Abstract
Increasing concentrations of air pollution have been shown to contribute to an enormity of adverse health outcomes worldwide, which have been observed in clinical, epidemiological, and animal studies as well as in vitro investigations. Recently, studies have shown that air pollution can affect the developing fetus via maternal exposure, resulting in preterm birth, low birth weight, growth restriction, and potentially adverse cardiovascular and respiratory outcomes. This review will provide a summary of the harmful effects of air pollution exposure on the developing fetus and infant, and suggest potential mechanisms to limit the exposure of pregnant mothers and infants to air pollution.
Keywords: air pollution, particulate matter, fetal, infant
Introduction
Rapid industrial growth and economic expansion in developing countries contributes to numerous adverse health consequences of air pollution exposure, with evidence implicating particulate matter (PM; component of air pollution) as the chief perpetrator of harmful health outcomes (Agency, 2006). Epidemiological studies have shown an association between PM exposure and adverse health outcomes for the past decade, particularly related to adult cardiovascular morbidity and mortality (Brook et al., 2004; Pope et al., 2004; Sun et al., 2010). This review will explore the growing literature on the effects of air pollution on fetal and infant development, including effects on cardiopulmonary disease, low birth weight (LBW), intrauterine growth retardation (IUGR), and pre-term birth. Given the vulnerability of immature organ systems to outside influences, the developing fetus and neonate may be at a greater risk for developing adverse health effects secondary to perinatal PM exposure (Choi et al., 2012; Pope, 2000). In addition, a longstanding line of evidence suggests that exposure to harmful levels of air pollutants accrued during sensitive periods of organ development may predispose an individual to developing certain adulthood cardiovascular pathologies (Bolton et al., 2012; Burton, 2009; Lacasana et al., 2005; Rocha et al., 2008; Sun et al., 2005{Bolton, 2012 #190).
This review will also focus on the potential impact of PM on pediatric outcomes, addressing vulnerabilities from fetal-life through infancy. Barker et al. (Barker and Osmond, 1986) initially proposed the concept that in utero variations in nutrient transfer from mother to child are related to LBW, ultimately resulting in adverse health outcomes later in life. Evidence detailed in this review expands upon this hypothesis, supporting the idea that environmental exposure to air pollution can similarly have harmful effects on the fetus. We aim to detail the harmful effects associated with fetal and infant PM exposure, and hopefully enhance international efforts to limit the exposure of pregnant mothers and children to PM and related air pollution sources.
PM sources and levels
Air pollution consists of a complex mixture of gases, liquids, and PM (Brook et al., 2004; Pope and Dockery, 2006). PM represents a diverse class of chemically and physically heterogeneous substances existing as separate particles (liquid droplets, solids, or semi-volatile materials) within the atmosphere (Agency, 2006). Human and biogenic sources emit PM into the ambient air, however human activity contributes the majority of primary PM present (Masih et al., 2010; Pandya et al., 2002; Wilhelm and Ritz, 2003). Motor vehicles, burning coal, residual oil, particles derived from the earth’s crust, and forest fires produce constituents of PM (Nelin et al., 2012). Other activities contributing to increased PM concentrations in the ambient air include wood and fossil fuel combustion, industrial processes, indoor cooking with biofuels, construction, and demolition activities (Agency, 2005).
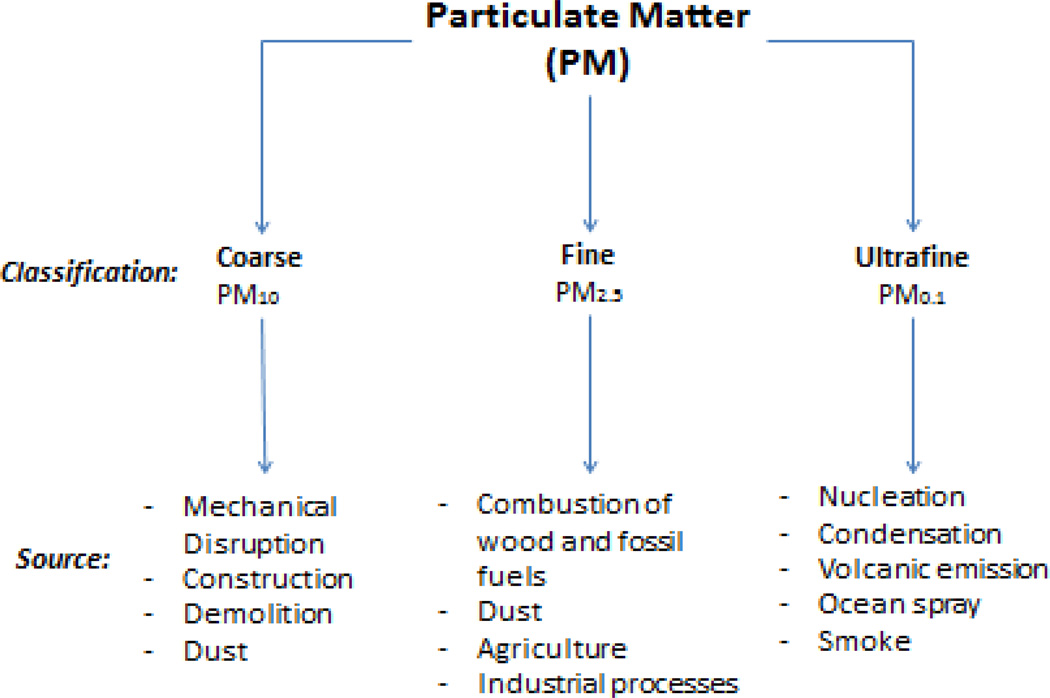
PM is normally expressed as the mass of particles within a cubic meter of air (micrograms per cubic meter (µg/m3)). PM in the ambient air contains three size ranges: coarse (PM 2.5–10 µm or PM10), fine (PM <2.5 µm or PM2.5), and ultrafine (PM <0.1 µm or PM0.1) particles (Sun et al., 2010), as shown in Figure 1. The present review focuses on PM2.5, as it has been the main focus of many scientific and legislative efforts stemming from its well documented and reproducible negative effects on human health (Brook, 2008). Despite the focus on PM2.5, it is critical to appreciate that particulate matter and air pollution exist as a heterogeneous mixture of gaseous and semi-volatile/volatile compounds, with biological toxicity based on the underlying chemical composition. This review also includes studies exploring constituents that contribute to air pollution, but are not classified as PM, such as NOx, polycyclic aromatic hydrocarbons, SOx, and tobacco smoke (Agency, 2006).
