Abstract
Ascorbic acid enhances synthesis of norepinephrine from dopamine in adrenal chromaffin cells by serving as a co-factor for chromaffin granule dopamine β-hydroxylase (DβH). However, there is controversy regarding in situ kinetics of the ascorbate effect in chromaffin cells, as well as whether they apply to neuronal cells. In this study we evaluated the stimulation of norepinephrine synthesis from dopamine in cultured SH-SY5Y neuroblastoma cells. These cells contained neither ascorbate nor norepinephrine in culture, but when provided with dopamine, they generated intracellular norepinephrine at rates that were stimulated several fold by intracellular ascorbate. Ascorbate-induced increases in norepinephrine synthesis in dopamine-treated cells were linear over 60 minutes, despite saturation of intracellular ascorbate. Norepinephrine accumulation after 60 minutes of incubation with 100 μM dopamine was half-maximal at intracellular ascorbate concentrations of 0.2 – 0.5 mM, which fits well with the literature Km for ascorbate of DβH using dopamine as a substrate. Moreover, these ascorbate concentrations were generated by initial extracellular ascorbate concentrations of less than 25 μM due to concentrative accumulation by the ascorbate transporter. Treatment with 100 μM dopamine acutely increased cellular superoxide generation, which was prevented by ascorbate loading, but associated with a decrease in intracellular ascorbate when the latter was present at concentrations under 1 mM. These results show that ascorbate promptly enhances norepinephrine synthesis from dopamine by neuronal cells, that it does so at physiologic intracellular concentrations in accord with the kinetics of DβH, and that it both protects cells from superoxide and by providing electrons to DβH.
Keywords: dopamine, norepinephrine, dopamine β-hydroxylase, oxidative stress, SH-SY5Y neuroblastoma cells
1. Introduction
Ascorbic acid (AA) has several functions in the brain and neurons. In addition to serving as an antioxidant, it also provides electrons for collagen synthesis, neuropeptide amidation, and catecholamine biosynthesis [1]. Regarding the latter, it enhances catecholamine biosynthesis at two steps. First, it recycles tetrahydrobiopterin that is required by tyrosine hydroxylase for synthesis of L-3,4-dyhydroxyphenylalanine (L-DOPA) [2], the first and rate-limiting step in the pathway [3]. It is also the major and likely the physiologic electron donor to dopamine β-hydroxylase (DβH) [4,5], which generates norepinephrine (NE) from dopamine (DA) in secretory granules. The role of AA in DβH function has been extensively studied in adrenal chromaffin granules (reviewed in [5]). In this process, AA donates an electron to the hydroxylation reaction and becomes the ascorbate radical, which is then recycled back to AA by electron transfer across the granule membrane from cytoplasmic AA via a cytochrome b561. The transfer is driven by ATP-dependent generation of a favorable proton gradient into the vesicle. Whereas electron transfer via this mechanism is efficient [5], chromaffin granules have very slow uptake of AA [6]. For example, cultured chromaffin cells deficient in AA readily took up AA over 2 h in culture, but AA did not significantly increase DβH activity over this time period [7]. Whether this slow activation applies to neuronal cells is unknown, since the only available study in cultured SK-N-SH neuroblastoma cells was performed after 6 h of treatment with AA [8]. In that study, 1 mM AA enhanced NE synthesis from radiolabeled DA by only about 50%. In addition to the question of duration of exposure to AA, it is also unknown whether any acute AA effects on NE synthesis occur at intracellular concentrations of AA in the physiologic range.
DA has mostly been studied in isolated neuronal cells not with regard to its conversion to NE by DβH, but with regard to oxidative stress that DA generates in cultured cells by redox cycling with molecular oxygen to its semiquinone and quinone forms [9–11]. This issue is relevant, since the DA concentrations in cells may reach 1 mM at the cell body [12] and as high as 50 mM at the nerve terminal synapse [12,13]. Most [14,15], but not all [11] studies show that DA toxicity is intracellular, since it can be prevented by blockers of DA uptake and is associated with intracellular quinone generation and melanin polymer formation [10]. In this regard, AA was found to substantially prevent cell death and pigment formation due to culture with DA [9–11,15], although it wasn’t always clear that this was due to intracellular AA. Studies of DA toxicity in neuronal cells have been performed over at least a 24 h interval and have not addressed whether AA might affect DA synthesis. It also isn’t known whether oxidative stress due to DA can be detected on shorter time frame, the nature of the oxidative stress generated, and whether this might be affected by AA.
In this work we sought to understand the effect of AA on DA conversion to NE in cultured SH-SY5Y neuroblastoma cells, which are known to express DβH [16] and to take up DA [17] and AA [18]. Our goals were to determine the efficiency of the AA effect, whether AA prevents short-term oxidative stress due to DA, and to establish the intracellular concentrations of AA, NE and DA resulting during NE synthesis from DA.
2. Materials and methods
2.1. Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied the L-ascorbic acid, DA, dehydroascorbate (DHA), dithiothreitol, NE, N-2-hydroxyethylpiperazine N′-2-ethanesulfonic acid (Hepes), TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman -2-carboxylic acid).
2.2. Cell Culture
SH-SY5Y neuroblastoma cells were obtained from the American Type Culture Collection) and were cultured in Dulbecco’s minimal essential medium containing 10% (v/v) fetal bovine serum, which was prepared by the Cell Culture Core of the Vanderbilt Diabetes Research and Training Center. Cells were cultured to confluence at 37 °C in humidified air containing 5% CO2. All experiments were performed in culture medium, with rinsing of the cells after the experiment 2 times in 2 ml of Krebs-Ringer-Hepes buffer (KRH) at 37 °C. KRH buffer consisted of 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4.
2.3. Assay of intracellular superoxide
After treatments as noted, cells in a 96-well plate format were rinsed twice in 0.2 ml of KRH, loaded with 10 μM dihydroethidium for 30 min at 37 °C, followed by measurement of 2-hydroxyethidium fluorescence with an excitation wavelength of 480nm and an emission wavelength of 570 nm on a BioTek H1 fluorescen t plate reader [19]. This method has a high specificity for superoxide detection[20], which was confirmed by inhibition of fluorescence using superoxide scavenger TEMPOL.
