Abstract
The Na+/Ca2+ exchanger (NCX) is thought to play an important role in the pathogenesis of pentylenetetrazole (PTZ)-induced tonic flexion in mice. Here, I investigated the expression of PTZ-induced generalized clonic and tonic-clonic seizures in rats, using two potent NCX reverse mode inhibitors, KB-R7943 and SN-6 for NCX subtypes 3 (NCX3) and 1 (NCX1), respectively. Pretreatment with KB-R7943 (3, 10, 30 mg/kg; p.o.) significantly reduced the expression of PTZ-induced generalized seizures with clonic and tonic-clonic components in 12–62% and 25–62% of the treated animals, respectively. In the remaining animals that exhibited seizures, KB-R7943 (3 mg/kg; p.o.) pretreatment significantly delayed the onset of the first seizure episode and reduced the seizure severity. Following pretreatment with SN-6 (0.3, 1, 3, 10, 30 mg/kg; p.o.), clonic and tonic-clonic PTZ-induced generalized seizures were reduced in 25–50% and 38–63% of treated animals, respectively. SN-6 (0.3, 1, 3, mg/kg; p.o.) also significantly reduced PTZinduced seizure severity scores, but did not alter seizure latencies. KB-R7943 (3, 30 mg/kg; p.o.) or SN-6 (3, 30 mg/kg; p.o.) administration potentiated the sub-anticonvulsant dose of diazepam (2.5 mg/kg; i.p.) that suppresses clonic and tonic-clonic PTZ-induced seizures. These findings suggested that Ca2+ influx via the NCX in reverse mode contributes to a neuronal hyperexcitability that leads to clonic and tonic-clonic generalized seizures and that the NCX1 and NCX3 isoforms may serve as novel molecular targets for seizure suppression.
Keywords: KB-7943, SN-6, anticonvulsant, hyperexcitability, clonic seizures, tonic-clonic seizures
1. Introduction
The Na+/Ca2+ exchanger (NCX) is a bidirectional membrane ion transporter that couples the counter-transport of Na+ and Ca2+ to regulate the levels of intracellular Ca2+ in various cell preparations (Blaustein and Lederer, 19991; Annunziato et al., 2004). Under physiological conditions when intracellular Ca2+ levels rise, the NCX couples the export of a Ca2+ ion to the import of three Na+ ions; this is known as the “forward” mode of NCX activity. However, when intracellular Na+ levels rise or strong membrane depolarization occurs, the exchanger reverses, exporting three Na+ ions for each imported Ca2+ ion; this is referred to as the “reverse” mode of NCX activity (Blaustein and Lederer, 19991; Annunziato et al., 2004). Elevated intracellular Ca2+ and altered Ca2+ homeostasis have been implicated in the pathogenesis of epilepsy. Thus, massive Ca2+ entry into the cell following activation of the NCX in reverse mode can disturb Ca2+ homeostasis, resulting in neuronal hyperexcitability that can lead to seizures. Three different isoforms of the NCX (NCX1, NCX2 and NCX3) have been characterized, cloned and detected in various tissues, including the central nervous system (Philipson and Nicoll, 1997; Quednau et al., 1997; Papa et al., 2003; Lytton, 2007). The NCX has been implicated in the pathophysiology of various neurological conditions, including Alzheimer’s disease (Bi et al., 2012; Sokolow et al., 2011), ischemia (Pignataro et al., 2004; Lee et al., 2005; Boscia et al., 2006), hypoxia (Secondo et al., 2007) and Parkinson’s disease (Ago et al., 2011). Although the role of the NCX in the pathogenesis of seizures and epilepsy remains poorly understood, we have reported that inhibition of Ca2+ influx via NCX activity in the reverse mode reduced the incidence of pilocarpine-induced limbic seizures and status epilepticus (Martinez and N’Gouemo, 2010). The NCX also plays an important role in the pathogenesis of generalized seizures because genetic deletion of the NCX1 isoform suppressed the tonic flexion component of pentylenetrazole (PTZ)-induced generalized seizures in mice (Saito et al., 2009). The NCX-3 isoform is also implicated in the pathogenesis of tonic-clonic seizures in Mongolian gerbils, a model of inherited epilepsy (Park et al., 2011). It remains unknown whether NCX in rats contributes to the pathogenesis of clonic and tonic-clonic components of PTZ-induced generalized seizures. Here, I sought to determine the extent to which pharmacological blockade of reverse mode NCX activity may alter the expression and severity of PTZ-induced generalized seizures in rats.