Figure 1. Sources and divisions of PM.
PM represents a class of heterogeneous substances that exist as discrete particles, combining to form one component of air pollution. PM can be divided into three different categories based on size range; coarse, fine, and ultrafine. Both human and biogenic sources produce constituents of PM, and PM exposure has become a growing field for research as many adverse health consequences have been related to PM exposure.
Adverse Birth Outcomes
The immature fetus is highly susceptible to toxicant exposure (Choi et al., 2012). This biological vulnerability is secondary to increased rates of cellular proliferation and growth, all in the setting of constantly changing metabolic and hormonal requirements. Any disruption in the efficiency of transplacental function in utero has the potential to negatively impact fetal growth and development, particularly during critical periods of organogenesis (Stevenson et al., 2003). Epidemiologic evidence suggests an association between PM10 and PM2.5 exposure during pregnancy and adverse birth outcomes, including increased infant mortality, LBW, IUGR, and preterm birth (Bell et al., 2010b; Rossner et al., 2011; Rudra et al., 2011). Similar studies have demonstrated no association between fetal air pollution exposure and LBW, suggesting that the correlation between exposure and effect is delicate and might be enhanced by external factors such as region, SES, and duration of exposure (Rossner et al., 2011). A growing body of literature investigating the link between PM exposure and adverse perinatal outcomes has emerged due to the increasing potential of exposure to PM during pregnancy. Chronic exposure to air pollution may disrupt biological mechanisms that regulate fetal growth and development; however, current evidence suggests that particulate air pollution exposure can only be associated with minimal, at best, adverse effects on birth outcome (Glinianiaia et al., 2004). The specific mechanism(s) of this effect remain relatively unknown (Figure 2). The effects of these and other clinical studies can be found in Table 1.
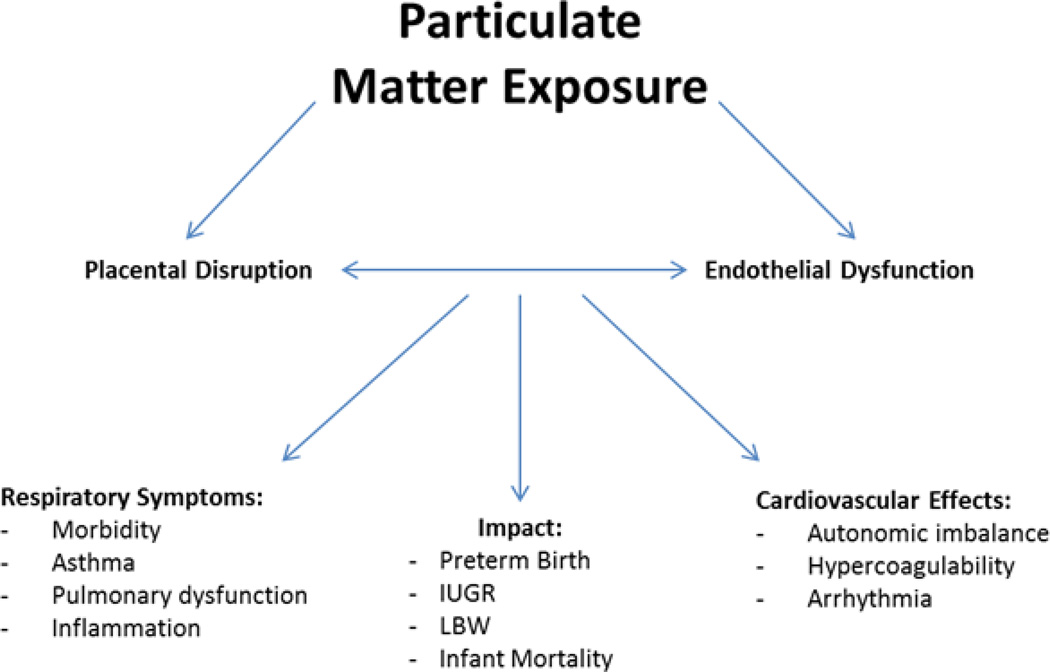
Figure 2. Pathways and impacts of PM exposure.
PM is a major constituent of air pollution that is comprised of particles exhibiting three different size ranges. A number of cardiovascular effects have been related to increased levels of PM exposure. Studies have also demonstrated that fetal PM exposure may result in a host of developmental conditions including Intrauterine Growth Retardation (IUGR), low birth weight (LBW), preterm birth, and infant mortality. The mechanisms of effect following PM exposure can be characterized by the onset of oxidative stress, which causes placental and endothelial dysfunction. This dysfunction can lead to the development of a number of cardiovascular and respiratory symptoms.
