Abstract
Aim
Yttrium-90 (90Y) radioembolization is a microembolic procedure. Hence, it is commonly used in hepatocellular carcinoma (HCC) patients with portal venous thrombosis (PVT). We analyzed liver function, imaging findings and treatment options (local/systemic) at disease progression following 90Y in HCC patients with PVT.
Methods
We treated 291 HCC patients with 90Y radioembolization. From this cohort, we included patients with liver-only disease, PVT and Child-Pugh (CP) score ≤7; this identified 63 patients with HCC and PVT (CP-A:35, CP-B7:27). Liver function, CP status and imaging findings at progression were determined in order to assess potential candidacy for systemic treatment/clinical trials. Survival, time-to-progression (TTP) and time-to-hepatic decompensation were performed using Kaplan-Meier methodology.
Results
Of 35 CP-A and 28 CP-B7 patients, 29 and 15 progressed, respectively. Median survival and TTP were 13.8 and 5.6 months in CP-A and 6.5 and 4.9 months in CP-B7 patients, respectively. Of the 29 CP-A patients who progressed, 45% maintained their CP status at progression (55% decompensated to CP-B). Of the 15 CP-B7 patients who progressed, 20% improved to CP-A, 20% maintained their CP score and 60% decompensated.
Conclusion
Knowledge of liver function and CP score of HCC with PVT progressing after 90Y is critically relevant information as these patients may be considered for systemic therapy/clinical trials. If strict CP-A status is mandated, our study demonstrated that 64% exhibited inadequate liver function and were ineligible for systemic therapy/clinical trials. An adjuvant approach using local therapy and systemic agents prior to progression should be investigated.
Keywords: hepatocellular carcinoma, portal venous thrombosis, progressive disease, yttrium-90, liver function
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 6th most common malignancy diagnosed world-wide.[1] Its incidence is increasing, and it is the 3rd most common cause of cancer-related mortality.[2] Late stage presentation, co-morbidities, and limited donor availability enables only 10% of patients to receive curative therapies.[3] 90Y radioembolization plays an important palliative role in the management of HCC by inducing tumor necrosis and delaying progression.[4–10]
One of the common indications for 90Y that has emerged since its introduction is HCC in the presence of portal venous thrombosis (PVT). This is a clinically relevant scenario, as 26–35% of HCC patients demonstrate vascular invasion at transplantation.[11] 90Y is a microembolic procedure and causes minimal occlusion of hepatic arteries; hence, it can be safely used in the setting of PVT without compromising blood flow to the hepatic parenchyma.[12] A recent study has suggested that the presence of PVT significantly increases the chances of extrahepatic spread of the tumor and decreases overall survival.[13] Assessing outcomes in this situation is therefore important; treating underlying PVT may theoretically translate into a lower rate of extrahepatic metastases. Unfortunately, despite promising response rates in PVT patients treated with 90Y, most patients will progress. This setting of disease progression represents a challenging clinical scenario as treatment options are scarce. The role of systemic agents was historically limited until two randomized trials demonstrated survival benefit with sorafenib.[14, 15] Both of these studies enrolled patients with advanced HCC who were ineligible for or had progressed on previous surgical/locoregional therapies. Interestingly, >95% of patients in these studies had Child-Pugh (CP) class A liver function. These trials led a consensus panel to recognize sorafenib as a standard-of-care for patients progressing on locoregional therapy (LRT).[16, 17]
The primary purpose of this study was to assess clinical/imaging progression of HCC patients with PVT treated with 90Y (Barcelona Clinic Liver Cancer [BCLC] C disease), as well as to investigate their candidacy for systemic treatment at disease progression. This analysis is of relevance since it explores the commonly-stated (but suboptimally studied) notion that patients with PVT may receive a LRT (in this case 90Y) followed by systemic agents at progression. This concept assumes that patient liver function (and CP score) at progression will be sufficiently maintained to permit use of systemic agents which, by 3 major consensus guidelines, should be reserved for CP-A patients.[16, 18, 19]
METHODS
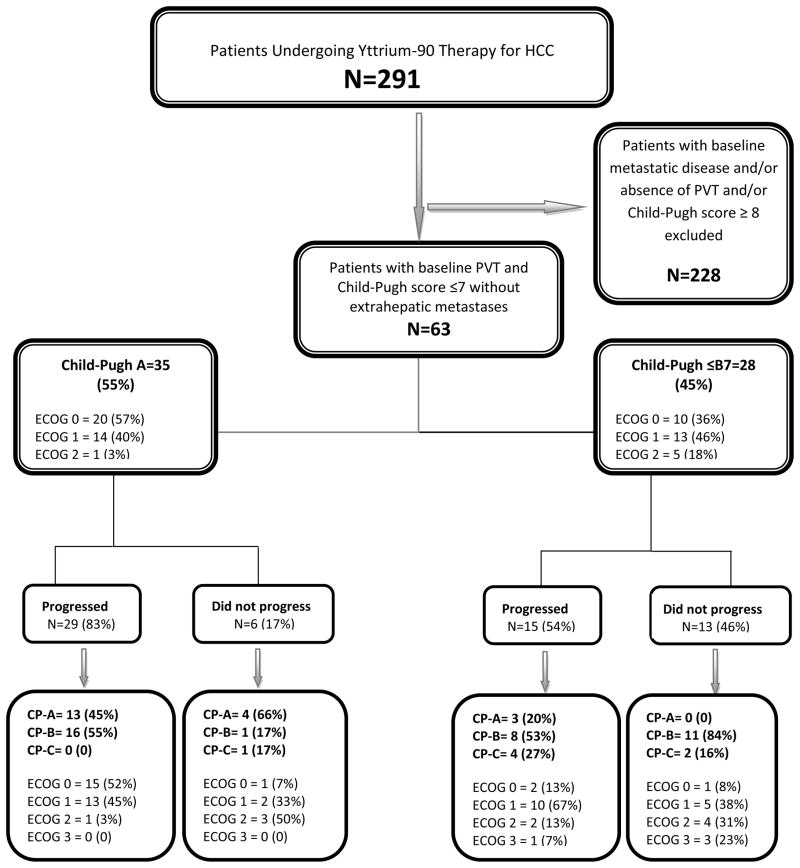
This analysis was approved by the Northwestern University Institutional Review Board and is Health Insurance Portability and Accountability Act complaint. The study is a subset analysis of a 291-patient cohort of consecutive HCC patients who were treated with 90Y radioembolization at our institution.[5] Patients were considered candidates for 90Y if they exhibited unresectable HCC (determined by transplant surgery) and bilirubin <3.0 mg/dL. For the purpose of isolating the appropriate cohort, we excluded patients who: 1) did not exhibit PVT, 2) demonstrated extrahepatic metastases, and 3) had a CP score of ≥8 (Figure 1: Study flow chart). We excluded patients with ≥CP-B8 and extrahepatic disease in order to reduce the confounding effect of liver function and extrahepatic metastases on survival, respectively. This resulted in the identification of a homogeneous 63 patient cohort who had PVT at baseline and preserved liver function (≤CP-B7). The patient population was further subdivided into two groups on the basis of CP status (CP-A and CP-B7). CP-B7 patients were included since this is a commonly used cut-off for clinical trials in HCC. Although patients were initially enrolled between January 2004 and December 2008, they have since then been prospectively followed and imaging findings updated; imaging/survival data were closed on December 28, 2011 to report mature outcomes. The data reported herein comply with research reporting standards for 90Y.[20]
