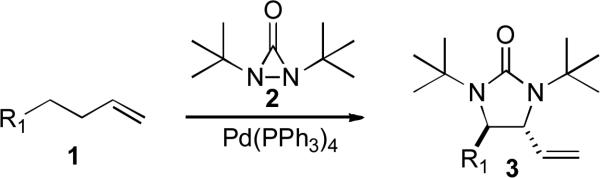
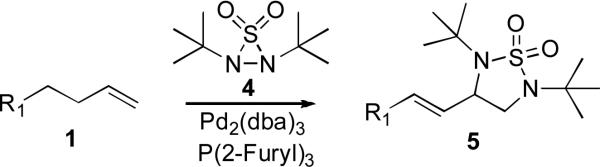
Metal-mediated and catalyzed diamination of olefins provides an effective approach to vicinal diamines which are very important functional moieties contained in various biologically active compounds and are widely used as chiral control elements in asymmetric synthesis.[1-6] Recently we reported various dienes and trienes can be regio- and stereoselectively diaminated using di-t-butyldiaziridinone (2)[7] as nitrogen source and Pd(0)[8] or Cu(I)[9] as catalyst. When readily available terminal olefins were reacted with di-t-butyldiaziridinone (2) and Pd(PPh3)4, the diamination occurred regio- and diastereoselectively at allylic and homoallylic carbons to form 3, likely via an in situ formed diene (Scheme 1).[10] However, when N,N-di-t-butylthiadiaziridine 1,1-dioxide (4)[11,12] is used as nitrogen source with Pd catalyst, terminal olefins are regioselectively diaminated at terminal carbons to form 5 via a different reaction mechanism (Scheme 2). Herein, we wish to report our preliminary results on this subject.
Scheme 1.
Scheme 2.
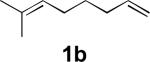
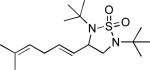
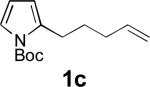
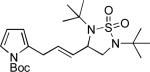
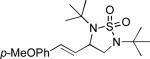
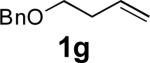
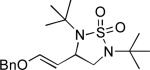
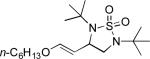
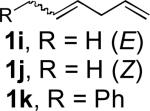
When terminal olefins such as 1-nonene were treated with 5 mol% Pd(PPh3)4 and N,N-di-t-butylthiadiaziridine 1,1-dioxide (4), no allylic and homoallylic diamination products similar to 3 were detected. Instead terminal diamination product 5 was formed in 34% conversion. The diamination process was further improved using 10 mol % Pd catalyst prepared from Pd2(dba)3 and tri-2-furylphosphine at higher reaction temperature. For example, treating 1-nonene (1a) with Pd2(dba)3 (0.05 equiv), tri-2-furylphosphine (0.3 equiv), and 4 (2.0 equiv) at 75 °C for 10 h gave terminal diamination product 5a in 68% yield (Table 1, entry 1). The diamination can be extended to a variety of terminal olefins (Table 1, entries 1-11) (the X-ray structure of 5f is shown in Figure 1). In all these cases, diamination products 5 were formed as major products along with small amounts of unidentified isomers. One possible isomer could result from the double bond migration of 5. In some cases (Table 1, entry 4), substantial amount of this type of isomer was observed.
Table 1.
Catalytic Dehydrogenative Diamination[a]
| Entry | Substrate (1) | Product (5) | Yield (%)[e] |
|---|---|---|---|
| 1 |
|
|
68 |
| 2 |
|
|
47 |
| 3 |
|
|
74 |
| 4 |
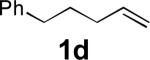
|
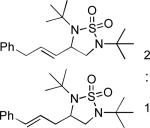
|
77 |
| 5[b] |
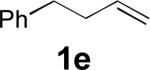
|
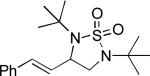
|
45 |
| 6 |
|
|
62 |
| 7 |
|
|
61 |
| 8 |
|
|
61 |
| 9[c] |
|
|
42 |
| 10[d] | 40 | ||
| 11[c] | (Z, E mixture, 6:1) | 66[f] |
All reactions were carried out with olefin (1.0 mmol), Pd2(dba)3 (0.050 mmol), tri-2-furylphosphine (0.30 mmol), and N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) (2.0 mmol) in benzene (0.25 mL) at 75 °C under argon for 10 h unless otherwise stated.
