Abstract
Background
We previously demonstrated that Cervarix® elicits antibody responses against vaccine-related types for which clinical efficacy was demonstrated (HPV-31 and -45). Here, we evaluated the kinetics of neutralization titers and avidity of Cervarix®-induced antibodies up to 36 months of follow-up in unexposed and HPV infected women.
Methods
A subset of women who participated in the Cost Rica HPV-16/18 Vaccine Trial had pre- and post-vaccination sera tested for antibody responses to HPV-16, -18, -31, -45, and -58 using a pseudovirion-based neutralization assay, and HPV-16 antibody avidity using an HPV-16 L1 VLP (virus-like particle)-based ELISA developed in our laboratory.
Results
In uninfected women, neutralizing antibody titers did not reach significance until after the 3rd dose for HPV-31 (month 12, p=0.009) and HPV-45 (month 12, p=0.003), but then persisted up to month 36 (HPV-31, p=0.01; HPV-45, p=0.002). Individuals infected with HPV-16 or HPV-31 at enrollment developed a significantly higher median antibody response to the corresponding HPV type after one dose, but there was not a difference between median titers after three doses compared to the HPV negative group. Median HPV-16 antibody avidity and titer increased over time up to month 12; however, the HPV-16 avidity did not correlate well with HPV-16 neutralizing antibody titers at each time point examined, except for month 6. The median avidity levels were higher in HPV-16 infected women at month 1 (p=0.04) and lower in HPV-16 infected women at month 12 (p=0.006) compared to the HPV negative women.
Conclusions
The persistence of cross-neutralization titers at month 36 suggests cross-reactive antibody responses are likely to persist long-term and are not influenced by infection status at enrollment. However, the weak correlation between avidity and neutralization titers emphasizes the need for examining avidity in efficacy studies to determine if high avidity antibodies play a critical role in protection against infection.
Keywords: Human papillomavirus, antibody, vaccine, avidity
1. Introduction
Human papillomavirus (HPV) L1 VLP (virus-like particle)-based vaccines elicit a strong antibody response to targeted HPV types, which is believed to be responsible for the strong efficacy reported in the vaccine trials [1–4]. We have recently demonstrated that Cervarix, which is composed of HPV-16 and HPV-18 L1 VLPs also induces neutralizing antibodies to vaccine-related types (HPV-31 and HPV-45), for which partial protection against cervical infection and neoplasm in young women has been described [2, 5], but not for types for which no efficacy was observed (HPV-52 and -58). Although the cross-neutralizing titers were typically about 100-fold lower than against the targeted types, the suggested cross-protection afforded by the vaccine may lead to a further reduction in cervical cancer [6]. However, the kinetics and durability of the anti-HPV-31 and anti-HPV-45 antibody responses following vaccination is still unknown and these factors may have direct implications in durability of efficacy against these types. A recent study from Einstein and colleagues compared the antibody responses to vaccine-targeted (HPV-16 and HPV-18) and vaccine-related types (HPV-31 and 45) over a 24 month period in women who were healthy, HPV DNA negative and seronegative at baseline for HPV type analyzed in women vaccinated with Cervarix® or Gardasil®[7]. Neutralizing antibody responses to HPV-31 and 45 were low with levels near the limit of detection of the assays.
Thus far, analyses of humoral immunity to the HPV vaccines have been limited to assessment of VLP binding titers and neutralizing activity, and little is known about antibody avidity following HPV vaccination. While the contribution of avidity maturation to protection against viral infections in general is still not well defined and controversial [8–10], the assessment of antibody avidity may provide a more complete view of the quality and function of systemic antibodies induced by vaccination. Lastly, there is little known about the effects of a type-specific cervical HPV infection at the time of vaccination on vaccine-induced antibody responses and duration of immunity.
To better understand the nature of the antibody responses induced by Cervarix®, here we investigated the kinetics of neutralizing anti-HPV-16, -18, -31, -45, and -58 antibody responses and anti-HPV-16 avidity up to 36 months of follow-up among women HPV negative and women HPV positive at baseline.