2.4 Assay of catecholamines
The catecholamines NE and DA were measured in cell extracts by high performance liquid chromatography as described [21]. Briefly, buffer or medium was removed following 2 KRH rinses of cells in 6-well culture plates. The cells were lysed in 0.5 ml of the HPLC mobile phase buffer, scraped from the plate, and centrifuged at 13, 000 × g for 1 min. Aliquots (0.1 ml) of the supernatant were taken for duplicate assays. The mobile phase consisted of 100 mM trichloroacetic acid, 10 mM sodium acetate, 0.1 mM EDTA, and 10.5% methanol, pH 3.8. The isocratic flow rate was 1 ml/minute, pumped by an ECS Model 582 pump. The column was an Absorbosphere C18, 5 μ (4.6 × 150 mm). Samples were injected on a Rheodyne model 7725i injector and catecholamines and AA were detected using an ESA Model 5011 analytic cell set at 0.5 volts on an ESA Model 5100A detector. Peak heights were quantified on a Shimadzu C-R5A integrator. Under these conditions, the void volume was 1.8 ml, AA eluted at 2.2 min, NE eluted at 4.0 min, and DA eluted at 11 min. Separation of AA and NE was such that there was no overlap in the chromatograms despite as much as a 60-fold higher AA concentration applied to the column (results not shown). The sensitivity for NE was 50 nM.
Intracellular concentrations of AA and catecholamines were calculated based on the intracellular space of 3-O-[3H]methylglucose in SH-SY5Y cells, which was 10.7 ± 3.4 μl/mg protein (N=24, ± SD) [18]. This space was measured as described previously for endothelial cells in culture [22]. Protein was measured by the Bradford method as described by the manufacturer (Bio-Rad Laboratories, Hercules, CA).
2.5. Combined assay of ascorbate and GSH
After incubations as indicated in 6-well plates, the medium was removed and the adherent cells were gently rinsed twice with 2 ml of ice-cold KRH. After removal of the last rinse the cell monolayer was treated with 0.1 ml of 25% metaphosphoric acid (w/v) for several minutes, followed by 0.35 ml of a buffer containing 0.1M Na2HPO4 and 0.05 mM EDTA, pH 8.0. Adherent material was scraped from the bottom of the plate, and the lysate was removed and centrifuged at 3 °C for 1 min at 13,000 × g. Duplicate aliquots of the supernatant were taken for assay of ascorbic acid by high performance liquid chromatography when measured with GSH (Fig. 5A) as previously described [23], except that tetrapentylammonium bromide was used as the ion pair reagent. In some experiments, ascorbate was also measured in 0.1 ml of the incubation medium by adding 0.1 ml of 25% metaphosphoric acid (w/v), mixing, neutralizing with 0.35 ml of the above phosphate/EDTA buffer, and centrifuging to remove insoluble material before assay of ascorbate. GSH was assayed in duplicate by the method of Hissin and Hilf [24]. Intracellular AA and GSH were calculated based on intracellular water space, as described above.
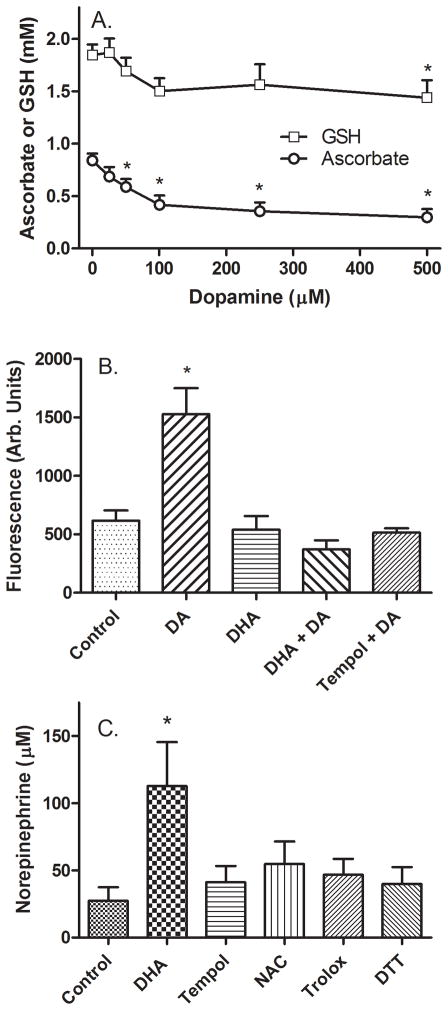
Figure 5. Generation of oxidative stress by DA in SH-SY5Y cells.
Panel A: Cells in culture were loaded with AA by treatment with 100 μM DHA for 30 minutes and then treated for an additional 120 minutes with the indicated concentration of DA. Cells were then rinsed twice in 2 ml of KRH and removed from the plate for assay of AA (Panel A, circles) and GSH (Panel A, squares). Data are shown from 5 experiments, with an “*” indicating p < 0.05 compared to cells not treated with DA by one-way repeated measures ANOVA. Panel B: Cells in culture were incubated at 37 °C and treated as indicated with 100 μM DHA (AA) or 50 μM TEMPOL for 30 minutes, followed by 100 μM DA. After 60 minutes, the cells were rinsed twice in KRH and dihydroethidium was added to a concentration of 10 μM. After an additional 30 minutes, the fluorescence of 2-hydroxyethidium was determined as described in Methods. Results are shown as relative fluorescence of 8 determinations, with an “*” indicating p < 0.05 compared to the control sample by one-way repeated measures ANOVA. Panel C: Cells in culture were treated without (Control) or with agents at the following concentrations: DHA, 50 μM; TEMPOL, 50 μM; N-acetylcysteine (NAC), 500 μM; Trolox, 250 μM; and dithiothreitol (DTT), 250 μM. After 30 minutes, DA was added to a concentration of 100 μM for an additional 60 minutes before 2 rinses in KRH and removal of the cells for assay of NE. Data are shown from 6 experiments, with an “*” indicating p < 0.05 compared to control by one-way repeated measures ANOVA.