The reaction was carried out at 75 °C for 22 h.
The reaction was carried out at 65 °C for 6 h.
The reaction was carried out at 50 °C for 20 h.
Isolated yield.
7% yield of (1Z, 3E) isomer was also isolated.
Figure 1.
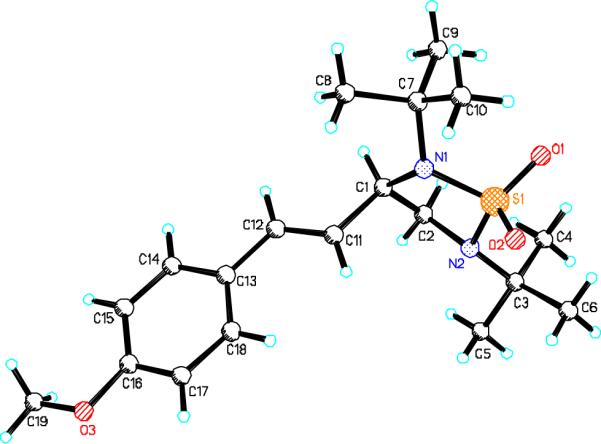
The X-ray structure of compound 5f
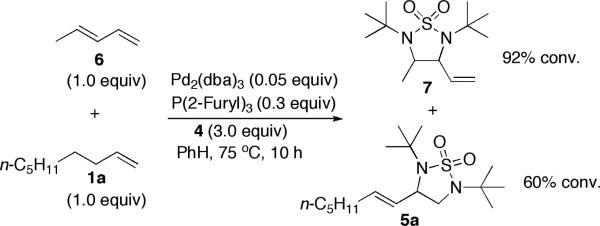
When a mixture of 1,3-pentadiene (6) and 1-nonene (1a) was subjected to the diamination conditions (Scheme 3), compounds 7 and 5a were formed, respectively. Diene 6 was diaminated predominately at the internal double bond [13] while terminal olefin 1a was diaminated at the terminal carbons. These results indicate that this diamination is unlikely to proceed via an in situ generated diene as in the case when di-t-butyldiaziridinone (2) was used as nitrogen source (Scheme 1).
Scheme 3.
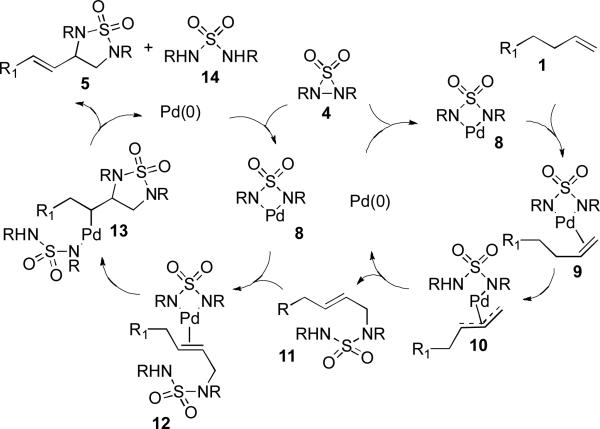
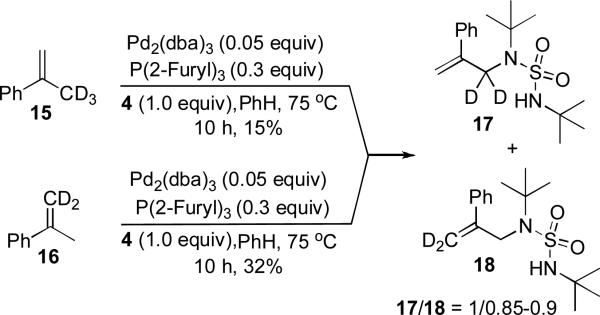
While a precise reaction mechanism awaits further study, a plausible catalytic cycle is shown in Scheme 4. Pd(0) first inserts into the N-N bond of 4 to form a four-membered Pd(II) species 8, which forms complex 9 with olefin 1. Removal of an allylic hydrogen of 9 forms π-allyl Pd complex 10,[14,15] which gives allyl sulfamide 11 and regenerates the Pd(0) catalyst after reductive elimination.[16] Subsequently, 11 complexes with 8 to form 12, which then undergoes a Pd(II)-catalyzed cyclization to form 13.[17] Finally, 13 undergoes a β-hydride elimination and reductive elimination to form product 5 and sulfamide 14 with regeneration of Pd(0) catalyst. In the case of 4-phenyl substituted 1-butenes (Table 1, entries 5 and 6), some amounts of dienes were formed from β-hydride elimination of π-allyl Pd complex 10,[18,19] which is consistent with the proposed mechanism. To further probe the allylic amination process, deuterium-labled α-methyl styrenes 15 and 16 were subjected to the reaction conditions. Although these two substrates were found to be less reactive due to steric effect, some amounts of allylic amination products were isolated, and the deuterium was almost equally distributed at terminal and allylic positions of the olefin in both cases (Scheme 5), which suggests that the initial allylic amination from π-allyl Pd complex 10 is a viable process (Scheme 4).