2. Materials and Methods
Sprague-Dawley rats (male, 150–200 g, Taconic, Germantown NY) were used in accordance with the NIH guidelines for use and care of laboratory animals and the Georgetown Animal Care and Use Committee approved all experiments. We minimized the number of animals used and their discomfort. Two doses of PTZ (50 or 60 mg/kg; Sigma Chemicals, St. Louis, MO) were tested to determine a minimum effective dose that was used in subsequent experiments. PTZ was dissolved in 0.9% saline and intraperitoneally (i.p.) injected to induce seizures. After PTZ injections animals were placed in clear plexiglass boxes for 60 min to monitor for the occurrence of seizure activity. Convulsive seizure behavior was classified as follows (Luttjohann et al., 2009, modified): stage 0, no response; stage 1, myoclonic jerks; stage 2, myoclonus (i.e., clonic seizures while the animal is lying on its belly); stage 3, bilateral forelimb clonic seizures without rearing; stage 4, forelimb clonic seizures and rearing; stage 5, tonic-clonic seizures. The acute PTZ model was chosen because generalized seizures induced by this method exhibits a gradual development over a period of approximately 2 min compared to seizures induced by electrical stimulation, which are characterized by an abrupt onset. To evaluate the role of the NCX in the development of PTZ-induced generalized seizures, I antagonized reverse mode NCX activity using two inhibitors, 2-[2- [4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea methanesulfonate (KB-R7943; Tocris Bioscience, Ellisville, MO) and the structurally related (2-[[4-[(4- nitrophenyl)methoxy]phenyl]methyl]-4-thiazoli dinecarboxylic acid ethyl ester (SN-6; Tocris Bioscience, Ellisville, MO). Animals were randomly separated into groups of 8 and those that only received the vehicle were used as controls. KB-R7943 (1, 3, 10, 30 mg/kg) and SN-6 (0.3, 1, 3, 10, 30 mg/kg) were dissolved in sterile water, filtered and administered 90 min before PTZ injections. KB-R7943 and SN-6 were given orally (p.o.) by gastric intubation in a volume of 0.2 ml/100 g body weight using an 18-gauge stainless steel feeding needle with a round tip (ball diameter 3 mm). The initial dose of 0.3 or 1 mg/kg was chosen based on published in vivo pharmacological studies and preliminary data (Martinez and N’Gouemo, 2010; Blokin et al., 2008). The 90 min timepoint was the most effective pretreatment window on seizure activity in our previous study (Martinez and N’Gouemo, 2010). In another series of experiments, I compared the anticonvulsant efficacy of KB-R7943 and SN-6 to diazepam (DZP), a clinically used anticonvulsant. DZP (2.5 and 5 mg/kg in 0.9% saline) was intraperitoneally injected into rats 90 minutes before the PTZ (60 mg/kg; i.p.) challenge. I also evaluated the extent to which a combination of either KB-R7943 (3 mg/kg; p.o.) or SN-6 (3 mg/kg; p.o.) with a sub-anticonvulsant dose of DZP (2.5 mg/kg; i.p.), administered 90 min before the PTZ (60 mg/kg; i.p.) challenge, prevented the occurrence of generalized seizures. At the end of the experiment, animals were euthanized with a lethal dose of Nembutal (100 mg/kg, i.p.). Following a given pharmacological pretreatment and PTZ challenge, animals that did not display class 1 seizures within the 60 min observation period were considered to be protected from seizure. For each group of animals, the incidences of clonic and tonic-clonic components of PTZ-induced generalized seizures were also recorded. The time intervals from the end of PTZ injections to the appearance of the first seizure episode were recorded, and this period was referred to as seizure latency. For each animal, the seizure severity score was also recorded.
