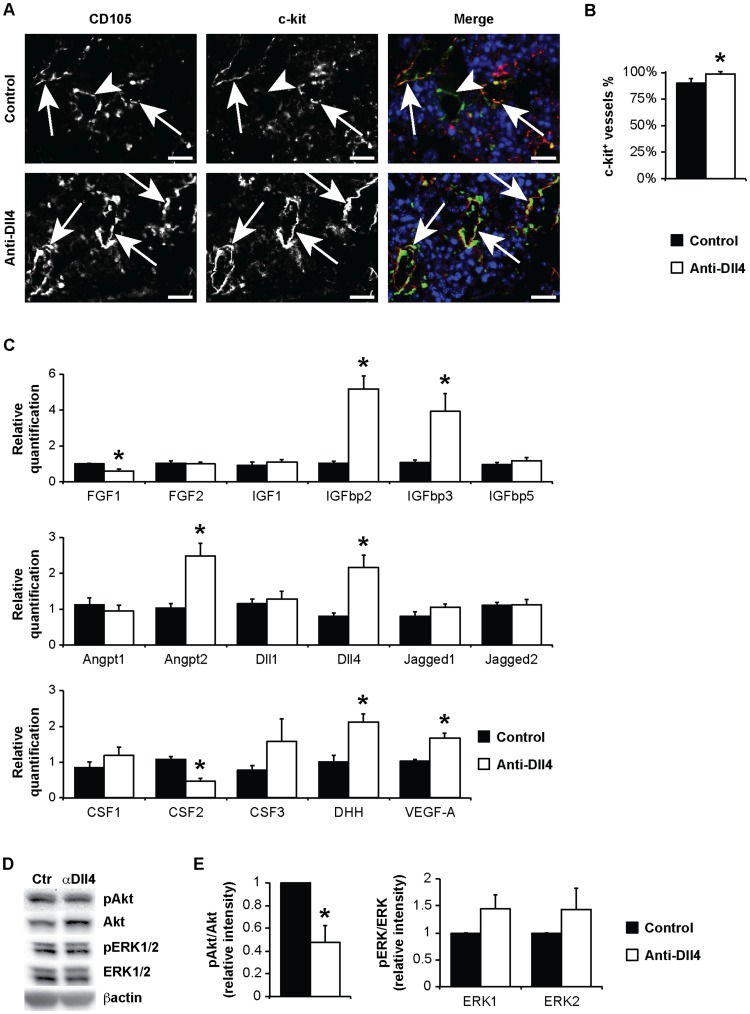
First, we characterized the BM endothelial phenotype induced by systemic anti-Dll4 blockade in more detail. We used a stem cell marker, c-kit, and found some BM vessels to be c-kit+ (Figure 2A). C-kit is unappreciated as a BM vessel marker, despite in vitro reports of c-kit expression in primary BM endothelial cells [57]. Some BM vessels were previously shown to express another stem cell marker, stem cell antigen-1 (Sca-1) [13], but its endothelial functions are still unknown. The overall percentage of c-kit+ vessels (assessed from double labeling with CD105) also increased in anti-Dll4 treated animals (Figure 2A, B).
Figure 2. Endothelial-specific effects of anti-Dll4 treatment.
(A) Immunofluorescence for CD105 and c-kit (Zeiss AxioImager.Z1). Arrowhead, CD105+c-kit− blood vessel; arrow, CD105+c-kit+ blood vessel. Bar = 20 µm. (B) c-kit+(CD105+) vessel percentage, per high power field (200x, Zeiss AxioImager.Z1), reveal an increase of c-kit+ BM vessel percentage in anti-Dll4 treated mice. (C) Angiocrine gene modulation was accessed by relative quantification of mRNA from total BM, revealing a decrease in FGF1 and CSF2 and an increase of IGFbp2, IGFbp3, Angpt2, Dll4, DHH and VEGF-A expression in anti-Dll4 treated mice. (D) HUVEC phosphorylation of Akt and ERK1/2, analysed by Western blotting. (E) Quantification of phosphorylation of Akt and ERK1/2 relative band intensity reveals a significant increase of Akt phosphorylation. Data are means ± s.e.m. *, p<0.05; n = 3.
Next, we searched for modulation of “angiocrine genes” and of MAPK and Akt signaling pathways in our system, since these were considered crucial for the instructive role exerted by the BM vascular niche in promoting hematopoietic recovery [29]. We performed qPCR analysis on a set of angiocrine genes, chosen because these are expressed depending on the activation state of BM endothelial cells [29] and because of their involvement in hematopoietic recovery and vascular remodeling (Figure 2C, S5).
Anti-Dll4 treated animals showed a significant decrease in BM expression of fibroblast growth factor 1 (FGF1) and colony stimulating factor 2 (granulocyte-macrophage, CSF2) and an increase in insulin-like growth factor binding protein 2 (IGFbp2), IGFbp3, angiopoietin 2 (Angpt2), Dll4, desert hedgehog (DHH) and vascular endothelial growth factor A (VEGF-A) (Figure 2C, S5A).
This increase in VEGF-A (but not SDF-1α or stem cell factor, SCF) mRNA levels was accompanied by increased VEGF-A protein levels in BM plasma, assessed by ELISA (Figure S5B).
In order to identify endothelial-specific angiocrine gene modulation, we treated HUVEC in vitro with anti-Dll4 antibody. Anti-Dll4 treatment resulted in a significant decrease in FGF1, CSF3, but not CSF2, and an increase in VEGF-A expression (Figure S5C). Genes whose expression was not changed in vivo were modulated in vitro, namely FGF2, CSF3, interleukin 6 (IL-6) and SCF (Figure S5C). Dll4 expression, however, was decreased in vitro, and increased in vivo (Figure S5C). The latter phenotypes can be interpreted as a non-endothelial cell-specific angiocrine gene modulation; another possibility is that the timing, activation state or endothelial cell identity of this in vitro assessment does not mimic BM endothelial cell characteristics.