Scheme 4.
A proposed catalytic cycle for diamination
Scheme 5.
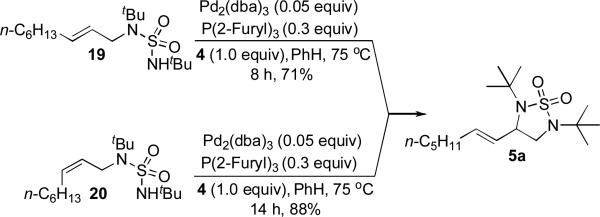
Clear identification and isolation of allyl sulfamide 11 from the crude reaction mixtures generally proved to be difficult. However, in the case of Table 1, entry 1, trace amounts of the allyl sulfamide in the reaction mixture could be detected by 1H NMR and GC-MS. Thus allyl sulfamides 19 and 20 were prepared and subjected to the reaction conditions (Scheme 6). These sulfamides indeed cyclized to form compound 5a in good yield. However, no cyclization was observed without di-t-butylthiadiaziridine 1,1-dioxide (4) (Scheme 6). These results are in agreement with the mechanism described in Scheme 4. The exact mechanism for the Pd(II)-catalyzed cyclization of 11 to form 5 awaits further study.
Scheme 6.
In summary, a variety of terminal olefins have been dehydrogenatively diaminated at terminal carbons using N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) as nitrogen source and Pd as catalyst, giving the diamination products in high regioselectivity. The diamination is likely to proceed via Pd-catalyzed allylic amination and subsequent cyclization. This diamination is mechanistically distinct from the previous process using di-t-butyldiaziridinone (2) as nitrogen source, thus resulting in different regioselectivity. Further efforts will be devoted to studies of the reaction mechanism, search for a more effective catalytic process, and expansion of the substrate scope as well as development of an asymmetric process.
Experimental Section
Procedure for preparation of N,N-di-t-butylthiadiaziridine 1,1-dioxide (4).[11]
To a suspension of sodium hydride (60%) (2.0 g, 50.0 mmol) in hexane (400 mL) was added N,N-di-t-butyl sulfamide (10.0 g, 48.0 mmol) over a period of 30 min. The resulting slurry was stirred at reflux under argon for 2 h. Upon cooling to -30 °C, t-butyl hypochlorite (5.43 g, 50.0 mmol) was added dropwise with lights off. The reaction mixture was stirred at -30 °C for 3 h and at 0 °C for 1 h in the dark. Cold ether (200 mL) was then added. The organic layers were washed with water (100 mL), dried over MgSO4, filtered, concentrated, and distilled under reduced pressure (95 °C, 8 mmHg) to give N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) as a colorless oil which turned into solid at room temperature (8.5 g, 86%).
