Abstract
The Ca2+ paradox represents a good model to study Ca2+ overload injury in ischemic heart diseases. We and others have demonstrated that contracture and calpain are involved in the Ca2+ paradox-induced injury. This study aimed to elucidate their roles in this model. The Ca2+ paradox was elicited by perfusing isolated rat hearts with Ca2+-free KH media for 3 min or 5 min followed by 30 min of Ca2+ repletion. The LVDP was measured to reflect contractile function, and the LVEDP was measured to indicate contracture. TTC staining and the quantification of LDH release were used to define cell death. Calpain activity and troponin I release were measured after Ca2+ repletion. Ca2+ repletion of the once 3-min Ca2+ depleted hearts resulted in almost no viable tissues and the disappearance of contractile function. Compared to the effects of the calpain inhibitor MDL28170, KB-R7943, an inhibitor of the Na+/Ca2+ exchanger, reduced the LVEDP level to a greater extent, which was well correlated with improved contractile function recovery and tissue survival. The depletion of Ca2+ for 5 min had the same effects on injury as the 3-min Ca2+ depletion, except that the LVEDP in the 5-min Ca2+ depletion group was lower than the level in the 3-min Ca2+ depletion group. KB-R7943 failed to reduce the level of LVEDP, with no improvement in the LVDP recovery in the hearts subjected to the 5-min Ca2+ depletion treatment; however, KB-R7943 preserved its protective effects in surviving tissue. Both KB-R7943 and MDL28170 attenuated the Ca2+ repletion-induced increase in calpain activity in 3 min or 5 min Ca2+ depleted hearts. However, only KB-R7943 reduced the release of troponin I from the Ca2+ paradoxic heart. These results provide evidence suggesting that contracture is the main cause for contractile dysfunction, while activation of calpain mediates cell death in the Ca2+ paradox.
Introduction
It is well documented that Ca2+ participates in numerous physiological functions in the heart, such as excitation-contraction coupling and excitability [1], [2], whereas abnormalities in Ca2+ homeostasis is a common phenomenon that occurs during progressive heart failure [3] and myocardial ischemia/reperfusion injury, i.e., thrombolysis treatment or percutaneous transluminal coronary angioplasty after acute thrombosis formation and restored circulation to the heart following the interruption of flow during open heart surgery [4]. To date, various studies have shown that Ca2+ overload leads to mechanical dysfunction, arrhythmias, and cell death [3], [4]. Therefore, it is important to unravel the mechanism in order to learn useful strategies for the prevention of ischemia/reperfusion injury.
The loss of Ca2+ homeostasis is easily reproduced by successive perfusion of hearts with Ca2+-free and Ca2+-containing media in the laboratory, which is termed the Ca2+ paradox [5], [6]. One of the most apparent changes after repletion of the once Ca2+-depleted hearts is diastolic dysfunction, or the development of contracture, which induces physical stress [7], [8], [9], [10]. This aspect is manifested by the formation of contraction bands, sustained cell shortening, or an elevated left ventricle end-diastolic pressure (LVEDP) in the tracing of left ventricle pressure [7], [8], [9]. The contracture is the accepted primary mediator, and it tears the sarcolemmal membrane apart from adjacent cells, leading to myoglobin, lactate dehydrogenase (LDH) and creatine kinase release, consequently resulting in cell death and heart dysfunction [5], [10]. However, some data cannot be explained by this theory. For example, in cultured cell models, which are free from mechanical interactions with adjacent cells, suppressing the Na+/Ca2+ exchanger (NCX) with SEA0400 decreased cell death induced by the Ca2+ paradox [11]. Therefore, it is possible that other mechanisms are involved in Ca2+ paradox-induced heart injury.
Calpains are intracellular cysteine proteases involved in numerous physiological and pathological phenomena, such as cell migration during wound closure or tumor invasion [12], [13]. Among the 15 members of the calpain family, the two best characterized calpains, known as μ-calpain and m-calpain, are expressed in the myocardium [13], [14], [15]. Although the amount of Ca2+ required for the in vitro activation of μ-calpain and m-calpain was different and m-calpain was regulated by binding to phosphatidylinositol 4,5-bisphosphate [12], [13], ample studies have shown that calpains are activated during ischemia/reperfusion, resulting in the cleavage of its substrates, such as Na+/K+-ATPase, α-fodrin, a prominent component of the membrane skeleton, and consequently heart injury [16], [17], [18], [19]. We and others have previously found that after Ca2+ repletion, calpains are activated and both μ- and m-calpain are anchored to the sarcolemmal membrane [20], [21]; moreover, the addition of MDL28170, an inhibitor of calpain, reduced the cleavage of α-fodrin and rescued myocardial dysfunction and cell death [20]. However, MDL28170 did not substantially reduce the level of LVEDP and the degradation of troponin I [20], a regulatory protein involved in maintaining the diastolic state. Based on these results, we hypothesized that calpain activation and contracture development were two independently important events in the cascade of the Ca2+ paradox, which resulted in multiple cell abnormalities that led to the heart injury.
In this study, KB-R7943, a selective inhibitor of NCX [22], [23], and MDL28170, an inhibitor of calpain, were used. We firstly compared the effects of KB-R7943 and MDL28170 on the recovery of LVEDP, an indicator of the contracture, in hearts subjected to the 3-min Ca2+ depletion treatment; we secondly analyzed the relationship between the contracture and contractile function recovery and between the contracture and tissue survival in the Ca2+ paradox. Thirdly, we prolonged the duration of Ca2+ depletion to 5 min to reduce the diminishing effects of KB-R7943 on the LVEDP and then evaluated its effects on the contractile function recovery and survival of tissue after Ca2+ repletion. Finally, we determined the effects of KB-R7943 and MDL28170 on calpain activity and troponin I release. These results suggest that contracture is the main cause for contractile dysfunction, while activation of calpain mediates cell death in the Ca2+ paradox.
Materials and Methods
Drugs and Chemicals
KB-R7943 and MDL28170 were purchased from Tocris Bioscience (Ellisville, MO, USA). The LDH enzyme-linked immunoassay (ELISA) kit was purchased from R&D Systems (Minneapolis, MN, USA). Rabbit anti-μ-calpain and -m-calpain antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Mouse anti-α-fodrin antibody was purchased from Enzo Life Sciences (Plymouth Meeting, PA, USA). A tetramethylrhodamine goat anti-rabbit IgG was purchased from Molecular Probes (Eugene, OR, USA). Triphenyltetrazolium chloride (TTC), 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI), Ponceau Red, and other chemicals were from Sigma (Shanghai, China).
Animals
Sprague-Dawley male rats of 250∼300 g body weight were obtained from the Laboratory Animal Centers at The Fourth Military Medical University. All experimental procedures described here were in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at The Fourth Military Medical University.