2. Materials and Methods
Study Population
Samples and data are from participants of the NCI-sponsored Costa Rica HPV Vaccine Trial (CVT) who have been described in detail[11].
I) Thirty-six month kinetics
To determine the kinetics of the antibody response to non-vaccine targeted HPV types induced by VLP vaccination, a group of women (n=157), were selected based on the following criteria: (i) HPV-16 and -18 L1 VLP vaccination (Cervarix®) according to a three dose schedule at months 0, 1, and 6; (ii) successful collection of serum at months 0 (prevaccination), 1 (1 month after 1st vaccine dose), 6 (6 months after 1st vaccine dose), 12 (6 months after all 3 doses were administered), 24, and 36; (iii) DNA negative at the cervix for HPV-16, -18, -31, -45, and -58 at enrollment (month 0). From this group of women, we randomly selected a subset of women (n=12) who were age and number of sexual partners matched for antibody testing. The sera was tested in the pseudovirion (PsV)-based neutralization assay to determine the levels of neutralizing antibodies to the vaccine-targeted types (HPV-16 and HPV-18), and phylogenetically-related HPV types (HPV-31 and HPV-45, for which cross-protection and cross-neutralization has been reported; and HPV-58, for which no evidence of cross-protection and cross-neutralization has been reported) were determined. All sera were also tested for HPV-16 antibody avidity.
II) Effect of prevalent HPV infection on vaccine induced antibody levels
To assess the effects of single HPV type-specific infections detected at month 0 on the antibody responses following vaccination, sera tested at months 0, 1, and 12 from women who met the following criteria: (i) HPV-16 and -18 L1 VLP vaccination (Cervarix®) according to a three dose schedule at months 0, 1, and 6; (ii) successful collection of serum at months 0 (prevaccination), 1 (1 month after 1st vaccine dose), and 12 (6 months after all 3 doses were administered); (iii) single HPV type-specific infection at enrollment (month 0): HPV-16 DNA positive (HPV16+, n=18), HPV-31 DNA positive (HPV31+, n=13), HPV-58 DNA positive (HPV58+, n=7). We randomly selected a subset of self-reported virgins (presumed HPV DNA negative, HPV-, n=15), which functioned as the control group. Sera were tested for HPV-16 antibody avidity and for functional amount of anti-HPV-16, -31, and -58 antibodies in the PsV-based neutralization assay. All participants provided informed consent, and the trial was approved by human subjects review committees at the National Cancer Institute (NCI) and Costa Rica.
Pseudovirion (PsV)-based neutralization assay
A PsV-based neutralization assay (SeAP-NA) was used to determine the antibody titers associated with each HPV type-specific PsV examined, as previously described[12]with slight modifications[13]. Each sample was tested in duplicate, and neutralization titers were calculated by linear interpolation and defined as the reciprocal of the dilution that caused 50% reduction in secreted alkaline phosphatase activity compared to control wells. The antibody titers below our lowest dilution (1/10) were arbitrarily given a value of 5. Due to the lack of availability of pseudovirions for other closely related vaccine types, we have focused this study on measuring the neutralizing antibody responses to HPV-16, -18, -31, -45, and -58.
Modified ELISA Avidity Assay Using Chaotropic Elution
We evaluated avidity of the anti-HPV16 antibodies only. Avidity to the VLPs of other types (HPV-31 and HPV-45) was not performed due to problems with producing these L1 VLPs in our laboratory, and the sensitivity of the ELISA-based avidity assay may not be effective in determining the avidity of antibodies with a low neutralization titer. The method of assessing the avidity of the anti-HPV-16 antibodies was previously reported [14]. Briefly, microtiter plates were coated with HPV-16 L1 VLP, and each serum sample was assayed at a single dilution, ranging from 1/100 to 1/100000 for all samples evaluated, which yields an absorbance reading of 1.0 ± 0.5 as previously determined in an HPV-16 ELISA. Guanidine-HCl (GuHCl) was added to the samples at various concentrations (0.5 to 3.5 M) to elute low avidity antibodies. The concentration of GuHCl, which reduced the optical density by 50% compared to sample wells without GuHCl treatment defined the Avidity Index. Samples with an HPV-16 L1 VLP ELISA unit (EU)/ml ≤2 were not subsequently tested with the avidity assay and were given a value of 0 as they were deemed to be negative.
