Abstract
Vaccine adjuvant-induced inflammation augments vaccine immunity in part by recruiting antigen presenting cells to vaccine draining lymph nodes. However, the role of one antigen presenting cell subtype, inflammatory monocytes, in regulating vaccine immunity in healthy animals has not been fully examined in detail. Therefore, vaccine-mediated monocyte recruitment and subsequent immune responses were investigated using murine vaccination models and in vitro assays. Recruitment of inflammatory monocytes to vaccine draining lymph nodes was rapid and mediated primarily by local production of MCP-1, as revealed by studies in MCP-1−/− mice. Interrupting monocyte recruitment to lymph nodes by either transient monocyte depletion or monocyte migration blockade led to marked amplification of both cellular and humoral immune responses to vaccination. These results were most consistent with the idea that rapidly mobilized inflammatory monocytes were actually suppressing vaccine responses. The suppressive nature of vaccine-elicited monocytes was confirmed using in vitro co-cultures of murine monocytes and T cells. Furthermore, it was determined that inflammatory monocytes suppressed T cell responses by sequestering cysteine, as cysteine supplementation in vitro and in vivo appreciably augmented vaccine responses. These findings indicated therefore that vaccination-elicited inflammation, while necessary for effective immunity, also generated potent counter-regulatory immune responses that were mediated primarily by inflammatory monocytes. Therefore, interrupting monocyte mediated vaccine counter-regulatory responses may serve as an effective new strategy for broadly amplifying vaccine immunity.
Keywords: adjuvant, CCR2, T cell, antibody, inflammation
Introduction
Much research effort has been devoted to developing vaccine adjuvants with greater potency and duration of action (1–6). However, at present, little is known regarding potential counter-regulatory mechanisms that may be generated by vaccination, or how these mechanisms may regulate vaccine responses.
Vaccination has been reported to trigger the mobilization and recruitment of inflammatory monocytes to vaccine draining LNs2 (7–10). One study identified expansion of this cell population following vaccination but did not identify the role these cells play in vaccine immunity (7). In another study, vaccine-elicited monocytes were found to increase IFN-γ production by T cells in draining LNs and it was concluded that inflammatory monocytes were important in augmenting vaccine responses (10). However, those studies did not specifically examine the vaccine regulatory effects of monocytes by depleting the cells or blocking their migration to LNs. Additionally, this work relied heavily on monitoring IFN-γ responses in CCR2 −/− mice which are known to lack the ability to produce IFN-γ (11). Other studies noted that high doses of vaccine adjuvant promoted mobilization of monocytes and neutrophils which dampened vaccine response through an inducible NO synthase dependent pathway (8–9).
Monocytes are recognized as key immune effector cells that can mediate protection against a number of different pathogens (12–15). At the same time, monocytes and macrophages are also associated with immune suppression and promotion of tumor growth and metastasis (7, 16–18). For example, studies in tumor bearing mice have shown that cancer-induced myeloid suppressor cells inhibited vaccine responses, which could be reversed by depleting myeloid cells (19–22). However, the role of inflammatory monocytes in regulating vaccine immunity in healthy animals has not been thoroughly studied, particularly with respect to the interaction between monocytes and T cells in vaccine draining LN. Since monocyte responses are regulated by both systemic and local cytokine signals, it is difficult to extrapolate from vaccination studies in animals with cancer to studies in healthy animals. Therefore, it remains an open question as to whether recruitment of inflammatory monocytes is essential for enhancement of vaccine responses, especially in healthy individuals without pre-existing expanded populations of immune suppressive myeloid cells as found in animals and humans with cancer.
To investigate monocyte regulation of vaccine immunity, healthy mice were vaccinated and the roles of local inflammation, chemokine release, and monocyte recruitment in controlling vaccine responses were assessed, including both cellular and humoral immune responses. Mice (C57Bl/6 wild type and CCR2−/−) were immunized using different vaccine adjuvants and monocyte recruitment to LN and vaccine immune responses were assessed. Monocyte effects on vaccine immunity were also assessed using monocyte depletion and migration blockade, while the direct interaction of vaccine elicited monocytes with T cells was explored using in vitro assays and inhibitors of known myeloid suppression pathways. These studies revealed an unexpected inhibitory role for vaccine-elicited monocytes in regulation of vaccine immunity. These inhibitory effects could be overcome using drugs that block monocyte migration or by providing exogenous cysteine. The findings suggest novel strategies for augmenting the effectiveness of conventional vaccines through manipulation of monocyte responses.
Materials and Methods
Animals and experimental manipulations
BALB/c, C57Bl/6, and ICR mice were purchased from Harlan laboratories (Denver, CO) and housed in micro-isolator cages in the laboratory animal facility at Colorado State University. Mice lacking expression of a functional CCR2 molecule (CCR2−/−) or CCL2 expression (CCL2−/−) on the C57Bl/6 background were obtained from Jackson Laboratories (Bar Harbor, ME). All animal procedures were approved by the Institutional Animal Care and Use Committee at Colorado State University. Liposomal clodronate was administered by tail vein injection of 200 ul of LC3, as described previously (23–24). Vaccination with OVA or HA4 was conducted by s.c. administration of 100 ul CLDC (cationic liposome-DNA complex)5 adjuvant plus 5 μg OVA or 1 μg HA protein, or 50 μl CLDC in the case of footpad injections. Animals were boosted once 10 days after the priming injection for Ova studies. For therapeutic vaccination with HA, animals were vaccinated every 7 days until sacrifice due to progressive tumor growth. Animals were treated with the spiropiperidine small molecule compound, RS102895 (25–26)(Sigma-Aldrich) delivered by i.p. injection at a dose of 5 mg/kg twice daily, starting the day before, the day of, and the day following vaccination and boost. N-acetylcysteine (NAC)6 (Sigma Aldrich) was administered at a concentration of 100 mg/kg i.p. immediately before and every 12 hours after vaccination for a total of 4 treatments for both the prime and the boost immunization.