Analysis of the incidences of PTZ-induced generalized clonic and tonic-clonic seizures was performed using a Chi-squared (χ2) test. The seizure latencies were analyzed using a one-way ANOVA with a Dunn’s post hoc test for multiple comparisons. Before using an ANOVA, the data were subjected to a normality test (i.e., the Shapiro- Wilk test) and a test for homogeneity of variances (i.e., the Levene’s test). Comparison of seizure severity scores was assessed with the Kruskal-Wallis rank test and the Dunn’s post hoc test. The cut-off for statistical significance was p<0.05. All data are presented as the mean ± S.E.M.
3. Results
The incidence of PTZ-induced seizures was first evaluated using two doses of PTZ (50 and 60 mg/kg; i.p.). At the 50 mg/kg dose, 25% of the animals (n=4) did not exhibit seizures, but clonic and tonic-clonic PTZ-induced seizures were observed in 75% and 50% of the animals, respectively. In contrast, all animals (n=4) treated with PTZ at the dose of 60 mg/kg exhibited generalized seizures. Therefore, for subsequent experiments PTZ was used at a dose of 60 mg/kg.
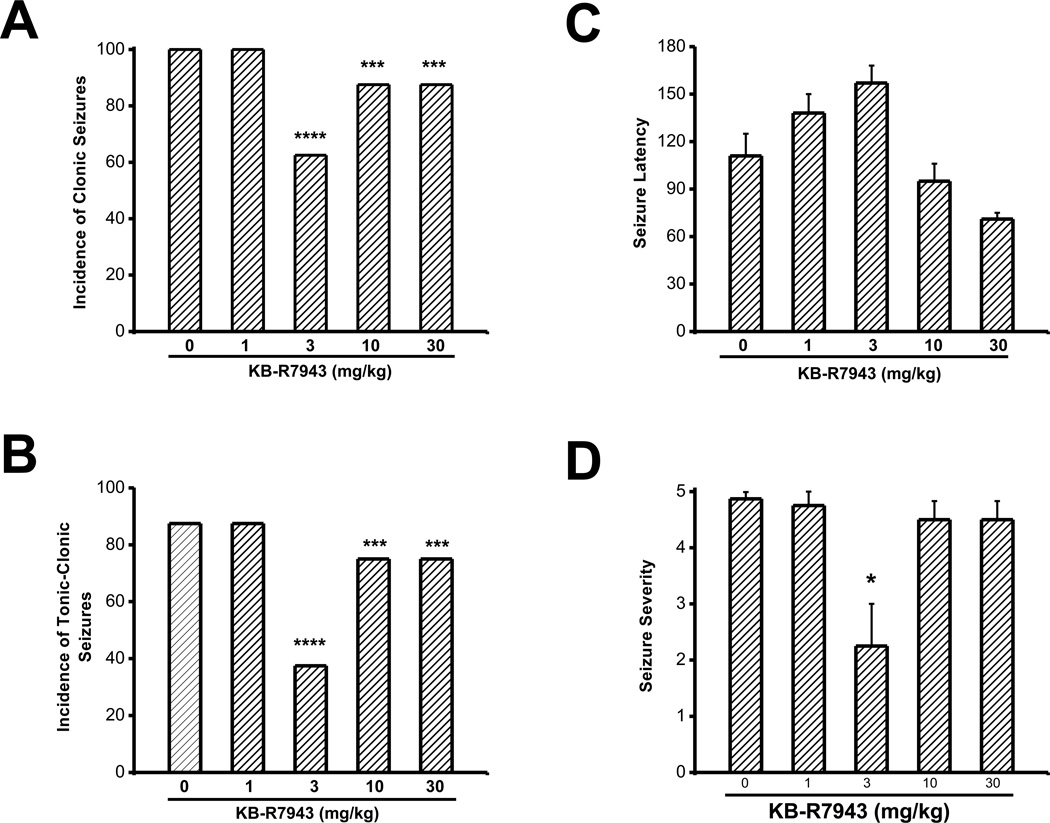
In the control group, the incidences of clonic and tonic-clonic PTZ-induced generalized seizures were 100% (8/8) and 87.5% (7/8), respectively. The latency to the first seizure episode was 111±14 s (n=8) and the seizure severity was 4.8±0.1 (n=8). KBR7943 pretreatment significantly reduced (χ2=85; p=0.0001) the incidence of clonic PTZ-induced generalized seizures. Compared to the control group, this reduction was observed in animals challenged with, 3 mg/kg (5/8; (χ2=43; p=0.000); Fig. 1A), 10 mg/kg (7/8 (χ2=11, p=0.001); Fig. 1A) and 30 mg/kg of PTZ (7/8,(χ2=11, p=0.001); Fig. 1A) but not at 1 mg/kg (8/8; Fig. 1A). Similarly, the incidence of tonic-clonic PTZ-induced generalized seizures was reduced following KB-R7943 pretreatment (χ2=85, p=0.0001). This reduction was observed following treatment with KB-R7943 at doses, 3 mg/kg (3/8 (χ2=57, p=0.0001; Fig. 1B), 10 mg/kg (6/8 (χ2=5, p=0.03); Fig. 1B) and 30 mg/kg (6/8 (χ2=5, p=0.03); Fig. 1B) when compared to the control group (6/8; Fig. 1B). In the remaining animals that exhibited PTZ-induced generalized seizures, KB-R7943 pretreatment significantly delayed the onset of the first seizure episode (F=9, p=0.0001; Fig. 1C). This delay was observed for a KB-R7943 dose of 3 mg/kg (157±12 s, n=5; Fig. 1C), but not 1 mg/kg (138 ±12 s, n=8), 10 mg/kg (91±11 s, n=7) or 30 mg/kg (71±4 s, n=7), when compared to the control group (111±14 s, n=8). Pretreatment with KB-R7943 also significantly reduced the seizure severity score (H=9.6; P=0.05). This effect was observed at a dose of 3 mg/kg (2.2±0.8, n=8, Fig. 1D), but not 1 mg/kg (4.8±0.3, n=8), 10 mg/kg (4±0.6, n=7) and 30 mg/kg (4.0±0.5, n=7) compared to the control group (4.9±01, n=8; Fig. 1C).
Figure 1.