Statistical Methods
SEAP-NA values (reciprocal of the dilution that caused 50% reduction in secreted alkaline phosphatase activity) were log transformed. Median antibody and avidity levels are presented. The Wilcoxon/Kruskal Wallis test was used to determine statistical significance between median titers and avidity levels by group. The spearman correlation coefficient was used to assess the correlation between HPV-16 neutralizing antibody titers and HPV-16 antibody avidity at months 0 (prevaccination), 1 (1 month after 1st vaccine dose), 6 (6 months after 1st vaccine dose), 12 (6 months after all 3 doses were administered), 24, and 36. A p-value <0.05 was considered significant for these analyses.
3. Results
A. Kinetics of Neutralizing Anti-HPV-31, -45, and -58 antibody titers
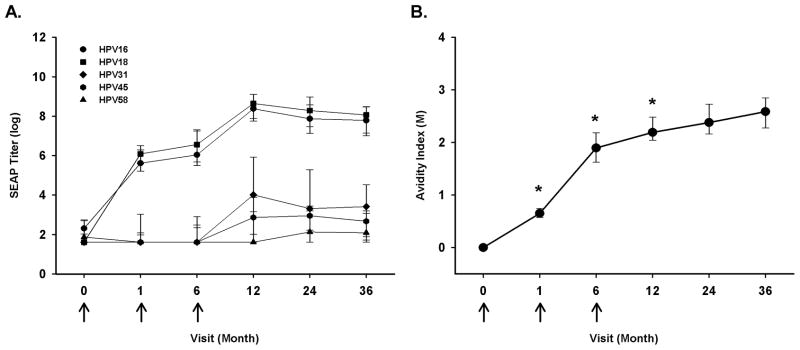
Here, we evaluated the longitudinal neutralizing antibody responses to vaccine types (HPV-16 and 18) and vaccine–related types (HPV-31, 45, and 58) over a period of 36 months of follow-up, specifically months 0, 1, 6, 12, 24, and 36 following enrollment. Anti-HPV-16 and anti-HPV-18 neutralizing antibody responses were similar to our previous report with antibody levels plateauing at month 12 (Figure 1A). Furthermore, the kinetics of anti-HPV-16 and anti-HPV-18 neutralizing antibody responses were consistent with ELISA-based measures of anti-HPV-16 and -18 antibody levels during vaccination and up to 36 months of follow up (data not shown). A significant anti-HPV-31 and -45 neutralizing antibody response over the baseline levels (enrollment) was only elicited following the administration of all three doses of Cervarix® at month 12 (HPV-31, p=0.009; HPV-45, p=0.003). This response was about 100-fold lower than the vaccine targeted types, but the neutralizing antibody levels were maintained over 36 months of follow-up. In contrast, Cervarix® did not induce a neutralizing antibody response to HPV-58, a phylogenetically more distant (compared to HPV31) relative of HPV-16 and for which there is no evidence of efficacy.
Figure 1.
Kinetic profile of neutralizing anti-HPV-16, -18, -31, -45, and -58 antibody responses and HPV-16 avidity following enrollment into HPV-16 and -18 L1 VLP vaccine trial among HPV uninfected women. Participants (n = 12) received three doses of Cervarix® at months 0, 1, and 6 (arrows). The participant’s neutralizing antibody responses (A) were evaluated in a pseudovirion-based neutralization assay from enrollment to 36 months post-enrollment. The lines represent median values at each visit, and the error bars represent the 25th to 75th percentiles. The participant’s anti-HPV-16 avidity response (B) was evaluated from enrollment to 36 months post-enrollment. The lines represent median values at each visit. Wilcoxon test was used to calculate the p-value (*-p<0.05) and represents the comparison between current visit and preceding visit.