Reagents, biochemicals, and cell lines
Liposomal clodronate was prepared in the lab as described previously (23). Cationic liposome-DNA complexes were also prepared in the lab as described previously (27). RS102895 was purchased from Sigma-Aldrich and dissolved in DMSO prior to dilution in water for injection. The A20-HA cell line and non-transfected A20 cells were kindly provided by Dr. Charles Drake (Johns Hopkins, Baltimore, MD). One million A20-HA cells were injected s.c. on the right flank 3 days prior to treatment initiation. Tumors were measured every other day using calipers. Biochemicals used to block monocyte suppression of T cell activation in vitro included 0.5M NAC (Sigma Aldrich, St. Louis MO), 55μM 2-ME (Gibco), 200μM Nor-NOHA (Cayman Chemical), 300μM L-NMMA (Cayman Chemical), functional grade neutralizing anti-mouse IL-10 (0.1μg/ml) and neutralizing anti-mouse TGFs (1μg/ml) (R&D systems), 20μM LoxBlock-1 (a novel 12/15-lipoxygenase inhibitor (28), also known as compound #5680672 (Chembridge Corp.), and 30mM indomethacin (Sigma Aldrich).
Antibody staining and flow cytometry
Directly labeled antibodies were purchased from either eBiosciences (San Diego, CA) or Becton-Dickinson (Bedford, MA) unless otherwise noted, and included the following clones: mouse CD11b (clone M1/70), mouse Ly6C (clone AL-21), mouse Ly6G (clone 1A8), mouse GR1 (clone RB6-8C5), mouse CD8 (clone 53-6.7), mouse/human B220 (clone RA3-6B2), mouse CD11c (clone N418), mouse CD20 (clone eBioL31), mouse CCR2 (clone 475301, R & D Systems, Minneapolis, MN), mouse CD45 (clone 30-F11). An unlabeled rabbit antibody to mouse xCT (Abcam, Cambridge, MA) was used together with goat α rabbit IgG (Jackson ImmunoResearch). Lymph nodes and spleen tissues were disrupted into single cell suspensions through cell strainers (BD Falcon) and washed twice with FACS buffer (PBS plus 2% FBS and 0.05% sodium azide). Prior to immunostaining, cells (approx 1 × 106 for most experiments) were first blocked with normal mouse serum and unlabeled anti-mouse CD16/32 to reduce non-specific antibody binding. Cells were incubated with antibodies in 96-well round bottom plates for 30 minutes at room temperature, then washed and incubated with streptavidin conjugates when necessary, then fixed in 1% paraformaldehyde prior to analysis. For identification of CD207+ cells, cells were fixed and permeabilized overnight prior to staining with CD207 (clone ebioL31). Flow cytometry was conducted using a Dako/CyanADP flow cytometer with Summit software (Little Rock, AR). Analysis was done with FlowJo software (Ashland, OR). Cell counts were conducted with an automated cell counter (Nexcelom Bioscience, Lawrence, MA).
Serology
Serum was collected via cardiac puncture into serum separator tubes (BD Falcon). Serum was stored at 4°C until use. ELISA plates for quantitation of anti-Ova titers were coated overnight with Ova, then washed and non-specific binding blocked using 3% bovine serum albumin (Calbiochem Darmstadt, Germany), for 1 hour. Antibody endpoint titers were determined using 10-fold dilutions of serum samples. Donkey anti-mouse IgG-HRP (Jackson Immunoresearch) was used to detect bound mouse IgG, followed by addition of TMB. ODs were determined using a BioTek Synergy HT (Winooski, VT) plate reader and analyzed with Microsoft excel software. Endpoint titer was determined at 3 standard deviations above control wells and plotted on a log scale.
Restimulation assays for cellular immune responses
Spleen cells (1 × 106 cells in 1 ml in 24-well plates) were processed as described above and stimulated with the indicated peptides or proteins for 72 hours: 50 μg/ml Ova, 10 μg/ml SIINFEKL (AnaSpec Fremont, CA), 1 μg/ml IYSTVASSL, 10 μg/ml SFERKEIFPKE, or with 1.3 × 104 live A20-HA cells for 72 hours. Supernatants were collected and analyzed for IFN-γ concentration, using a commercial ELISA kit following manufacturer’s instructions (R&D systems). MCP-1 was measured using a commercially available kit (R&D systems) following manufacturer’s instructions.
In vivo tracking of labeled liposomes or Ova
Bodipy labeled PBS liposomes were prepared as described previously (23) and delivered i.v. in the tail vein in a volume of 200 μl. 5 μg OVA-Allophycocyanin (Invitrogen Carlsbad, CA) was injected in the footpad in 50 μl CLDC. Spleen and LN tissues were collected 12 hours after liposome administration for analysis by flow cytometry.
Monocyte suppression of T cells
Mice were vaccinated in the footpad and 24 hours later, popliteal LNs were collected and pooled from approximately 10–12 mice per experiment. Live monocytes were sorted on a Mo-Flo cell sorter at the Colorado State University Cytometry Core facility. Positive gates for monocyte analysis included low-side scatter cells that were CD11b+ and Gr-1+, while Ly6G+ cells (neutrophils) were excluded from analysis. Purified monocytes were incubated with naive spleen cells at 3 different ratios of monocytes to T cells (1:10, 1:100, 1:1,000) in HL-1 media containing 1% each of FBS, pennicillin/streptomycin, and L-glutamine (29). Prior to incubation with purified monocytes, naive spleen cells were stained with 2.5μM CFSE (Invitrogen) for 10 minutes at 37°c and then the T cells were activated with 5 μg/ml Con A for 4 days. In the indicated experiments, compounds for blocking monocyte cellular pathways were added at the time monocytes and T cells were mixed. After 96h of incubation, cells were washed and stained with anti-CD3 antibody and proliferation of CFSE+ CD3+ cells was determined using flow cytometry. For in vivo assessment of T cell response in the presence or absence of inflammatory monocytes, popliteal LNs were collected 24h after footpad vaccination, T cells activated with Con A, then CFSE labeled and proliferation assessed 4 days later, using flow cytometry, as noted above.
Statistical Analyses
Statistical comparisons between those data sets with two treatment groups were done using a non-parametric t-test (Mann-Whitney test). Comparisons between 3 or more groups were done using ANOVA, followed by Tukey multiple means post-test. Tumor growth data was analyzed by repeated measures two-way ANOVA, followed by the Bonferroni post test. Analyses were conducted using Prism5 software (GraphPad, La Jolla, CA). For all analyses, statistical significance was determined for p<0.05.
