Abstract
Skilled motor actions are associated with handedness and neuroanatomical specializations in humans. Recent reports have documented similar neuroanatomical asymmetries and their relationship to hand preference in some nonhuman primate species, including chimpanzees and capuchin monkeys. We investigated whether capuchins displayed significant hand preferences for a tool use task and whether such preferences were associated with motor-processing regions of the brain. Handedness data on a dipping tool-use task and high-resolution 3T MRI scans were collected from 15 monkeys. Capuchins displayed a significant group-level left-hand preference for this type of tool use, and handedness was associated with asymmetry of the primary motor cortex. Left-hand preferent individuals displayed a deeper central sulcus in the right hemisphere. Our results suggest that capuchins show an underlying right-hemisphere bias for skilled movement.
Keywords: hemispheric specialization, laterality, handedness
One of the most pronounced behavioral asymmetries in humans is hand dominance, with a majority of individuals (approximately 90%) expressing right-handedness for fine motor tasks [Coren & Porac, 1977; Annett 2006]. This left-hemisphere dominance for skilled movement such as tool use is associated with structural and functional asymmetries of the primary motor cortex, primary somatosensory cortex and cerebellum [Lewis, 2006]. Functional brain asymmetries in nonhuman primates often correspond to those seen in humans. As in humans, the most marked expression of this is seen in hand dominance. Chimpanzees, macaques, and capuchin monkeys display individual hand dominance for various skilled motor tasks [Hopkins 2007], though none of these species demonstrate the degree of population-level bias observed in humans.
Tool use and gestural communication can be considered goal-directed, manipulative acts. Bradshaw and Nettleton [1982] and Gibson [1993] proposed that advanced tool use preceded gestural communication, and may have played a role in its evolution. This suggests that a common neurological substrate should be involved in both skills [Frey, 2008]. Indeed, functional neuroimaging studies of humans indicate that similar neural substrates are involved in both retrieving and planning actions of the hand (as required in tool use) and planning intransitive gestures – the left parietal and dorsal premotor cortices [Kroliczak & Frey 2009]. Thus, neurological evidence exists to support the link between the origins of tool use and gestural communication. Furthermore, this would suggest that neuranatomical asymmetries would be present in species that display handedness for advanced tool use but do not show gestural communication, such as capuchins.
Capuchins share with humans, macaques and chimpanzees key characteristics that contribute to manual control: corticospinal terminals in the ventral horn of the spinal cord innervating digits of the hand, opposable (or laterally opposable) thumb and precision grips, complex manipulation, and the use of feeding tools in the wild. Humans, macaques and capuchins also possess a well-developed parietal Brodmann area 2 and Brodmann area 5 – cortical regions that receive input from the hand [Padberg et al., 2007]. Capuchins are noted for their high degree of manipulative propensities and extractive foraging habits, which are analogous to complex manipulative skills demonstrated by humans and chimpanzees [Parker & Gibson, 1977]. Whether or not capuchins express a tendency towards population-level handedness is not clear, with some research groups reporting population-level preferences [e.g., Spinozzi et al., 1998] and others not [e.g., Fragaszy et al. 2004; Lilak & Phillips, 2008; Westergaard & Suomi, 1996]. However, individual capuchins do display strong and significant preferences for a given hand in specific tasks, particularly in those tasks requiring bimanual coordination [Fragaszy & Mitchell, 1990; Lilak and Phillips, 2008; Limongelli et al., 1994; Westergaard & Suomi, 1993, 1996]. One frequently used task of bimanual coordination in nonhuman primates is the TUBE task. Handedness on this task is correlated with neuroanatomical structures associated with cortical motor areas representing hand in chimpanzees and capuchins [Hopkins & Cantalupo, 2004; Phillips & Sherwood, 2005]. Whether such relationships are present in capuchins with more complex skilled movements of the hand, seen in tool-using tasks, is unknown. Therefore, the aim of this study was to assess the relationship between hand preference on a tool use task and neuroanatomical asymmetry of primary motor cortical area in capuchins. A demonstration of group-level handedness for an advanced tool use task and associated neuroanatomical asymmetry in motor processing areas in capuchins would provide further support for the hypothesis that the origins of tool use and communicative gesture are linked.