B. HPV-16 avidity
As with the longitudinal anti-HPV-16 neutralizing antibody response, the anti-HPV-16 avidity response significantly increased with sequential visits up to month 12 (Figure 1B). Therefore, avidity maturation continued to occur even after the third dose of Cervarix®. The avidity of the antibodies remained relatively constant between months 12 and 36, indicating that there was apparently no further selection for or against high avidity plasma cells. Perhaps surprisingly, the anti-HPV-16 avidity responses only correlated with the anti-HPV-16 neutralizing antibody levels at month 6 (ρ=0.72, p=0.008) from the participants. All other time points analyzed did not have a significant correlation between the anti-HPV-16 avidity responses and anti-HPV-16 neutralizing antibody levels. Avidity to vaccine-related types was not evaluated in this study due to the lack of these VLPs in our laboratory. However, when considering the low titers of neutralizing antibodies, frequency of responders, and sensitivity of the ELISA-based avidity assay, one might expect difficulties in detecting avidity maturation to HPV-31 and HPV-45 VLPs.
C. Effects of type-specific HPV infection on neutralizing antibody responses
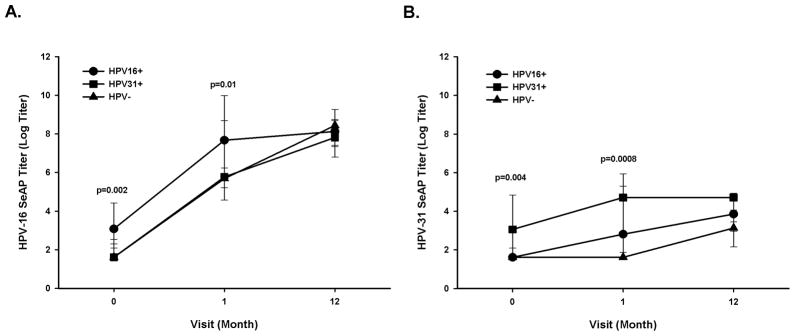
Next, we evaluated whether women with evidence of detectable HPV infection at the cervix, and thus presumably immunologically-primed at the time of vaccination, would develop a higher neutralizing antibody response against the homologous type compared to HPV DNA negative self-reported virgins (HPV-), following vaccination with Cervarix®. We focused on measuring the anti-HPV-16 and phylogenetically-related anti-HPV-31 antibody responses from same-type and related-type infections. The anti-HPV-16 neutralizing antibody responses increased significantly from month 0 to month 1, following one dose of vaccine for all three groups: women HPV-16 positive (HPV16+, p<0.0001), women HPV-31 positive (HPV31+, p=0.0003), and self-reported virgins, (HPV- p<0.0001), yet there was only a significant increase in anti-HPV-16 neutralizing titer from month 1 to month 12 for the participants within the HPV- group (p<0.0001), but not for the HPV16+ (p=0.7) and HPV31+ (p=0.1) groups. Furthermore, the HPV16+ group had significantly higher titers, due to pre-existing immunity to HPV-16 than the HPV- group at month 0 (median titers: HPV16+, 22; HPV-, 5; p=0.002) and month 1(median titers: HPV16+, 2152; HPV-, 294; p=0.01) (Figure 2A). However, at month 12 the anti-HPV-16 titers were equivalent between the HPV16+ and HPV- groups (median titers: HPV16+, 3385; HPV-, 4616; p=0.8). Lastly, the anti-HPV-16 neutralizing antibody responses induced by vaccination were unaffected from enrollment to month 12 by HPV-31 infection at enrollment when compared to the HPV- group (Figure 2A).
Figure 2.
Effects of HPV-16 and -31 infections at enrollment on Cervarix®- induced anti-HPV-16 and anti-HPV-31 neutralizing antibody responses. Participants with an HPV-16 infection (HPV16+, n = 18), HPV-31 infection (HPV31+, n = 13), or HPV DNA negative, self-reported virgins (HPV-, n=15) at enrollment were evaluated for anti-HPV16 (A) and anti-HPV-31 (B) neutralizing antibody responses via pseudovirion-based neutralization assay at enrollment (month 0), month 1, and month 12. The lines represent median values at each visit, and the error bars represent the 25th to 75th percentiles. The p-value was calculated based on the Wilcoxon test and represents the comparison between HPV- and HPV16+ at each visit in panel A and between HPV- and HPV31+ at each visit in panel B.