Results
Vaccination induces recruitment of CCR2+ inflammatory monocytes to draining LNs
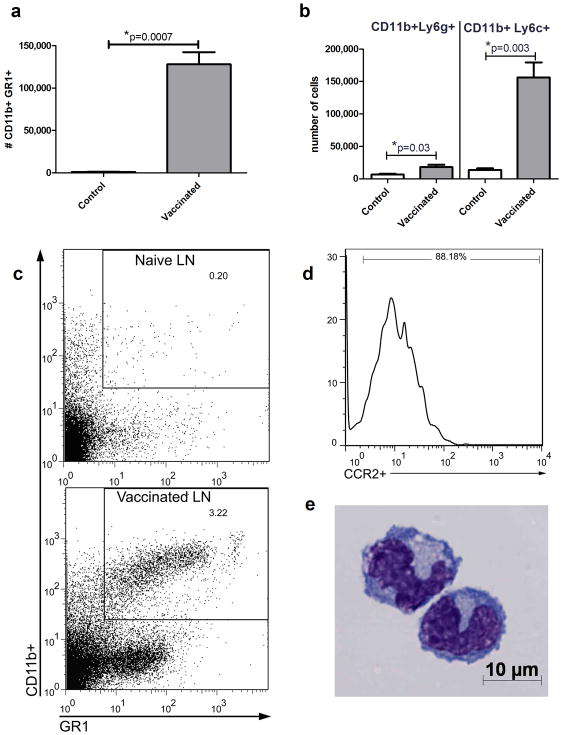
C57Bl/6 mice (n =5 per group) were vaccinated in the footpad using a liposome-based adjuvant and 2 μg of Ova (27, 30). Within 2 hours of immunization, CD11b+Gr-1+ myeloid cells rapidly appeared in the bloodstream and their numbers in circulation remained elevated for at least 72h following vaccination, and then declined (data not shown). In addition, the numbers of CD11b+Gr-1+ cells were markedly increased (up to 30-fold) in vaccine draining LNs within 24h of vaccination (Figure 1a). The vaccine elicited myeloid cells in LNs consisted primarily of CD11b+Ly6Chi cells (monocytes), with much smaller numbers of CD11b+Ly6G+ cells (neutrophils) (Figure 1b). Representative flow cytometry plots of Ly6G−CD11b+GR1+ cells are depicted in figure 1c. In addition, nearly all of the CD11b+Ly6C+ cells in vaccine draining LNs also expressed CCR2, a phenotype most consistent with inflammatory monocytes (31) (Figure 1d). Cytologically, Ly6G−CD11b+Gr-1+ cells purified from vaccine-draining LNs exhibited morphology consistent with monocytes (Figure 1e).
Figure 1. Vaccine-elicited myeloid cells migrate rapidly to vaccine draining nodes.
1a. Recruitment of CD11b+Gr-1+ cells in vaccine-draining LNs was assessed 24 hours after immunization by flow cytometry. Naive LNs were included as controls. 1b. Total CD11b+Gr-1+ cells were subdivided into neutrophilic and monocytic populations (i.e., CD11b+Ly6g+ and CD11b+Ly6c+, respectively). Error bars represent SEM, n=5. Statistical significance was determined using a Mann-Whitney test, with * = p < 0.05. Experiments were repeated 3 times with similar results. 1c. Representative flow plots of CD11b+Gr-1+ cells from a naive LN or a vaccine draining LN collected 24 hours after vaccination. (Ly6g+ cells were excluded from the analysis). 1d. Representative histogram of CCR2 expression by Ly6g−CD11b+Gr-1+ cells from Figure 2c. 1e. Representative photomicrograph of inflammatory monocytes purified by flow sorting from vaccine draining LNs 24 hours after immunization.
Induction of chemokine release by vaccination and effects on humoral immunity
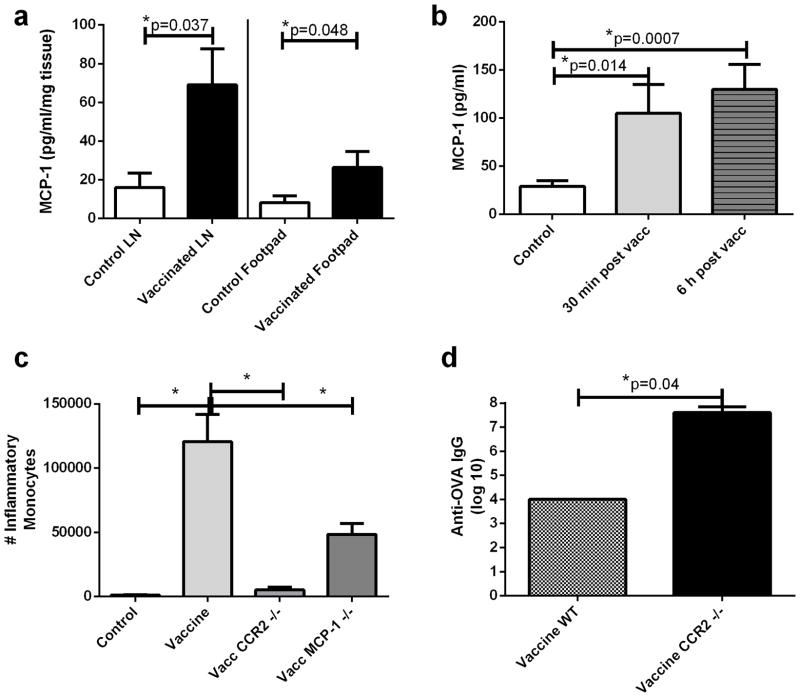
The signals responsible for mobilization and recruitment of inflammatory monocytes following vaccination were investigated next, focusing on the role of MCP-1, as suggested by previous studies (32). Significant increases in MCP-1 release were observed by tissues collected from the vaccination site as well as by draining LN tissues (Figure 2a). Additionally, MCP-1 concentrations were significantly increased in plasma of vaccinated animals within 30 minutes of vaccination, and remained elevated for at least 6 hours thereafter (Figure 2b). To determine whether the MCP-1/CCR2 pathway was involved in inflammatory monocyte recruitment in response to vaccination, CCR2−/− and MCP-1−/− mice were vaccinated at the footpad and vaccine draining LN collected 24 hours later for analysis by flow cytometry. Compared to wild type mice, CCR2−/− and MCP-1−/− mice exhibited significantly reduced numbers of inflammatory monocytes in vaccine draining LNs compared to wild type animals (Figure 2c). The effects of monocyte recruitment on vaccine immunity were also examined using CCR2−/− mice. We observed that CCR2−/− mice generated significantly higher Ova specific antibody titers than did wild type animals (Figure 2d). CCR2 −/− are not capable of mounting a detectable IFN-γ response, therefore this endpoint was not examined in this experiment (11). These results suggested that vaccine induced recruitment of inflammatory monocytes inhibited vaccine-induced humoral immunity.