Method
Subjects
Handedness data and in vivo magnetic resonance images were collected from 15 adult and juvenile brown capuchin monkeys (Cebus apella; male n = 8, female n = 7). Subjects were housed at The College of Wooster (Wooster, Ohio), Hiram College (Hiram Ohio) and the Southwest National Primate Research Center (San Antonio, Texas). Subjects ranged in age from 4 – 22 years (M = 10.3, SD = 6.7), and were socially housed in large, enriched enclosures. While adult capuchins have been shown to display a stronger lateral bias than juveniles [Westergaard and Suomi, 1993], to our knowledge only one longitudinal study has been conducted to determine if the direction of hand preference changes during development. Westergaard et al. [1998] reported that hand preference at 23 to 24 weeks was positively correlated with hand preference at 47 to 48 weeks. All were born in captivity and had been socially housed since birth. This research complied with protocols approved by the IACUC at each institution, adhered to the legal requirements governing research with nonhuman primates in the United States, and adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates.
Behavioral Data
Hand preference was determined on a dipping tool use task, which required subjects to insert straw into a small hole to dip for a preferred food substance (such as applesauce or yogurt). A poly-vinyl-chloride (PVC) tube, 28 cm in length and 2.5 cm in diameter, was drilled with three equally spaced 2mm diameter holes (which were smaller than the subjects’ fingers). Food was placed inside the tube and a cap secured on each end. This apparatus was then attached to a fixed substrate. In order to retrieve the food, subjects had to insert straw, which was readily available in the enclosure, into one of the holes and then bring the straw to the mouth [see Figure 1 and Supplementary video]. The hand used for inserting the straw was defined as the dominant hand and the frequency of left and right hand responses within the trial period was recorded. Each subject was tested four times, with each test session lasting a maximum of 30 min. A minimum of 50 responses were obtained from each subject.
Figure 1.

Photograph showing a capuchin monkey using her left hand while engaged in the tool use task.
Handedness index (HI) scores were determined for each subject by using the formula (#R −#L)/(#R + #L), where #R is the number of instances in which the right hand was used and #L equals the number of instances in which the left hand was used. The HI produces scores ranging from − 1.0 to +1.0, with negative scores indicating a preference for the left hand and positive scores indicating a preference for the right hand. More extreme absolute scores (ABS-HI) reflect a stronger preference for a preferred hand. A mean handedness index (MHI) was calculated for each individual by taking the average HI across all test sessions. To determine if the hand preference of an individual was significantly different from chance, z-scores were calculated based on the total frequency in left and right hand use. Subjects with z-scores greater than 2.54 were classified as right-handed; subjects with z-scores less than −2.54 were classified as left-handed.
MRI Procedure and Image Quantification Method
Subjects were transported to the Research Imaging Institute (RII), University of Texas Health Science Center at San Antonio (San Antonio, Texas) or to the Neuroscience Imaging Center (NIC; Pittsburgh, Pennsylvania) for the imaging procedure, and anesthetized following standard protocols used at the facilities.
At the RII, subjects were initially immobilized by ketamine (7 mg/kg) and dexmeditomidine (.06 mg/kg) injection IM. After preanesthetizing the animal with the stated doses of ketamine and dexmeditomidine, subjects were orotracheally intubated with a cuffed or uncuffed endotracheal tube of the proper size (estimated to be between 2.0 and 4.0 mm). One to 2.5% isoflurane mixed with oxygen/air blend was administered through the endotracheal tube to maintain anesthesia. Animals were placed on a pressure ventilator. At the NIC, after preanesthetizing subjects with ketamine (7 mg/kg) and dexmeditomidine (.06 mg/kg) injection IM and atropine (0.05 mg/kg) injection SQ, subjects were then given a bolus of propofol (2–5 mg/kg) intravenously; a constant intravenous drip of 250–330 μg/kg/min of propofol maintained anesthesia. All subjects remained anesthetized throughout the MR procedure (approximately 25 minutes) and respiration rate, heart rate, and oxygen consumption were continually monitored.
Once subjects were anesthetized, they were placed in a Siemens 3T Trio (Research Imaging Institute) or Siemens 3T Allegra Scanner (Neuroscience Imaging Center) and heads fitted inside a 12 cm head coil specifically designed for non-human primates. Sagittal T1-weighted 3D MPRAGE images were acquired through the entire brain using a gradient echo protocol (pulse repetition = 1500 ms, echo time = 3.04 ms, and a 256 × 256 matrix). Slices were obtained as 0.5 mm thick contiguous sections. Subjects were allowed to fully recover from the anesthesia before return transport.