2.6 Data Analysis
Results are shown as mean + standard error. Statistical comparisons were made using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA). Except where noted, differences between treatments were assessed by two-way analysis of variance (ANOVA) with post-hoc testing using the Bonferroni multiple comparisons test. A value of p < 0.05 was considered significant.
3. Results
3.1. Intracellular AA rapidly stimulates DA conversion to NE by SH-SY5Y cells
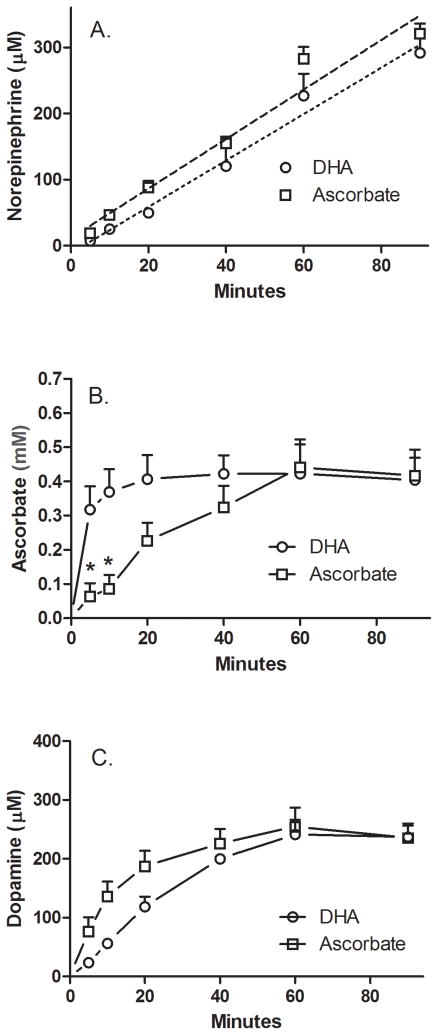
The time dependence of the effect of intracellular AA on NE synthesis was evaluated with the results shown in Fig. 1. In this experiment, either AA or DHA were added at the same time as 100 μM DA to SH-SY5Y cells in culture and the intracellular concentrations of NE, AA, and DA were followed over 90 minutes. The rationale for comparing effects of AA and its two-electron oxidized form DHA is that they enter cells on two different transporters (SVCT2 and GLUT-type glucose transporter, respectively), that DHA is rapidly reduced to AA in the cell [18,25], and that extracellular AA is very low with DHA loading so that intracellular AA effects are studied. For both AA and DHA, NE was generated in similar concentrations in a linear manner with a tendency to decrease at the 90 minute time point (Fig. 1A). Intracellular concentrations of AA increased to the same steady-state levels at 60 minutes with both forms of the vitamin, but this was reached much more rapidly with DHA loading (Fig. 1B). Uptake of DA also plateaued, but not until after about 40 min and was statistically no different with either form of the vitamin (two-way ANOVA) (Fig. 1C). No AA was detected in the medium outside DHA-treated cells (results not shown). For both AA and DHA, intracellular AA and DA concentrations stabilized, whereas NE synthesis continued unabated. Most importantly, the results show that progressive NE generation begins soon after addition of substrate and co-factor in these cells.
Figure 1. Time-dependent stimulation by AA of DA conversion to NE by SH-SY5Y cells in culture.
Cells in culture were incubated with 100 μM concentrations of AA (circles) or DHA (squares) followed immediately by 100 μM DA. Incubations were carried out in culture and at the times indicated, the wells were rinsed twice with 2 ml of KRH and the cells were removed from the plate for assay of NE (Panel A), AA (Panel B), and DA (Panel C) as described under Methods. Results are shown from 3 experiments for DHA loading and 5 experiments for AA loading, with an “*” indicating p < 0.05 compared to DHA-treated cells at the same time point.
3.2 AA enhances NE synthesis at low intracellular DA concentrations
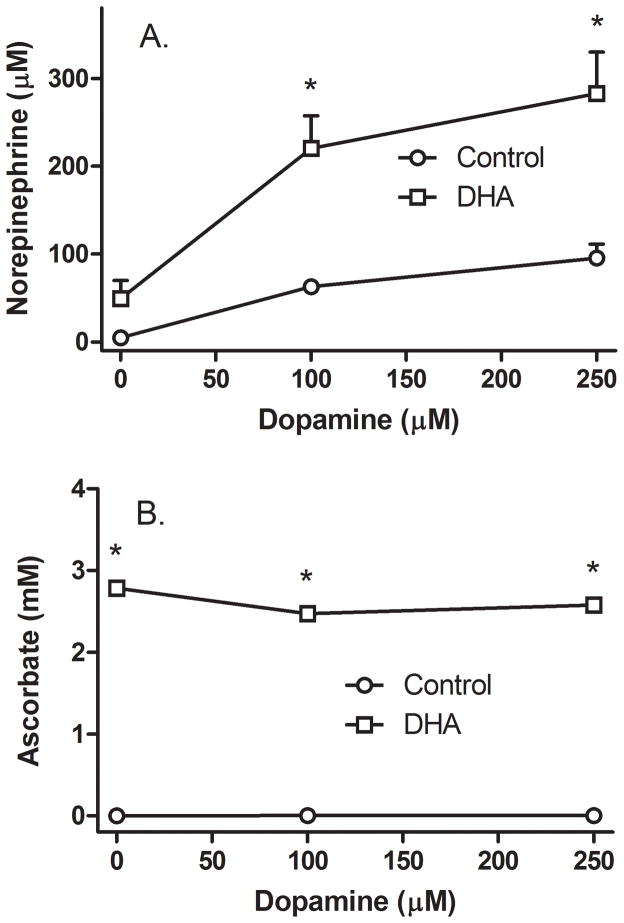
Since the increase in intracellular AA in response to DHA loading was rapid and thus at steady-state for most of the 90 minute time course in Fig. 1B, and since AA loading with DHA did not require or generate extracellular AA, most of the remainder of the experiments in this study were performed using loading of intracellular AA with DHA rather than AA itself. The concentration dependence of relatively high DA concentrations on NE generation was first evaluated, as shown in Fig. 2. Untreated SH-SY5Y cells in culture generated little or no NE in the absence of DA over 60 min in culture (Fig. 2A, circle at zero DA), even though the culture medium contained 100 μM L-tyrosine. Incubation of cells with increasing amounts of DA for 60 min progressively increased intracellular NE (Fig. 2A, circles). To examine the effects of a relatively high intracellular AA concentration on NE synthesis, cells were loaded with 500 μM DHA for 30 min before addition of DA, which resulted in intracellular AA concentrations of about 3 mM (Fig. 2B, squares). Although these trended downward, they were not significantly affected by increasing DA concentrations. NE generation in response to AA loading increased several fold from that due to DA alone (Fig. 2A, squares). In the absence of DA, AA loading increased intracellular NE in each of the 7 experiments performed, but overall variability between experiments precluded statistical significance (Fig. 2A, square at zero DA).