After characterizing angiocrine gene modulation, we searched for alterations of Akt and ERK1/2 signaling pathways induced by anti-Dll4 treatment. In light of the theory supported by Kobayashi et al., the fine-tuning between Akt and MAPK activation in BM endothelial cells balances self-renewal vs. differentiation of HSPCs. We found that treatment of HUVEC with anti-Dll4 decreased Akt phosphorylation, but did not induce significant changes in ERK1/2 activation (Figure 2D, E), which supports the notion that reduced Akt and equal MAPK promotes the maintenance of the HSPCs pool [29].
These data suggest that modulating the BM vascular niche by anti-Dll4 treatment increases c-kit+ vessels and affects BM endothelial cells activation state and angiocrine factors production.
Anti-Dll4 Treatment Perturbs Hematopoietic Recovery Following Irradiation
Having shown systemic anti-Dll4 treatment affected BM endothelial cells in vivo and in vitro, including angiocrine gene modulation, next we explored the hematopoietic effects of anti-Dll4 treatment in BM hematopoietic recovery following myeloablation.
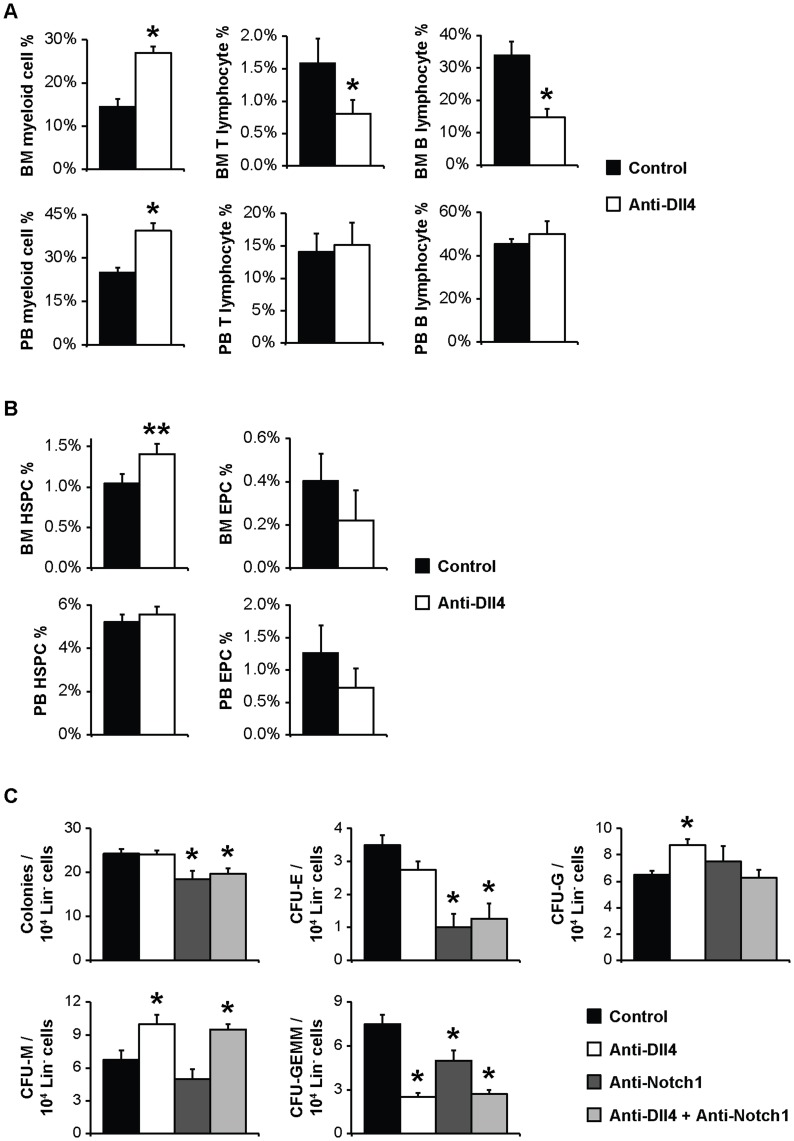
Both BM and PB from anti-Dll4 treated mice showed increased myeloid cell content (CD11b+) (Figure 3A). The BM lymphocytic compartment was also affected by the anti-Dll4 treatment; there was a significant decrease in both CD3+ T and B220+ B lymphocytes, with no significant changes in the PB (Figure 3A).
Figure 3. Anti-Dll4 treatment perturbs hematopoiesis following irradiation.
(A) Flow cytometric analysis of the percentage of myeloid (CD11b+) cells, T lymphocytes (CD3+ cells), and B lymphocytes (B220+) in the BM and PB, revealing an increase in both myeloid BM and PB content and a decrease in T and B lymphocyte BM content in anti-Dll4 treated mice. (B) Flow cytometric analysis of the percentage of stem/progenitor cells, namely HSPCs (Sca1+Flk1−) and EPCs (Sca1+Flk1+), revealing that anti-Dll4 treatment does not significantly affect these populations, notwithstanding the trend (p = 0.07) towards and increase of BM HSPCs. Data are means ± s.e.m. *, p<0.05, **, p = 0.07; data represents one of three experiments in which n = 3. (C) Colony counts from methylcellulose culture of Lin- cord blood-derived cells reveal anti-Dll4 treatment in vitro induces an increased HSPCs potential to differentiate to the myeloid lineage (CFU-G and CFU-M), an effect independent upon anti-Notch1 treatment. Anti-Notch1 treatment, independent of combined anti-Dll4 treatment, induces a decrease in HSPCs potential to differentiate to the erythrocytic lineage (CFU-E), and decreased HSPCs differentiation potential (total colony number). All treatments reduced multipotent HSPCs (CFU-GEMM). Data are means ± s.e.m. *, p<0.05; n = 4.
In contrast, anti-Dll4 treatment did not seem to affect BM progenitor cell populations. As shown in Figure 3B, there were no significant changes in the percentage of BM or PB EPCs (Sca1+Flk1+) or HSPCs (Sca1+Flk1−), with a trend for increased BM HSPCs (p = 0.07) in anti-Dll4 treated mice.