Langendorff-perfused Rat Heart
As described previously [20], the hearts were quickly removed and retrogradely perfused through the aorta in a non-circulating Langendorff apparatus (Radnoti Glass Technology Inc., Monrovia, CA, USA) at a constant pressure of 80 mm Hg. Krebs-Henseleit (KH) was used as a perfusion solution and contained 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.25 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose (pH 7.4, 37°C). The solution was equilibrated with 95% O2/5% CO2. A water-filled latex balloon-tipped catheter was carefully placed into the left ventricle through the left atrium and adjusted to a left ventricular end-diastolic pressure (LVEDP) of approximately 5 mm Hg by inflating the balloon. The distal portion of the catheter was connected to a computer via a transducer (Model 100 BP-Biopac System Inc., Goleta, CA, USA). Data processing was performed using AcqKnowledge software (version 3.8.1). The index of myocardial contractile function was determined with the left ventricular developed pressure (LVDP), which was calculated from the difference between the left ventricular peak systolic pressure and end-diastolic pressure. The LVEDP was used to reflect the diastolic function, or the degree of contracture occurring at the end of the experiment. A 10-min equilibration period was allowed before the experiments started.
Study Groups and Experimental Protocol
The experiments were randomly divided into 9 groups. Every group had 6 hearts. For the normal control (Group 1), the hearts were perfused for 45 min with normal KH solution. For the drug control (Group 2 and 3), the hearts were treated with 10 µM KB-R7943 or MDL-28170 for 8 min followed by perfusion with normal KH solution. In the 3-min Ca2+ depletion protocol, the heart was successively perfused with Ca2+-free KH solution for 3 min and KH solution containing 1.25 mM Ca2+ for 30 min without (Group 4) or with KB-R7943 (Group 5) and MDL28170 (Group 6). In the 5-min Ca2+ depletion protocol, the heart was depleted of Ca2+ for 5 min without (Group 7) or with KB-R7943 (Group 8) and MDL28170 (Group 9).
For the Ca2+-free KH solution, Ca2+ was omitted, and 0.1 mM EDTA was added to ensure the removal of any contaminating Ca2+. A 10 µM concentration of KB-R7943 or MDL28170 was added during the 1-min perfusion prior to Ca2+ depletion, during the entire period of Ca2+ depletion, and for the first 2 min of Ca2+ repletion. The concentrations of KB-R7943 and MDL28170 used in this study were based on previous studies [23], [24], [25], [26].
Myocardial Infarct Size Determination
At the end of experiments, the heart was frozen, cut into slices perpendicular to the apex-base axis, incubated in 1.0% TTC for 20 min at 37°C, and then immersed in 10% formalin overnight. Viable myocardium was stained brick red and the absence of TTC staining demarcated the areas of irreversibly injured myocardium. The area of myocardial injury was determined by a computerized planimetry technique (OPTIMAS v. 5.2, BioScan Inc., Edmonds, WA, USA) and expressed as a percentage of the total area [20].
LDH and Troponin I Release Assay
The coronary effluent was collected during the 30 min of Ca2+ repletion, and LDH, an indicator of cell death [27], [28], was measured by an ELISA kit (R&D Systems, Minneapolis, MN, USA). The amount of LDH released during the 30 min of Ca2+ repletion was normalized against the wet weight of the heart and expressed as IU/g.
Cardiac troponin I in the coronary effluent was measured with the current version of the AccuTnI assay (Beckman Coulter Inc, Fullerton, CA, USA). The Access Immunoassay System is a random access, bench-top analyzer that has been described in detail in previous studies [29], [30].
In vitro Calpain Activity Assay
The frozen left ventricle tissue was homogenized in an ice-cold Tris-buffered saline solution containing 20 mM Tris-HCl (pH 7.3), 1 mM EGTA, 150 mM NaCl, and 1% Triton X-100 and centrifuged for 15 min at 15,000×g. The supernatant was collected and stored at −70°C until use. Calpain activity was measured by fluorometry (BioTek Synergy HT Microplate Reader) using Suc-LLVY-AMC (Calbiochem) as a substrate, as previously described [16], [17]. The release of the fluorescent product AMC was assessed using excitation and emission wavelengths of 380 nm and 460 nm, respectively. The calpain inhibitor MDL28170 at a concentration of 10 µM was used to determine the specificity of the assay.
Western Blot Analysis
Two minutes after Ca2+ repletion, the heart was removed, and the left ventricular myocardium was frozen in liquid nitrogen and stored at −70°C until use. Approximately 100 mg of frozen tissue was homogenized, using a Bio-Gen PRO200 homogenizer, for 30 s at 4°C in 0.5 ml of lysis buffer containing 50 mM Tris-HCl (pH 7.3), 150 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, with 1% Triton X-100 and 1% protease inhibitor cocktail. The lysates were centrifuged for 15 min at 12,000×g, and the resulting supernatant was aliquoted and stored at −70°C. Total protein concentrations were determined using the Bradford protein assay kit, and 40 µg of the protein was used to evaluate the calpain activity by Western blot using a monoclonal antibody against α-fodrin, which recognizes the calpain-cleaved fragment (approximately 150 kDa), as described previously [18], [31]. The samples were resolved using an 8% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. The membranes were incubated with a primary antibody against α-fodrin. An anti-mouse IgG peroxidase conjugate was used as a secondary antibody. The protein bands were detected by chemiluminescence and quantified using the Image Lab software package (Bio-Rad Laboratories, Herts, UK). The membranes were stained with Ponceau Red to confirm that equal amounts of protein were loaded.
Immunofluorescence Histochemistry and Confocal Analysis
Two minutes after Ca2+ repletion, the left ventricle tissue was excised, fixed in 4% paraformaldehyde at 4°C overnight, embedded in paraffin, and cut into 3-µm-thick sections. After deparaffinization and rehydration, the sections were stained with anti-μ-calpain (1∶100) and anti-m-calpain antibody (1∶25) at 4°C overnight, followed by a 60-min incubation at room temperature with a TRITC-labeled goat anti-rabbit IgG second antibody. DAPI was used to label the nuclei. The sections were examined using a laser-scanning confocal microscope equipped with the FV10-ASW system (Olympus FV1000). The images were analyzed using Image-Pro Plus 5.0 software (Media Cybernetics Inc, Rockville, MD 20850, USA) to quantify the percentage of membrane-anchored calpain, as described previously [32]. Briefly, the sarcolemmal membrane sheets were individually outlined, and the average pixel intensity within each sheet was determined. The background intensity was determined using cytosolic areas. The ratios between the membranes and the background above 120% were considered positive results. At least 300 sarcolemmal membrane sheets were examined for each experimental condition by individuals unaware of the group designations.
Statistical Analysis
All data are presented as the mean  SEM. Group comparisons were performed using ANOVA followed by the Tukey’s test. Statistical analyses were performed using GraphPad Prism v. 4 (GraphPad software, Inc., La Jolla, CA, USA). Two-tailed P<0.05 was considered statistically significant.
SEM. Group comparisons were performed using ANOVA followed by the Tukey’s test. Statistical analyses were performed using GraphPad Prism v. 4 (GraphPad software, Inc., La Jolla, CA, USA). Two-tailed P<0.05 was considered statistically significant.