Figure 2. Ascorbate effects on NE generation from relatively high DA concentrations.
Cells in culture were incubated in the absence (circles) or presence (squares) of 500 μM DHA, followed in 30 minutes by addition of the indicated concentration of DA. After another 60 minutes in culture the cells were rinsed twice in 2 ml of KRH and removed from the plate for assay of NE (Panel A) and AA (Panel B) as described under Methods. Results are shown from 6 experiments, with an “*” indicating p < 0.05 compared to cells not treated with AA at the same DA concentration by two-way ANOVA. Both concentrations of DA significantly enhanced NE generation compared to cells not treated with DA in the presence or absence of AA (one-way repeated measures ANOVA, Tukey’s post-hoc test, p < 0.05).
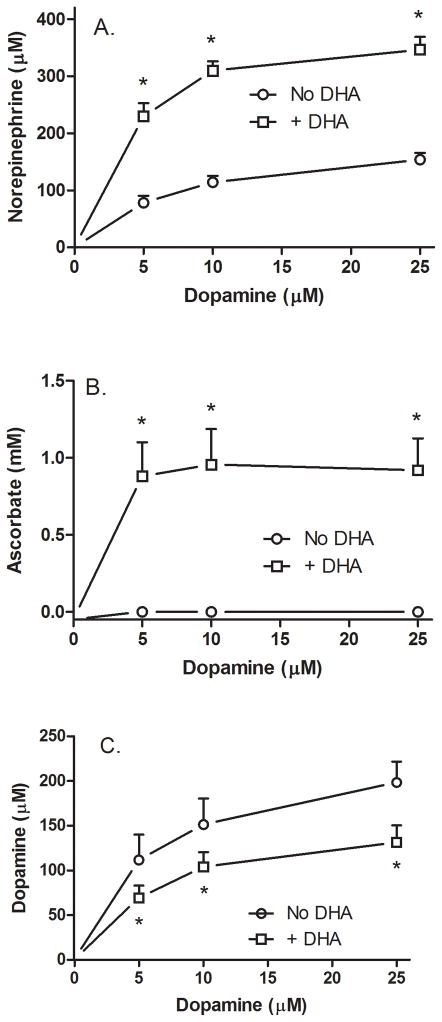
It was also of interest to test the effect of intracellular AA on NE and DA levels in cells at much lower concentrations of DA and AA, since this would provide an indication of the affinity/access of DβH to its substrate and whether AA-induced synthesis of NE would impact intracellular DA. In the experiment shown in Fig. 3, cells were treated without or with 100 μM DHA for 30 min, followed by DA for 60 min. As shown in Panel A, NE from DA alone showed a modest and significant upward trend (circles) that was doubled or tripled by the presence of intracellular AA (squares). These cells had robust NE generation to levels about the same as generated at much higher intracellular AA concentrations in the experiment shown in Fig. 2. This variance is perhaps explained by the fact that the cells in the Fig. 3 experiment were of an earlier passage in culture, although other factors related to different lots of fetal calf serum could also have contributed. Such variability in NE synthesis in SK-N-SH cells has been noted previously [26]. AA concentrations of just under 1 mM were achieved with loading of 100 μM DHA, again showing the marked accumulation against a concentration gradient. These levels were not affected by the low concentrations of DA used. Intracellular DA concentrations rose as expected with increasing extracellular DA, although not linearly (Fig. 3C, circles). Indeed, the presence of intracellular AA decreased intracellular DA by about one-third at all three DA concentrations (Fig. 3C, squares). Despite lower intracellular DA at the end of the experiment, compared to DA treatment alone, cells loaded with AA by DHA treatment cells were able to maintain increased NE synthesis, presumably by drawing down the intracellular DA. Although AA-induced NE formation decreased intracellular DA, the final intracellular DA concentration was still many-fold greater than the initial extracellular DA concentrations (Fig 3C). Neither AA nor NE was detected in the culture medium (results not shown).
Figure 3. Ascorbate effects on NE generation from relatively low DA concentrations.
Cells in culture were incubated in the absence (circles) or presence (squares) of 100 μM DHA, followed in 30 min by addition of the indicated concentration of DA. After another 60 minutes in culture the cells were rinsed twice in 2 ml of KRH and removed from the plate for assay of NE (Panel A), AA (Panel B), and DA (Panel C) as described under Methods. Results are shown from 4 experiments, with an “*” indicating p < 0.05 compared to cells not treated with AA at the same DA concentration.