Table 1.
Summary of epidemiological data outlining the effects resulting from increased PM exposure. The literature demonstrates a strong correlation between fetal PM exposure and developmental defects, most notably IUGR, LBW, and preterm birth. Studies have ranged in geographic location from North America, Europe, and Asia, demonstrating a widespread relationship between PM exposure and fetal effects.
| Reference | Location | Fetal Effect |
|---|---|---|
| Dejmek et al. (1999) | Northern Bohemia | Adjusted Odds Ratio (AOR) of IUGR^: PM10→1.62medium / 2.64high PM2.5→1.26medium / 2.11high |
| Ritz et al. (2000) | Southern California | Adjusted Risk Ratio (RR) preterm birth: PM10 (50µg increase) → 1.20 CO (3-ppm increase) → 1.13 *Exposure 6 months before pregnancy |
| Chen et al. (2001) | Washoe County, NV | No relationship between LBW^^ and any exposure; Increase in PM10 exposure during 3rd trimester → 11 g LBW |
| Ha et al. (2001) | Seoul, South Korea | AOR for LBW: CO → 1.08 (1.04–1.12) NO2 → 1.07 (1.03–1.11) SO2 → 1.06 (1.02–1.10) TSP → 1.04 (1.00–1.08) |
| Maroziene & Grazuleviciene et al. (2002) | Kaunas, Lithuania | AOR for LBW: Formaldehyde3rd tertile → 1.84 (1.12–3.03) AOR for preterm birth: NO2 high → 1.68 (1.15–2.46) *First trimester exposures only |
| Bobak et al. (2003) | Czechoslovakia | AOR for LBW: SO2 → 1.20 (1.11–1.30) TSP → 1.15 (1.07–1.24) AOR for Preterm birth: SO2 → 1.27 (1.16–1.39) TSP → 1.18 (1.05–1.31) |
| Lee B.E. et al. (2003) | Seoul, South Korea | LBW increase: CO exposure during months 2–5 of pregnancy SO2 exposure during months 3–5 of pregnancy NO2 exposure during months 3–5 of pregnancy PM10 exposure during months 2–4 of pregnancy |
| Jedrychowski et al. (2004) | Poland | 40 µg/m3 increase in PM2.5 exposure → 140.3 g LBW |
| Parker et al. (2005) | California | AOR for SGA^^^: PM2.5 → 1.26 (1.03–1.50) CO → no effect |
| Salam et al. (2005) | California | 12-ppb increase in 24 hr O3 → 47.2 g LBW 1.4-ppm increase in CO → 21.7 g LBW and 20% increase IUGR |
| Suh et al. (2008) | Seoul, South Korea | Odds Ratio (OR) for preterm birth: Combined effects of GSTM1 genotype & PM10 exposure → 6.22 (2.14–18.08) |
| Wu et al. (2009) | Southern California | OR preeclampsia: Highest NOx → 1.33 (33% increase) Highest PM2.5 → 1.42 (42% increase) OR preterm birth: Highest NOx → 2.28 (128% increase) Highest PM2.5 → 1.81 (81% increase) |
| Bell et al. (2010) | Massachusetts Connecticut | Increase in exposure to PM2.5 constituents → LBW |
| Carbajal-Arroyo et al. (2011) | Mexico City, Mexico | 38.7 µg/m3 increase PM10 → 5.3% increase respiratory mortality with 1 day lag, 10% increase respiratory mortality with 2 day lag *O3 associated with respiratory mortality in low SES |
(IUGR - Intrauterine Growth Retardation, <10th percentile of birth weight; LBW - Low Birth Weight, <2500 g; SGA - Small for gestational age, weight below the 10th percentile; Preterm Birth - <37 weeks gestation; TSP – Total Respirable Particles).
Placental Function and IUGR
A study by Veras et al. (Veras et al., 2008) evaluated the effects of PM, a mixture with a majority composed of PM2.5,exposure on functional morphology of the placenta. They showed that PM exposure during pregnancy induced changes in multiple placental compartments, including the maternal vascular space, fetal capillaries, and surface exchange areas. More importantly, these alterations in placental function were associated with a higher incidence of low birth weight among exposed fetuses.
Numerous epidemiological studies have documented the correlation between PM exposure and low birth weight. Salam et al. (Salam et al., 2005) demonstrated that increased ozone exposure throughout pregnancy, increased CO exposure throughout the first trimester of pregnancy, and increased PM10 exposure during the third trimester of pregnancy are all associated with lower birth weight.
Although tobacco smoke and PM exposure are not equitable, the similarities in effect are worthy of consideration. Among 362 nonsmoking Polish women with singleton pregnancies, the birth weight of the children from mothers exposed to 50 µg/ml was 140.3 g less than the birth weight of children from mothers exposed to 10 µg/ml (Jedrychowski et al., 2004). A study in Seoul, South Korea demonstrated that exposure to elevated levels of CO, PM10, SO2, and NO2 between the second and fifth months of pregnancy contributed to LBW (Lee et al., 2003). Bell et al. (Bell et al., 2010a) revealed that pregnant women exposed to high concentrations of PM2.5 have children with LBW, particularly exposure during the third-trimester. Parker et al. (Parker et al., 2005) showed a connection between increased exposure to PM2.5 throughout a full nine months of pregnancy and reduced birth weight. However, no association was evident between increased CO exposure and reduced birth weight among the observed population.