We also evaluated the effect of vaccination on anti-HPV-31 neutralizing antibody responses in participants with either HPV-31 or HPV-16 infection at enrollment. The anti-HPV-31 neutralizing antibody titers did not increase from month 0 to month 1 for HPV- (p=1.0) or HPV31+ (p=0.2), but did increase for HPV16+ (p=0.03) group. Additional doses of Cervarix® did not significantly increase the anti-HPV-31 neutralizing antibody titers among the HPV16+ group from month 1 to month 12 (p=0.3). Despite the lack of boosting effect of Cervarix® on HPV-31 responses in individuals with HPV-31 infection, HPV31+ group had overall significantly higher anti-HPV-31 neutralizing antibody titers due to pre-existing immunity to HPV-31 than the HPV- group (median titers at month 0: HPV31+, 21; HPV-, 5; p=0.004; median titers at month 1: HPV31+, 111; HPV-, 5; p=0.0008) (Figure 2B).
D. Effects of type-specific HPV infection on anti-HPV-16 avidity
The anti-HPV-16 median avidity levels were higher in HPV-16 infected women than in HPV DNA negative after one dose of Cervarix® (HPV16+, 1.3; HPV-, 0.8; p=0.04), but was lower after three doses of Cervarix® (HPV16+, 1.8; HPV-, 2.4; p=0.006) as shown in Figure 3. Furthermore, we assessed whether an HPV-31 infection prior to vaccination would affect the anti-HPV-16 avidity response after one and three doses of Cervarix®, and, as illustrated in Figure 3, there was not a significant difference in anti-HPV-16 avidity levels after receiving either one or three doses of Cervarix® compared to the HPV- group.
Figure 3.
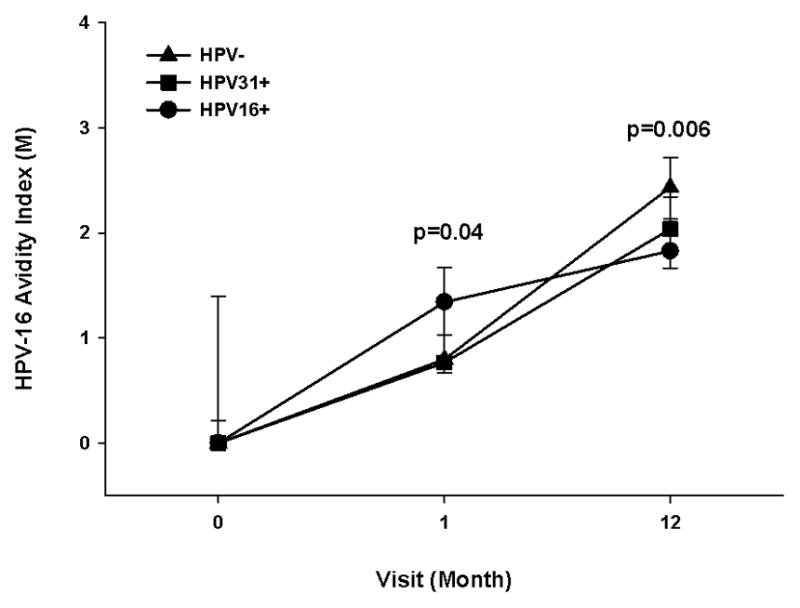
Effects of HPV-16 infection at enrollment on Cervarix® -induced anti-HPV-16 avidity response. Participants with an HPV-16 infection (HPV16+, n=18), HPV-31 infection (HPV31+, n=13), and HPV DNA negative, self-reported virgins (HPV-, n=15) at enrollment had serum tested for avidity at enrollment (month 0), months 1 and 12. The lines represent median values at each visit, and the error bars represent the 25th to 75th percentiles. The Wilcoxon test was used to calculate the p value between uninfected and infected individuals at each visit (infection based on enrollment HPV DNA assessment). A p-value <0.05 was considered significant, and the indicated p values are based on the comparison between the HPV16+ and HPV- groups at the same visit.