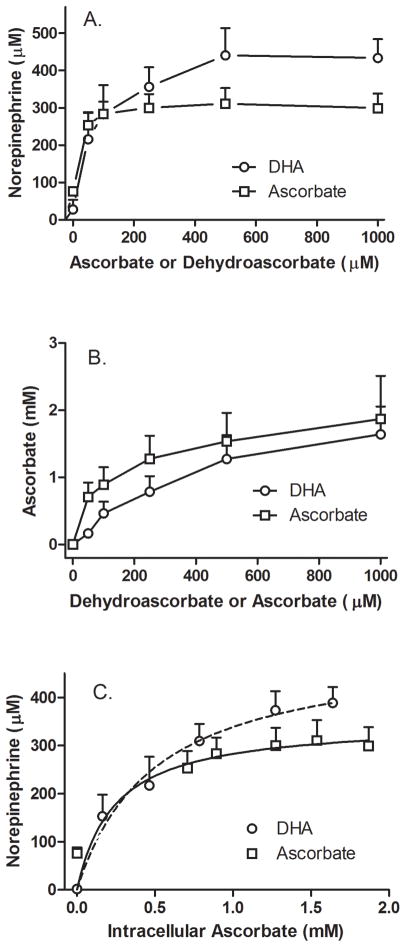
3.3. Increasing intracellular AA stimulates of NE generation
To correlate intracellular AA with NE generation, cells were treated in culture for 30 min with increasing concentrations of DHA (circles) or AA (squares), followed by addition of 50 μM DA for an additional 60 min (Fig. 4). For both forms of the vitamin, this resulted in a marked increase in intracellular NE at added loading concentrations as low as 50 μM that plateaued above 250 μM for each form of the vitamin (Fig. 4A). As expected, intracellular AA also increased and this occurred to a similar extent for both AA and DHA (Fig. 4B). When intracellular NE was plotted as a function of measured intracellular AA, NE generation was a saturable function of the intracellular AA concentration (Fig. 4C). Fitting these results to hyperbolic models showed that with DHA loading the AA concentration at which the intracellular NE concentration was half-maximal was 0.5 mM. With AA loading, intracellular AA and NE plateaued more abruptly, making an estimate of the half-maximal NE concentration inaccurate, although the calculated value was 0.2 mM, clearly in the range of that with DHA. These results confirm that relatively low intracellular AA concentrations (under 1 mM) markedly stimulate NE generation in DA-treated cells compared to cells lacking AA.
Figure 4. AA concentration-dependence of NE generation from DA in SH-SY5Y cells.
Cells in culture were incubated with increasing concentrations of DHA (circles) or AA (squares), followed in 30 minutes by addition of 50 μM DA. After another 60 minutes in culture the cells were rinsed twice in 2 ml of KRH and removed from the plate for assay of NE (Panel A) and AA (Panel B) as described under Methods. In Panel C, the cellular contents of NE (Panel A) were plotted as a function of the measured intracellular AA concentrations at each loading concentration of each form of the vitamin (Panel B). The dashed lines in Panel C show hyperbolic fits to the data. For DHA loading, the calculated AA concentration at which NE accumulation was half-maximal was 0.5 mM, with a maximum of 506 μM. For AA loading, these values were 0.24 mM and 350 μM, respectively. Results are shown from experiments with each form of AA.
3.4 Short-term DA treatment induces an oxidative stress in SH-SY5Y cells
To test whether relatively high concentrations of DA cause oxidative stress in SH-SY5Y cells, cells were loaded with 100 μM DHA. After 90 minutes in the absence of DA, intracellular AA concentrations were about 0.8 mM. Addition of increasing concentrations of DA for the last 60 minutes of this incubation progressively decreased intracellular AA to about 50% of control at 500 μM DA (Fig. 5A, circles). A significant decrease in intracellular AA was apparent at a DA concentration of 50 μM. This contrasts with the results seen when cells were loaded with 500 μM DHA prior to DA, where intracellular AA levels were about 3 mM and a slight but insignificant decrease in intracellular AA due to DA was noted (Fig. 2B). GSH was also measured in the cells in the experiment shown in Fig. 5A (squares) and found to decrease with increasing DA, although significance was reached only at the highest DA concentration.
To assess the extent to which DA caused an oxidative stress, cells treated with 0 or 100 μM DHA for 30 min were exposed to 0 or 100 μM DA for an additional 60 min. After rinsing to remove extracellular DHA and DA, the cells were treated for an additional 30 min with 10 μM dihydroethidium and taken for assay of superoxide as 2-hydroxyethidium fluorescence. As shown in Fig. 5B, DA more than doubled superoxide levels, whereas increasing intracellular AA by DHA loading alone had no effect. Increasing the intracellular AA concentration completely prevented the increase in superoxide generation by the cells. Treating cell with the cell-penetrant antioxidant TEMPOL also prevented the increase in superoxide due to DA. These results show that DA acutely increases superoxide generation in SH-SY5Y cells and that AA and TEMPOL prevent this increase. They also suggest that at least part of the decrease in intracellular AA due to DA was related to prevention superoxide generation.
Although TEMPOL decreased superoxide generation in response to DA in the experiment of Fig. 5B, neither it nor several other antioxidants increased NE generation by the cells (Fig. 5C). In this experiment, cells were incubated with concentrations of the antioxidants known to be effective in decreasing oxidative stress for 30 min followed by addition of DA and measurement of NE 60 min later. Only AA loading with DHA increased NE generation.
4. Discussion
Although AA has long been known to be the physiologic co-factor for DβH in adrenal chromaffin cells [5], its role in promoting or even regulating NE synthesis from DA in isolated neuronal cells has not been elucidated. In the absence of added AA and DA and despite the fact that the culture medium contained 100 μM L-tyrosine, SH-SY5Y neuroblastoma cells did not generate detectable amounts of intracellular NE in culture. Incubation over 60 min with increasing concentrations of exogenous DA alone did cause a gradual but modest increase in intracellular NE, in line with previous results showing that DA itself can support a low level of NE synthesis by purified bovine adrenal DβH [27,28]. Loading cells with AA alone over 90 min by treatment with DHA caused a small and variable increase in intracellular NE. When cells were provided with both DA and AA, however, they showed a marked increase in NE generation, supporting the notion that DβH is functional in these cells, but that they lack both substrate and co-cofactor for its efficient synthesis.