Results
KB-R7943 Rescued Heart Contractile Function and Tissue Survival after a 3-min Ca2+ Depletion More Effectively than MDL28170, with a Marked Drop in LVEDP
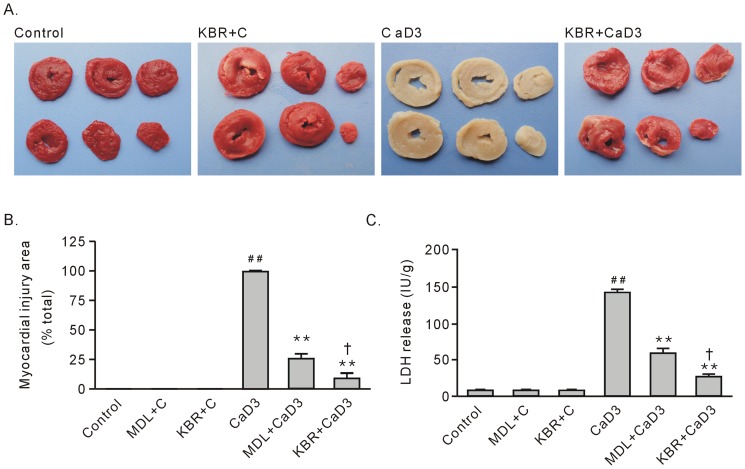
As we had stated previously [20], the 3-min Ca2+ depletion resulted in no viable tissue, as shown by TTC staining, and an increase in LDH release compared with the control group (Figure 1, A–C). Treatment with 10 µM KB-R7943, administered 1 min before the Ca2+ depletion, during the Ca2+ depletion, and the first 2 min after the Ca2+ repletion almost completely abolished the tissue injury by the 3-min Ca2+ depletion treatment, with a small myocardial injury area marked by TTC staining and significant decreases in the release of LDH (Figure 1, A–C).
Figure 1. The effects of treatment with 10 µM KB-R7943 or 10 µM MDL28170 on the survival of heart tissue after the 3-min Ca2+ depletion treatment.
A. Representative TTC staining for hearts subjected to the 3-min Ca2+ depletion treatment in the absence or presence of KB-R7943. TTC-positive staining represents viable tissue. B. Group results on the myocardial injury area, which is expressed as a percentage of the total area. C. Group results on LDH release. Each bar represents the mean±SEM, n = 6. ## P<0.01 vs. the control, **P<0.01 vs. CaD3, † P<0.01 vs. MDL+CaD3. Abbreviations: MDL+C, MDL28170 control; KBR+C, KB-R7943 control; CaD3, 3-min Ca2+ depletion group; MDL, MDL28170; and KBR, KB-R7943.
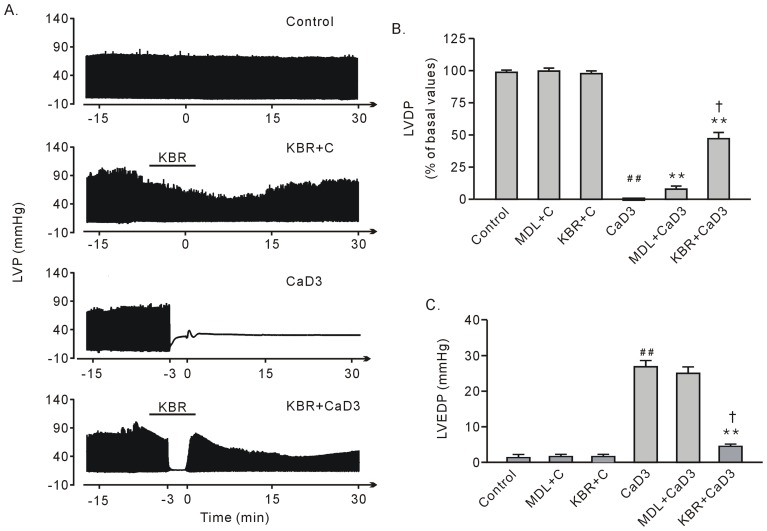
After Ca2+ repletion, the heart exhibited no contractile ability, characterized by diminishing LVDP. The LVEDP significantly increased at the end of the perfusion, suggesting contracture had occurred. Treatment with 10 µM KB-R7943 significantly rescued heart contractile function with LVEDP dropping in level (Figure 2, A–C). Finally, LVDP decreased with KB-R7943 treatment; however, it returned to normal levels after the drug was washed out (Figure 2, A and B).
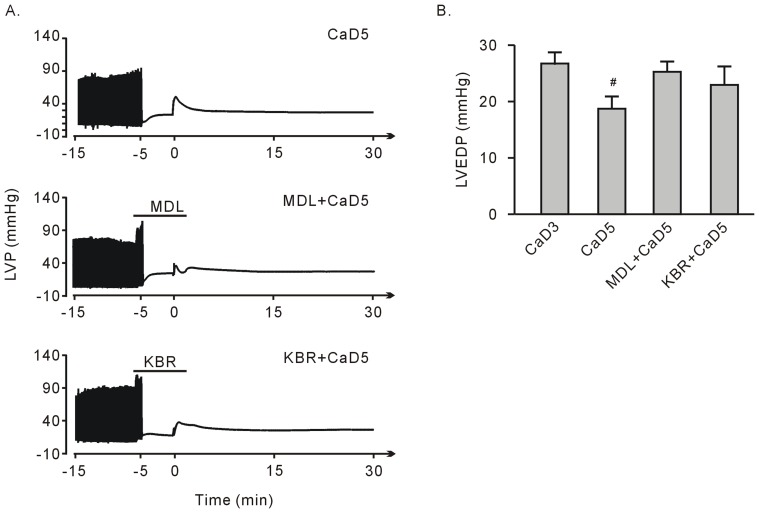
Figure 2. The effects of treatment with 10 µM KB-R7943 or 10 µM MDL28170 on the recovery of heart function after the 3-min Ca2+ depletion treatment.
A. Representative left ventricular pressure tracing in the hearts subjected to the 3-min Ca2+ depletion treatment in the presence or absence of KB-R7943. B. Group results on the recovery of LVDP at the end of perfusion. C. Group results on LVEDP at the end of perfusion. Each bar represents the mean±SEM, n = 6. ## P<0.01 vs. the control, **P<0.01 vs. with CaD3, † P<0.01 vs. MDL+CaD3. Please refer to Figure 1 for the abbreviations.