Images were spatially realigned into standard anatomical orientation with the transaxial plane parallel to the anterior commissure-posterior commissure (AC-PC) line and perpendicular to the interhemispheric fissure. Computer files for individual subjects were numerically coded prior to measurement to prevent observer bias, and the individual performing the tracings was blind to the handedness data. Morphometric measurements of depth of the central sulcus were performed using Analyze 10.0 (Analyze Direct, Overland Park, Kansas, USA). The central sulcus can be used to localize the motor hand area of the primary motor cortex. Measurement of central sulcus depth followed the methodology of Phillips and Sherwood [2005]. Briefly, central sulcus depth was measured as the intrasulcal length of the precentral gyrus as viewed in horizontal sections. Only the portion of the precentral gyrus representing the hand was selected for measurement because asymmetries in central sulcus depth at this location have previously been shown to correlate significantly with handedness in human males and capuchin males [Amunts, Jäncke, Mohlberg, Steinmetz, & Zilles, 2000; Phillips & Sherwood, 2005]. This region constituted the series of sections located between 40 – 60% of the total dorsoventral length of the central sulcus as measured starting at the first dorsal section containing the central sulcus. In each section, the intrasulcal length of the precentral gyrus was manually traced from both hemispheres [see Figure 2]. The asymmetry quotient (AQ = R−L/[(R+L) x 0.5]) of central sulcus depth was then calculated for each section. Finally, a summary AQ for the region of hand representation was calculated as the mean of all AQ measurements in each section, for each subject.
Figure 2.
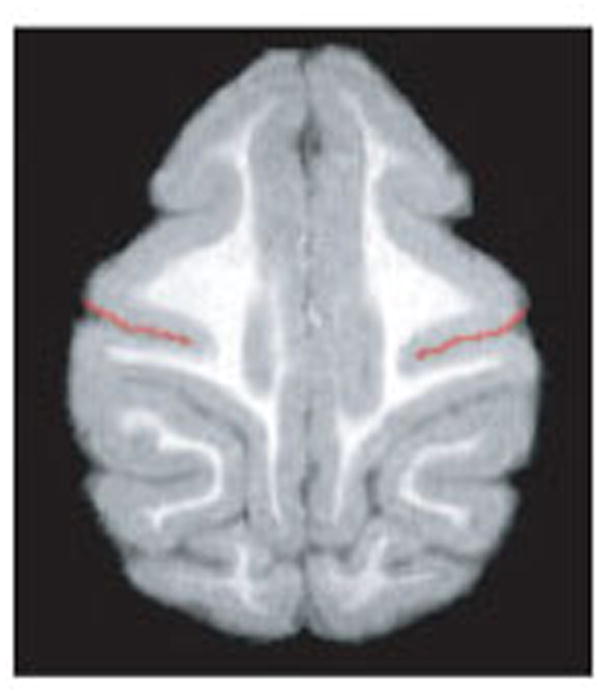
Central sulcus depth was measured as the intrasulcus length of the precentral gyrus as viewed in horizontal sections.
Results
The hand preference results for the tool use task are provided in Table 1. The internal consistency (Cronbach’s alpha) was .84, indicating subjects showed high test-retest consistency for hand preference. All but two subjects exhibited a strong unambiguous preference to use one hand as dominant, as determined by a z score for HI exceeding +/− 2.54. Eleven subjects displayed a left-hand preference, three subjects displayed a right-hand preference, and one subject was ambipreferent. The mean HI across all subjects for this task was −.46 (SEM = .18). A one-sample t-test indicated a group-level left-hand preference for the tool task, t(14) = −2.52, P = .024.
Table 1.
Handedness distribution and central sulcus asymmetry quotient (AQ) for capuchin monkeys on a dipping tool use task.
| Subject | Sex | HI | z-score | Handedness Classification | Central Sulcus AQ |
|---|---|---|---|---|---|
| Georgia | F | .26 | 9.41 | R | −11.53 |
| Gizmo | F | −.98 | −11.41 | L | 19.73 |
| Jake | F | −.98 | −11.97 | L | 8.82 |
| Ellie | F | −.88 | −7.73 | L | −22.98 |
| Noel | F | −.78 | −13.64 | L | −6.28 |
| Luuloa | F | −.68 | −9.41 | L | 17.78 |
| Dee | F | 1.00 | 5.39 | R | −2.23 |
| Alou | M | −.66 | −13.57 | L | .90 |
| Carlos | M | −.52 | −4.1 | L | 7.00 |
| DiMaggio | M | −.90 | −7.32 | L | −.20 |
| Sosa | M | −.97 | −12.26 | L | 6.00 |
| Vincent | M | −1.00 | −14.59 | L | 10.80 |
| Koufax | M | −1.00 | −13.48 | L | 14.20 |
| Shoeless | M | .21 | −0.68 | A | −2.60 |
| Pip | M | .95 | 6.13 | R | −3.60 |
R = right-handed, L = left-handed, and A = ambiguously handed.