After characterizing the global alterations in hematopoiesis upon anti-Dll4 treatment, we performed in vitro CFU assays, counting single-cell derived colonies, which represent either multipotent (CFU-granulocyte-erythrocyte-macrophage-megakaryocyte, CFU-GEMM), bipotent (CFU-granulocyte-macrophage) or unipotent (CFU-monocyte, CGU-M, CFU-granulocyte, CFU-G, or CFU-erythrocyte, CFU-E) [47], [48]. These assays allowed us to evaluate if the hematopoietic effects seen with anti-Dll4 treatment could also be due to direct effects on hematopoietic elements, namely in their differentiation capacity.
For that, we sorted BM HSPCs (Lin-Sca1+) from anti-Dll4 treated and control mice and cultured these in methylcellulose in vitro [47], [48]. In accordance with the lack of change in HSPCs frequency seen after anti-Dll4 treatment (Figure 3B), this did not affect HSPCs CFU potential, or colony number (Figure S6).
Next, we also assessed the direct effects of anti-Dll4 treatment on HSPCs, by treating naïve HSPCs with anti-Dll4 in vitro, in CFU assays. We further sought to determine whether Notch1 was the receptor involved, by blocking Notch1 using a monoclonal antibody either alone or in conjugation with anti-Dll4. We induced cord blood HSPCs’ (Lin-) differentiation in methylcellulose in the presence of either PBS, anti-Dll4, anti-Notch1, or the 2 neutralizing antibodies together. As shown in Figure 3C, anti-Dll4 treatment shifted differentiation towards the myeloid lineage (increased CFU-M and CFU-G colonies), an effect independent of anti-Notch1 treatment, as anti-Notch1 did not affect CFU-M or CFU-G colony number. Anti-Dll4 treatment reduced multipotent HSPCs (CFU-GEMM colonies), as did anti-Notch1 and the conjugation of both antibodies, indicating that anti-Dll4 treatment reduced multipotent HSPCs by reducing Notch1-mediated Notch signalling. Anti-Notch1, alone or combined with anti-Dll4, decreased HSPCs potential to differentiate into the erythroid lineage (CFU-E), and decreased HSPCs differentiation potential (total colony number). Both treatments reduced multipotent HSPCs (CFU-GEMM) (Figure 3C).
Taken together, these data suggest that besides affecting the BM vascular niche, anti-Dll4 treatment also perturbs hematopoietic cell differentiation and commitment.
Anti-Dll4 Treatment of Donor BM Improves Hematopoietic Recovery Following Transplantation into Lethally Irradiated Recipients
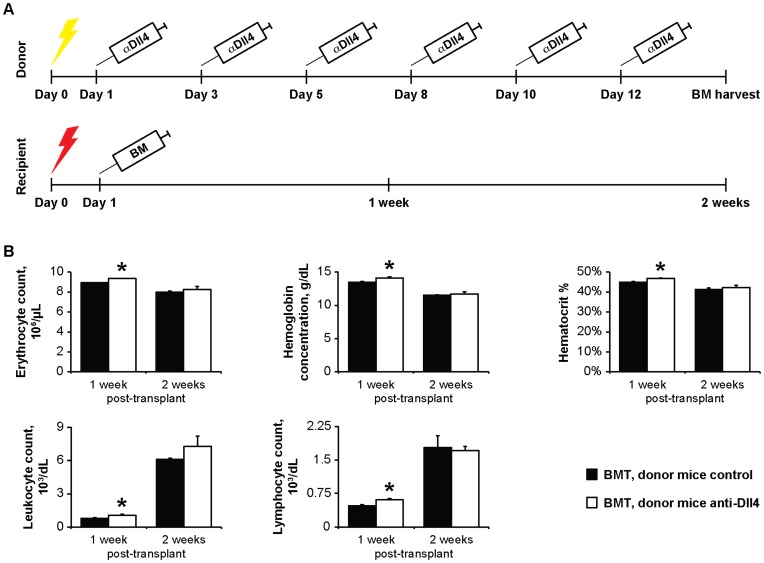
Next, we assessed whether the BM changes induced by anti-Dll4 treatment affected the efficiency of BM hematopoietic recovery in a transplant setting. For this purpose, we lethally irradiated recipient mice, which were subsequently transplanted with BM from untreated or anti-Dll4 treated mice (Figure 4A). Mice that received BM from anti-Dll4 treated mice showed evidence of improved hematopoietic recovery following lethal myeloablation (significantly faster recovery of leukocytes, hematocrit and lymphocytes), assessed by CBC (Figure 4B).
Figure 4. Anti-Dll4 treatment of donor BM improves hematopoietic recovery following transplantation into lethally irradiated recipients.
(A) Schematic representation of the BMT. Yellow lightening bolt, sub-lethal irradiation; red lightening bolt, lethal irradiation. (B) Erythrocyte, hemoglobin, hematocrit, leukocyte and lymphocyte quantifications were assessed by PB cell blood counts. Data shows donor anti-Dll4 treated mice induces faster recovery of different hematological parameters day 1 week after transplantation. Data are means ± s.e.m. *, p<0.05; n = 3.
These data suggest that treatment of BM donor mice with anti-Dll4 improves hematopoietic recovery following lethal myeloablation.
Discussion
The data presented in this paper show that systemic Dll4 blockade induces qualitative changes in the BM vasculature (which becomes more heterogeneous), which may be favorable in a BMT setting. A number of studies have proven the relevance of the BM vascular niche in hematopoiesis, but the heterogeneity of the BM vasculature has only been recently objectively assessed, clearly suggesting further detailed studies are needed to understand the importance of the different BM vessels for normal BM function [4]–[9], [13], [26], [29], [58]–[60].