IUGR refers to infants who fail to achieve their in utero growth potential (Resnik, 2002). It is important to identify fetuses with IUGR due to their increased perinatal mortality rates, which can be in excess of six to ten times that of normal growing peers. The risk of poor developmental outcomes extends beyond the perinatal period, as children born with IUGR demonstrate below average neurodevelopmental outcomes that persist through later childhood and into adolescence (Bernstein et al., 2000). A number of studies have shown an association between PM exposure during pregnancy and intrauterine growth restriction (IUGR). Parker et al. (Parker et al., 2005) showed that pregnant women who reside in areas with the highest PM2.5 concentrations delivered infants with lower birth weights (approximately 30 g less) than age-matched peers exposed to low-levels of PM. Despite inherent limitations to this study, including the failure to account for maternal exposure to PM outside the primary residence, this work suggests a deleterious effect of PM exposure on the developing fetus. Additional work by Chen et al. (Chen et al., 2000) showed that a 10 µg/m3 increase in mean PM10 exposure during the third trimester of pregnancy was associated with a decrease in BW of 11 g (95% C.I, 2.3–19.8). Dejmek et al. (Dejmek et al., 1999) conducted a study in an extremely polluted area of the Northern Bohemian region of the Czech Republic that assessed correlations between the impact of PM10 exposure and development of IUGR. This study suggested that exposure to elevated levels of PM2.5 during the early stages of pregnancy may cause negative fetal growth effects, including IUGR. Salam et al. (Salam et al., 2005) investigated the relationship between CO exposure during the first trimester and O3 exposure during the third trimester. They proposed that a 1.4 ppm and 12 ppb increase in exposure, respectively, to these pollutants resulted in a 20% increased risk of IUGR compared to normal exposure levels.
Long-term exposure to particulate air pollution has been associated with increased inflammatory states in humans. A 3.91 µg/m3 cross-sectional exposure increase in PM2.5 was associated with increases in high sensitivity C-reactive protein (CRP) and fibrinogen levels, marking systemic inflammation (Hertel et al., 2010). Similarly, Lee et al. (Lee et al., 2011) demonstrated that increased exposure to PM10, PM2.5, and ozone was correlated with an increase in CRP levels during the early stages of pregnancy. Specifically, a 4.6 µg/m3 increase in PM2.5 was associated with an odds ratio of 1.47. Heightened inflammatory states in pregnant mothers are associated with adverse fetal and neonatal outcomes, including intrauterine death, premature delivery, fetal neurological injury, and neonatal sepsis (Goldenberg et al., 2005). Recent evidence suggests that PM exposure during pregnancy may contribute to pro-inflammatory states in exposed mothers, with adverse downstream effects on the developing fetus in utero (Elovitz and Wang, 2004). Numerous studies cite heightened inflammatory states in the fetus as the cause of worse neurodevelopmental outcomes; specifically, increased serum IL-6 levels have repeatedly been linked to deleterious health outcomes, including an increased risk for cerebral palsy and other adverse neurodevelopmental outcomes (Adams-Chapman and Stoll, 2006; Gomez et al., 1998; Nelson et al., 2003; Romero et al., 1998; Wu et al., 2009b). The basis for this fetal response may be the transfer of maternal cytokines, such as IL-6, across the placenta. However, other possible causes may include altered placental vascular function and inhibition of placental oxygen transfer due to competition from elevated CO exposure (Bell et al., 2007).
These findings are consistent with the growing body of literature suggesting that even minor alterations in placental morphology can dramatically impact fetal embryogenesis, manifesting as IUGR, with the potential for dramatic short- and long-term negative effects on infant morbidity and mortality (Grafe, 1994). Future research efforts are needed to better clarify the potential role of PM exposure during pregnancy on maternal and fetal systemic inflammatory responses and the impact on brain injury in the newborn.
Infant Mortality
The association between ambient air pollution exposure during pregnancy and infant mortality is well documented (Bobak, 2000; Woodruff et al., 1997). A landmark study by Woodruff et al. (Woodruff et al., 1997) separated levels of PM10 exposure among pregnant mothers into three classifications: high, medium, and low levels. After controlling for demographic and environmental factors, the study showed a 10% increase in infant mortality among pregnant mothers in the high PM10 exposure group (OR 1.10, and 95% 1.04, 1.16) compared to low PM exposure. Additional work by Loomis et al. (Loomis et al., 1999) found that a 10 µg/m3 increase in the average level of PM2.5 during the 3–5 days preceding death was associated with a 6.9% increase in infant death. Additionally, a study conducted in Mexico City between 1997 and 2005 demonstrated that a 38.7 µg/m3 increase in ambient PM10 levels led to slightly increased respiratory-related infant mortality, while demonstrating that an increase in infant mortality due to increased PM10 exposure only affected infants with medium to low socioeconomic status (Carbajal-Arroyo et al., 2011). Studies that additionally link health outcomes with socioeconomic status may prove useful in creating a platform for legislative efforts aimed to ameliorate these disparities and ultimately increase overall public health.
Preterm Birth and Stillbirth
Preterm births comprise up to 75% of neonatal morbidity and 70% of neonatal deaths, with surviving infants at risk for long-term neurodevelopmental delays, pulmonary dysfunction, and ophthalmologic disorders (Behrman, 2007). Previous work demonstrated that maternal exposure to increased PM10 concentrations during birth is associated with an increased risk for preterm birth. Specifically, for every 10 µg/m3 increase in PM10 concentration, the risk for preterm birth increased by 6–15% (Huynh et al., 2006). A subsequent study by Ritz et al. (Ritz et al., 2000) evaluated the effect of PM10 exposure on the incidence of preterm birth in 97,518 neonates in Southern California. This study found that for each 50 µg/ml increase in ambient PM concentration, there was a 20% increase in preterm birth. Wu et al. (Wu et al., 2009a) conducted a study of 81,186 singleton births in Southern California, using a line-source dispersion model to approximate individual exposure to air pollutants. They showed that women experienced a 128% increased risk in very preterm birth when subjected to the highest NOx exposure quartiles during pregnancy. Additionally, the incidence of preeclampsia rose by 33% when subjected to the highest exposure quartiles of NOx, and by 42% when subjected to the highest exposure quartiles of PM2.5 during pregnancy.