As there was a clear bias in this group for the use of the left hand for tool use, to compare asymmetry of the central sulcus across handedness groups, we dichotomously categorized subjects as either left-hand preferent (n = 11) or right-hand preferent (n = 4). [Note that all subjects with positive HI values are classified as right-hand preferent.] Central sulcus AQ was significantly different (Mann Whitey U, Z = 1.96, P = 0.050) between left-hand preferent and right-hand preferent individuals [see Figure 3] on the tool use task. Left-hand preferent monkeys displayed a deeper central sulcus in the right hemisphere.
Figure 3.
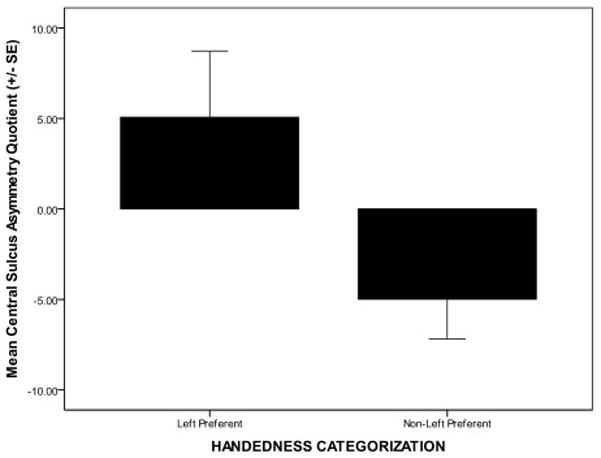
Mean asymmetry quotient [AQ = R − L/(R + L) * .5] where R and L represent central sulcus depth in the right and left hemisphere, respectively. Positive values indicate right-hemipshere bias, negative values indicate left-hemisphere bias. Central sulcus asymmetry is significantly different between left-hand preferent and right-hand preferent capuchins on a dipping tool use task.
Discussion
We report two novel and significant findings in this report. First, we provide evidence of a group-level left-hand preference for capuchins in a dipping tool-use task. Additionally, hand preference was significantly associated with asymmetry of primary motor cortex for the region representing hand. Left-hand preferent and right-hand preferent monkeys differed on lateralization of the primary motor cortex, with left-hand preferent monkeys having a deeper central sulcus on the right hemisphere. These results are similar to previous findings of a relationship between asymmetry of the central sulcus and hand preference on a bimanual coordinated task in male capuchins [Phillips & Sherwood, 2005]. That study, using a small (n = 6) sample of male capuchins, reported left-handed monkeys on the TUBE task showed a deeper central sulcus in the contralateral hemisphere, whereas right-handed monkeys had a more symmetrical depth of the central suclus.
Interestingly, wild chimpanzees also show population left-handedness for termite fishing [Lonsdorf & Hopkins, 2005], and captive male chimpanzees display a bias toward left hand use in a simulated termite fishing task that requires a precise guiding motion [Hopkins et al., 2009]. The left-hand preference in both capuchins and male chimpanzees may reflect spatial characteristics of the dipping task. Fagot and Vauclair [1988) and MacNeilage et al. [1987] have proposed that the left hand is preferred for precise, visually guided manipulative actions.
Studies of chimpanzees and capuchins have shown there is little consistency of hand preference across various motor tasks, except for instances where solving different tasks requires similar motor actions (Hopkins & Pearson, 2000; Hopkins, Taglialatela, Leavens, Russell, & Schapiro, 2010; Lilak & Phillips, 2008). Thus, we would not expect individuals to have consistent hand preferences on the tool use and TUBE task as these utilize different motor actions. This was indeed the case (Phillips, unpublished data; r = .14, p = 0.62).
Wild capuchins from several study sites have been reported to use tools for feeding, including the use of sticks as probes, leaves as sponges, and rocks as hammer stones [Chevalier-Skolnikoff, 1990; Fernandes, 1991; Phillips, 1998; Fragaszy et al., 2004]. The dipping tool use task used in this investigation has a natural counterpart: critically endangered blonde capuchins (Cebus flavius) of the Atlantic Forest use sticks to fish for above ground termite nests and spontaneously modify the sticks [Souto et al., 2011].