We describe that systemic targeting Dll4, which has previously been shown to confine to particular vascular ECs (called “tip cells”), changes the vascular identity in the BM. Following myeloablation, we applied an anti-Dll4 treatment, similar to what is currently being performed in phase I clinical trials to treat patients with solid malignancies. This treatment resulted in different vascular alterations in the BM, as shown by increased CD31, VE-Cadherin and c-kit+ cells, without quantitative changes in CD105+, VEGFR3+, SMA+ or lectin+ vessels. The global BM vessel identity is therefore altered upon anti-Dll4 treatment.
Interestingly, CD31 is indispensable for several stages of hematopoiesis, endothelial cells survival and angiogenesis, which in turn are all crucial for hematopoietic recovery following myeloablation [13], [26], [61]–[67]. VE-Cadherin is also required for hematopoiesis and angiogenesis [55], [68], [69]. The role of c-kit, in ECs, however, is unknown; studies assessing its role in angiogenesis and, more specifically, in the BM microenvironment, will be required for proper interpretation of the data presented in this paper.
Regarding the modulation of “angiocrine genes”, besides the increase in CD31+ BM vessels previously described, we detected a significant increase in IGFbp2, IGFbp3, Angpt2, DHH and VEGF-A and a decrease in FGF1 and CSF2 expression in whole BM extracts from anti-Dll4 treated animals (Figure 2C). Even though FGF1, which is decreased upon anti-Dll4 treatment, prevents vessel regression, IGFbp3, Angpt2 and VEGF-A, which are increased, are modulators of vascular survival and re-growth, which, as previously mentioned, is crucial for hematopoietic recovery following myeloablation [70]–[72].
Despite the decrease of CSF2, which is associated with a decrease in the myeloid lineage, IGF1 induces proliferation and differentiation of myeloid lineage cells [73], and DHH is important for granulocyte differentiation/proliferation in the BM [74]; moreover, the myeloid modulation we describe may be due to a direct effect of anti-Dll4 treatment on hematopoietic cells (Figure 3C). Both HSPCs and myeloid cells are reported to express Dll4 [32]. We show that anti-Dll4 treatment of HSPCs in vitro increases CFU-M and CFU-G number, independently of Notch1 modulation, but decreases multipotential progenitor cell-derived CFUs, similar to anti-Notch1 treatment (Figure 3C).
VEGF-A blocks both B and T lymphopoiesis [75]–[77]. The altered BM lymphocyte content observed might either be simply due to the increased myeloid content, to the VEGF-A increase in the BM, to the direct effect of anti-Dll4 in lymphoid cells, and/or to the effect of Dll4 inhibition in secondary hematopoietic organs, such as the thymus and spleen, which were previously shown to express Dll4 [35], [49], [56], [78]–[80].
Regarding the signaling pathways that are proposed to trigger the EC role in hematopoiesis, we observed a decrease of Akt activation, without significant changes in Erk1/2 (Figure 2D, E) after exposing EC to anti-Dll4. It should be noted that Dll4 has been shown to modulate the MAPK activation on a stimulus-depending manner [81]; the technical constraints to study EC-specific signaling pathways activation in vivo led us to an in vitro study, which may not completely mirror the in vivo systemic effects of anti-Dll4, nor the proper stimulus acting in different BM microenvironments.
In vivo assessments showed both HSPC phenotype and function (reconstitution potential) were not impaired by systemic anti-Dll4 treatment, and in vitro differentiation of HSPCs collected from the BM of anti-Dll4 treated mice was also not impaired, meaning HSPCs are unaffected by in vivo anti-Dll4 treatment (Figure 3B, 4B and S6).
Systemic anti-Dll4 treatment of donor mice in a setting of BMT resulted in a mild, but significant, accelerated hematopoietic recovery of recipient mice (Figure 4) [7], [13]. For a successful BMT, HSPCs must home and engraft in the BM, a process for which BM ECs are essential [11]–[13]. In this study, we transplanted whole BM mononuclear cells; this fraction includes BM ECs, which were previously shown to incorporate in the BM vasculature [12]. Interestingly, vascular CD31 and VE-Cadherin regulate the transition of HSPCs between blood and BM [65], [68]. The increased VE-Cadherin and CD31-positive BM vessels from anti-Dll4 treated donor mice may have enhanced the homing of HSPCs in recipient mice, thereby leading to an overall faster hematopoietic recovery. Interestingly, we have also observed an increase in Dll4 expression in the BM of anti-Dll4 treated donor mice (Figure 2C). Given that anti-Dll4 treatment was performed only in donor mice, and not in recipients, the transplanted cells may have increased Dll4 protein levels. Remarkably, in vitro data have shown that increasing Dll4 signaling in HSPCs increases erythroid commitment and HSPCs proliferation, induces commitment and complete maturation to the T cell lineage, and maintains HSPCs stemness [32], [34], [82], [83]. In our BMT model, these effects were transient, because the treatment was not maintained thoughout the process of hematopoietic recovery; therefore, the lymphoproliferative disease that mice overexpressing Dll4 in the hematopoietic lineage are expected to develop was not observed (evidenced by the long term survival of recipient mice) [33], [35]. Alternatively, or in addition, IGFbp2 and IGFbp3 showed increased expression following anti-Dll4 treatment; these factors, by stabilizing IGF1, may contribute towards the effects of anti-Dll4 in promoting hematopoietic recovery following BMT [84].
Together, our data shows that targeting Dll4 alters the vascular identity in the BM, mildly affects hematopoiesis, and promotes a faster hematopoietic recovery after BMT. We have characterized the BM vascular niche and provide evidence of its heterogeneity, which may create different microenvironments within the BM. This assessment may be particularly interesting to explore, as relevant information regarding the functional characterization of hematopoietic stem cell niches can be obtained. We further suggest anti-Dll4 blockade may be an interesting therapeutic approach in a BMT setting.