Figure 2. Effects of MCP-1 and CCR2 expression on monocyte migration and vaccine responses.
2a. Footpad tissues (left) and vaccine draining LN (right) were removed 2 hours after footpad immunization and incubated in complete medium for 24 hours. Supernatants were analyzed for MCP-1 concentration by ELISA and normalized to tissue weight (mg). 2b. Mice were vaccinated and plasma was collected 30 minutes and 6 hours later for assessment of MCP-1 concentrations by ELISA. 2c. Inflammatory monocytes in vaccine draining LNs were quantified in wild type, CCR2−/−, or MCP-1 −/− mice 24 hours after vaccination. 2d. Wild type or CCR2−/−mice were vaccinated twice, 10 days apart with Ova, using CLDC adjuvant, as noted in Methods. Anti-Ova IgG titers in serum were determined 12 days following the second immunization. Error bars represent SEM, n=5. * Statistical significance was determined using one-way ANOVA, followed by Tukey multiple means comparison test, with *= p < 0.05, in 2b–c. Statistical significance was determined using a Mann-Whitney test, with * = p < 0.05. for 2a and 2d. Similar results were obtained in one additional independent experiment.
Monocyte depletion at the time of immunization significantly amplifies vaccine immunity
Experiments were conducted next to assess the effects of blocking monocyte recruitment to LNs on vaccine responses. Monocyte depletion was accomplished using either LC or the CCR2 small molecule antagonist, RS102895 (23–24, 33–34). Mice (n = 5 per group) were vaccinated s.c. between the shoulder blades or in the footpad using a cationic liposome adjuvant, CLDC, (27, 30) admixed with 5 μg or 2 μg ovalbumin protein, respectively. Liposomal clodronate or RS102895 was administered at the time of vaccination. Vaccination (with or without concurrent monocyte depletion or blockade) was repeated in 10 days and vaccine-induced immune responses were assessed 12 days after the booster immunization.
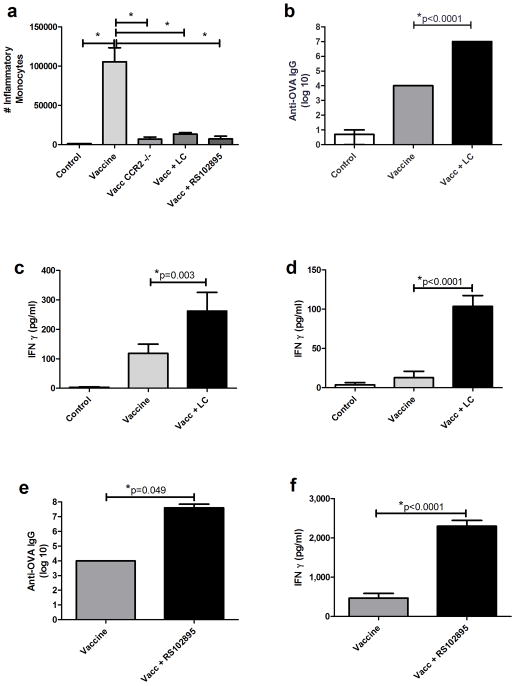
Concurrent vaccination and treatment with LC or RS102895 significantly reduced numbers of inflammatory monocytes in vaccine draining LNs collected 24 hours after vaccination (Figure 3a). Langerhan’s DC were monitored at draining LNs as well. Liposomal clodronate did not affect the number of Langerhan’s DC arriving at the LN via lymphatic drainage nor did LC or RS102895 alter the amount of Ova antigen arriving at the draining LN as measured by florescent tracking experiments (data not shown). Monocyte depletion with LC at the time of immunization also resulted in marked increases in anti-Ova antibody titers (Figure 3b). Moreover, spleen cells collected from vaccinated mice that concurrently received LC produced significantly more IFN-γ following ex vivo re-stimulation with Ova protein (Figure 3c) or Ova-MHC I peptide, SIINFEKL (Figure 3d). Vaccination and concurrent treatment with RS102895 also resulted in significant increases in antibody responses to vaccination (Figure 3e). Spleen cells from vaccinated, RS102895-treated mice also produced significantly greater amounts of IFN-γ following ex vivo re-stimulation with Ova (Figure 3f). LC-mediated enhancement of vaccine response was also tested with other anjuvants. Vaccination with incomplete freund’s adjuvant, Alhydrogel, monophosphoryl lipid A, or Poly I:C were all enhanced with concurrent monocyte depletion using LC (data not shown). Thus, depletion of monocytes, or migration blockade to draining LNs at the time of vaccination elicited marked and broad enhancement of vaccine immunity in healthy animals.
Figure 3. Effects of monocyte depletion or migration blockade on vaccine responses.
3a. Inflammatory monocytes in vaccine draining LNs were quantified 24 hours following administration of LC or the CCR2 antagonist RS102895, as described in Methods. CCR2 −/− and naive animals were included as controls. 3b. Anti-Ova IgG titers were determined in vaccinated mice that were concurrently treated with LC. 3c. Spleen cells from animals in 3b were re-stimulated with Ova (50 μg/ml) for determination of IFN-γ release. 3d. Spleen cells from vaccinated and LC-treated or untreated mice were also restimulated with the class I restricted Ova peptide, SIINFEKL (10 μg/ml), for 24 hours and IFN-γ release quantified by ELISA. 3e. Anti-Ova IgG titers were determined in vaccinated mice that were concurrently treated with RS102895. 3f. Spleen cells from animals in 3e were re-stimulated with Ova (50 μg/ml) for determination of IFN-γ release. Error bars represent SEM, n=5. * Statistical significance was determined using one-way ANOVA, followed by Tukey multiple means comparison test, with *= p < 0.05, in 3a–d. Statistical significance was determined using a Mann-Whitney test, with * = p < 0.05. for 3e and 3f. Similar results were obtained in one additional independent experiment.