The increase in NE synthesis when cells were treated with AA and DA was prompt and progressive over 90 min, whether AA loading was achieved by incubation with DHA or AA. This differs from the response observed with cultured AA-depleted adrenal chromaffin cells, in which AA stimulated DβH-dependent tyramine conversion to octopamine only after several hours [7]. Although AA uptake into chromaffin cells was rapid [7,29,30], its entry into chromaffin granules is known to be very slow [6,30]. This was considered to account for the long lag-phase in the AA effect on octopamine synthesis by DβH [7]. Our finding that AA enhanced NE generation from DA much more rapidly in SH-SY5Y cells suggests that intracellular AA or its reducing equivalents had ready access to DβH in this cell type. A potential caveat is that DHA does enter chromaffin granules rapidly compared to AA [6] and we loaded cells with DHA most experiments. Thus, DHA could have diffused into secretory granules and accounted for the rapid response. Still, our finding that DHA and AA had similar linear time-dependent increases in NE generation despite more rapid increases in intracellular AA with DHA than AA loading shows that NE generation is rapid with AA itself. Our result could be explained by a cytoplasmic location for at least some functional DβH. This is certainly not the case for chromaffin cells [5], and also unlikely for SH-SY5Y cells, which are known to have large dense core vesicles that on fractionation contain newly synthesized NE and DβH immunoreactivity [31]. Another possibility is that the vesicles containing DβH in SH-SY5Y cells also contain an AA transporter to facilitate AA entry into the vesicles. Indeed, we have previously reported that the SVCT2 is localized to punctate vesicular structures in cultured hippocampal neuronal axons [32]. Whether neuronal secretory vesicles contain functional SVCT2 is a question for future studies, since it could impact the well-known release of AA at the synapse during neurotransmission [33].
There is controversy regarding the in situ affinity of DβH for AA in chromaffin cells. Initial studies in cultured adrenal chromaffin cells and purified chromaffin granules found a relatively high apparent Km of 15–17 mM for AA [34], which was subsequently confirmed and attributed to negative cooperativity of DβH at higher AA concentrations [35]. However, other studies in adrenal chromaffin granules found a Km of 0.6 mM [36], which was similar to that of 0.5–0.6 mM found in extracted and purified bovine DβH [4,37]. The main difference between these studies was the assay of DβH using conversion of tyramine to octopamine in the experiments reporting a high Km for AA, compared to use of DA as the substrate for the low Km studies. Our finding of an apparent intracellular Km of 0.5 mM or less for ascorbate in intact SH-SY5Y cells also using DA as substrate supports the results of Dhariwal, et al. [36] and their contention that the natural substrate DA should be used to assess the affinity of DβH for AA. It is important to note in this coupled compartmental system that the concentration of extracellular AA generating half-maximal NE appearance in the cell was about 100 μM, which fits with the measured CSF AA concentration in humans [38,39].
It is also relevant to consider the in situ kinetics of DA with regard to NE generation by DβH. DA was concentrated many-fold in SH-SY5Y cells over that present in the medium during 60 minutes of incubation. DA uptake was likely mediated by the NE transporter [40], which is known to be expressed in SK-N-SH cells, from which SH-SY5Y cells were derived [41]. Since the affinity of the NE transporter for NE and presumably DA is about 700 nM in SH-SY5Y cells [42], it will be saturated at the DA concentrations used in these studies. Nonetheless, it is able to concentrate DA in the cells relative to DA present in the incubation medium. The apparent Km of DβH for DA in brain extracts is 2 mM [37], which is higher than apparently saturating total cell DA concentrations reached in the present studies. It is thus likely that DβH in secretory vesicles is exposed to much higher concentrations of DA due to concentrative uptake into the vesicles on the VMAT2, which has a Km for DA of about 25 μM [43].
When cells were loaded with DA concentrations of 10 to 25 μM, AA loading decreased intracellular DA by one-third compared to cells loaded with DA alone. This was likely due to consumption of DA by its conversion to NE, which occurs on an equimolar basis [4]. Although about twice as much NE was generated by AA from DA compared to the decrease in intracellular DA, the difference probably reflects continued uptake of DA from the medium over the 60 minutes of incubation.
The key factor in enhancing the effect of AA on SH-SY5Y cell DβH is the ability to concentrate AA from the extracellular medium. This was achieved in the present studies either by rapid uptake and reduction of DHA to AA as noted earlier, or by uptake of AA itself. It is likely that AA is the major form of the vitamin entering neurons under normal conditions, since DHA is very unstable at physiologic pH [44,45]. Inhibitor studies suggested that concentrative AA transport into these cells is mediated by the SVCT2 [18], which they express as a ~50 kilodalton protein [46]. The apparent Km for AA transport into SH-SY5Y cells is about 110 μM [18]. This fits the range of extracellular AA concentrations that generate half-maximal intracellular AA concentrations in the present study, which in turn generate half-maximal intracellular NE concentrations. Given CSF AA concentrations in humans of 100–200 μM noted above, the extracellular AA concentration will directly affect and thus regulate both intracellular AA and NE generation.
In addition to facilitating NE synthesis, intracellular AA also prevented superoxide generation by DA. Although superoxide is the expected initial product of redox cycling of DA with molecular oxygen, only its downstream toxicity had been previously documented after as decreased mitochondrial function with increased lipid peroxidation [10] and decreased cell viability [11,15]. AA at low millimolar concentrations is capable of scavenging superoxide directly [47] and it can recycle the semiquinone radical of DA [48], which would halt further oxidation of DA to its toxic quinone form. Although the antioxidant TEMPOL also prevented superoxide generation from DA, neither TEMPOL nor other antioxidants facilitated NE generation from DA. This result confirms and extends those of previous studies in which thiols did not enhance the activity of DβH in chromaffin cell cultures [49] or in purified chromaffin DβH [4]. Since potent intracellular antioxidants failed to affect NE synthesis from DA, it seems likely that this effect of AA was not a major factor in its ability to stimulate NE generation from DA.
When loaded with 100 μM AA, SH-SY5Y cells can maintain AA concentrations for at least 16 h in culture of 1.5–2 mM [18], which are similar to those of GSH (1.0–1.5 mM) seen in the present and the previous study. Although GSH is typically considered the major low molecular weight thiol-based antioxidant in these cells [11,18], the results of our previous study with glutamate toxicity show that AA is a more sensitive indicator of oxidative stress than is GSH [18]. This was also evident in the present study, since treating cells loaded to 0.8 mM AA with 100 μM DA for 60 min decreased intracellular AA by 50%, but only modestly decreased GSH. This is not to say that AA is a better antioxidant in these cells than GSH, but more likely that the cells have a high capacity to recycle GSH from GSSG via glutathione reductase. On the other hand, when cells were loaded to 3 mM with AA, the relative decrease in AA was smaller and showed only a downward trend. A caveat here is that AA is also oxidized in the DβH reaction and this will contribute to its decrease. Nonetheless, the ability of AA to prevent superoxide generation due to DA suggests that at least part of the decrease in AA is due to scavenging superoxide.