Supporting Information
Anti-Dll4 blockade interferes with the BM vascular niche. (A) Immunohistochemistry for VEGFR3 and SMA counterstained with Mayer’s haemalum (Leica DMD 108). Immunofluorescence for lectin (Leica LSM 510). Bar = 20 µm. (B) Relative quantification of mRNA from total BM reveals an increase in VE-Cadherin, but not CD31, expression in anti-Dll4 treated mice. (C) Relative quantification of mRNA from HUVEC reveals an increase in VE-Cadherin, but not CD31, expression in anti-Dll4 treated cells. Data are means ± s.e.m. *, p<0.05; n = 3.
(TIF)
The use of different endothelial cell markers reveal different types of BM vessels. Immunohistochemistry for CD105, VE-Cadherin, SMA and CD31 counterstained with Mayer’s haemalum (LEICA DMD 108). Stable vessels are SMA+ (a, c), CD105high (a) or CD105low (c, f), VE-Cadherinhigh (a, c, f), and CD31+ (f). Sinusoids are SMA- (b, d, e), CD105+ (b, d, e, g, h), VE-Cadherin+ (b, e) or VE-Cadherin- (d), and CD31+ (g) or CD31- (h). Bar = 10 µm.
(TIF)
Endothelial cell-specific Dll4 blockade interferes with the BM vascular niche. (A) Immunohistochemistry for CD31, CD105 and VE-Cadherin counterstained with Mayer’s haemalum (Leica DMD108). Bar = 20 µm. (B) CD31, CD105 and VE-Cadherin-positive vessel count, per high power field (400x, Leica DMD108), reveal an increase of CD31 and VE-Cadherin-positive BM vessels in VECad-Cre-ERT2Dll4lox/lox mice. (C) Flow cytometry analysis of the percentage of megakaryocytes (CD41+ cells) in the BM shows an increase of BM megakaryocyte cell percentage in mice. Data are shown as means ± s.e.m. *, p<0.05; n = 11.
(TIF)
Therapeutic anti-Dll4 blockade interferes with the hepatic vascular niche. (A) Macroscopic observation of the liver of anti-Dll4 treated mice reveals an obvious disruption in tissue architecture. Bar = 2 mm. (B) Histology of the liver reveals anti-Dll4 treatment promotes severe centrolobular sinusoidal dilation (arrows), with multifocal hepatocyte regeneration foci (arrowheads), as compared to the normal liver morphology observed in control mice; hematoxilin-eosin staining (Leica DMD 108). Bar = 25 µm. Data are means ± s.e.m. *, p<0.05; data represents one of three experiments in which n = 3.
(TIF)
Endothelial-specific effects of anti-Dll4 treatment. (A) Angiocrine gene modulation was assessed by relative quantification of mRNA from total BM. None of the displayed genes is modulated in vivo by anti-Dll4 treatment. (B) Bone marrow VEGF-A, SDF-1α and SCF levels, as determined by ELISA. (C) Angiocrine gene modulation was assessed in vitro by relative quantification of mRNA from HUVEC. HUVEC subjected to anti-Dll4 treatment decreases FGF1 and increases VEGF-A expression, similar to total BM from anti-Dll4 treated mice. CSF3, but not CSF2, expression is decreased upon in vitro anti-Dll4 treatment. FGF2 and Dll4 are significantly decreased, and IL-6 and SCF are significantly increased in anti-Dll4 treated cells. Data are means ± s.e.m. *, p<0.05; n = 3.
(TIF)
Anti-Dll4 treatment does not perturb colony forming (CFU) potential of Lin-Sca1+ hematopoietic precursor cells. Colony counts from methylcellulose culture of Lin-Sca1+ sorted cells reveal anti-Dll4 treatment in vivo does not affect intrinsic stem cell’s ability to differentiate into different hematopoietic lineages. Data are means ± s.e.m. *, p<0.05; n = 3.
(TIF)
Antibodies list.
(XLS)
Primers list.
(XLS)
Acknowledgments
The authors would like to thank other members of the Angiogenesis Lab for their input and suggestions. The Program for Advanced Medical Education is sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia, Portugal.
Funding Statement
This work was supported by FCT PTDC/SAU-ORG/113617/2009 (www.fct.pt). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schofield R (1978) The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4: 7–25. [PubMed] [Google Scholar]
- 2. Zhang J, Niu C, Ye L, Huang H, He X, et al. (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841 doi:10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 3. Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, et al. (2004) Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell 118: 149–161 doi:10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4. Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH, Terhorst C, et al. (2005) SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell 121: 1109–1121 doi:10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 5. Xie Y, Yin T, Wiegraebe W, He XC, Miller D, et al. (2008) Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457: 97–102 doi:10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 6. Celso Lo C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, et al. (2008) Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457: 92–97 doi:10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, et al. (2010) Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6: 251–264 doi:10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura Y, Ding B, Imai N, Nolan DJ, Butler JM, et al. (2011) c-Kit-Mediated Functional Positioning of Stem Cells to Their Niches Is Essential for Maintenance and Regeneration of Adult Hematopoiesis. PLoS ONE 6: e26918 doi:10.1371/journal.pone.0026918.g006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462 doi:10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, et al. (1995) Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 86: 3353–3363. [PubMed] [Google Scholar]
- 11. Kopp HG, Avecolla ST, Hooper AT, Shmelkov SV, Ramos CA, et al. (2005) Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood 106: 505–513 doi:10.1182/blood-2004–11–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, et al. (2007) Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood 109: 2365–2372 doi:10.1182/blood-2006–05–022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, et al. (2009) Engraftment and Reconstitution of Hematopoiesis Is Dependent on VEGFR2-Mediated Regeneration of Sinusoidal Endothelial Cells. Stem Cell 4: 263–274 doi:10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, et al. (2009) Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood 113: 2104–2107 doi:10.1182/blood-2008–06–162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamorte S, Remédio L, Dias S (2009) Communication between bone marrow niches in normal bone marrow function and during hemopathies progression. Hematol Rep 1. doi:10.4081/hr.2009.e14.