Inflammatory monocyte depletion or blockade increases the efficacy of cancer vaccination in a murine B cell lymphoma model
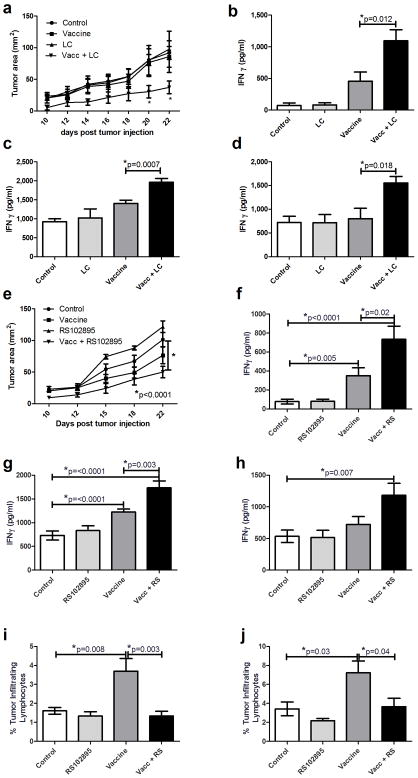
Next, studies were conducted to determine whether monocyte depletion or blockade amplified therapeutic immunity to a tumor vaccine. Using a murine A20-HA tumor challenge model (35–36), BALB/c mice were immunized with recombinant HA protein (1 μg) once weekly, with or without concurrent administration of LC. Tumor growth was significantly reduced in mice that received vaccination plus LC treatment (Figure 4a). Spleen cells from vaccinated and LC treated mice also produced significantly greater amounts of IFN-γ when re-stimulated ex vivo with MHC class I or II peptides from HA (Figure 4b and 4c) or with live A20-HA cells (Figure 4d). Weekly vaccination plus treatment with RS102895 also significantly reduced tumor growth rates compared to mice that were vaccinated only or treated only with RS102895 (Figure 4e). Re-stimulation with MHC I or MHC II HA peptides or with live A20-HA cells resulted in significant increases in IFN-γ responses by spleen cells from animals vaccinated and concurrently treated with RS102895 (Figure 4f, 4g, 4h). Numbers of inflammatory monocytes or myeloid derived suppressor cells were significantly increased in tumor tissues of vaccinated mice compared to control mice, whereas this increase was significantly attenuated in animals that received RS102895 during vaccination (Figure 4i, j respectively). These results indicated therefore that inflammatory monocyte depletion or migration blockade could significantly amplify early therapeutic vaccine immunity in a tumor challenge model.
Figure 4. Effects of vaccination and monocyte depletion or blockade on tumor growth and anti-tumor immunity.
4a. Mice with s.c implanted A20-HA tumors (n = 5 per group) were vaccinated weekly with a vaccine comprised of 1 ug HA protein in CLDC adjuvant, administered s.c., as described in Methods. Treatment groups included untreated mice, mice that received vaccine alone, mice that were treated with LC only, or mice that were vaccinated and treated with LC. The vaccine was administered on day 3, 10, and 17 after tumor injection and tumor growth was monitored every other day starting on day 10. Statistical significance was determined using two-way ANOVA with Bonferroni post test. 4b–d. On day 22 after tumor injection, mice were euthanized and spleen cells were collected and restimulated with the MHC I (4b) or MHC II (4c) restricted HA peptides, or with live A20HA cells at an effector to target ratio of 1:75 (4d) and monitored for IFN-γ release. 4e. Mice with s.c implanted A20-HA tumors were vaccinated weekly with a vaccine comprised of 1 ug HA protein in CLDC adjuvant, administered s.c. Treatment groups included untreated mice, mice that received vaccine alone, mice that were treated with RS102895 alone administered i.p., or mice that were vaccinated and treated with RS102895. The vaccine was administered on day 3, 10, and 17 after tumor injection. Statistical significance was determined using two-way ANOVA with Bonferroni post test. 4f–h. On day 22, mice were euthanized and spleen cells were restimulated with the MHC I (4f) or MHC II (4g) restricted HA peptides, or with live A20HA cells at an effector to target ratio of 1:75 (4h) and monitored for IFN-γ release. 4i–j. Tumors were collected and analyzed by flow cytometry for evaluation of tumor infiltrating populations of inflammatory monocytes (CD11b+Ly6chi) (4i) or myeloid derived suppressor cells (CD11b+GR1+) (4j). Error bars represent SEM, n=10. Statistical significance was determined using one-way ANOVA, followed by Tukey multiple means comparison test, with *= p < 0.05. These experiments were repeated in one additional independent experiment.
Vaccine elicited inflammatory monocytes directly suppressive to T cells
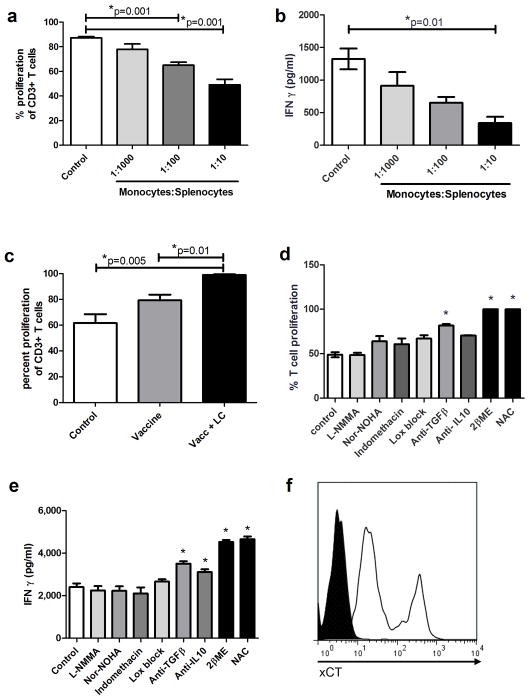
Experiments were conducted to determine whether vaccine-elicited inflammatory monocytes had direct T cell suppressive properties. Ly6G−CD11b+Gr-1+ myeloid cells were purified by flow cytometry from pooled LNs 24 hours after footpad immunization and the purified monocytes were then titrated into cultures of mitogen activated naive spleen cells. T cell proliferation was significantly suppressed in a dose-dependent fashion following addition of purified inflammatory monocytes (Figure 5a). Vaccine-elicited monocytes also significantly suppressed IFN-γ release from activated T cells (Figure 5b). We also observed that mitogen induced T cell proliferation was significantly increased in LN cells that were collected from mice that were vaccinated and concurrently received LC in vivo to deplete vaccine elicited monocytes (Figure 5c).
Figure 5. Vaccine elicited monocytes directly suppress T cell activation and proliferation.