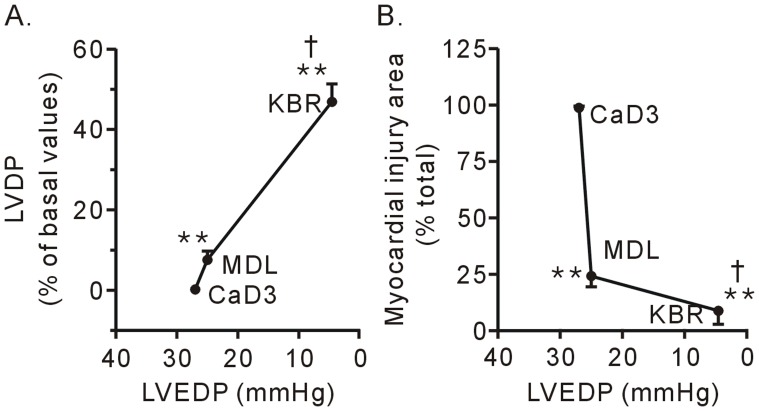
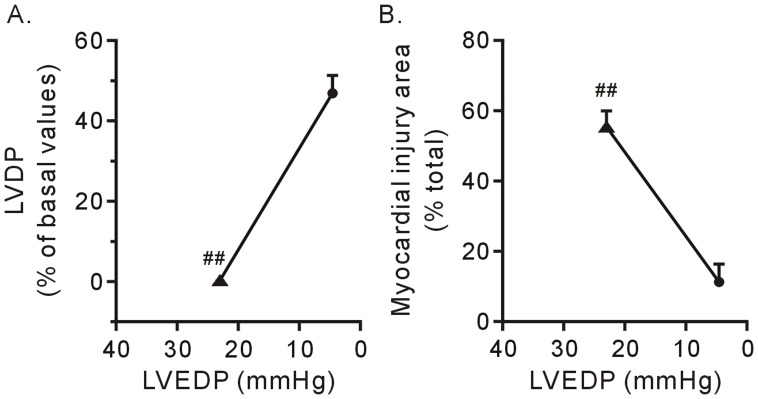
Compared to the calpain inhibitor MDL28170, KB-R7943 strongly decreased the level of LVEDP, which was well correlated with improved contractile function recovery (Figure 2 and 3A) and tissue survival (Figure 1 and 3B). These results confirmed that contracture is the main cause for heart injury in the Ca2+ paradox. Moreover, the results suggest that, in contrast to calpain, contracture is responsible for contractile dysfunction in the Ca2+ paradox.
Figure 3. The contractile function recovery (A) and improvement in cell survival (B) after treatment with 10 µM KB-R7943 (KBR) was correlated to the recovery of LVEDP in the hearts subjected to the 3-min Ca2+ depletion treatment.
Each bar represents the mean±SEM, n = 6. **P<0.01 vs. the 3-min Ca2+ depletion group (CaD3), † P<0.01 vs. MDL28170 (MDL).
KB-R7943 Failed to Rescue Contractile Function after the 5-min Ca2+ Depletion but Still Preserved Surviving Tissue, Although Failing to Reduce the LVEDP
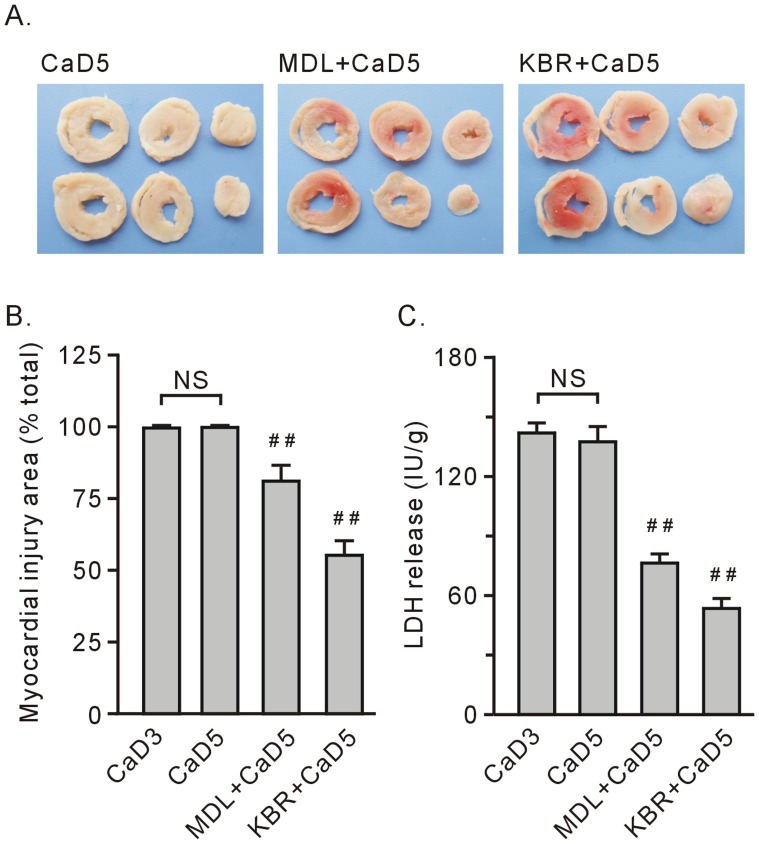
Compared to the 3-min Ca2+ depletion treatment, repletion of the once 5-min Ca2+ depleted hearts did not impose any obvious alterations in TTC staining and LDH release at the end of experiment (Figure 1 and 4). Treatment with KB-R7943 or 10 µM MDL28170 still significantly preserved surviving tissue, although these effects became weakened (Figure 4, A–C).
Figure 4. Treatment with 10 µM KB-R7943 (KBR) or 10 µM MDL28170 (MDL) preserved heart tissue survival after the 5-min Ca2+ depletion treatment.
A. Representative heart tissue slice stained with TTC. B. Group results on the myocardial injury area, which was expressed as a percentage of the total area. C. Group results on LDH release. Each bar represents the mean±SEM, n = 6. ## P<0.01 vs. the 5-min Ca2+ depletion group (CaD5). NS stands for no significant difference between the 3-min Ca2+ depletion group (CaD3) and CaD5.
After repletion of the once 5-min Ca2+ depleted hearts, there was no contractile performance as indicated by the vanishing LVDP, a finding similar to that of the 3-min Ca2+ depletion treatment (Figure 2 and 5A). The LVEDP significantly increased at the end of perfusion, although it was below the level after the 3-min Ca2+ depletion (Figure 5B). Treatments with either 10 µM KB-R7943 or 10 µM MDL28170 failed to rescue the LVDP, with no alterations in the LVEDP (Figure 5, A and B).
Figure 5. Treatment with 10 µM KB-R7943 (KBR) or 10 µM MDL28170 (MDL) failed to rescue heart function recovery after the 5-min Ca2+ depletion treatment.
A. Representative left ventricular pressure tracing. B. Group results on the LVEDP at the end of perfusion. Each bar represents the mean±SEM, n = 6. Compared to the 3-min Ca2+ depletion group (CaD3), # P<0.05.
Figure 6 shows the relationships between the contracture and contractile function recovery and between the contracture and surviving tissue in the hearts subjected to different durations of Ca2+ depletion. In the hearts subjected to the 3-min Ca2+ depletion treatment, treatment with 10 µM KBR7943 reduced the LVEDP back to normal levels during Ca2+ repletion and significantly improved the contractile function recovery of the heart. In contrast, treatment with 10 µM KBR7943 failed to reduce the LVEDP, and the effects on contractile function recovery and tissue survival were diminished in the hearts subjected to the 5-min Ca2+ depletion treatment (Figure 6, A and B).