Our results, showing a group-level hand preference in capuchins for tool use, support the position that handedness resulted from the increased demands associated with feeding and tool use [Wundram, 1986]. While there is no population-wide hand preference seen for the TUBE task [Fragaszy, Visalberghi, & Fedigan, 2004; Lilak & Phillips, 2008; Westergaard & Suomi, 1996; but see Spinozzi, Castorina, & Truppa, 1998 for a conclusion of right-handedness in the TUBE task], several studies have indicated that capuchins show stronger left-hand preferences in certain tool-using tasks, specifically tasks requiring the use of probing tools [Anderson, Degiorgio, Lamarque, & Fagot, 1996; Westergaard, 1991; Westergaard & Suomi, 1993]. Contrary to this pattern, Souto et al. [2011] reported capuchins always used the right hand to insert a stick into the termite nest. However, caution must be exercised in interpretation, as only three of six study animals were observed to engage in this behavior, and an extremely limited number of instances (eight, out of 72 days) were observed. Determining whether the wild blonde capuchins display handedness for termite fishing will be important for comparison with handedness data in laboratory-reared Cebus monkeys.
Our results indicate that capuchins show an underlying right-hemisphere bias for skilled tool use, and that this is associated with structural asymmetry of the primary motor cortex. As capuchins do not display communicative gestures, these results do not support the hypothesis that asymmetries for skilled motor actions arose after neuroanatomical asymmetries for language, as proposed by Corballis [2002]. However, that capuchin monkeys show neuroanatomical specialization for tool use in the absence of gestural communication is consistent with the hypothesis that tool use and gestural communication have common neural origins, as has been proposed by Frey [2008]. While chimpanzees do show relationships between handedness for tool use and gestural communication with language area homologues (inferior frontal gyrus and planum temporale) [Hopkins et al. 2007; Meguerditchian et al., 2012; Taglialatela et al., 2006], whether similar relationships exist between handedness for tool use or gestural communication and asymmetry of the primary motor cortex is unknown. As Hopkins and Cantalupo [2004] reported that handedness on the TUBE task correlated with primary motor cortex asymmetries [but not the planum temporale or FO frontal operculum], we expect chimpanzees would have such asymmetries associated with handedness for goal-directed actions such as tool use and gestural communication. Determining whether chimpanzees also show such a relationship will be important to further evaluate the hypothesis that tool use and gestural communication have common neural origins.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institute of Neurological Disorders and Stroke, grant NS070717–01, and a CTSA Imaging Supplement (parent grant UL1RR025767) to KAP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke or the National Institutes of Health.
References
- Amunts K, Jäncke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–312. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Degiorgio C, Lamarque C, Fagot J. A multi-task assessment of hand lateralization in capuchin monkeys (Cebus apella) Primates. 1996;37:97–103. [Google Scholar]
- Annett M. The distribution of handedness in chimpanzees: estimating right shift in Hopkins’ sample. Laterality. 2006;11:101–109. doi: 10.1080/13576500500376500. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettleton NC. Language lateralization to the dominant hemisphere: tool use, gesture and language in hominid evolution. Current Psychological Reviews. 1992;2:171–192. [Google Scholar]
- Chevalier-Skolnikoff S. Tool use by wild Cebus monkeys at Santa Rosa National Park, Costa Rica. Primates. 1990;31:375–383. [Google Scholar]
- Coren S, Porac C. Fifty centuries of right-handedess: the historical record. Science. 1977;198:631–632. doi: 10.1126/science.335510. [DOI] [PubMed] [Google Scholar]
- Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary motor cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006;44:2582–2586. doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Handedness and bimanual coordination in the lowland gorilla. Brain, Behavior and Evolution. 1988;32:89–95. doi: 10.1159/000116536. [DOI] [PubMed] [Google Scholar]
- Fernandes MEB. Tool use and predation of oysters (Crassostrea rhizophorae) by the tufted capuchin, Cebus apella apella, in brackish water mangrove swamp. Primates. 32:529–531. [Google Scholar]
- Fragaszy DM, Mitchell SR. Hand preference and performance on unimanual and bimanual tasks in capuchin monkeys (Cebus apella) Journal of Comparative psychology. 1990;104:275–282. doi: 10.1037/0735-7036.104.3.275. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philosophical Transactions of the Royal Society B. 2008;363:1951–1957. doi: 10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KR. The evolution of lateral asymmetries, language, tool-use, and intellect. In: Bradshaw J, Rogers L, editors. American Journal of Physical Anthropology. Vol. 92. 1993. pp. 123–124. [Google Scholar]