Representative Dehydrogenative Diamination Procedure (Table 1, Entry 1)
A Pyrex tube charged with Pd2(dba)3 (0.046 g, 0.050 mmol) and tri-2-furylphosphine (0.070 g, 0.30 mmol) was evacuated and then filled with argon three times. Upon addition of benzene (0.25 mL), the resulting mixture was stirred at 75 °C for 15 min. 1-Nonene (1a) (0.126 g, 1.0 mmol) was added, followed by N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) (0.413 g, 2.0 mmol). The reaction mixture was stirred at 75 °C for 10 h, and purified by flash chromatography [silica gel; toluene first to remove the yellow dibenzylideneacetone, then petroleum ether-EtOAc (40/1 to 30/1) ] to give diamination product 5a as a colorless oil (0.225 g, 68%).
Representative Procedure for Allylic Amination of α-Methyl Styrene (Scheme 5)
A Pyrex tube charged with Pd2(dba)3 (0.023 g, 0.025 mmol) and tri-2-furylphosphine (0.035 g, 0.15 mmol) was evacuated and then filled with argon three times, followed by addition of benzene (0.125 mL). After the resulting mixture was stirred at 75 °C for 15 min, α-methyl styrene 16 (0.060 g, 0.50 mmol) and N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) (0.103 g, 0.50 mmol) were added successively. The reaction mixture was stirred at 75 °C for 10 h and purified by flash chromatography [silica gel; toluene first to remove the yellow dibenzylideneacetone, then petroleum ether-EtOAc (30/1)] to give allylic amination products 17 and 18 as a white solid (0.052 g, 32%).
Representative Procedure for Cyclization of Allyl Sulfamide (Scheme 6)
A Pyrex tube charged with Pd2(dba)3 (0.018 g, 0.020 mmol) and tri-2-furylphosphine (0.028 g, 0.12 mmol) was evacuated and then filled with argon three times, followed by addition of benzene (0.1 mL). After the resulting mixture was stirred at 75 °C for 15 min, allyl sulfamide 20 (0.133 g, 0.40 mmol) and N,N-di-t-butylthiadiaziridine 1,1-dioxide (4) (0.083 g, 0.40 mmol) were added successively. The reaction mixture was stirred at 75 °C for 14 h and purified by flash chromatography [silica gel; toluene first to remove the yellow dibenzylideneacetone, then petroleum ether-EtOAc (30/1 to 20/1)] to give cyclization product 5a as a colorless oil (0.116 g, 88%).
Footnotes
We are grateful to the generous financial support from the General Medical Sciences of the National Institutes of Health (GM083944-01) and the Camille and Henry Dreyfus Foundation.
References
- 1.For leading reviews, see: Lucet D, Gall TL, Mioskowski C. Angew. Chem. Int. Ed. 1998;37:2580. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L.Mortensen MS, O'Doherty GA. Chemtracts: Organic Chemistry. 2005;18:555.Kotti SRSS, Timmons C, Li G. Chem. Biol. Drug. Des. 2006;67:101. doi: 10.1111/j.1747-0285.2006.00347.x.
- 2.For examples of metal-mediated diaminations, see: Co: Becker PN, White MA, Bergman RG. J. Am. Chem. Soc. 1980;102:5676. Hg: Barluenga J, Alonso-Cires L, Asensio G. Synthesis. 1979:962. Mn: Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J. Org. Chem. 1985;50:3647. Os: Chong AO, Oshima K, Sharpless KB. J. Am. Chem. Soc. 1977;99:3420.Muñiz K. Eur. J. Org. Chem. 2004:2243.Muñiz K, Nieger M. Synlett. 2003:211.Muñiz K, Nieger M. Chem. Commun. 2005:2729. doi: 10.1039/b502150b. Pd: Bäckvall J-E. Tetrahedron Lett. 1978:163. Tl: Aranda VG, Barluenga J, Aznar F. Synthesis. 1974:504.
- 3.For recent Cu(II)-mediated intramolecular diamination, see: Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250. doi: 10.1021/ja053335v.Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035. doi: 10.1021/ol0706713.
- 4.For Rh(II), Fe(III)-catalyzed diamination with TsNCl2, see: Li G, Wei H-X, Kim SH, Carducci MD. Angew. Chem. Int. Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I.Wei H-X, Kim SH, Li G. J. Org. Chem. 2002;67:4777. doi: 10.1021/jo0200769.