- 16. Patel NS, Li J-L, Generali D, Poulsom R, Cranston DW, et al. (2005) Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Research 65: 8690–8697 doi:10.1158/0008–5472.CAN-05–1208. [DOI] [PubMed] [Google Scholar]
- 17. Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, et al. (2006) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444: 1032–1037 doi:10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 18. Ridgway J, Zhang G, Wu Y, Stawicki S, Liang W-C, et al. (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444: 1083–1087 doi:10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 19. Real C, Remédio L, Caiado F, Igreja C, Borges C, et al. (2011) Bone Marrow-Derived Endothelial Progenitors Expressing Delta-Like 4 (Dll4) Regulate Tumor Angiogenesis. PLoS ONE 6: e18323 doi:10.1371/journal.pone.0018323.g007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A Multiple-Ascending-Dose Study of the Safety and Tolerability of REGN421(SAR153192) in Patients With Advanced Solid Malignancies - Full Text View - ClinicalTrials.gov (2012) A Multiple-Ascending-Dose Study of the Safety and Tolerability of REGN421(SAR153192) in Patients With Advanced Solid Malignancies - Full Text View - ClinicalTrials.gov: 1–3.
- 21.A Phase 1 Dose Escalation Study of OMP-21M18 in Subjects With Solid Tumors - Full Text View - ClinicalTrials.gov (2012) A Phase 1 Dose Escalation Study of OMP-21M18 in Subjects With Solid Tumors - Full Text View - ClinicalTrials.gov: 1–3.
- 22. Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, et al. (1989) Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem 37: 315–321 doi:10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- 23. Galmiche MC, Koteliansky VE, Brière J, Hervé P, Charbord P (1993) Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood 82: 66–76. [PubMed] [Google Scholar]
- 24. Bianco P, Riminucci M, Gronthos S, Robey PG (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19: 180–192 doi:10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 25. Bautch VL (2011) Stem cells and the vasculature. Nature Medicine 17: 1437–1443 doi:10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- 26. Kopp H-G, Avecilla ST, Hooper AT, Rafii S (2005) The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 20: 349–356 doi:10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 27. Vainchenker W, Deschamps JF, Bastin JM, Guichard J, Titeux M, et al. (1982) Two monoclonal antiplatelet antibodies as markers of human megakaryocyte maturation: immunofluorescent staining and platelet peroxidase detection in megakaryocyte colonies and in in vivo cells from normal and leukemic patients. Blood 59: 514–521. [PubMed] [Google Scholar]
- 28. Kostyak JC, Naik MU, Naik UP (2012) Calcium- and integrin-binding protein 1 regulates megakaryocyte ploidy, adhesion, and migration. Blood 119: 838–846 doi:10.1182/blood-2011–04–346098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kobayashi H, Butler JM, O'Donnell R, Kobayashi M, Ding B-S, et al. (2010) Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature 12: 1046–1056 doi:10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lauret E, Catelain C, Titeux M, Poirault S, Dando JS, et al. (2004) Membrane-bound Delta-4 Notch ligand reduces the proliferative activity of primitive human hematopoietic CD34+CD38low cells while maintaining their LTC-IC potential. Leukemia 18: 788–797 doi:10.1038/sj.leu.2403288. [DOI] [PubMed] [Google Scholar]
- 31. Lahmar M, Catelain C, Poirault S, Dorsch M, Villeval J-L, et al. (2008) Distinct effects of the soluble versus membrane-bound forms of the notch ligand delta-4 on human CD34+CD38low cell expansion and differentiation. Stem Cells 26: 621–629 doi:10.1634/stemcells.2007–0428. [DOI] [PubMed] [Google Scholar]
- 32. Karanu FN, Murdoch B, Miyabayashi T, Ohno M, Koremoto M, et al. (2001) Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood 97: 1960–1967. [DOI] [PubMed] [Google Scholar]
- 33. Dorsch M, Zheng G, Yowe D, Rao P, Wang Y, et al. (2002) Ectopic expression of Delta4 impairs hematopoietic development and leads to lymphoproliferative disease. Blood 100: 2046–2055. [PubMed] [Google Scholar]
- 34. Poirault-Chassac S, Six E, Catelain C, Lavergne M, Villeval J-L, et al. (2010) Notch/Delta4 signaling inhibits human megakaryocytic terminal differentiation. Blood 116: 5670–5678 doi:10.1182/blood-2010–05–285957. [DOI] [PubMed] [Google Scholar]
- 35. Yan XQ, Sarmiento U, Sun Y, Huang G, Guo J, et al. (2001) A novel Notch ligand, Dll4, induces T-cell leukemia/lymphoma when overexpressed in mice by retroviral-mediated gene transfer. Blood 98: 3793–3799. [DOI] [PubMed] [Google Scholar]
- 36. Hozumi K, Mailhos C, Negishi N, Hirano KI, Yahata T, et al. (2008) Delta-like 4 is indispensable in thymic environment specific for T cell development. Journal of Experimental Medicine 205: 2507–2513 doi:10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohtashami M, Shah DK, Nakase H, Kianizad K, Petrie HT, et al. (2010) Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. The Journal of Immunology 185: 867–876 doi:10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 38. Todd RF, Nadler LM, Schlossman SF (1981) Antigens on human monocytes identified by monoclonal antibodies. J Immunol 126: 1435–1442. [PubMed] [Google Scholar]
- 39. Wang J, Sun Q, Morita Y, Jiang H, Groß A, et al. (2012) A Differentiation Checkpoint Limits Hematopoietic Stem Cell Self-Renewal in Response to DNA Damage. Cell 148: 1001–1014 doi:10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 40. Coffman RL, Weissman IL (1981) B220: a B cell-specific member of th T200 glycoprotein family. Nature 289: 681–683. [DOI] [PubMed] [Google Scholar]
- 41. Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF (1980) Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci USA 77: 1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241: 58–62. [DOI] [PubMed] [Google Scholar]
- 43. Christensen JL, Weissman IL (2001) Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA 98: 14541–14546 doi:10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967 doi:10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, et al. (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Medicine 5: 434–438 doi:10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 46. Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, et al. (2011) Acrolein Inhalation Prevents Vascular Endothelial Growth Factor-Induced Mobilization of Flk-1+/Sca-1+ Cells in Mice. Arteriosclerosis, Thrombosis, and Vascular Biology 31: 1598–1606 doi:10.1161/ATVBAHA.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakahata T, Ogawa M (1982) Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci USA 79: 3843–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Broxmeyer HE, Lee M-R, Hangoc G, Cooper S, Prasain N, et al. (2011) Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood 117: 4773–4777 doi:10.1182/blood-2011–01–330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ishida W, Fukuda K, Sakamoto S, Koyama N, Koyanagi A, et al. (2011) Regulation of experimental autoimmune uveoretinitis by anti-delta-like ligand 4 monoclonal antibody. Invest Ophthalmol Vis Sci 52: 8224–8230 doi:10.1167/iovs.11–7756. [DOI] [PubMed] [Google Scholar]
- 50. Bassil R, Zhu B, Lahoud Y, Riella LV, Yagita H, et al. (2011) Notch Ligand Delta-Like 4 Blockade Alleviates Experimental Autoimmune Encephalomyelitis by Promoting Regulatory T Cell Development. The Journal of Immunology 187: 2322–2328 doi:10.4049/jimmunol.1100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunamura M, Yagita H (2008) Neutralizing monoclonal antibody against human Dll4.