Suh et al. (Suh et al., 2008) evaluated the link between PM10 exposure and preterm birth in an effort to identify the role of genetic susceptibility to PM10 effects. The authors found that exposure to high levels of PM during the third trimester of pregnancy was associated with an increased risk for preterm birth, and this risk was magnified in the setting of an underlying glutathione (GSTM1) null genotype. This study suggests that individuals with underlying genetic predisposition to oxidative injury may be at an increased risk for preterm birth following ambient PM10 exposure, and that reducing PM10 exposure prior to the third trimester of pregnancy may result in a reduction in the incidence of preterm birth. Understanding the genetic polymorphisms that place a mother and fetus at increased risk for poor pregnancy outcomes following PM exposure will allow for better stratification of a mother’s risk, better surveillance of PM concentrations, and guide potential interventional strategies.
In addition to preterm birth, evidence exists linking exposure to particulate air pollution with stillbirth (Bobak, 2000; Faiz et al., 2012; Mavalankar et al., 1991). If fetal death occurs within 20 weeks of pregnancy, it is classified as a stillbirth, with the odds of this occurring ~1 in 160 (2007). Mishra et al. (Mishra et al., 2005) reported an association between pollution from indoor cooking with biofuels and incidence of stillbirth. The study specifically states women using biofuels to cook have a significantly greater chance of experiencing a stillbirth than women using cleaner sources of energy (OR= 1.44; 95% CI: 1.04, 1.97). A study in Taiwan revealed an association between stillbirth and other major constituents of air pollution such as SO2 and PM10 (Hwang et al., 2011). Hwang et al. (Hwang et al., 2011) specifically demonstrated that increased incidence of stillbirth was associated with a 1-ppb increase in SO2 exposure during the first trimester (adjusted OR = 1.02; 95% confidence interval (CI), 1.00–1.04) and a 10-µg/m3 increase in PM10 exposure during both the first and second gestational months (adjusted OR = 1.02; 95% CI, 1.00–1.05; adjusted OR = 1.02; 95% CI, 1.00–1.04).
Particulates generated from the use of biofuels in the home as a result of cooking in poorly ventilated conditions have similarly been associated with LBW. Mishra et al. (Mishra et al., 2004) demonstrated this correlation in a study of 3,559 childbirths in Zimbabwe between 1994 and 1999. On average, LBW was associated with mothers who used wood, dung, or straw to cook rather than natural gas and electricity. A similar study conducted in rural Guatemala indicated LBW is directly associated with domestic use of wood fuel in the home. Of the 1,717 women and newborn children included in the rural Guatemalan study, children with mothers using wood to cook on open fires had the lowest birth weight (2,819 g), followed by exposure to chimney stove cooking (intermediate low birth weight), while children born to mothers who used gas and electricity for cooking demonstrated the healthiest average birth weight (2,948 g) (Boy et al., 2002).
Animal Studies
The fetal effects of air pollution exposure can be evaluated through the use of animal models, as pregnant mice can be exposed to a known concentration of pollutant and the offspring can be examined. The time-course of PM exposure can also be manipulated, i.e. mice can be exposed prior to pregnancy, during pregnancy, or the resulting progeny can be exposed as neonates. In addition, animals can be exposed to known concentrations of specific components of air pollution such as PM10, PM2.5, and PM0.1, either alone or in combination.
Results from animal studies have recapitulated the outcomes of epidemiological studies. Exposure of female mice to various concentrations of diesel exhaust during the four months prior to pregnancy resulted in decreased body weight of the offspring, increased abortion rates, abnormal development of female offspring, and adverse nesting behaviors (Tsukue et al., 2002). In a model of endometriosis induced pre- and post-natally, lesions were increased in rats exposed to diesel exhaust as compared to controls (Umezawa et al., 2008). In addition, placental morphology has been shown to change with PM exposure, as mice exposed to PM prior to and during pregnancy had increased fetal capillary proliferation and decreased maternal blood spaces, perhaps explaining the cause of low birth weights (Damaceno-Rodrigues et al., 2009; Veras et al., 2008).
Animal exposure studies have also revealed pathology that is difficult to measure in humans. The effects of maternal exposure on the nervous system of animals have been evidenced by reduced locomotion and dopamine turnover in mice maternally exposed to PM (Yokota et al., 2009), as well as altered sleep patterns in rats maternally exposed to ozone (Haro and Paz, 1993). However, the latter study was challenged by an additional study which showed that maternal ozone exposure did not change any endpoints of behavior in the offspring (Petruzzi et al., 1994). Genetic mutations have been shown in several studies, whereby pregnant mice exposed to DEP orally had an increase in DNA deletions of the offspring (Reliene et al., 2005). These results have also been challenged, as other biomarkers and cognitive tests were unaltered in mice prenatally exposed to DEP (Hougaard et al., 2008). An altered cardiovascular phenotype from gestational exposure to PM has been shown, as oxidative stress markers in mice exposed prenatally to PM were increased (Damaceno-Rodrigues et al., 2009).