KB-R7943 suppresses the expression of PTZ-induced generalized seizures. The effects of various doses of KB-R7943 were evaluated on the incidence of PTZ (60 mg/kg, i.p.)-induced generalized seizures. KB-R7943 (3, 10 and 30 mg/kg; p.o.) pretreatment reduced the incidence of clonic (A) and tonic-clonic seizures (B). KB-R7943 at the dose of 3 mg/kg significantly delayed the onset of seizures (C) and reduced the seizure severity (D). Data represent mean ± S.E.M. *P<0.05, ***P<0.001, ****P<0.0001.
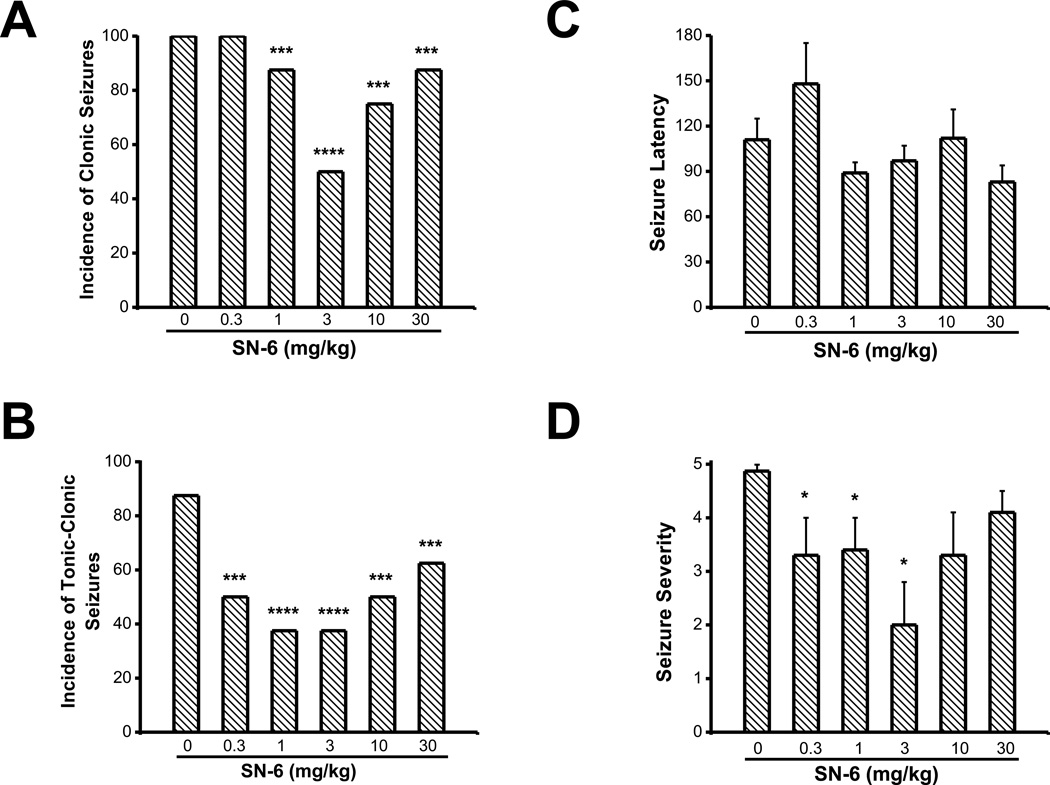
To verify the results of KB-R7943 I evaluated the effects of SN-6 on PTZ-induced generalized seizures. SN-6 pretreatment significantly reduced the incidence of PTZ-induced generalized clonic seizures (χ2=127, p=0.0001). This effect was observed for the doses above 0.3 mg/kg (1 mg/kg, 7/8 (χ2=11, p=0.001); 3 mg/kg, 4/8 (χ2=64, p=0.0001); 10 mg/kg, 6/8, (χ2=26, p=0.0001); 30 mg/kg, 8/8 (χ2=11, p=0.001); Fig. 2A) compared to the control group (8/8). SN-6 pretreatment also markedly reduced the incidence of PTZ-induced tonic-clonic seizures (χ2=108, p=0.0001). This effect was also observed at all doses tested (1 mg/kg, 3/8 (χ2=87, p=0.0001); 3 mg/kg, 3/8 (χ2=87, p=0.0001); 10 mg/kg, 4/8, (χ2=64, p=0.0001); 30 mg/kg, 5/8 (χ2=30, p=0.0001); Fig. 2B), compared to the control group (8/8). In the remaining animals that exhibited seizures, SN-6 pretreatment did not significantly increase the seizure latency (148±27 s, n=8) at the dose of 0.3 mg/kg compared to controls (111±14 s, n=8); Fig. 2C). No changes in seizure latencies were observed with doses above 0.3 mg/kg (1 mg/kg, 89±7 s, n=8; 3 mg/kg, 97±10 s, n=4; 10 mg/kg, 112±19 s, n=6; 30 mg/kg, 83±11 s, n=7, compared to controls, 111±14 s, n=12). Nevertheless, the seizure severity scores were significantly reduced following SN-6 pretreatment (H=9.6, P=0.05). This effect was observed at a dose of 3 mg/kg (2±0.8, n=8; Fig. 2D) but not 1 mg/kg (3.4±0.6, n=7), 10 mg/kg (3.3±0.8, n=6) and 30 mg/kg (4±0.8, n=8) compared to the control group (4.9±0.1, n=8; Fig. 2D).
Figure 2.
SN-6 alters the expression of PTZ-induced generalized seizures. The effects of various doses of SN-6 were evaluated on the incidence of PTZ (60 mg/kg, i.p.)-induced generalized seizures. SN-6 pretreatment (0.3, 1, 3, 10 and 30 mg/kg; p.o.) reduced the incidence of clonic (A) and tonic-clonic seizures (B). SN-6 at the dose of 3 mg/kg nonsignificantly enhanced the seizure latency (C). SN-6 at the doses of 0.3, 1 and 3 mg/kg significantly reduced the seizure severity (D). Data represent mean ± S.E.M. *P<0.05, ***P<0.001, ****P<0.0001.
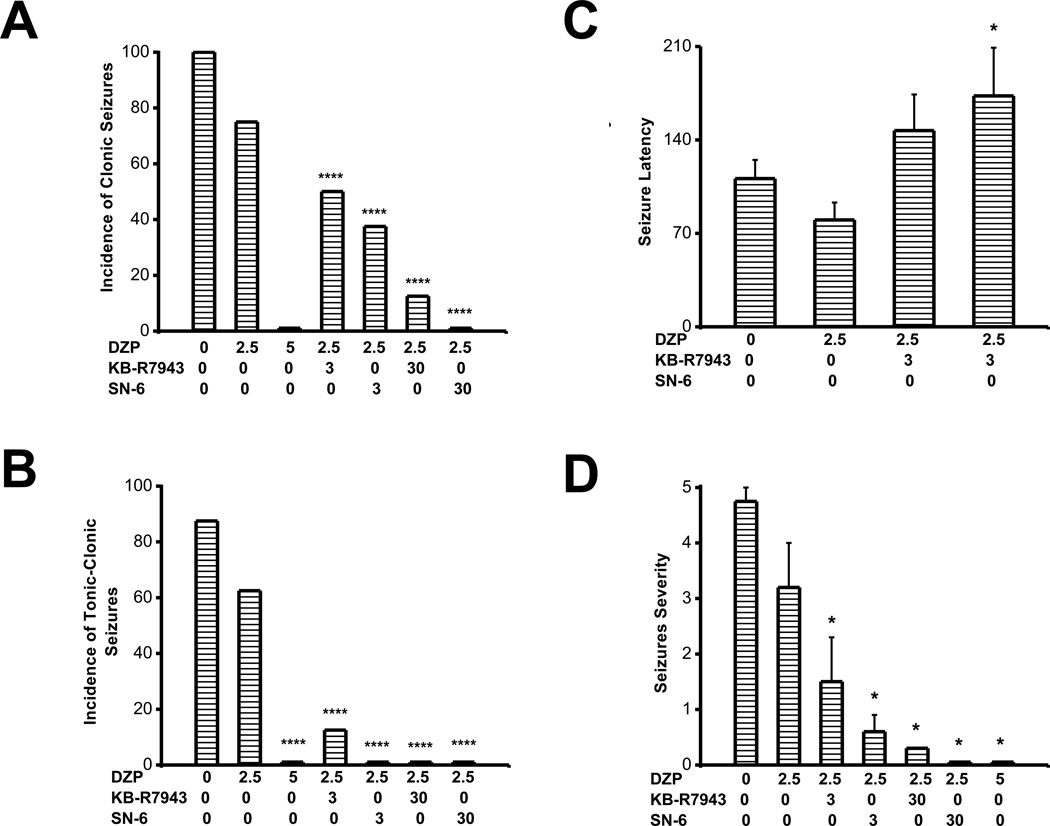
Diazepam, a clinically used anticonvulsant was used to validate the PTZ model and compare its efficacy to that of KB-R7943 and SN-6. Pretreatment with DZP, at the dose of 2.5 mg/kg, reduced the incidence of clonic (6/8 (χ2=26.3, p=0.0001); Fig. 3A and tonic-clonic (5/8 (χ2=14, p=0.0001); Fig. 3B) seizures compared to the control group, but failed to alter the seizure latency (90±12 s, n=4; controls, 111±14 s, n=8; Fig. 3C) and seizure severity score (3.2±0.8, n=8; controls, 4.8±0.1, n=8; Fig. 3D). At the dose of 5 mg/kg, diazepam completely suppressed the occurrence of clonic and tonic-clonic component of PTZ-induced generalized seizures (Fig. 3A,B). I also evaluate the extent to which a combination of KB-R7943 (3 mg/kg; p.o.) and DZP (2.5 mg/kg; i.p.), as well as SN-6 (3 mg/kg; p.o.) and DZP (2.5 mg/kg; i.p.) affects the development of PTZ-induced generalized