Figure 6. Impairment of contractile function recovery (A) and tissue survival (B) by KB-R7943 was correlated to its blunted effects on LVEDP in the hearts subjected to the 5-min Ca2+ depletion.
Each bar represents the mean±SE, n = 6. The circle refers to the 3-min Ca2+ depletion group, and the triangle refers to the 5-min Ca2+ depletion group; ## P<0.01 vs. the 3-min Ca2+ depletion group.
Both MDL28170 and KB-R7943 Attenuated Ca2+ Repletion-induced Increases in Calpain Activity in the 3-min and 5-min Ca2+ Depleted Hearts
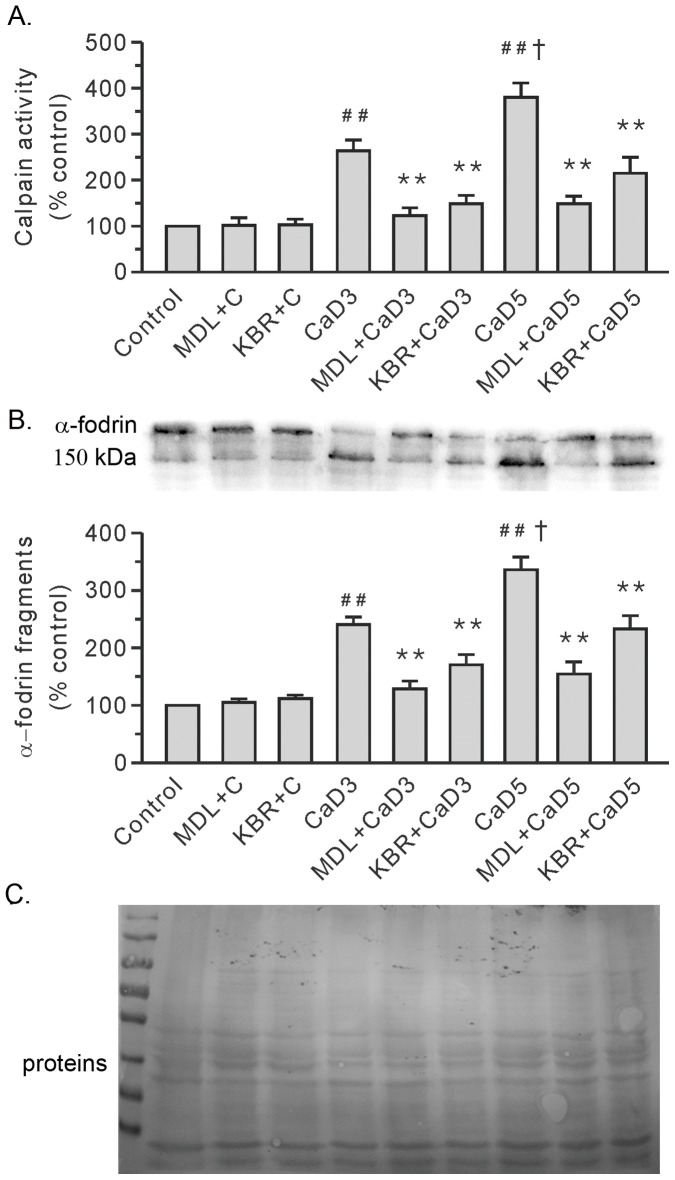
We measured the calpain activity to delineate the role of calpain in the heart injury induced by the Ca2+ paradox. Compared to the control group, repletion of the once 3-min Ca2+ depleted hearts induced an increase in calpain activity (Figure 7A). Moreover, the hearts in the 5-min Ca2+ depletion group exhibited higher levels of calpain activity than the levels in the 3-min Ca2+ depleted group (Figure 7A). The documented cleavage of α-fodrin, a well-known calpain substrate, further substantiated the activation of calpain (Figure 7B). More importantly, KB-R7943, like MDL28170, significantly attenuated Ca2+ repletion-induced increases in calpain activity in the 3-min and 5-min Ca2+ depleted hearts (Figure 7). Both KB-R7943 and MDL28170 had no effect on the control hearts.
Figure 7. Treatment with 10 µM KB-R7943 (KBR) or 10 µM MDL28170 (MDL) reduced the activity of calpain in the Ca2+ paradoxic hearts.
A. Group results on calpain activity by using Suc-LLVY-AMC. B. Representative blots for α-fodrin are shown at the top, along with the densitometric analysis result (bottom). C. Ponceau Red staining of the membrane. The values were expressed as arbitrary units relative to the control, which was given a value of 1. Each bar represents the mean±SEM, n = 6. ## P<0.01 vs. the control, † P<0.01 vs. the 3-min Ca2+ depletion group (CaD3), **P<0.01 vs. the corresponding group without the drug. MDL+C, MDL28170 control; KBR+C, KB-R7943 control; and CaD5, 5-min Ca2+ depletion group.
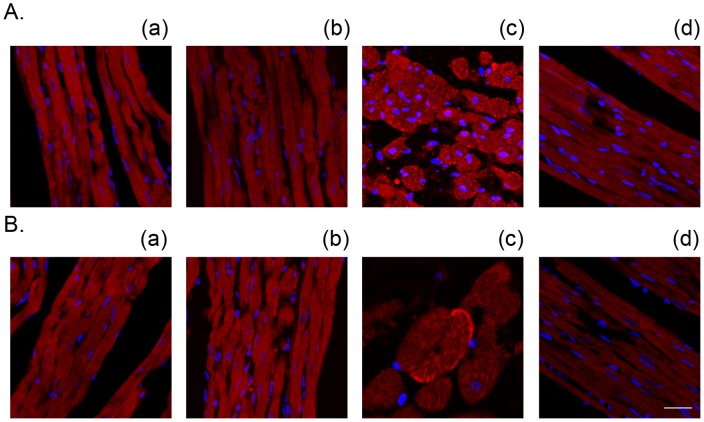
We previously illustrated that repletion of the once 3-min Ca2+ depleted hearts induced an increase in membrane-anchored calpain [20]. Therefore, we evaluated the effects of KB-R7943 by confocal imaging. Similar to the calpain inhibitor MDL28170, treatment with 10 µM KB-R7943 blocked the alterations of both μ- and m-calpain, confirming that activation of calpain is an important factor contributing to heart injury by the Ca2+ paradox (Figure 8 and Table 1). In contrast to the hearts in the 3-min Ca2+ depletion group where μ-calpain was predominantly activated (Figure 8), both μ- and m-calpain were anchored to the sarcolemmal membrane in the hearts in the 5-min Ca2+ depletion group (Figure 9 and Table 2), reflecting increased activity of calpain in the 5-min Ca2+ depletion group. Nevertheless, these effects were attenuated by both KB-R7943 and MDL28170 (Figure 8 and 9, Table 1 and 2). Neither KB-R7943, nor MDL28170 had an effect in the control hearts (Figure 8 and 9, Table 1 and 2).