Overall, examination of animals exposed to PM in utero has been limited. Future studies should examine the effects of maternal PM exposure on different organ systems, as well as examine the mechanisms involved in the transfer of pollutants to the fetus. Oxidative stress is a potential mechanism of the fetal insult resulting from pollutant exposure, but current research is severely limited. Studies have only been able to associate increased oxidative stress in utero in response to pollutant exposure (Damaceno-Rodrigues et al., 2009), or associate oxidative markers with an altered fetal measures (Rossner et al., 2011). Strong links between air pollution and oxidative stress, and how this causes adverse fetal outcomes needs to be examined before conclusions can be drawn. In addition, specific components of air pollution can be isolated, which has been done in certain studies, revealing that manganese sulfate concentrations are increased in the fetus following maternal inhalation (Dorman et al., 2005), and that maternal inhalation of sulfur dioxide affects aggression of the young (Fiore et al., 1998). Overall, animal studies have not only reaffirmed human epidemiological data, they have also suggested changes in placental morphology and the nervous system, while genetic mutations following PM exposure have been demonstrated by other studies.
Conclusion
In response to the growing body of literature suggesting an association between PM exposure and negative health outcomes, the Environmental Protection Agency has developed a series of ambient air monitoring sites with mass and chemical speciation measurement capabilities. These monitoring sites provide information on regional differences in PM mass and concentration, as well as the impact of potential effect modifiers (weather, size, composition). These monitoring sites also provide valuable opportunities for scientists and legislators to measure and quantify PM concentration, serving as a stimulus for potential areas of intervention in cities failing to comply with federal standards for PM concentration.
Studies exploring the health effects of air pollution, specifically PM, are difficult to summarize since measurement techniques and definitions have changed over time. Additionally, the harmfulness of PM of equal size depends on its chemical composition, defined as the combination of chemical agents present in PM. Additional difficulty in examining the effects of air pollution arises with the large amount of confounding factors that can influence the type and amount of air pollution exposure, as well as fetal outcomes. Considering that the studies mentioned above originate from different geographic regions with various PM sources, composition, average levels, ranges, and co-pollutant exposures, as well as in mothers with different economic states, other health concerns and different ages, there appears to be a consistent, albeit small, association between PM exposure and adverse health outcomes. However, when this small association is evaluated at a population level, the health impact could be substantial.
Despite a growing body of epidemiological evidence suggesting an association between PM and adverse health consequences in the young, conclusions about mechanisms must await additional research efforts. Specifically, representative animal studies are needed to clarify the following: 1) establishment of early biomarkers of PM toxicity, both in utero and ex vivo; 2) investigate potential interactions between PM components and other determinants of air pollution (i.e. investigate for possible synergistic effects of PM with other air pollutants); 3) better define the range of concentrations, including the spatial and temporal variability of PM throughout the US, in an effort to establish regional and local air quality standards. These studies will help our understanding of the relationship between the environment and some of the most susceptible individuals, children.
Acknowledgments
Sources of Funding
This manuscript was supported in part by a Scientific Development Grant from the American Heart Association (#0835298N) and the National Institutes of Health (#R01ES019923-01 and R01ES019923-01S1) to LEW.
List of Acronyms
- AOR
adjusted odds ratio
- DEP
diesel exhaust particles
- CRP
C-reactive protein
- GST1
glutathione S-transferase
- IL-6
interleukin-6
- IUGR
intrauterine growth restriction
- LBW
low birth weight
- NOx
oxides of nitrogen
- OR
odds ratio
- PM
particulate matter
- PM0.1
ultrafine particles, diameter <0.1 µm
- PM2.5
fine particles, diameter <2.5 µm
- PM10
coarse particles, diameter 2.5 –10 µm
- RR
adjusted risk ratio
- SES
socioeconomic status
- SOX
oxides of sulfur
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACOG Committee Opinion No. 383: Evaluation of stillbirths and neonatal deaths. Obstet Gynecol. 2007;110:963–966. doi: 10.1097/01.AOG.0000263934.51252.e0. [DOI] [PubMed] [Google Scholar]
- Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19:290–297. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- Agency E. National Summary of Particulate Matter Emission. 2005 http://wwwepagov/air/emissioncom.