seizures. Co-administration of KB-R7943 (3 mg/kg; p.o.) and DZP (2.5 mg/kg; i.p.) significantly reduced the incidence of clonic seizures (χ2=67, p=0.0001; Fig. 3A), nearly suppressed the occurrence of tonic-clonic seizures (χ2=118, p=0.0001; Fig. 3B), non-significantly increased the seizure latency (Fig. 3C) and significantly reduced the seizure severity (H=9, p=0.01; Fig. 3D), compared to controls. Co-administration of KB-R7943 (3 mg/kg; p.o.) and DZP (2.5 mg/kg; i.p.) also significantly reduced the incidence of clonic seizures (χ2=12, p=0.0001; Fig. 3A) and tonic-clonic seizures (χ2=51, p=0.0001; Fig. 3B), compared to the DZP (2.5 mg/kg; i.p.) group. At the dose of 30 mg/kg, KB-R7943 markedly potentiated the effect of DZP (2.5 mg/kg; i.p.) by reducing the incidence of clonic seizures (1/8, (χ2=12, p=0.0001); Fig. 3B) and suppressing the expression of tonic-clonic seizures (0/8, (χ2=153, p=0.0001); Fig. 3B), compared to controls. This treatment also significantly reduced the incidence of clonic (χ2=76, p=0.0001; Fig. 3A) and tonic-clonic seizures (χ2=89, p=0.0001; Fig. 3B), compared to the DZP (2.5 mg/kg; i.p.) group. Similarly, co-administration of SN-6 (3 mg/kg; p.o.) and DZP (2.5 mg/kg; p.o.) significantly reduced the incidence of clonic seizures (χ2=94, p=0.0001; Fig. 3A), completely suppressed the occurrence of tonic-clonic seizures (χ2=223, p=0.0001; Fig. 3B), non-significantly increased the seizure latency (Fig. 3C) and significantly reduced the seizure severity (H=13, p=0.001; Fig. 3D), compared to controls. In the remaining animals that exhibited seizures, co-administration of SN-6 and DZP significantly (F=8.7, p=0.03) increased the seizure latency (173±36 s, n=3) compared to DZP alone (111±14 s, n=8; Fig. 3C). Co-administration of SN-6 (3 mg/kg; p.o.) and DZP (2.5 mg/kg; p.o.) also significantly reduced the incidence of clonic seizures (χ2=26, p=0.0001; Fig. 3A), completely suppressed the occurrence of tonic-clonic seizures (χ2=89, p=0.0001; Fig. 3B), compared to DZP (2.5 mg/kg; i.p.) group. At the dose of 30 mg/kg, SN-6 markedly potentiated the effect of DZP (2.5 mg/kg; i.p.) by suppressing both clonic (0/8, χ2=196, p=0.0001; Fig. 3B) and expression of tonic-clonic (0/8, χ2=153, p=0.0001; Fig. 3B) PTZ-induced seizures, compared to controls. This treatment also significantly reduced the incidence of clonic (χ2=117, p=0.0001; Fig. 3A) and tonic-clonic seizures (χ2=89, p=0.0001; Fig. 3B), compared to the DZP (2.5 mg/kg; i.p.) group.
Figure 3.
KB-R7943 and SN-6 potentiate the sub-effective dose of DZP. Rats were subjected to DZP pretreatment at the dose of 2.5 and 5 mg/kg. Another groups of rats received a combination of KB-R7943 (3 mg/kg) and DZP (2.5 mg/kg) or SN-6 (3 mg/kg) and DZP (2.5 mg/kg). DZP pretreatment at 5 mg/kg but not at 2.5 mg/kg completely suppressed clonic (A) and tonic-clonic seizures (B). KB-R7943 and SN-6 potentiate the effects of 2.5 mg/kg of DZP against clonic (A) and tonic-clonic (B) seizures. Note that the SN-6 and DZP combination was as effective as 5 mg/kg of DZP in completely suppressing tonic-clonic seizures. KB-R7943 or SN-6 combined with DZP (2.5 mg/kg) non-significantly increased the seizure latency (C), but significantly reduced the seizure severity (D). Note that the combination of SN-6 and DZP completely suppressed motor seizures. Data represent mean ± S.E.M. *P<0.05, ****P<0.0001.