- 5.For a recent Pd(II)-catalyzed intermolecular diamination of conjugated dienes, see: Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J. Am. Chem. Soc. 2005;127:7308. doi: 10.1021/ja051181d.
- 6.For recent Pd(II) and Ni-catalyzed intramolecular diamination of olefins, see: Streuff J, Hövelmann CH, Nieger M, Muñiz K. J. Am. Chem. Soc. 2005;127:14586. doi: 10.1021/ja055190y.Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem. Int. Ed. 2007;46:7125. doi: 10.1002/anie.200702160.Muñiz K. J. Am. Chem. Soc. 2007;129:14542. doi: 10.1021/ja075655f.Muñiz K, Hövelmann CH, Streuff J. J. Am. Chem. Soc. 2008;130:763. doi: 10.1021/ja075041a.Hövelmann CH, Streuff J, Brelot L, Muñiz K. Chem. Commun. 2008:2334. doi: 10.1039/b719479j.
- 7.Greene FD, Stowell JC, Bergmark WR. J. Org. Chem. 1969;34:2254. [Google Scholar]
- 8.a Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:762. doi: 10.1021/ja0680562. [DOI] [PubMed] [Google Scholar]; b Xu L, Du H, Shi Y. J. Org. Chem. 2007;72:7038. doi: 10.1021/jo0709394. [DOI] [PubMed] [Google Scholar]; c Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:11688. doi: 10.1021/ja074698t. [DOI] [PubMed] [Google Scholar]; d Xu L, Shi Y. J. Org. Chem. 2008;73:749. doi: 10.1021/jo702167u. [DOI] [PubMed] [Google Scholar]
- 9.Yuan W, Du H, Zhao B, Shi Y. Org. Lett. 2007;9:2589. doi: 10.1021/ol071105a. [DOI] [PubMed] [Google Scholar]
- 10.a Du H, Yuan W, Zhao B, Shi Y. J. Am. Chem. Soc. 2007;129:7496. doi: 10.1021/ja072080d. [DOI] [PubMed] [Google Scholar]; b Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For a leading reference on 4, see: Timberlake JW, Alender J, Garner AW, Hodges ML, Özmeral C, Szilagyi S, Jacobus JO. J. Org. Chem. 1981;46:2082.
- 12.For a Cu(I)-catalyzed diamination with 4, see: Zhao B, Yuan W, Du H, Shi Y. Org. Lett. 2007;9:4943. doi: 10.1021/ol702061s.
- 13.Trace amounts of diamination product at the terminal carbons were formed for diene 6.
- 14.For leading reviews on generation of π-allyl Pd complexes from olefins with PdX2, see: Trost BM. Acc. Chem. Res. 1980;13:385.Åkermark B, Zetterberg K. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E.-i., editor. John Wiley & Sons, Inc.; New York: 2002. p. 1875.
- 15.For a recent book on Pd chemistry, see: Tsuji J. Palladium Reagents and Catalysts: New Perspective for the 21st Century. John Wiley & Sons Ltd; 2004.
- 16.For a recent report on Pd(OAc)2-catalyzed allylic amination of terminal olefins, see: Reed SA, White MC. J. Am. Chem. Soc. 2008;130:3316. doi: 10.1021/ja710206u.
- 17.For leading reviews on Pd(II)-catalyzed cyclization of N and O nucleophile to olefins, see: Hegedus LS. Tetrahedron. 1984;40:2415.Stoltz BM. Chem. Lett. 2004;33:362.Sigman MS, Schultz MJ. Org. Biomol. Chem. 2004;2:2551. doi: 10.1039/B409127M.Wolfe JP, Thomas JS. Curr. Org. Chem. 2005;9:625.Zeni G, Larock RC. Chem. Rev. 2006;106:4644. doi: 10.1021/cr0683966.Kotov V, Scarborough CC, Stahl SS. Inorg. Chem. 2007;46:1910. doi: 10.1021/ic061997v.
- 18.Internal diamination products from the resulting dienes were also observed.
- 19.For leading reviews on formation of dienes from π-allyl Pd complexes, see: a ref. 14a; Shimizu I. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E.-i., editor. John Wiley & Sons, Inc.; New York: 2002. p. 1981.