5a. Inflammatory monocytes were purified by flow sorting from vaccine draining LNs 24 hours after vaccination and titrated at 3 ratios of monocytes : splenocytes (1:10, 1:100, 1:1,000) into cultures containing CFSE-labeled spleen cells that were then activated in vitro with Con A (5 μg/ml). Proliferation of CD3+ T cells was assessed 4 days later by flow cytometry. 5b. Supernatants from cultures in 5b were analyzed by ELISA for IFN-γ release. 5c. Twenty-four hours after vaccination (with or without LC treatment), LN cells were harvested from the draining LN and stimulated in vitro with Con A. CD3+ T cell proliferation was assessed 4 days later by flow cytometry. 5d. T cells were stained with CFSE and stimulated to proliferate with Con A (5 μg/ml) for 96 hours. Some cultures also were incubated with L-NMMA (300 μM), Nor-NOHA (200μM), indomethacin (30 mM), Lox Block (20 μM), neutralizing antibodies against TGF-β (1 μg/ml) or IL-10 (0.1 μg/ml), 2βME (55 μM), or NAC (0.5 M). T cell proliferation was assessed by flow cytometry 4 days later. 5e. Supernatants were collected after 96 hour incubation and analyzed for IFNγ release by ELISA. Error bars represent SEM, n=5. Statistical significance was determined using one-way ANOVA, followed by Tukey multiple means comparison test, with *= p < 0.05. 5f. Vaccine elicited monocytes were analyzed for cell surface expression of the cystine importer, xCT, by flow cytometry. Filled histogram represents isotype control, open histogram represents xCT stained monocytes.
Mechanism of vaccine-elicited monocyte suppression of T cells
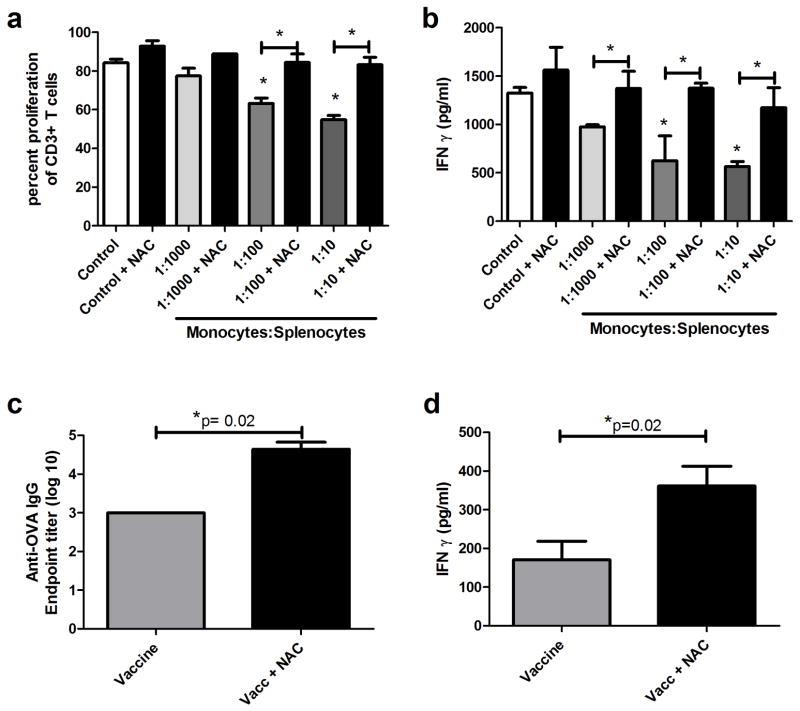
Purified vaccine-elicited monocytes were co-cultured with naive T cells, along with inhibitors of known myeloid suppression pathways (18, 37). When monocyte-T cell co-cultures were treated with an inhibitor of the NO synthase pathway (L-NMMA); (Movahedi et al., 2008), with an arginase inhibitor (Nor-NOHA); (38) or with Lox block® for blockade of lipoxygenase pathways 12 and 15 (28), there was no effect on monocyte mediated suppression of T cell proliferation or IFN-γ release (Figure 5d and 5e). Similarly, inhibition of prostaglandin release by treatment with indomethacin (Veltman et al., 2010) also failed to reverse T cell suppression by monocytes (Figure 5d and 5e). Neutralization of IL-10 and TGF-β with specific antisera had only modest affects on reversing monocyte-mediated T cell suppression (39–40) (Figure 5d and 5e). However, addition of an exogenous source of cysteine (NAC) or reduction of extracellular cystine to cysteine using 2-ME both significantly reversed monocyte-mediated suppression of T cell proliferation and cytokine release (Figure 5d and 5e). In addition, we found that inflammatory monocytes in vaccine-draining LNs expressed high levels of the xCT amino acid transporter molecule, which has been reported to be critically involved in cystine uptake by myeloid cells (37)(Figure 5f). These data suggested that extracellular cysteine, an important amino acid required for T cell activation, was involved in monocyte mediated T cell suppression. We also observed that addition of NAC nearly completely rescued T cell proliferation and IFN-γ responses over a wide range of monocyte: T cell ratios (Figure 6a and 6b). However, we cannot entirely exclude based on these experiments that the known anti-oxidant properties of NAC may play some role in the observed vaccine enhancement effects observed here. However, we do note that many of the other inhibitors used in the in vitro experiments such as indomethacin and lipoxygenase inhibitors also have anti-oxidant properties, yet did not alter T cell responsiveness.
Figure 6. Vaccine elicited monocyte-induced suppression of T cell activation and proliferation, can be reversed by addition of exogenous cysteine.
6a. Flow purified monocytes were added to activated T cells (as in 5a), with or without the addition of 0.5 mM N-acetylcysteine (NAC) as a cysteine source, and T cell proliferation was assessed using flow cytometry. 6b. Supernatants from cultures in 6a were analyzed by ELISA for IFN-γ release. * Statistical significance was determined using one-way ANOVA, followed by Tukey multiple means comparison test, with *= p < 0.05. Similar results were obtained in one additional independent experiment. 6c. Mice were vaccinated against Ova with or without concurrent NAC treatment (100 mg/kg, i.p., administered every 12 hours for 4 treatments). This treatment was also administered for the boost immunization. Serum was collected 12 days after boost and monitored for Anti-Ova IgG. 6d. Splenocytes from mice in 6c were stimulated ex vivo with 50 μg/ml ova and monitored for IFNγ release by ELISA. Error bars represent SEM, n=5. Statistical significance was determined using a Mann-Whitney test, with * = p < 0.05.