4. Discussion
The present study demonstrates that inhibition of the reverse mode of NCX with KB-R7943 or SN-6 preferentially suppressed the occurrence of tonic-clonic component of PTZ-induced generalized seizures and significantly reduced the scores of seizure severity. A combination of KB-R7943 or SN-6 with a sub-anticonvulsant dose of DZP suppressed PTZ-induced convulsive behaviors. Both KB-R7943 and SN-6 preferentially inhibit the reverse mode activity of the NCX, suggesting that during seizures NCX may operate in the reverse mode and that blockade of NCX-mediated Ca2+ entry into a cell may be a potentially useful anticonvulsant approach for investigating seizure behavior in the acute PTZ seizure model.
Administration of PTZ induces a progression of convulsive behaviors that leads to myoclonic jerks and ultimately to generalized tonic-clonic seizures (Luttjohann et al., 2009). The delayed onset of PTZ-induced seizures following KB-R7943 pretreatment may reflect an inhibitory effect on neuronal hyperexcitability and propagation of seizure activity. Alternatively, the increased seizure latency could reflect an effect of KB-R7943 on the brain’s uptake of PTZ, as studies in the brain report a high affinity uptake of KB-R7943 (Matsuda et al., 2001; Miyata et al., 2002). NCX antagonists such as KB-R7943 could inhibit the uptake of PTZ in the brain by altering the brain blood barrier, although currently there are no studies to support this hypothesis. The neural circuits responsible for induction of tonic seizures are located mainly in the brainstem, while clonic seizures arise because of the neural circuitry in the forebrain. It is therefore tempting to suggest that inhibition of NCX activity in the brainstem may contribute to the prevention of the tonic-clonic component of PTZ-induced generalized seizures.
Studies show that the NCX plays an important role in the pathogenesis of seizures. Genetic deletion of the NCX1 suppressed the occurrence of PTZ-induced tonic seizures in mice (Saito et al., 2009). Similarly, downregulation of the NCX3 protein expression was found in the hippocampus of the Mongolian gerbil, an inherited model of tonic-clonic epilepsy (Park et al., 2011). We have previously reported that inhibition of the reverse mode of NCX also reduced the incidence of pilocarpine-induced seizures and status epilepticus (Martinez and N’Gouemo, 2010). KB-R7943 and SN-6 predominantly inhibit the NCX3 and NCX1 isoforms (Iwamoto and Shigekawa, 1998; Iwamoto, 2004), which suggests that these isoforms are particularly important potential targets for anticonvulsants in models of acute tonic-clonic seizures, inherited tonic-clonic epilepsy, limbic seizures and status epilepticus. However, the role of NCX activity in the control of neuronal excitability is not yet completely understood. The NCX is an electrogenic transporter that exchanges three Na+ ions for every Ca2+ ion. Thus, the reverse mode of the NCX is expected to hyperpolarize neurons via two mechanisms at least, including the net efflux of positive charge per cycle and the activation of Ca2+-dependent K+ channels due to increases of intracellular Ca2+. The contribution of the direct electrogenic effect of the NCX in the genesis of hyperpolarization is not well known. Given this unknown, the hyperpolarizing effects of the NCX in reverse mode may be mediated by activation of Ca2+-activated K+ channels, rather than by the direct electrogenic effect of the NCX. Blockade of Ca2+-activated K+ channels can induced spontaneous seizures however, I did not observe signs of hyperexcitability, such as Straub tail and spontaneous seizures following administration of KB-R7943 and SN-6 at any tested dose. Therefore, I suggest that inhibition of the electrogenic effect of the NCX in reverse mode contributes to the anticonvulsant effect of KB-R7943 and SN-6. PTZ-induced neuronal hyperexcitability may trigger a massive Na+ influx that contributes to significant increase in intracellular Na+ thereby activating the reverse mode activity of the NCX, causing entry rather that expulsion of Ca2+ which can initiated and/or sustained seizure activity. The colocalization of Na+ channels and NCX may represent the molecular mechanism underlying neuronal hyperexcitability associated with activation of the reverse mode activity of the NCX.
In the present study, I found that 3 mg/kg of KB-R7943 or SN-6 was the most effective dose in reducing the incidence and severity of PTZ-induced generalized seizures. A dose-dependent effect was found with lower doses (0.3–3 mg/kg) of SN-6. Increasing doses of KB-R7943 and SN-6 resulted in less or no effective anticonvulsant effects. These findings indicate that the effects of KB-R7943 and SN-6 on the NCX are complex and cannot be explained by a conventional dose-effect relationship. It has been reported that the IC50 for KB-R7943 inhibition of NCX reverse and forward mode is 2 µM and >30 µM, respectively. Similarly, SN-6 up to 30 µM inhibits NCX reverse mode but did not significantly affects the forward mode (Iwamoto et al., 2004). Thus, it is possible that 0.3- 3 mg/kg doses of KB-R7943 or SN-6 suppress PTZ-induced generalized seizures when NCX was operating exclusively in the reverse mode. However, when doses are increased, the forward mode is then inhibited, in addition to the reverse mode, resulting in a reduction or loss of the anticonvulsant effect. The lack of or less effective anticonvulsant effects seen with higher doses of NCX inhibitors may also be due to nonspecific effects other that inhibition of NCX activity. Multiple lines of evidence indicate that, in addition to antagonizing the NCX, KB-R7943 at relatively high concentrations also partially antagonizes various voltage- and ligand-gated channels including, K+ channels, L-type Ca2+ channels, N-methyl-D-aspartate receptors, canonical transient receptor potential channels and nicotinic acetylcholine receptors (Sobolevsky et al., 1999; Pintado et al., 2000; Birinyi et al., 2005; Ouardouz et al., 2005; Kraft, 2007). Similarly, SN-6 also affects these membrane ionic currents but less potently than KB-R7943 (Niu et al., 2007) and at concentrations >30 µM (Iwamoto et al., 2004). Evidence indicates that blockade of NMDA receptors can prevent tonic-clonic but not clonic PTZ-induced generalized seizures in rats (Velisek et al., 1990, 1991). Similarly, blockade of L-type Ca2+ channels also suppressed PTZ-induced generalized seizures in rats (Kamal et al., 1990). However, blockade of the reverse mode of NCX attenuates both clonic and tonic-clonic PTZ-induced seizures. In view of these observations, it is unlikely that mechanisms unrelated (e.g., blockade of NMDA receptors and L-type Ca2+ channels) to NCX reverse mode activity may contribute to the anticonvulsant potential of NCX antagonists.