4. Discussion
HPV-16/18 antibody responses to Cervarix® vaccination are known to be robust and to last for many years [2, 3]. We and others previously showed that anti-HPV antibody responses to vaccine-related (HPV-31 and HPV-45) types are also induced by vaccination with the bivalent HPV L1 VLP vaccine[5, 7, 15, 16], but require three doses and are typically 100-fold lower than observed for the vaccine targeted types, HPV-16/18. Here, we have extended those previous findings by showing the kinetics of the antibody response to vaccine cross-related types and that anti-HPV-31 and -45 antibodies plateau and remain elevated for up to 3 years. HPV-31 and HPV-45 contribute to approximately 4% and 6%, respectively, of cervical cancers worldwide, so a durable cross-protective response induced by Cervarix® may potentially translate into an additional clinical benefit on women’s health[17].
The kinetic findings of neutralizing anti-HPV-31 antibodies up to 24 months from Einstein et al. are similar to our results; however, Einstein et al. infrequently detected neutralizing anti-HPV-45 antibodies, which is in contrast to our results. One reason for the discrepancy may be the method of defining the lower limit of detection for the HPV-31 and HPV-45 pseudovirion-based neutralization assays[7]. Interestingly, the neutralizing antibody levels did not reach significance over baseline (enrollment) until after all three doses were received, which further supports the findings from Kreimer et al., who described good type-specific but no cross-type protection in women receiving just one or two doses of Cervarix®[19].
One of the main highlights of this manuscript is that we show for the first time that the HPV-16 avidity response gradually increased after each dose of Cervarix® and peaked after receiving three doses. These results are consistent with the avidity responses following other vaccinations such as Hepatitis B virus vaccine [20] and influenza vaccine [9], although in contrast with previous studies of vesicular stomatitis virus infection in mice that suggested that maximum avidity maturation was rapidly reached following initial exposure to a capsid antigen[10].
We further observed that the anti-HPV-16 neutralization antibody responses and avidity responses were significantly elevated at month 1, following the first dose of vaccination amongst vaccine recipients, who were HPV-16 DNA positive at enrollment compared to HPV DNA negative, self-described virgin vaccinees, which is consistent with a typical immune memory recall response in the context of previous antigenic priming. However, following the completion of the vaccination series, there was no benefit to having a pre-existing immune response to HPV-16 as the HPV16+ group developed similar neutralizing antibody titers and significantly lower avidity levels compared to HPV- group after receiving 3 doses of Cervarix® by month 12. One caveat with our interpretation of the results is the assumption that each participant has only been infected with one type of HPV, but it is possible for the participants to have been infected with the same type of HPV or other HPV type months to years prior which may have influenced our results. Although, the avidity levels amongst the HPV-16 DNA positive women were significantly lower than the HPV- group, we also examined the avidity levels in a group of women who were HPV-16 DNA negative and sexually experienced, and the avidity levels at month 12 from this group were similar to the HPV-16 DNA positive women (data not shown). However, it remains to be determined whether the difference in avidity levels at month 12 is biologically significant. Future studies with larger sample sizes will be needed to confirm and better understand the impact of infection in avidity of antibodies induced by vaccination.
Interestingly, there was a lack of correlation between anti-HPV-16 neutralizing antibody levels and anti-HPV-16 avidity for nearly all of the time points evaluated, except at month 6. It may be that neutralizing activity of VLP antibodies are relatively independent of their avidity once a threshold level is reached following primary vaccination. Due to the limited sample size (n=12) at each time point, future studies will be needed to confirm our findings.