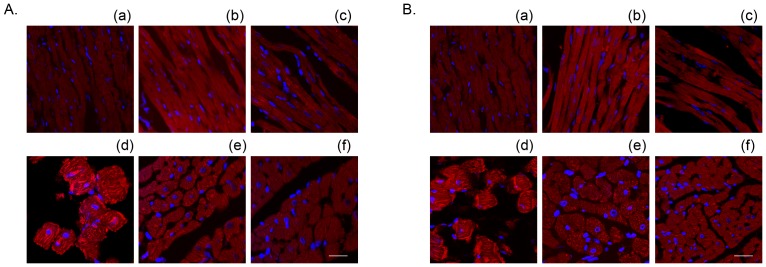
Figure 8. Treatment with 10 µM KB-R7943 attenuated the Ca2+ repletion-induced membrane anchoring of both μ- (A) and m-calpain (B) in the 3-min Ca2+ depletion group.
The nuclei were counterstained with DAPI (blue). (a) control, (b) KB-R7943 control, (c) Ca2+ paradox, (d) Ca2+ paradox with KB-R7943. Scale bar, 5 µm.
Table 1. The effects of treatment with 10 µM KB-R7943 or 10 µM MDL28170 on the percentage of calpain membrane-positive cells in the hearts subjected to the 3-min Ca2+ depletion and 2-min Ca2+ repletion.
| control | MDL+C | KBR+C | CaD3 | MDL+CaD3 | KBR+CaD3 | |
| % μ-calpain positive cells | 0 | 0 | 0 | 23.2±3.1## | 7.6±1.1** | 3.5±0.4** |
| % m-calpain positive cells | 0 | 0 | 0 | 3.1±0.4## | 2.2±0.5 | 0.8±0.1** |
The data are documented as the mean±SEM, n = 4.
P<0.01 vs. the control,
P<0.01 vs. the corresponding group without the drug. KBR+C, KB-R7943 control; MDL+C, MDL28170 control; CaD3, 3-min Ca2+ depletion group; MDL, MDL28170; and KBR, KB-R7943.
Figure 9. Treatment with 10 µM KB-R7943 or 10 µM MDL28170 attenuated the Ca2+ repletion-induced membrane anchoring of both μ- (A) and m-calpain (B) in the 5-min Ca2+ depletion group.
(a)–(c) represent the control, MDL28170 and KB-R7943 control hearts, respectively, (d)-(f) represent the 5-min Ca2+ depletion, with MDL28170 and with KB-R7943, respectively. Scale bar, 5 µm.
Table 2. The effects of treatment with 10 µM KB-R7943 or 10 µM MDL28170 on the percentage of calpain membrane-positive cells in the hearts subjected to the 5-min Ca2+ depletion and 2-min Ca2+ repletion.
| control | MDL+C | KBR+C | CaD3 | CaD5 | MDL+CaD5 | KBR+CaD5 | |
| % μ-calpain positive cells | 0 | 0 | 0 | 23.2±3.1## | 34.1±4.5## | 11.6±1.5** | 5.5±0.8** |
| % m-calpain positive cells | 0 | 0 | 0 | 3.1±0.4## | 40.1±5.5##, † | 12.3±1.4** | 8.2±0.7** |
The data are documented as the mean±SEM, n = 4.
P<0.01 vs. the control,
P<0.01 vs. CaD3,
P<0.01 vs. the corresponding group without the drug. KBR+C, KB-R7943 control; MDL+C, MDL28170 control; CaD3, 3-min Ca2+ depletion group; CaD5, 5-min Ca2+ depletion group; MDL, MDL28170; and KBR, KB-R7943.
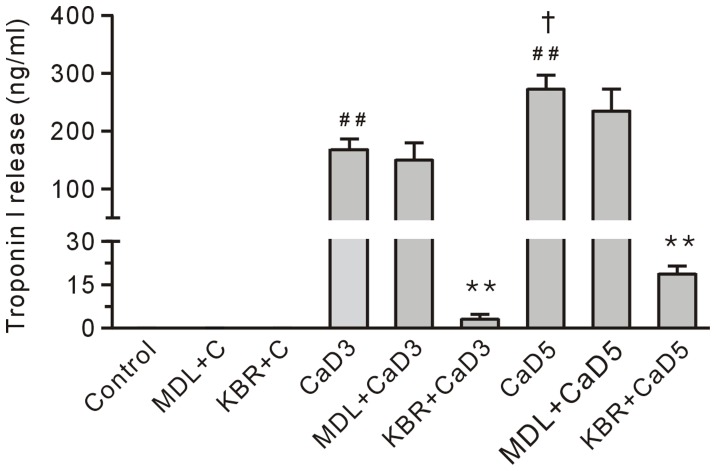
KB-R7943, but not MDL28170, Reduced Ca2+ Repletion-induced Troponin I Release in the 3-min and the 5-min Ca2+ Depleted Hearts
Compared to the control group, the level of troponin I in the coronary effluent in the 3-min Ca2+ depletion group was significantly increased (Figure 10), suggesting that the loss of troponin I may result in the development of a contracture. Furthermore, the amount of troponin I in the coronary effluent in the 5-min Ca2+ depletion group was higher than the level in the 3-min Ca2+ depletion group (Figure 10), which was consistent with the data showing that the hearts in the 5-min Ca2+ depletion group had more severe diastolic dysfunction and contracture (Figure 5). Treatment with 10 µM KB-R7943 significantly reduced the loss of troponin I in the Ca2+ paradoxic heart; however, treatment with 10 µM MDL28170 had no effect (Figure 10), indicating calpain was not involved in the development of the contracture in the Ca2+ paradoxic heart.
Figure 10. Treatment with 10 µM KB-R7943 (KBR), but not 10 µM MDL28170 (MDL) reduced the level of troponin I in the coronary effluent from the Ca2+ paradoxic hearts.
Each bar represents the mean±SEM, n = 6. ## P<0.01 vs. the control, † P<0.01 vs. the 3-min Ca2+ depletion group (CaD3), **P<0.01 vs. the corresponding group without the drug. MDL+C, MDL28170 control; KBR+C, KB-R7943 control; and CaD5, 5-min Ca2+ depletion group.
Discussion
It is generally accepted that the development of the contracture is the primary event in the Ca2+ paradox that leads to cell death and heart dysfunction [5], [10]. The most interesting findings in the present study were 1) compared with the calpain inhibitor MDL28170, KB-R7943 was more effective at rescuing heart contractile dysfunction and tissue death after the 3-min Ca2+ depletion, and this was accompanied by reduced LVEDP levels to a greater extent; 2) After prolonging the duration of Ca2+ depletion to 5 min, KB-R7943 failed to decrease the level of LVEDP and rescue contractile dysfunction, but the treatment still preserved surviving tissue against Ca2+ repletion and inhibited calpain activity. These results provide, for the first time, evidence suggesting that contracture is the main cause for contractile dysfunction, and activation of calpain mediates cell death in the Ca2+ paradox.