In the present study, I found that both KB-R7943 and SN-6 potentiates the noneffective dose of DZP in suppressing PTZ-induced generalized seizures. DZP acts through the GABA receptor to suppress seizures, therefore the present findings suggest that the NCX isoforms might be located on GABAergic neurons, thus increasing GABA release and subsequent neuronal inhibition that leads to seizure suppression. Future studies should evaluate how NCX antagonists potentiate the effects of DZP on PTZinduced seizures and determine whether NCX isoforms are exclusively located on GABAergic neurons.
Here using an acute PTZ seizure model, I show that antagonizing the reverse mode of NCX activity to inhibit Ca2+ influx play a substantial role in suppressing seizures and may serve as a potentially useful approach toward developing an anticonvulsant drug that may work via a GABA/benzodiazepine/NCX mechanism. Understanding how NCX reverse mode inhibitors alter seizure generation and propagation can provide new insights toward the understanding of neuronal hyperexcitability that leads to seizures and the development of selective NCX antagonists. Such development would be a useful therapeutic approach for the treatment of tonic-clonic generalized seizures and epilepsy.
Highlights.
-
►
Normal: the NCX forward mode transports Ca2+ out and Na+ into the neuron
-
►
PTZ-induced seizures: the NCX reverse mode transports Na+ out and Ca2+ into the neuron
-
►
NCX inhibitors reduced the incidence and severity of seizures
NCX inhibitors potentiate the sub-effective dose of diazepam in suppressing seizures
Acknowledgments
This publication was made possible by Public Health Service grants NS047193 and AA020073 from the National Institutes of Health (NIH) and its contents are the responsibility of the author and do not necessarily represent the official views of NIH. The author wish to thank NIH/NIDDK STEP-UP students Obinna Achucho, Vanessa Niba, Michaella Wilson and Tylar Clark for technical assistance.
Abbreviations
- DZP
diazepam
- NCX
sodium/calcium exchanger
- PTZ
pentylenetetrazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Conflict of Interest
The author has no conflict of interest.
References
- Ago Y, Kawasaki T, Nashida T, Ota Y, Cong Y, Kitamoto M, Takahashi T, Takuma K, Matsuda T. SEA400, a specific Na+/Ca2+ exchange inhibitor, prevents dopaminergic neurotoxicity in an MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2011;61:1441–1451. doi: 10.1016/j.neuropharm.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Annunziato L, Pignataro G, DiRengo GF. Pharmacology of brain Na+/Ca2+exchanger: from molecular biology to therapeutic perspectives. Pharmacol. Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Bi X-H, Lu C-M, Liu Q, Zhang Z-X, Zhao H-L, Yu J, Zhang J-W. A 14 bp indel variation in the NCX1 gene modulates the age at onset in late-onset Alzheimer’s disease. J. Neural Transm. 2012;119:383–386. doi: 10.1007/s00702-011-0696-4. [DOI] [PubMed] [Google Scholar]
- Birinyi P, Acsai K, Bányász T, Tóth A, Horváth B, Virág L, Szentandrássy N, Magyar J, Varró A, Fülöp F, Nánási PP. Effects of SEA0400 and KB-R7943 on Na+/Ca2+exchange current and L-type Ca2+current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2005;372:63–70. doi: 10.1007/s00210-005-1079-x. [DOI] [PubMed] [Google Scholar]
- Blaustein M, Lederer J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Blokhin IO, Vlasov TD, Galagudza MM, Nifontov EM, Petrishchev NN. Role of the sodium-calcium exchanger in the myocardial protection against ischemia-reperfusion injury. Ross. Fiziol. Zh. Im. IM. Sechenova. 2008;94:284–292. [PubMed] [Google Scholar]
- Boscia F, Gala R, Pignataro G, de Bartolomeis A, Cicale M, Ambesi-Impiombato A, DiRenzo G, Annunziato L. Permanent focal braisn ischemia induces isofrom-dependent changes in the pattern of Na+/Ca2+exchange gene expression in the ischemic core, periinfarct area, and intact brain regions. J. Cereb. Blood Flow Metab. 2000;26:503–517. doi: 10.1038/sj.jcbfm.9600207. [DOI] [PubMed] [Google Scholar]
- Iwamoto T. Forefront of Na+/Ca2+exchanger studies: molecular pharmacology of Na+/Ca2+exchange inhibitors. J. Pharmacol Sci. 2004;96:27–32. doi: 10.1254/jphs.fmj04002x6. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Shigekawa M. Differential inhibition of Na+/Ca2+exchanger isoforms by divalent cations and isothiourea derivative. Am. J. Physiol. 1998;275:C423–C430. doi: 10.1152/ajpcell.1998.275.2.C423. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Inoue Y, Ito K, Sakaue T, Kita S, Katsuragi T. The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4(4- nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol. Pharmacol. 2004;66:45–55. doi: 10.1124/mol.66.1.45. [DOI] [PubMed] [Google Scholar]
- Kamal JA, Nadig RS, Joseph T, David J. Effect of calcium channel blockers on experimentally induced seizures in rats. Indian. J. Exp. Biol. 1990;28:605–608. [PubMed] [Google Scholar]