Here, we were only able to examine the HPV-16 avidity responses following vaccination with Cervarix® due to production problems of HPV-31 and HPV-45 L1 VLPs in our laboratory; however, it will be of interest in future studies to optimize avidity assays for vaccine-related types (HPV-31 and HPV-45) to determine whether avidity maturation patterns will mimic vaccine types. Considering the low neutralizing antibody levels from the vaccine-related types, the ELISA-based avidity assay for these types may need to be replaced by a more sensitive method such as surface plasmon resonance (SPR) based technology.
In conclusion, Cervarix® induces a significant increase in anti-HPV-31 and anti-HPV-45 neutralization antibody levels following three doses of vaccine, and levels persisted for three years. Furthermore, a homologous infection at time of the vaccination and thus, pre-existing immunity did not influence the overall antibody response achieved after the full vaccination course except for avidity for which levels were lower at 12-months for those positive at entry. Larger and longer studies are needed to determine the minimum protective anti-HPV antibody levels in vivo, the role of high avidity antibodies in vaccine efficacy and natural history, and the long-term effects of pre-existing HPV infection on vaccine-induced immunogenicity.
Highlights.
Evaluated kinetics of Cervarix®-induced neutralization and avidity antibodies.
Evaluated effects of homologous HPV infection on Cervarix®-induced antibody levels.
3 doses of Cervarix® induces significant levels of vaccine-related HPV antibodies.
HPV-16 avidity has similar kinetic profile as neutralizing antibodies.
Following 3 doses of Cervarix®, HPV infection had little effect on antibody levels.
Acknowledgments
We would like to thank Tadahito Kanda for the plasmid used to generate the HPV58 pseudovirions, Susana Pang and Cynthia Thompson for their help with pseudovirion production as well as the Costa Rica Vaccine Trial (CVT) group members. The names and affiliations of investigators in the Costa Rica Vaccine Trial (CVT) group are given below.
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica:
Mario Alfaro (Cytopathologist);
Manuel Barrantes (Field Supervisor);
M. Concepción Bratti (co-Investigator);
Fernando Cárdenas (General Field Supervisor);
Bernal Cortés (Specimen and Repository Manager);
Albert Espinoza (Head, Coding and Data Entry);
Yenory Estrada (Pharmacist);
Paula González (co-Investigator);
Diego Guillén (Pathologist);
Rolando Herrero (co-Principal Investigator);
Silvia E. Jiménez (Trial Coordinator);
Jorge Morales (Colposcopist);
Luis Villegas (Colposcopist);
Lidia Ana Morera (Head Study Nurse);
Elmer Pérez (Field Supervisor);
Carolina Porras (co-Investigator);
Ana Cecilia Rodríguez (co-Investigator);
Libia Rivas (Clinical coordinator).
University of Costa Rica, San José, Costa Rica:
Enrique Freer (Director, HPV Diagnostics Laboratory);
José Bonilla (Head, HPV Immunology Laboratory);
Alfonso García-Piñeres (Immunologist);
Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory);
Ivannia Atmella (Microbiologist, Immunology Laboratory);
Margarita Ramírez (Microbiologist, Immunology Laboratory).
US National Cancer Institute, Bethesda, MD:
Allan Hildesheim (co-Principal Investigator & NCI co-Project Officer);
Aimée R. Kreimer (co-Investigator);
Mahboobeh Safaeian (co-Investigator)
Douglas R. Lowy (HPV Virologist);
Nora Macklin (Trial Coordinator);
Mark Schiffman (Medical Monitor & NCI co-Project Officer);
John T. Schiller (HPV Virologist);
Mark Sherman (QC Pathologist);
Diane Solomon (Medical Monitor & QC Pathologist);
Sholom Wacholder (Statistician).
SAIC-Frederick, Inc., NCI-Frederick, Frederick, MD:
Ligia Pinto (Head, HPV Immunology Laboratory);
Troy Kemp (Scientist, HPV Immunology Laboratory).
Women’s and Infants’ Hospital, Providence, RI:
Claire Eklund (QC Cytology);
Martha Hutchinson (QC Cytology).
Georgetown University, Washington, DC:
Mary Sidawy (Histopathologist).