- Agency EP. National Ambient air quality standards for particulate matter: proposed rule. Fed Regist. 2006;71:2620–2708. [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Behrman R. Preterm Birth: Causes, Consequences, and Prevention. 2007;1:1–67. [PubMed] [Google Scholar]
- Bell ML, Belanger K, Ebisu K, Gent JF, Lee HJ, Koutrakis P, Leaderer BP. Prenatal Exposure to Fine Particulate Matter and Birth Weight. Epidemiology. 2010a;21:884–891. doi: 10.1097/EDE.0b013e3181f2f405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K, Ebisu K, Gent JF, Lee HJ, Koutrakis P, Leaderer BP. Prenatal exposure to fine particulate matter and birth weight: variations by particulate constituents and sources. Epidemiology. 2010b;21:884–891. doi: 10.1097/EDE.0b013e3181f2f405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein I, Horbar J, Badger G, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am J Obstet Gynecol. 2000;182:198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environmental Health Perspective. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012 doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- Boy E, Bruce N, Delgado H. Birth weight and exposure to kitchen wood smoke during pregnancy in rural Guatemala. Environ Health Perspect. 2002;110:109–114. doi: 10.1289/ehp.02110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of ambient particulate air pollution. Clin Sci (Lond) 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Casicio W. Air pollution and cardivascular disease: a statment for healthcare professionals from the expert panel on population and prevention science of the american heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Burton A. Children's health: methylation links prenatal PAH exposure to asthma. Environ Health Perspect. 2009;117:A195. doi: 10.1289/ehp.117-a195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal-Arroyo L, Miranda-Soberanis V, Medina-Ramon M, Rojas-Bracho L, Tzintzun G, Solis-Gutierrez P, Mendez-Ramirez I, Schwartz J, Romieu I. Effect of PM(10) and O(3) on infant mortality among residents in the Mexico City Metropolitan Area: a case-crossover analysis, 1997–2005. J Epidemiol Community Health. 2011;65:715–721. doi: 10.1136/jech.2009.101212. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang W, Jennison B, Goodrich A, Omaye S. Air pollution and birth weight in Northern Nevada. Inhalation toxicology. 2000;14:141–157. doi: 10.1080/089583701753403962. [DOI] [PubMed] [Google Scholar]
- Choi H, Wang L, Lin X, Spengler JD, Perera FP. Fetal window of vulnerability to airborne polycyclic aromatic hydrocarbons on proportional intrauterine growth restriction. PLoS One. 2012;7:e35464. doi: 10.1371/journal.pone.0035464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaceno-Rodrigues NR, Veras MM, Negri EM, Zanchi A, Rhoden CR, Saldiva P, Dolhnikoff M, Caldini EG. Effect of pre- and postnatal exposure to urban air pollution on myocardial lipid peroxidation levels in adult mice. Inhal Toxicol. 2009;21:1129–1137. doi: 10.3109/08958370902798430. [DOI] [PubMed] [Google Scholar]
- Dejmek J, Selevan SG, Benes I, Solansky I, Sram RJ. Fetal growth and maternal exposure to particulate matter during pregnancy. Environ Health Perspect. 1999;107:475–480. doi: 10.1289/ehp.99107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, McElveen AM, Marshall MW, Parkinson CU, James RA, Struve MF, Wong BA. Tissue Manganese Concentrations in Lactating Rats and Their Offspring Following Combined in Utero and Lactation Exposure to Inhaled Manganese Sulfate. Toxicol Sci. 2005;84:12–21. doi: 10.1093/toxsci/kfi060. [DOI] [PubMed] [Google Scholar]
- Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- Faiz AS, Rhoads GG, Demissie K, Kruse L, Lin Y, Rich DQ. Ambient air pollution and the risk of stillbirth. Am J Epidemiol. 2012;176:308–316. doi: 10.1093/aje/kws029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Petruzzi S, Dell'Omo G, Alleva E. Prenatal SulfurDioxide Exposure Induces Changes in the Behavior of Adult Male Mice During Agonistic Encounters. Neurotoxicol Teratol. 1998;20:543–548. doi: 10.1016/s0892-0362(98)00003-8. [DOI] [PubMed] [Google Scholar]
- Glinianiaia S, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and pregnancy outcomes: a review of the literature. Epidemiology. 2004;15:36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32:523–559. doi: 10.1016/j.clp.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Romero R, Ghezzi F, Yoon B, Mazor M, Berry S. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- Grafe M. The correlation of prenatal brain damage with placental pathology. J Neuropathol Exp Neurol. 1994;53:407–415. doi: 10.1097/00005072-199407000-00013. [DOI] [PubMed] [Google Scholar]
- Haro R, Paz C. Effects of ozone exposure during pregnancy on ontogeny of sleep in rats. Neurosci Lett. 1993;164:67–70. doi: 10.1016/0304-3940(93)90859-j. [DOI] [PubMed] [Google Scholar]
- Hertel S, Viehmann A, Moebus S, Mann K, Brocker-Preuss M, Mohlenkamp S, Nonnemacher M, Erbel R, Jakobs H, Memmesheimer M, Jockel KH, Hoffmann B. Influence of short-term exposure to ultrafine and fine particles on systemic inflammation. Eur J Epidemiol. 2010;25:581–592. doi: 10.1007/s10654-010-9477-x. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, Wallin H. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Particle and Fibre Toxicology. 2008;5 doi: 10.1186/1743-8977-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M, Woodruff TJ, Parker JD, Schoendorf KC. Relationsips between air pollution and preterm birth in California. Paediatr Perinat Epidemiol. 2006;20:454–461. doi: 10.1111/j.1365-3016.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- Hwang BF, Lee YL, Jaakkola JJ. Air pollution and stillbirth: a population-based case-control study in Taiwan. Environ Health Perspect. 2011;119:1345–1349. doi: 10.1289/ehp.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated Risk for Altered Fetal Growth Resulting from Exposure to Fine Particles during Pregnancy: An Epidemiologic Prospective Cohort Study in Poland. Environ Health Perspect. 2004;112:1398–1402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasana M, Esplugues A, Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. Eur J Epidemiol. 2005;20:183–199. doi: 10.1007/s10654-004-3005-9. [DOI] [PubMed] [Google Scholar]
- Lee BE, Ha EH, Park HS, Kim YJ, Hong YC, Kim H, Lee JT. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Hum Reprod. 2003;18:638–643. doi: 10.1093/humrep/deg102. [DOI] [PubMed] [Google Scholar]