- 52.Sunamura M, Yagita H (2008) Anti-Dll4 binding protein. UK Patent Application: 1–85.
- 53. Sekine C, Koyanagi A, Koyama N, Hozumi K, Chiba S, et al. (2012) Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes. Arthritis Research & Therapy 14: R45 doi:10.1186/ar3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakamura-Ishizu A, Morikawa S, Shimizu K, Ezaki T (2008) Characterization of sinusoidal endothelial cells of the liver and bone marrow using an intravital lectin injection method. J Mol Histol 39: 471–479 doi:10.1007/s10735–008–9186-x. [DOI] [PubMed] [Google Scholar]
- 55. Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, et al. (2004) Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nature Medicine 10: 64–71 doi:10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 56. Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, et al. (2010) Chronic DLL4 blockade induces vascular neoplasms. Nature 463: E1–E1 doi:10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 57. Candal FJ, Rafii S, Parker JT, Ades EW, Ferris B, et al. (1996) BMEC-1: a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc Res 52: 221–234 doi:10.1006/mvre.1996.0060. [DOI] [PubMed] [Google Scholar]
- 58.Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, et al.. (2012) Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. doi:10.1182/blood-2011–05–353201. [DOI] [PMC free article] [PubMed]
- 59. Pitchford SC, Lodie T, Rankin SM (2012) VEGFR1 stimulates a CXCR4-dependent translocation of megakaryocytes to the vascular niche, enhancing platelet production in mice. Blood 120: 2787–2795 doi:10.1182/blood-2011–09–378174. [DOI] [PubMed] [Google Scholar]
- 60. Li XM, Hu Z, Jorgenson ML, Slayton WB (2009) High Levels of Acetylated Low-Density Lipoprotein Uptake and Low Tyrosine Kinase With Immunoglobulin and Epidermal Growth Factor Homology Domains-2 (Tie2) Promoter Activity Distinguish Sinusoids From Other Vessel Types in Murine Bone Marrow. Circulation 120: 1910–1918 doi:10.1161/CIRCULATIONAHA.109.871574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, et al. (1993) Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 262: 1580–1582. [DOI] [PubMed] [Google Scholar]
- 62. Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, et al. (1995) CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. The Journal of Cell Biology 130: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu Y, Welte T, Michaud M, Madri JA (2007) PECAM-1: a multifaceted regulator of megakaryocytopoiesis. Blood 110: 851–859 doi:10.1182/blood-2006–05–022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dhanjal TS, Pendaries C, Ross EA, Larson MK, Protty MB, et al. (2007) A novel role for PECAM-1 in megakaryocytokinesis and recovery of platelet counts in thrombocytopenic mice. Blood 109: 4237–4244 doi:10.1182/blood-2006–10–050740. [DOI] [PubMed] [Google Scholar]
- 65. Ross EA, Freeman S, Zhao Y, Dhanjal TS, Ross EJ, et al. (2008) A Novel Role for PECAM-1 (CD31) in Regulating Haematopoietic Progenitor Cell Compartmentalization between the Peripheral Blood and Bone Marrow. PLoS ONE 3: e2338 doi:10.1371/journal.pone.0002338.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, et al. (1999) Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. Journal of Cell Science 112 (Pt 12): 1989–1997. [DOI] [PubMed] [Google Scholar]
- 67. Cao G, O'Brien CD, Zhou Z, Sanders SM, Greenbaum JN, et al. (2002) Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol, Cell Physiol 282: C1181–C1190 doi:10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- 68. van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FPJ, et al. (2002) Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol 168: 588–596. [DOI] [PubMed] [Google Scholar]
- 69. Nelson CM, Chen CS (2003) VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. Journal of Cell Science 116: 3571–3581 doi:10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 70. Chang K-H, Chan-Ling T, McFarland EL, Afzal A, Pan H, et al. (2007) IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA 104: 10595–10600 doi:10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lofqvist C, Chen J, Connor KM, Smith ACH, Aderman CM, et al. (2007) IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci USA 104: 10589–10594 doi:10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, et al. (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60 doi:10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 73. Heemskerk VH, Daemen MA, Buurman WA (1999) Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Reviews 10: 5–14. [DOI] [PubMed] [Google Scholar]
- 74. Lau C-I, Outram SV, Saldaña JI, Furmanski AL, Dessens JT, et al. (2012) Regulation of murine normal and stress-induced erythropoiesis by Desert Hedgehog. Blood 119: 4741–4751 doi:10.1182/blood-2011–10–387266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, et al. (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101: 4878–4886 doi:10.1182/blood-2002–07–1956. [DOI] [PubMed] [Google Scholar]