The effects of NAC supplementation on vaccine responses were also assessed in vivo. Immunized mice were concurrently treated with NAC administered i.p. at 100 mg/kg and vaccine responses were compared to those of mice that were only vaccinated. Mice treated with NAC at the time of vaccination had significantly increased antibody titers compared to mice that were only vaccinated (Figure 6c). In addition, NAC-treated and vaccinated mice generated significantly greater quantities of IFN-γ from spleen cells following antigen stimulation ex vivo (Figure 6d). These data are therefore consistent with the hypothesis that cysteine sequestration by vaccine-elicited inflammatory monocytes was the primary mechanism of T cell suppression and vaccine counter-regulation.
Discussion
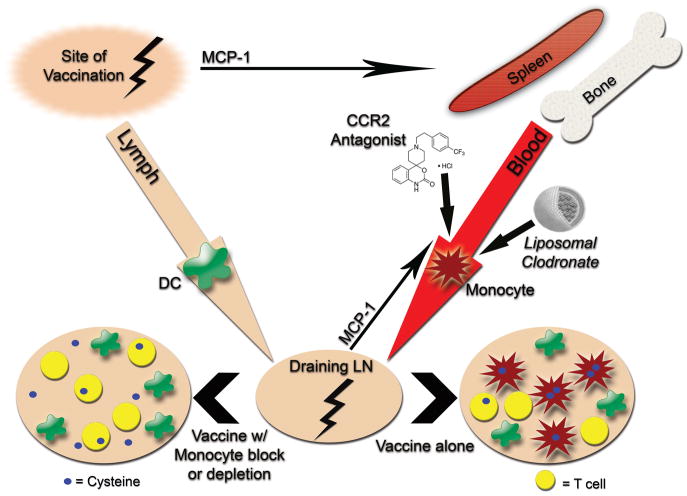
The results of the studies presented here provide strong evidence for a previously undescribed immune suppressive and paradoxical counter-regulatory role for inflammatory monocytes in vaccine immunity. Our studies showed that inflammatory monocytes could be rapidly recruited to vaccine draining LNs in an MCP-1-dependent fashion and once there, monocytes potently suppressed local T cell responses in LNs by sequestering cysteine. These conclusions were based on evidence that 1) immune responses to vaccination were significantly enhanced in CCR2−/− mice, 2) immune responses to vaccination were significantly enhanced when inflammatory monocytes were depleted or their migration blocked, 3) purified vaccine-elicited monocytes directly suppressed T cell responses in vitro, and 4) addition of exogenous cysteine nearly completely reversed the T cell suppressive effects of inflammatory monocytes, both in vitro and in vivo. Therefore, we propose a model wherein inflammation associated with vaccination triggers local production of MCP-1 and induces rapid immune counter-regulatory responses that are mediated by inflammatory monocytes recruited to vaccine draining LNs (Figure 7).
Figure 7.
Proposed model of inflammatory monocyte-mediated counter regulatory immune responses to vaccination and the role of monocyte depletion or blockade in amplifying vaccine immunity.
Inflammatory monocytes are likely to be generated anytime local inflammation develops, resulting in MCP-1 production. Indeed, we saw that depletion of monocytes during vaccination with a variety of adjuvants enhanced vaccine immunity. In fact, it was previously reported that exposure to even very low doses of TLR agonists was sufficient to mobilize inflammatory monocytes from the bone marrow (41). It is likely therefore that inflammatory monocytes move into circulation shortly after local release of MCP-1 and migrate to sites of inflammation to serve an important homeostatic function. While this response may be helpful during inflammatory disease states, we show that the counter regulatory response elicited by these monocytes can limit immune responses during vaccination. Therefore, transient blockade of monocytes during the vaccination process is an attractive therapeutic target that warrants further study.
Vaccine-elicited inflammatory monocytes appear to use a relatively unusual mechanism to suppress T cell responses. Whereas tumor-generated monocytic myeloid suppressor cells have typically been reported to suppress T cell function by production of arginase or NO, vaccine-elicited inflammatory monocytes suppressed T cell responses by depleting extracellular cysteine, most likely by sequestering its precursor molecule cystine. This mechanism of T cell suppression was reported recently for tumor-elicited myeloid suppressor cells (37). Since T cells require extracellular cysteine for activation and proliferation, the loss of available cysteine following monocyte sequestration of cystine results in significant suppression of T cell function (see Figure 6). This mechanism differs from that reported by Martino et al., which identified NO as the causative component of BCG- elicited, myeloid cell-mediated immunosuppression (9). Interestingly, Martino and colleagues did not report finding these cells in the draining LN earlier than 3 days after vaccination whereas we identify vaccine elicited monocytes in the node within 24 hours of vaccination suggesting that early responding monocytes that suppress through a cysteine dependent mechanism may actually be a different population than that reported by Martino.
Additionally, our results differ from those of Nakano et al, who concluded that inflammatory monocytes elicited by vaccination actually enhanced immune responses. The immunization models used in our studies and those used by Nakano et al.(10) were not identical, but we believe the discrepancy can be best explained by the fact that Nakano et al., did not directly evaluate T cell or B cell responses to vaccination, nor did they examine how monocyte blockade or depletion affected those responses. The Nakano publication also noted that since the CCR2 −/− mice lacked a sufficient IFN-γ response then monocytes must be important in augmenting vaccine immunity. However, the known IFN-γ production defect in CCR2−/− mice would have made it difficult to directly compare IFN-γ production following vaccination of wild type versus CCR2−/− mice (11).
We believe the monocyte-mediated vaccine counter-regulatory immune responses described here are broadly relevant, since they were found to occur following immunization with a variety of adjuvants and antigens. Recently Friedlender et al., reported that a population of CD11c+ cells were recruited via a CCL2 dependent mechanism following vaccination and augmented cancer immunotherapy (42). While the vaccine elicited monocytes described in our study did not express CD11c+, it remains possible that they may alter their phenotype over time and therefore may represent be a less differentiated population of cells than those reported by Fridlender et al. In addition, the phenotype of vaccine-elicited inflammatory monocytes may differ significantly in normal animals as in our studies versus their phenotype in animals that already have cancer, as in the Fridlender studies. Finally, the monocyte migration response reported here was most likely TLR independent since it was also observed following immunization with alum adjuvant, though the TLR independence of the effect was not formally examined in our study. Many inflammatory stimuli can induce MCP-1 production and therefore signaling via CCR2 may be the common thread linking vaccine adjuvants and counter-regulatory immune responses mediated by inflammatory monocytes (43–47). Our data indicated that MCP-1 was the major chemokine required to mobilize and recruit inflammatory monocytes following vaccination.