DDL Diagnostic Laboratory, the Netherlands:
Wim Quint (Virologist, HPV DNA Testing);
Leen-Jan van Doorn (HPV DNA Testing).
Financial support. The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored by the NCI, and conducted with support from the Ministry of Health of Costa Rica. The project was funded by the NCI Intramural Research Program and the National Institutes of Health Office for Research on Women’s Health (ORWH). Vaccine was provided for our trial by GlaxoSmithKline Biologicals (GSK Bio, Rixensart, Belgium), under a Clinical Trials Agreement with the NCI. GSK Bio also provided support for aspects of the trial associated with regulatory submission needs of the company under grant FDA BB-IND 7920. Laboratory testing was performed at the NCI-sponsored SAIC-Frederick, Inc. HPV Immunology Laboratory in Frederick, MD. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The NCI and Costa Rica investigators make final editorial decisions on this and subsequent publications; GSK Bio has the right to review and comment. This project has also been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Potential conflicts of interest: Troy J. Kemp, No conflict; Allan Hildesheim, No conflict; Mahboobeh Safaeian, No conflict; Kerri J. Penrose, No conflict; Yuanji Pan, No conflict; Carolina Porras, No conflict; John T. Schiller, listed as inventor on US government–owned patents covering the papillomavirus virus-like-particle–based vaccine technology. These patents have been licensed co-exclusively to Merck and GlaxoSmithKline; Douglas R. Lowy, listed as inventor on US government–owned patents covering the papillomavirus virus-like-particle–based vaccine technology. These patents have been licensed co-exclusively to Merck and GlaxoSmithKline; Rolando Herrero, No conflict; and Ligia A. Pinto, No conflict.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muñoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. The Lancet. 2009;373(9679):1949–57. doi: 10.1016/S0140-6736(09)60691-7. 2009/6/12. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009 Jul 25;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 3.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006 Dec 4;95(11):1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007 Jun 30;369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 5.Kemp TJ, Hildesheim A, Safaeian M, Dauner JG, Pan Y, Porras C, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011 Mar 3;29(11):2011–4. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaards JA, Coupe VM, Meijer CJ, Berkhof J. The clinical benefit and cost-effectiveness of human papillomavirus vaccination for adult women in the Netherlands. Vaccine. 2011 Nov 8;29(48):8929–36. doi: 10.1016/j.vaccine.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 7.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccin. 2011 Dec;7(12):1359–73. doi: 10.4161/hv.7.12.18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006 Mar;6(3):231–43. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 9.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011 Jun 1;3(85):85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roost HP, Bachmann MF, Haag A, Kalinke U, Pliska V, Hengartner H, et al. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1257–61. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008 Sep 2;26(37):4795–808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008 Jul 4;26(29–30):3608–16. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safaeian M, Ghosh A, Porras C, Lin SW, Rodriguez AC, Schiffman M, et al. Direct Comparison of HPV16 Serological Assays Used to Define HPV-Naive Women in HPV Vaccine Trials. Cancer Epidemiol Biomarkers Prev. 2012 Sep;21(9):1547–54. doi: 10.1158/1055-9965.EPI-12-0558. [DOI] [PubMed] [Google Scholar]
- 14.Dauner JG, Pan Y, Hildesheim A, Kemp TJ, Porras C, Pinto LA. Development and application of a GuHCl-modified ELISA to measure the avidity of anti-HPV L1 VLP antibodies in vaccinated individuals. Mol Cell Probes. 2012 Apr;26(2):73–80. doi: 10.1016/j.mcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto LA, Viscidi R, Harro CD, Kemp TJ, Garcia-Pineres AJ, Trivett M, et al. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006 Sep 30;353(2):451–62. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007 Jul-Aug;3(4):109–15. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 17.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010 Nov;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 18.Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Oct;12(10):781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 19.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011 Oct 5;103(19):1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegrist CA, Pihlgren M, Tougne C, Efler SM, Morris ML, AlAdhami MJ, et al. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004 Dec 16;23(5):615–22. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]