In the current study, we found that both KB-R7943 and MDL28170 improved the survival of heart tissue from the 3-min and 5-min Ca2+ depletion treatment, which was accompanied with a reduction in the spike in calpain activity and the degradation of α-fordin. These results support the notion that the activation of calpain is a critical event leading to tissue death in the Ca2+ paradox. Furthermore, our data, consistent with previous studies, identified membrane-anchored μ- and m-calpain in the Ca2+ paradoxical hearts, suggesting that this step triggers a strong activation of this protease [12], [18]. Thus, it would be interesting to identify the function of calpain and the underlying activation mechanism. In addition, previous studies showed that mitochondria become swollen in shape and functionally defective in the Ca2+ paradox [8], [33]. Further studies are needed to clarify the relationship between calpain and proteins related to mitochondria in the Ca2+ paradox because mitochondria controls the production of high energy phosphate and cell death, which are regulated by Ca2+ [34].
Contracture developing in the Ca2+ paradox include the following major characteristics: 1) abnormalities in the sarcomere structure, i.e., some degree of A-band compression and apparent thickening of the Z-bands; 2) contracture force development, which is characterized by an increase in the LVEDP performance and consequently disruption of the sarcolemmal membrane [5], [7], [8], [9], [10]. In the present study, it was found that the intensity of the contracture in the 5-min Ca2+ depletion group is weaker than the contracture intensity in the 3-min Ca2+ depletion group. This lesser intensity may be due to the amount of Ca2+ in the sarcoplasmic reticulum is decreased by the prolonged Ca2+-free superfusion time [35]. More importantly, our data revealed that there was a positive relationship between contractile function recovery and the level of LVEDP (Figure 3 and 6), suggesting that contracture results in contractile dysfunction in the Ca2+ paradox. Compared to MDL 28710, an inhibitor of calpain, KB-R7943 had greatly decreased the level of LVEDP and improved cell survival. We interpret from our results that force stress from the contracture and the activation of calpain are two key nodes in the Ca2+ paradox cascade, which results in hearts injury.
An interesting observation in this study is that KB-R7943 rescued contractile dysfunction after the 3-min Ca2+ depletion, which was accompanied with a decreased release of troponin I, a regulatory protein involved in maintaining the diastolic state [36], [37]. This is in contrast to previous studies showing that nifedipine or verapamil had no effects on the recovery of mechanical activity [38]and the data showing that the canonical transient receptor potential channels mediate Ca2+ influx [35]. Our data support the view that NCX mediates Ca2+ overload in the Ca2+ paradox [10], [39]. More importantly, our result suggests that loss of troponin I is an important step for inducing the development of the contracture in the Ca2+ paradoxic hearts. KB-R7943 failed to rescue heart contractile dysfunction after the 5-min Ca2+ depletion treatment, compared to the corresponding data in the 3-min Ca2+ depletion group. The data measuring the level of troponin I in the coronary effluent showed that, although KB-R7943 reduced the release of troponin I in the Ca2+ paradoxic hearts, the amount of troponin I release in the 5-min Ca2+ depletion with KB-R7943 group was still higher than the amount released in the control hearts (Figure 10). This may explain the failure of KB-R7943 to rescue the mechanical performance of the heart in the 5-min Ca2+ depletion group. In addition, we cannot rule out the possibility that other calcium channels, i.e., the canonical transient receptor potential channels [35] may contribute to Ca2+ overload in the Ca2+ paradox.
In this study, we found that MDL28170 did not affect the release of troponin I from the Ca2+ paradoxic hearts, supporting the notion that activation of calpain mediates tissue death, but not contractile dysfunction. In addition, the ubiquitin proteasome system has been reported to mediate sarcomere degradation [40]; thus, further studies are needed to clarify whether this system participates in the Ca2+ overload-induced troponin I release. Figures 1 and 4 show that the area of surviving tissue in the 5-min Ca2+ depletion with MDL28170 group was smaller than the area in the corresponding 3-min Ca2+ depletion group. We suggest that the disruption of the sarcolemmal membrane caused by contracture aggravates calpain-mediated tissue death.
These results are clinically relevant because left ventricular diastolic dysfunction with a normal ejection fraction currently represents 40% to 50% of all heart failure cases [41], 44% to 75% in patients operated for coronary artery disease or aortic stenosis [42]. The left ventricular diastolic dysfunction is linked to an increased incidence of mortality and higher healthcare expenditures. Clinical and laboratory studies showed that loss of Ca2+ homeostasis and the subsequent development of contractures are some of the important factors for diastolic dysfunction [3], [4], [43]. Coincidently, calpain activated by Ca2+ overload has been implicated as an target for treating myocardial ischemia/reperfusion injury [18], [19]. In this study, we suggest that blocking both the development of diastolic dysfunction and the activation of calpain concurrently may be a therapeutic approach in limiting myocardial injury, especially in heart failure and cardiac surgery, and improving the survival of patients.
In summary, the present study provides the first evidence suggesting that the contracture is the main cause for contractile dysfunction, while activation of calpain mediates tissue death in the Ca2+ paradox. Further studies should focus on unfolding the molecular mechanism underlying the development of the contracture in the Ca2+ paradox.
Acknowledgments
The authors thank Haifeng Zhang for his technical support.