- Kraft R. The Na+/Ca2+exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem. Biophys. Res. Commun. 2007;361:230–236. doi: 10.1016/j.bbrc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Lee C, Dhalla NS, Hryshko LV. Therapeutic potential of novel Na+-Ca2+exchange inhibitors in attenuating ischemia-reperfusion injury. Can. J. Cardiol. 2005;21:509–516. [PubMed] [Google Scholar]
- Luttjohann A, Fabene PF, van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009;98:579–586. doi: 10.1016/j.physbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Lytton J. Na+/Ca2+exchangers: three mammalian gene families control Ca2+transport. Biochem. J. 2007;406:365–382. doi: 10.1042/BJ20070619. [DOI] [PubMed] [Google Scholar]
- Martinez Y, N’Gouemo P. Blockade of the sodium calcium exchanger exhibits anticonvulsant activity in a pilocarpine model of acute seizures in rats. Brain Res. 2010;1366:211–216. doi: 10.1016/j.brainres.2010.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma N, et al. SEA0400, a novel and selective inhibitor of Na+-Ca2+exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J. Exp. Pharmacol. Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- Miyata A, Zipes DP, Hall S, Rubart M. KB-R7043 prevents acute, atrial fibrillation-induced shortening of atrial refractoriness in anesthetized dogs. Circulation. 2002;106:1410–1419. doi: 10.1161/01.cir.0000028587.85711.f6. [DOI] [PubMed] [Google Scholar]
- Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urshida T, Hayashi H, Kimura J. Characterization of SN-6, a novel Na+/Ca2+exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur. J. Pharmacol. 2007;573:161–169. doi: 10.1016/j.ejphar.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Zamponi GW, Barr W, Kiedrowski L, Stys PK. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+exchange and L-type Ca2+channels. Neuropharmacology. 2005;48:566–575. doi: 10.1016/j.neuropharm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Papa M, Canitano A, Boscia F, Castaldo P, Sellitti S, Prozig H, Taglialatela M, Annunziato L. Differential expression of Na2+-Ca2+exchanger transcripts and proteins in rat brain regions. J. Comp. Neurol. 2003;461:31–48. doi: 10.1002/cne.10665. [DOI] [PubMed] [Google Scholar]
- Park D-K, Park K-H, Ko J-S, Kim D-S. Alteration in NCX-3 immunoreactivity within the gerbil hippocampus following spontaneous seizures. BMB Rep. 2011;44:306–311. doi: 10.5483/BMBRep.2011.44.5.306. [DOI] [PubMed] [Google Scholar]
- Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Ann. Rev. Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Gala R, Cuomo O, Tortiglione A, Giaccio L, Castaldo P, Sirabella R, Matrone C, Canitano A, Amoroso S, DiRenzo G, Annunziato L. Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke. 2004;35:2566–2570. doi: 10.1161/01.STR.0000143730.29964.93. [DOI] [PubMed] [Google Scholar]
- Pintado AJ, Herrero CJ, García AG, Montiel C. The novel Na(+)/Ca(2+) exchange inhibitor KB-R7943 also blocks native and expressed neuronal nicotinic receptors. Br. J. Pharmacol. 2000;130:1893–1902. doi: 10.1038/sj.bjp.0703519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednau BT, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997;272(Cell Physiol.):C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- Saito R, Kaneko E, Tanaka Y, Honda K, Matsuda T, Baba A, Komuro I, Kita S, Iwamoto T, Tanako Y. Involvement of Na+/Ca2+exchanger in pentylenetetrazole-induced convulsion by use of Na+/Ca2+exchanger knockout mice. Biol. Pharm. Bull. 32:1928–1930. doi: 10.1248/bpb.32.1928. [DOI] [PubMed] [Google Scholar]
- Secondo A, Stainano RI, Scorziello A, Sirabella R, Boscia F, Adornetto A, Valsecchi V, Molinaro P, Canzoniero LM, Di Renzo G, Annunziato L. BHK cells transfected with NCX3 are more resistant to hypoxia followed by regeneration that those transfected with NCX1 and NCX2: possible relationship with mitochondrial membrane potential. Cell Calcium. 2007;42:521–535. doi: 10.1016/j.ceca.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Khodorov BI. Blockade of NMDA channels in acutely isolated rat hippocampal neurons by the Na+/Ca2+exchange inhibitor KB-R7943. Neuropharmacology. 1999;38:1235–1242. doi: 10.1016/s0028-3908(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Sokolow S, Luu SH, Headley AJ, Hanson AY, Kim T, Miller A, Vinters CA, Vinters HV, Gylys KH. High levels of synaptosomal Na(+)-Ca(2+) exchnagers (NCX1, NCX2, NCX3) co-localized with amyloid-beta in human cerebral cortex affected by Alzheimer's disease. Cell Calcium. 2011;49:208–216. doi: 10.1016/j.ceca.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísek L, Kusá R, KulovanV M, Mares P. Excitatory amino acid antagonists and pentylenetetrazol-induced seizures during ontogenesisIThe effects of 2-amino-7- phosphonoheptanoate. Life Sci. 1990;46:1349–1357. doi: 10.1016/0024-3205(90)90334-n. [DOI] [PubMed] [Google Scholar]
- Velísek L, Veresová S, Pôbisová H, Mares P. Excitatory amino acid antagonists and pentylenetetrazol-induced seizures during ontogenesis. I. The effects of MK-801. Psychopharmacology. 1991;104:510–514. doi: 10.1007/BF02245658. [DOI] [PubMed] [Google Scholar]