- 76. Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, et al. (2007) Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110: 624–631 doi:10.1182/blood-2007–01–065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fragoso R, Igreja C, Clode N, Henriques A, Appleton C, et al. (2008) VEGF signaling on hematopoietic precursors restricts B-lymphoid commitment in vitro and in vivo. Exp Hematol 36: 1329–1336 doi:10.1016/j.exphem.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 78. Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, et al. (2000) Dll4, a novel Notch ligand expressed in arterial endothelium. Genes & Development 14: 1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 79. Itoi M, Tsukamoto N, Amagai T (2006) Expression of Dll4 and CCL25 in Foxn1-negative epithelial cells in the post-natal thymus. International Immunology 19: 127–132 doi:10.1093/intimm/dxl129. [DOI] [PubMed] [Google Scholar]
- 80. Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, et al. (2008) Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. Journal of Experimental Medicine 205: 2515–2523 doi:10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Harrington LS, Sainson RCA, Williams CK, Taylor JM, Shi W, et al. (2008) Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res 75: 144–154 doi:10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 82. Dando JS, Tavian M, Catelain C, Poirault S, Bennaceur-Griscelli A, et al. (2005) Notch/Delta4 Interaction in Human Embryonic Liver CD34+CD38 −Cells: Positive Influence on BFU-E Production and LTC-IC Potential Maintenance. Stem Cells 23: 550–560 doi:10.1634/stemcells.2004–0205. [DOI] [PubMed] [Google Scholar]
- 83. La Coste de A, Six E, Fazilleau N, Mascarell L, Legrand N, et al. (2005) In vivo and in absence of a thymus, the enforced expression of the Notch ligands delta-1 or delta-4 promotes T cell development with specific unique effects. J Immunol 174: 2730–2737. [DOI] [PubMed] [Google Scholar]
- 84. Tian ZG, Woody MA, Sun R, Welniak LA, Raziuddin A, et al. (1998) Recombinant human growth hormone promotes hematopoietic reconstitution after syngeneic bone marrow transplantation in mice. Stem Cells 16: 193–199 doi:10.1002/stem.160193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-Dll4 blockade interferes with the BM vascular niche. (A) Immunohistochemistry for VEGFR3 and SMA counterstained with Mayer’s haemalum (Leica DMD 108). Immunofluorescence for lectin (Leica LSM 510). Bar = 20 µm. (B) Relative quantification of mRNA from total BM reveals an increase in VE-Cadherin, but not CD31, expression in anti-Dll4 treated mice. (C) Relative quantification of mRNA from HUVEC reveals an increase in VE-Cadherin, but not CD31, expression in anti-Dll4 treated cells. Data are means ± s.e.m. *, p<0.05; n = 3.
(TIF)
The use of different endothelial cell markers reveal different types of BM vessels. Immunohistochemistry for CD105, VE-Cadherin, SMA and CD31 counterstained with Mayer’s haemalum (LEICA DMD 108). Stable vessels are SMA+ (a, c), CD105high (a) or CD105low (c, f), VE-Cadherinhigh (a, c, f), and CD31+ (f). Sinusoids are SMA- (b, d, e), CD105+ (b, d, e, g, h), VE-Cadherin+ (b, e) or VE-Cadherin- (d), and CD31+ (g) or CD31- (h). Bar = 10 µm.
(TIF)
Endothelial cell-specific Dll4 blockade interferes with the BM vascular niche. (A) Immunohistochemistry for CD31, CD105 and VE-Cadherin counterstained with Mayer’s haemalum (Leica DMD108). Bar = 20 µm. (B) CD31, CD105 and VE-Cadherin-positive vessel count, per high power field (400x, Leica DMD108), reveal an increase of CD31 and VE-Cadherin-positive BM vessels in VECad-Cre-ERT2Dll4lox/lox mice. (C) Flow cytometry analysis of the percentage of megakaryocytes (CD41+ cells) in the BM shows an increase of BM megakaryocyte cell percentage in mice. Data are shown as means ± s.e.m. *, p<0.05; n = 11.
(TIF)
Therapeutic anti-Dll4 blockade interferes with the hepatic vascular niche. (A) Macroscopic observation of the liver of anti-Dll4 treated mice reveals an obvious disruption in tissue architecture. Bar = 2 mm. (B) Histology of the liver reveals anti-Dll4 treatment promotes severe centrolobular sinusoidal dilation (arrows), with multifocal hepatocyte regeneration foci (arrowheads), as compared to the normal liver morphology observed in control mice; hematoxilin-eosin staining (Leica DMD 108). Bar = 25 µm. Data are means ± s.e.m. *, p<0.05; data represents one of three experiments in which n = 3.
(TIF)
Endothelial-specific effects of anti-Dll4 treatment. (A) Angiocrine gene modulation was assessed by relative quantification of mRNA from total BM. None of the displayed genes is modulated in vivo by anti-Dll4 treatment. (B) Bone marrow VEGF-A, SDF-1α and SCF levels, as determined by ELISA. (C) Angiocrine gene modulation was assessed in vitro by relative quantification of mRNA from HUVEC. HUVEC subjected to anti-Dll4 treatment decreases FGF1 and increases VEGF-A expression, similar to total BM from anti-Dll4 treated mice. CSF3, but not CSF2, expression is decreased upon in vitro anti-Dll4 treatment. FGF2 and Dll4 are significantly decreased, and IL-6 and SCF are significantly increased in anti-Dll4 treated cells. Data are means ± s.e.m. *, p<0.05; n = 3.
(TIF)
Anti-Dll4 treatment does not perturb colony forming (CFU) potential of Lin-Sca1+ hematopoietic precursor cells. Colony counts from methylcellulose culture of Lin-Sca1+ sorted cells reveal anti-Dll4 treatment in vivo does not affect intrinsic stem cell’s ability to differentiate into different hematopoietic lineages. Data are means ± s.e.m. *, p<0.05; n = 3.
(TIF)
Antibodies list.
(XLS)
Primers list.
(XLS)