- Hopkins WD. The Evolution of Hemispheric Specialization in Primates. London: Academic Press; 2007. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Canatlupo C. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes): Implication for theories on the evolution of language. Psychological Science. 2007;18:971–977. doi: 10.1111/j.1467-9280.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. Handedness for tool use in captive chimpanzees (Pan troglodytes): Sex differences, performance, heritability and comparison to the wild. Behaviour. 2009;146:1463–1483. doi: 10.1163/156853909X441005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Russell JL, Nir TM, Schaeffer J. Cortical representation of lateralized grasping in chimpanzees (Pan troglodytes): A combined MRI and PET study. PLoS ONE. 2010;5:e13383. doi: 10.1371/journal.pone.0013383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G, Frey SH. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cerebral Cortex. 2009;19:2396–2410. doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. The Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Lilak AL, Phillips KA. Consistency of hand preference across low-level and high-level tasks in capuchin monkeys (Cebus apella) American Journal of Primatology. 2008;70:254–260. doi: 10.1002/ajp/20485. [DOI] [PubMed] [Google Scholar]
- Linomgelli L, Sonetti MG, Visalberghi E. Hand preference of tufted capuchins (Cebus apella) in tool-using tasks. In: Anderson JRJ, Herrenschmidt N, Roeder J, Thierry B, editors. Current Primatology. III. Strasbourg: Universite Louis Pasteur Press; 1994. pp. 9–15. Behavioural Neuroscience, Physiology and Reproduction. [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population-level handedness for tool use. Proceedings of the National Academy of Sciences. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Meguerditchian M, Gardner MJ, Schapiro S, Hopkins WD. The sound of one hand clapping: handedness and perisylvian neural correlates of a communicative gesture in chimpanzees. Proceedings of the Royal Society Biology. 2012;279:1959–1966. doi: 10.1098/rspb.2011.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Franca JG, Cooke DF, Soares JGM, Rosa MGP, Fiorani M, Jr, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. The Journal of Neuroscience. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker ST, Gibson KR. Object manipulation, tool use, and sensorimotor intelligence as feeding adaptations in Cebus monkeys and great apes. Journal of Human Evolution. 1977;6:623–641. [Google Scholar]
- Phillips KA. Tool use in wild capuchin monkeys (Cebus albifrons trinitatis) American Journal of Primatology. 1998;46:259–261. doi: 10.1002/(SICI)1098-2345(1998)46:3<259::AID-AJP6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hopkins WD. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella) Neuropsychologia. 2007;45:2333–2339. doi: 10.1016/j.neuropsychologia.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys (Cebus apella) Behavioral Neuroscience. 2005;119:1701–1704. doi: 10.1037/0735-7044.119.6.1701. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CS, Lilak AL. Corpus callosum morphology in capuchin monkeys is influenced by sex and handedness. PLoS ONE. 2007;2(8):e792. doi: 10.1371/journal.pone.0000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto A, Bione CBC, Bastos M, Bezerra BM, Fragaszy D, Schiel N. Critically endangered blonde capuchins fish for termites and use new techniques to accomplish the task. Biology Letters. 2012 doi: 10.1098/rsbl.2011.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinozzi G, Castorina M, Truppa V. Hand preferences in unimanual and coordinated bimanual tasks by tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1998;112:183–191. [Google Scholar]
- Taglialatela J, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetries in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard GC. Hand preference in the use and manufacture of tools by tufted capuchin (Cebus apella) and lion-tailed macaque (Macaca silenus) monkeys. Journal of Comparative Psychology. 1991;105:172–176. doi: 10.1037/0735-7036.105.2.172. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Byrne G, Suomi SJ. Early lateral bias in tufted capuchins (Cebus apella) Developmental Psychobiology. 1998;32 (1):45–40. doi: 10.1002/(sici)1098-2302(199801)32:1<45::aid-dev5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference in capuchin monkeys (Cebus apella) varies with age. Primates. 1993;34:295–299. doi: 10.1159/000156726. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi S. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) Journal of Comparative Psychology. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]
- Wundram IJ. Cortical motor asymmetry and hominid feeding strategies. Human Evolution. 1986;1:183–187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