The key role played by the MCP-1/CCR2 signaling pathway in regulating vaccine suppression by monocytes may make it possible to use specific chemokine receptor antagonists as a new type of “vaccine adjuvant-adjuvant”. For example, administration of a CCR2 antagonist at the time of vaccination significantly enhanced vaccine humoral and cellular immune responses (see Figure 3). Thus, it should be possible to substantially improve vaccine effectiveness using chemokine receptor antagonists already under development for treatment of inflammatory disorders in humans (48–49). Finally, vaccine amplification by administration of chemokine receptor antagonists and/or concurrent administration of cysteine may be particularly attractive as strategies to boost vaccine responses in difficult to vaccinate populations such as the very young and the elderly.
Acknowledgments
The authors would like to thank Dr. Scott Hafeman for preparing the liposomal clodronate used for these studies and Mrs. Leslie Armstrong for assistance with flow sorting. The authors would also like to thank Dr. Charles Drake at Johns Hopkins University for kindly providing the A20-HA cell line.
Footnotes
This work was supported in part by a grant from the Skippy Frank Translational Medicine and Life Sciences Fund, by a CSU Infectious Diseases Research Center grant, and by NIH grant U54 AI065357-05.
LN(s), Lymph node(s)
LC, Liposomal Clodronate
HA, Hemagglutinin
CLDC, cationic liposomal DNA complexes
NAC, N-acetyl cysteine
References
- 1.Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Petrilli V, Tschopp J, O’Neill LA, Lavelle EC. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27:3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 6.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 7.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 9.Martino A, Badell E, Abadie V, Balloy V, Chignard M, Mistou MY, Combadiere B, Combadiere C, Winter N. Mycobacterium bovis bacillus Calmette-Guerin vaccination mobilizes innate myeloid-derived suppressor cells restraining in vivo T cell priming via IL-1R-dependent nitric oxide production. J Immunol. 2010;184:2038–2047. doi: 10.4049/jimmunol.0903348. [DOI] [PubMed] [Google Scholar]
- 10.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters W, Dupuis M, Charo IF. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J Immunol. 2000;165:7072–7077. doi: 10.4049/jimmunol.165.12.7072. [DOI] [PubMed] [Google Scholar]
- 12.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodyear A, Jones A, Troyer R, Bielefeldt-Ohmann H, Dow S. Critical protective role for MCP-1 in pneumonic Burkholderia mallei infection. J Immunol. 2009;184:1445–1454. doi: 10.4049/jimmunol.0900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 15.Czuprynski CJ, Campbell PA, Henson PM. Killing of Listeria monocytogenes by human neutrophils and monocytes, but not by monocyte-derived macrophages. J Reticuloendothel Soc. 1983;34:29–44. [PubMed] [Google Scholar]
- 16.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 17.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 21.Song X, Ye D, Liu B, Cui J, Zhao X, Yi L, Liang J, Song J, Zhang Z, Zhao Q. Combination of all-trans retinoic acid and a human papillomavirus therapeutic vaccine suppresses the number and function of immature myeloid cells and enhances antitumor immunity. Cancer Sci. 2009;100:334–340. doi: 10.1111/j.1349-7006.2008.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Deventer HW, Burgents JE, Wu QP, Woodford RM, Brickey WJ, Allen IC, McElvania-Tekippe E, Serody JS, Ting JP. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 2010;70:10161–10169. doi: 10.1158/0008-5472.CAN-10-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafeman S, London C, Elmslie R, Dow S. Evaluation of liposomal clodronate for treatment of malignant histiocytosis in dogs. Cancer Immunol Immunother. 2010;59:441–452. doi: 10.1007/s00262-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 25.Rehni AK, Singh TG. Involvement of CCR-2 chemokine receptor activation in ischemic preconditioning and postconditioning of brain in mice. Cytokine. 2012;60:83–89. doi: 10.1016/j.cyto.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Rehni AK, Singh N. Ammonium pyrrolidine dithiocarbamate and RS 102895 attenuate opioid withdrawal in vivo and in vitro. Psychopharmacology (Berl) 2012;220:427–438. doi: 10.1007/s00213-011-2489-8. [DOI] [PubMed] [Google Scholar]
- 27.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999;163:1552–1561. [PubMed] [Google Scholar]
- 28.van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res. 2008;86:904–909. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton MJ, Banath JP, Lam V, Lepard NE, Krystal G, Bennewith KL. Serum inhibits the immunosuppressive function of myeloid-derived suppressor cells isolated from 4T1 tumor-bearing mice. Cancer Immunol Immunother. 2011;61:643–654. doi: 10.1007/s00262-011-1125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 31.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpe S, Cameroni E, Moepps B, Thelen S, Apuzzo T, Thelen M. CCR2 Acts as Scavenger for CCL2 during Monocyte Chemotaxis. PLoS One. 2012;7:e37208. doi: 10.1371/journal.pone.0037208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo JI, Pan H, Oh S, Lim DJ, Moon SK. Spiral ligament fibrocyte-derived MCP-1/CCL2 contributes to inner ear inflammation secondary to nontypeable H. influenzae-induced otitis media. BMC Infect Dis. 2010;10:314. doi: 10.1186/1471-2334-10-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirzadegan T, Diehl F, Ebi B, Bhakta S, Polsky I, McCarley D, Mulkins M, Weatherhead GS, Lapierre JM, Dankwardt J, Morgans D, Jr, Wilhelm R, Jarnagin K. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275:25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 35.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding ZC, Blazar BR, Mellor AL, Munn DH, Zhou G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 2010;115:2397–2406. doi: 10.1182/blood-2009-11-253336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunt SK, V, Clements K, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang LC, Heitjan D, Snyder LA, Albelda SM. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010;70:109–118. doi: 10.1158/0008-5472.CAN-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002;169:2026–2033. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 44.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 45.Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su TP, Wang JQ, Buch S. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kindrachuk J, Jenssen H, Elliott M, Townsend R, Nijnik A, Lee SF, Gerdts V, Babiuk LA, Halperin SA, Hancock RE. A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine. 2009;27:4662–4671. doi: 10.1016/j.vaccine.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 47.Shahrara S, Pickens SR, Mandelin AM, 2nd, Karpus WJ, Huang Q, Kolls JK, Pope RM. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 49.Xia M, Sui Z. Recent developments in CCR2 antagonists. Expert Opin Ther Pat. 2009;19:295–303. doi: 10.1517/13543770902755129. [DOI] [PubMed] [Google Scholar]