Funding Statement
This work was supported by Natural Science Foundation of China (grant no. 30971196 and 81100082). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415: 198–205. [DOI] [PubMed] [Google Scholar]
- 2. Fozzard HA (2002) Cardiac sodium and calcium channels: a history of excitatory currents. Cardiovasc. Res. 55: 1–8. [DOI] [PubMed] [Google Scholar]
- 3. Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, et al. (2010) Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Talukder MA, Zweier JL, Periasamy M (2009) Targeting calcium transport in ischaemic heart disease. Cardiovasc. Res. 84: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piper HM (2000) The calcium paradox revisited: an artefact of great heuristic value. Cardiovasc. Res. 45: 123–127. [DOI] [PubMed] [Google Scholar]
- 6. Zimmerman AN, Hulsmann WC (1966) Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature 211: 646–647. [DOI] [PubMed] [Google Scholar]
- 7. Vander Heide RS, Altschuld RA, Lamka KG, Ganote CE (1986) Modification of caffeine-induced injury in Ca2+-free perfused rat hearts. Relationship to the calcium paradox. Am. J. Pathol. 123: 351–364. [PMC free article] [PubMed] [Google Scholar]
- 8. Singal PK, Matsukubo MP, Dhalla NS (1979) Calcium-related changes in the ultrastructure of mammalian myocardium. Br. J. Exp. Pathol. 60: 96–106. [PMC free article] [PubMed] [Google Scholar]
- 9. Vander Heide RS, Angelo JP, Altschuld RA, Ganote CE (1986) Energy dependence of contraction band formation in perfused hearts and isolated adult myocytes. Am. J. Pathol. 125: 55–68. [PMC free article] [PubMed] [Google Scholar]
- 10. Daly MJ, Elz JS, Nayler WG (1987) Contracture and the calcium paradox in the rat heart. Circ. Res. 61: 560–569. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi K, Takahashi T, Suzuki T, Onishi M, Tanaka Y, et al. (2003) Protective effects of SEA0400, a novel and selective inhibitor of the Na+/Ca2+ exchanger, on myocardial ischemia-reperfusion injuries. Eur. J. Pharmacol. 458: 155–162. [DOI] [PubMed] [Google Scholar]
- 12. Leloup L, Shao H, Bae YH, Deasy B, Stolz D, et al. (2010) m-Calpain activation is regulated by its membrane localization and by its binding to phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 285: 33549–33566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol. Rev. 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 14. Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, et al. (2007) Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ. Res. 100: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki K, Hata S, Kawabata Y, Sorimachi H (2004) Structure, activation, and biology of calpain. Diabetes 53 Suppl 1S12–18. [DOI] [PubMed] [Google Scholar]
- 16. Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J (2005) Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ. Res. 97: 465–473. [DOI] [PubMed] [Google Scholar]
- 17. Chen M, He H, Zhan S, Krajewski S, Reed JC, et al. (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J. Biol. Chem. 276: 30724–30728. [DOI] [PubMed] [Google Scholar]
- 18. Hernando V, Inserte J, Sartorio CL, Parra VM, Poncelas-Nozal M, et al. (2010) Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J. Mol. Cell. Cardiol. 49: 271–279. [DOI] [PubMed] [Google Scholar]
- 19. Mani SK, Balasubramanian S, Zavadzkas JA, Jeffords LB, Rivers WT, et al. (2009) Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 297: H1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bi SH, Jin ZX, Zhang JY, Chen T, Zhang SL, et al. (2012) The calpain inhibitor MDL28170 protects against Ca2+ paradox in rat hearts. Clin. Exp. Pharmacol. Physiol. 39: 385–392. [DOI] [PubMed] [Google Scholar]
- 21. Gaitanaki C, Papazafiri P, Beis I (2003) The calpain-calpastatin system and the calcium paradox in the isolated perfused pigeon heart. Cell. Physiol. Biochem. 13: 173–180. [DOI] [PubMed] [Google Scholar]
- 22. Iwamoto T, Watano T, Shigekawa M (1996) A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 271: 22391–22397. [DOI] [PubMed] [Google Scholar]
- 23. Satoh H, Ginsburg KS, Qing K, Terada H, Hayashi H, et al. (2000) KB-R7943 block of Ca2+ influx via Na+/Ca2+ exchange does not alter twitches or glycoside inotropy but prevents Ca2+ overload in rat ventricular myocytes. Circulation 101: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 24. Donkor IO (2000) A survey of calpain inhibitors. Curr. Med. Chem. 7: 1171–1188. [DOI] [PubMed] [Google Scholar]
- 25. Schafer C, Ladilov Y, Inserte J, Schafer M, Haffner S, et al. (2001) Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc. Res. 51: 241–250. [DOI] [PubMed] [Google Scholar]
- 26. Inserte J, Barba I, Hernando V, Garcia-Dorado D (2009) Delayed recovery of intracellular acidosis during reperfusion prevents calpain activation and determines protection in postconditioned myocardium. Cardiovasc. Res. 81: 116–122. [DOI] [PubMed] [Google Scholar]
- 27. Shan L, Li J, Wei M, Ma J, Wan L, et al. (2010) Disruption of Rac1 signaling reduces ischemia-reperfusion injury in the diabetic heart by inhibiting calpain. Free Radic. Biol. Med. 49: 1804–1814. [DOI] [PubMed] [Google Scholar]
- 28. Sapia L, Palomeque J, Mattiazzi A, Petroff MV (2010) Na+/K+-ATPase inhibition by ouabain induces CaMKII-dependent apoptosis in adult rat cardiac myocytes. J. Mol. Cell. Cardiol. 49: 459–468. [DOI] [PubMed] [Google Scholar]
- 29. Venge P, Lindahl B, Wallentin L (2001) New generation cardiac troponin I assay for the access immunoassay system. Clin Chem 47: 959–961. [PubMed] [Google Scholar]
- 30. Jin ZX, Zhou JJ, Xin M, Peng DR, Wang XM, et al. (2007) Postconditioning the human heart with adenosine in heart valve replacement surgery. Ann. Thorac. Surg. 83: 2066–2072. [DOI] [PubMed] [Google Scholar]
- 31. Yoshida K, Inui M, Harada K, Saido TC, Sorimachi Y, et al. (1995) Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin (nonerythroid spectrin or fodrin) by calpain. Circ. Res. 77: 603–610. [DOI] [PubMed] [Google Scholar]
- 32. Wada T, Hori S, Sugiyama M, Fujisawa E, Nakano T, et al. (2010) Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 298: E881–888. [DOI] [PubMed] [Google Scholar]
- 33. Makazan Z, Saini-Chohan HK, Dhalla NS (2009) Mitochondrial oxidative phosphorylation in hearts subjected to Ca2+ depletion and Ca2+ repletion. Can. J. Physiol. Pharmacol. 87: 789–797. [DOI] [PubMed] [Google Scholar]
- 34. Murgia M, Giorgi C, Pinton P, Rizzuto R (2009) Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J. Mol. Cell. Cardiol. 46: 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kojima A, Kitagawa H, Omatsu-Kanbe M, Matsuura H, Nosaka S (2010) Ca2+ paradox injury mediated through TRPC channels in mouse ventricular myocytes. Br. J. Pharmacol. 161: 1734–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villars PS, Hamlin SK, Shaw AD, Kanusky JT (2004) Role of diastole in left ventricular function, I: Biochemical and biomechanical events. Am. J. Crit. Care 13: 394–403. [PubMed] [Google Scholar]
- 37. MacGowan GA, Du C, Cowan DB, Stamm C, McGowan FX, et al. (2001) Ischemic dysfunction in transgenic mice expressing troponin I lacking protein kinase C phosphorylation sites. Am. J. Physiol. Heart Circ. Physiol. 280: H835–843. [DOI] [PubMed] [Google Scholar]
- 38. Nayler WG, Perry SE, Elz JS, Daly MJ (1984) Calcium, sodium, and the calcium paradox. Circ. Res. 55: 227–237. [DOI] [PubMed] [Google Scholar]
- 39. Chatamra KR, Chapman RA (1996) The effects of sodium-calcium exchange inhibitors on protein loss associated with the calcium paradox of the isolated Langendorff perfused guinea-pig heart. Exp. Physiol. 81: 203–210. [DOI] [PubMed] [Google Scholar]
- 40. Willis MS, Schisler JC, Portbury AL, Patterson C (2009) Build it up-Tear it down: protein quality control in the cardiac sarcomere. Cardiovasc. Res. 81: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, et al. (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 42. Apostolakis EE, Baikoussis NG, Parissis H, Siminelakis SN, Papadopoulos GS (2009) Left ventricular diastolic dysfunction of the cardiac surgery patient; a point of view for the cardiac surgeon and cardio-anesthesiologist. J. Cardiothorac. Surg. 4: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barry WH, Peeters GA, Rasmussen CA Jr, Cunningham MJ (1987) Role of changes in [Ca2+]i in energy deprivation contracture. Circ. Res. 61: 726–734. [DOI] [PubMed] [Google Scholar]