In conclusion, intracellular AA stimulates NE generation from DA in SH-SY5Y cells in a time- and concentration-dependent manner from extracellular AA concentrations in the physiologic range of expected AA concentrations in the CSF. This stimulation is associated with prevention of DA-dependent superoxide generation that might otherwise be toxic to the cells. These two actions of AA suggest that intracellular AA might both regulate NE synthesis and prevent attendant toxicity of DA.
Highlights.
Vitamin C rapidly and efficiently stimulates norepinephrine synthesis
Physiologic intracellular vitamin C levels regulate norepinephrine synthesis
Vitamin C prevents superoxide generation in response to dopamine
Acknowledgments
This work was supported by NIH grant NS 057674 and by the Vanderbilt Diabetes Research and Training Center (DK 020593). JMM was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
Abbreviations used
- ANOVA
analysis of variance
- AA
ascorbic acid
- CSF
cerebrospinal fluid
- DHA
dehydroascorbate
- DβH
dopamine β-hydroxylase
- L-DOPA
L-3,4-dihydroxyphenylalanine
- DA
dopamine
- Hepes
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- KRH
Krebs-Ringer Hepes
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrison FE, May JM. Vitamin C function in the brain: Vital role of the ascorbate transporter (SVCT2) Free Radic Biol Med. 2009;45:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone KJ, Townsley BH. The effect of L-ascorbate on catecholamine biosynthesis. Biochem J. 1973;131:611–613. doi: 10.1042/bj1310611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallet J. The TiPS/TINS Lecture. Catecholamines: from gene regulation to neuropsychiatric disorders. Trends Neurosci. 1996;19:191–196. doi: 10.1016/s0166-2236(96)10029-1. [DOI] [PubMed] [Google Scholar]
- 4.Levin EY, Levenberg B, Kaufman S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1960;235:2080–2086. [PubMed] [Google Scholar]
- 5.Diliberto EJ, Jr, Daniels AJ, Viveros OH. Multicompartmental secretion of ascorbate and its dual role in dopamine beta-hydroxylation. Am J Clin Nutr. 1991;54:1163S–1172S. doi: 10.1093/ajcn/54.6.1163s. [DOI] [PubMed] [Google Scholar]
- 6.Tirrell JG, Westhead EW. The uptake of ascorbic acid and dehydroascorbic acid by chromaffin granules of the adrenal medulla. Neuroscience. 1979;4:181–186. doi: 10.1016/0306-4522(79)90227-6. [DOI] [PubMed] [Google Scholar]
- 7.Menniti FS, Knoth J, Diliberto EJ., Jr Role of ascorbic acid in dopamine beta-hydroxylation. The endogenous enzyme cofactor and putative electron donor for cofactor regeneration. J Biol Chem. 1986;261:16901–16908. [PubMed] [Google Scholar]
- 8.Richards ML, Sadee W. Human neuroblastoma cell lines as models of catechol uptake. Brain Res. 1986;384:132–137. doi: 10.1016/0006-8993(86)91228-x. [DOI] [PubMed] [Google Scholar]
- 9.Pardo B, Mena MA, Fahn S, Garcia de YJ. Ascorbic acid protects against levodopa-induced neurotoxicity on a catecholamine-rich human neuroblastoma cell line. Mov Disord. 1993;8:278–284. doi: 10.1002/mds.870080305. [DOI] [PubMed] [Google Scholar]
- 10.Lai CT, Yu PH. Dopamine- and L-beta-3,4-dihydroxyphenylalanine hydrochloride (L-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem Pharmacol. 1997;53:363–372. doi: 10.1016/s0006-2952(96)00731-9. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Pei L, Li S, Wang M, Liu F. Extracellular dopamine induces the oxidative toxicity of SH-SY5Y cells. Synapse. 2008;62:797–803. doi: 10.1002/syn.20554. [DOI] [PubMed] [Google Scholar]
- 12.Anden NE, Hfuxe K, Hamberger B, Hokfelt T. A quantitative study on the nigro-neostriatal dopamine neuron system in the rat. Acta Physiol Scand. 1966;67:306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohen G. Monoamine oxidase, hydrogen peroxide, and Parkinson’s disease. Adv Neurol. 1987;45:119–125. [PubMed] [Google Scholar]
- 14.Simantov R, Blinder E, Ratovitski T, Tauber M, Gabbay M, Porat S. Dopamine-induced apoptosis in human neuronal cells: inhibition by nucleic acids antisense to the dopamine transporter. Neuroscience. 1996;74:39–50. doi: 10.1016/0306-4522(96)00102-9. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Santos C, Ferrer I, Santidrian AF, Barrachina M, Gil J, Ambrosio S. Dopamine induces autophagic cell death and alpha-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res. 2003;73:341–350. doi: 10.1002/jnr.10663. [DOI] [PubMed] [Google Scholar]
- 16.Oyarce AM, Fleming PJ. Multiple forms of human dopamine beta-hydroxylase in SH- SY5Y neuroblastoma cells. Arch Biochem Biophys. 1991;290:503–510. doi: 10.1016/0003-9861(91)90573-2. [DOI] [PubMed] [Google Scholar]
- 17.Guo JT, Chen AQ, Kong Q, Zhu H, Ma CM, Qin C. Inhibition of vesicular monoamine transporter-2 activity in alpha-synuclein stably transfected SH-SY5Y cells. Cell Mol Neurobiol. 2008;28:35–47. doi: 10.1007/s10571-007-9227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May JM, Li L, Hayslett K, Qu ZC. Ascorbate transport and recycling by SH-SY5Y neuroblastoma cells: Response to glutamate toxicity. Neurochem Res. 2006;31:785–794. doi: 10.1007/s11064-006-9077-z. [DOI] [PubMed] [Google Scholar]
- 19.Nazarewicz R, Bikineyeva A, Harrison DG, Dikalov S. Rapid and Specific Measurements of Superoxide Using Fluorescence Spectroscopy. FASEB J. 2012;26:578.3. doi: 10.1177/1087057112468765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 21.Lindsey JW, Jung AE, Narayanan TK, Ritchie GD. Acute effects of a bicyclophosphate neuroconvulsant on monoamine neurotransmitter and metabolite levels in the rat brain. J Toxicol Environ Health A. 1998;54:421–429. doi: 10.1080/009841098158827. [DOI] [PubMed] [Google Scholar]