- Lee PC, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011;22:524–531. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Castillejos M, Gold DR, McDonnell W, Borja-Aburto VH. Air pollution and infant mortality in Mexico City. Epidemiology. 1999;10:118–123. [PubMed] [Google Scholar]
- Masih A, Saini R, Singhvi R, Taneja A. Concentrations, sources, and exposure profiles of polycyclic aromatic hydrocarbons (PAHs) in particulate matter (PM(10)) in the north central part of India. Environ Monit Assess. 2010;163:421–431. doi: 10.1007/s10661-009-0846-4. [DOI] [PubMed] [Google Scholar]
- Mavalankar DV, Trivedi CR, Gray RH. Levels and risk factors for perinatal mortality in Ahmedabad, India. Bull World Health Organ. 1991;69:435–442. [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Dai X, Smith KR, Mika L. Maternal exposure to biomass smoke and reduced birth weight in Zimbabwe. Ann Epidemiol. 2004;14:740–747. doi: 10.1016/j.annepidem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Mishra V, Retherford RD, Smith KR. Cooking smoke and tobacco smoke as risk factors for stillbirth. Int J Environ Health Res. 2005;15:397–410. doi: 10.1080/09603120500288913. [DOI] [PubMed] [Google Scholar]
- Nelin TD, Joseph AM, Gorr MW, Wold LE. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett. 2012;208:293–299. doi: 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K, Grether J, Dambrosia J, Walsh E, Kohler S, Satyanarayana G. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–607. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- Pandya R, Solomon G, Kinner A, Balmes J. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ Health Perspect. 2002;110:103–112. doi: 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ, Basu R, Shoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–128. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- Petruzzi S, Musi B, Bignami G. Acute and chronic sulphur dioxide (SO2) exposure: an overview of its effects on humans and laboratory animals. Annali-Istituto Superiore Di Sanita. 1994;30:151. [PubMed] [Google Scholar]
- Pope C. Epidemiology of fine particulate air pollution and mortality: effects by cause, age, and socioeconomic status. Journal of Epidemiology Community Health. 2000;108:713–723. doi: 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thurston GD. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. Journal of Air Waste Management. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Reliene R, Hlavacova A, Mahadevan B, Baird WM, Schiestl RH. Diesel exhaust particles cause increased levels of DNA deletions after transplacental exposure in mice. Mutat Res Lett. 2005;570:245–252. doi: 10.1016/j.mrfmmm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F, Chapa C, Fruin S. Effect of Air Pollution on Preterm Birth Among Children Born in Southern California Between 1989 and 1993. Epidemiology. 2000;11:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Rocha ESIR, Lichtenfels AJ, Amador Pereira LA, Saldiva PH. Effects of ambient levels of air pollution generated by traffic on birth and placental weights in mice. Fertil Steril. 2008;90:1921–1924. doi: 10.1016/j.fertnstert.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Romero R, Gomez R, Ghezzi F, Yoon B, Mazor M, Edwin S. A fetal system inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- Rossner P, Jr, Tabashidze N, Dostal M, Novakova Z, Chvatalova I, Spatova M, Sram RJ. Genetic, biochemical, and environmental factors associated with pregnancy outcomes in newborns from the Czech Republic. Environ Health Perspect. 2011;119:265–271. doi: 10.1289/ehp.1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra CB, Williams MA, Sheppard L, Koenig JQ, Schiff MA. Ambient carbon monoxide and fine particulate matter in relation to preeclampsia and preterm delivery in western Washington State. Environ Health Perspect. 2011;119:886–892. doi: 10.1289/ehp.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam MT, Millstein J, Li YF, Lurmann FW, Margolis HG, Gilliland FD. Birth Outcomes and Prenatal Exposure to Ozone, Carbon Monoxide, and Particulate Matter: Results from the Children’s Health Study. Environ Health Perspect. 2005;113:1638–1644. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K, Benitz W, Sunshine P. Fetal and neonatal brain injury: mechanisms, management, and the risks of brain injury. 3rd edition. 2003. pp. 30–58. [Google Scholar]
- Sun Q, Hong X, Wold LE. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. 2010;121:2755–2765. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jing X. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. Journal of American Medical Association. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Tsukue N, Tsubone H, Suzuki AK. Diesel exhaust affects the abnormal delivery in pregnant mice and the growth of their young. Inhal Toxicol. 2002;14:635–651. doi: 10.1080/08958370290084548. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Sakata C, Tabata M, Tanaka N, Kudo S, Takeda K, Ihara T, Sugamata M. Diesel Exhaust Exposure Enhances the Persistence of Endometriosis Model in Rats. J Health Sci. 2008;54:503–507. [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Caldini EG, Ribeiro A, Mayhew TM, Saldiva P, Dolhnikoff M. Particulate Urban Air Pollution Affects the Functional Morphology of Mouse Placenta. Biol Reprod. 2008;79:578–584. doi: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. Residential proximity to traffic and adverse birth outcomes in Los Angeles county, California, 1994–1996. Environ Health Perspect. 2003;111:207–216. doi: 10.1289/ehp.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T, Grillo J, Schoendorf K. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ Health Perspective. 1997;105 doi: 10.1289/ehp.97105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, Ritz B. Association between Local Traffic-Generated Air Pollution and Preeclampsia and Preterm Delivery in the South Coast Air Basin of California. Environ Health Perspect. 2009a;117:1773–1779. doi: 10.1289/ehp.0800334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Croen LA, Torres AR, Van De Water J, Grether JK, Hsu NN. Interleukin-6 genotype and risk for cerebral palsy in term and near-term infants. Ann Neurol. 2009b;66:663–670. doi: 10.1002/ana.21766. [DOI] [PubMed] [Google Scholar]
- Yokota S, Mizuo K, Moriya N, Osiho S, Sugawara I, Takeda K. Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neurosci Lett. 2009;449:38–41. doi: 10.1016/j.neulet.2008.09.085. [DOI] [PubMed] [Google Scholar]