- 22.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 23.May JM, Qu ZC, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 24.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 26.Seitz G, Gebhardt S, Beck JF, Bohm W, Lode HN, Niethammer D, Bruchelt G. Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neurosci Lett. 1998;244:33–36. doi: 10.1016/s0304-3940(98)00129-3. [DOI] [PubMed] [Google Scholar]
- 27.Levin EY, Kaufman S. Studies on the enzyme catalyzing the conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1961;236:2043–2049. [PubMed] [Google Scholar]
- 28.Stewart LC, Klinman JP. Characterization of alternate reductant binding and electron transfer in the dopamine beta-monooxygenase reaction. Biochemistry. 1987;26:5302–5309. doi: 10.1021/bi00391a013. [DOI] [PubMed] [Google Scholar]
- 29.Diliberto EJ, Jr, Heckman GD, Daniels AJ. Characterization of ascorbic acid transport by adrenomedullary chromaffin cells. Evidence for Na+-dependent co-transport. J Biol Chem. 1983;258:12886–12894. [PubMed] [Google Scholar]
- 30.Levine M, Morita K, Pollard H. Enhancement of norepinephrine biosynthesis by ascorbic acid in cultured bovine chromaffin cells. J Biol Chem. 1985;260:12942–12947. [PubMed] [Google Scholar]
- 31.Goodall AR, Danks K, Walker JH, Ball SG, Vaughan PF. Occurrence of two types of secretory vesicles in the human neuroblastoma SH-SY5Y. J Neurochem. 1997;68:1542–1552. doi: 10.1046/j.1471-4159.1997.68041542.x. [DOI] [PubMed] [Google Scholar]
- 32.Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res. 2007;85:1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- 33.Rebec GV, Pierce RC. A vitamin as neuromodulator: Ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 34.Menniti FS, Knoth J, Peterson DS, Diliberto EJ., Jr The in situ kinetics of dopamine beta-hydroxylase in bovine adrenomedullary chromaffin cells. Intravesicular compartmentation reduces apparent affinity for the cofactor ascorbate. J Biol Chem. 1987;262:7651–7657. [PubMed] [Google Scholar]
- 35.Stewart LC, Klinman JP. Cooperativity in the dopamine beta-monooxygenase reaction. Evidence for ascorbate regulation of enzyme activity. J Biol Chem. 1991;266:11537–11543. [PubMed] [Google Scholar]
- 36.Dhariwal KR, Washko P, Hartzell WO, Levine M. Ascorbic acid within chromaffin granules. In situ kinetics of norepinephrine biosynthesis. J Biol Chem. 1989;264:15404–15409. [PubMed] [Google Scholar]
- 37.Sperk G, Galhaup I, Shlogl E, Hortnagl H, Hornykiewicz O. A sensitive and reliable assay for dopamine beta-hydroxylase in tissue. J Neurochem. 1980;35:972–976. doi: 10.1111/j.1471-4159.1980.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 38.Reiber H, Ruff M, Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin Chim Acta. 1993;217:163–173. doi: 10.1016/0009-8981(93)90162-w. [DOI] [PubMed] [Google Scholar]
- 39.Lönnrot K, Metsä-Ketelä T, Molnár G, Ahonen JP, Latvala M, Peltola J, Pietilä T, Alho H. The effect of ascorbate and ubiquinone supplementation on plasma and CSF total antioxidant capacity. Free Radic Biol Med. 1996;21:211–217. doi: 10.1016/0891-5849(95)02207-4. [DOI] [PubMed] [Google Scholar]
- 40.Seitz G, Stegmann HB, Jager HH, Schlude HM, Wolburg H, Roginsky VA, Niethammer D, Bruchelt G. Neuroblastoma cells expressing the noradrenaline transporter are destroyed more selectively by 6-fluorodopamine than by 6-hydroxydopamine. J Neurochem. 2000;75:511–520. doi: 10.1046/j.1471-4159.2000.0750511.x. [DOI] [PubMed] [Google Scholar]
- 41.Lode HN, Bruchelt G, Seitz G, Gebhardt S, Gekeler V, Niethammer D, Beck J. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of monoamine transporters in neuroblastoma cell lines: correlations to meta-iodobenzylguanidine (MIBG) uptake and tyrosine hydroxylase gene expression. Eur J Cancer. 1995;31A:586–590. doi: 10.1016/0959-8049(95)00039-l. [DOI] [PubMed] [Google Scholar]
- 42.Amano T, Aoki S, Setsuie R, Sakurai M, Wada K, Noda M. Identification of a novel regulatory mechanism for norepinephrine transporter activity by the IP3 receptor. Eur J Pharmacol. 2006;536:62–68. doi: 10.1016/j.ejphar.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31:483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drake BB, Smythe CV, King CG. Complexes of dehydroascorbic acid with three sulfhydryl compounds. J Biol Chem. 1942;143:89–98. [Google Scholar]
- 45.Tolbert BM, Ward JB. Dehydroascorbic acid. In: Seib PA, Tolbert BM, editors. Ascorbic Acid: Chemistry, Metabolism, and Uses. Washington, D.C: American Chemical Society; 1982. pp. 101–123. [Google Scholar]
- 46.Li X, Huang J, May JM. Ascorbic acid spares alpha-tocopherol and decreases lipid peroxidation in neuronal cells. Biochem Biophys Res Commun. 2003;305:656–661. doi: 10.1016/s0006-291x(03)00836-2. [DOI] [PubMed] [Google Scholar]
- 47.Jackson TS, Xu AM, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- 48.Sakagami H, Satoh K, Ida Y, Hosaka M, Arakawa H, Maeda M. Interaction between sodium ascorbate and dopamine. Free Radic Biol Med. 1998;25:1013–1020. doi: 10.1016/s0891-5849(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 49.Levine M. Ascorbic acid specifically enhances dopamine beta-monooxygenase activity in resting and stimulated chromaffin cells. J Biol Chem. 1986;261:7347–7356. [PubMed] [Google Scholar]