Figure 1.
Evaluation and Staging
Diagnostic criteria for HCC included biopsy or radiographic findings as defined by guidelines.[16] Enhancing portal vein thrombus (on imaging) associated with an HCC was deemed to represent malignant vascular invasion. Baseline staging was performed by CP (liver function), United Network for Organ Sharing (UNOS TNM) (tumor size/number) and BCLC classification systems (composite of liver function, tumor size/number and symptoms). The decision to treat with 90Y was consensus-based during weekly HCC conference represented by medical oncology, hepatology, transplant surgery and interventional radiology.
90Y Radioembolization
Pre-treatment mesenteric angiography and technetium-99m macroaggregated albumin scans were performed to assess gastrointestinal flow and lung shunting.[21] Bilobar disease was treated on a lobar basis at 30-day intervals. Patients were discharged 2–6 hours after treatment without the need for hospitalization per published standards.[21] 90Y radioembolization was performed with glass-based microspheres (Nordion, Canada). 38 of 63 received >1 90Y treatments (2 treatments: N=23, 3 treatments N=12, 4 treatments: N=2, 5 treatments: N=1).
Analysis of Progression
Patients were followed using cross-sectional imaging one month following treatment and at scheduled 2–3 month intervals on protocol. The imaging modality remained consistent for each patient. The scans were analyzed on the basis of World Health Organization (WHO) size and European Association for Study of the Liver (EASL) necrosis criteria (Supplementary Table 1). [7, 14, 22–25] Scans were analyzed for different patterns of progression and observation of any of the following constituted time-to-progression (TTP) endpoints: 1) WHO progressive disease (PD) of target/index lesion, 2) EASL PD of target/index lesion, 3) development of new lesions, 4) development of extrahepatic metastases and, 5) progression of pre-existing PVT length and width. PVT progression was declared when any of these findings were observed: 1) dramatic PVT increase in length and/or width or, 2) extension in the branch order of PVT involvement (ex: extension from segmental to lobar or lobar to main PVT). The date of progression was determined on the basis of earliest scan demonstrating progression by any of the patterns cited above, even if this required histologic confirmation at a later date (e.g. biopsy confirmation of new lung metastasis). Patterns of progression were also analyzed without a time limit, thereby incorporating any progression in post-90Y period until the end of follow-up or death. Patients could exhibit progression by more than 1 pattern listed above; all were captured and reported.
Clinical staging at progression
Physical examination, laboratory reports and follow-up scans were analyzed at the time of imaging progression to determine CP score. Laboratory tests coincided with imaging (+/− 2 days). This analysis would permit assessment of: 1) patient suitability for systemic agents/clinical trials on the basis of liver function and, 2) long-term effect of 90Y on liver function compared to baseline. For patients who did not progress, the analysis was performed on the day of last follow-up. Performance status was also assessed at the time of progression.
Imaging and Survival Analysis
Radiologic response was assessed by WHO and EASL guidelines and response in PVT, separately for CP-A and CP-B7 patients. Median survival (from date of first treatment) and TTP were also analyzed separately for CP-A and CP-B7 patients. Survival from the date of progression was also determined; this information is of relevance since it approximates survival in the advanced and progressive HCC setting following progression. TTP was defined as time from first 90Y to the scan demonstrating any method of progression described above. Median imaging follow-up from last scan to death/end-of-follow up was calculated. Time-to-hepatic decompensation (TTHD), defined as an increase from any baseline CP class to >B7, was also performed.
At the time of closure of date, 61 out 63 patients had died. The two patients alive at the time of data closure were both CP-A.
Statistics
Data were summarized using descriptive statistics (median and range for continuous variables; count and frequency for categorical variables). Median survival, TTP, TTHD were determined using Kaplan-Meier methodology[26]. Statistical analyses were performed using MedCalc for Windows, version 9.5.0.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
Patient Sample and Baseline Characteristics
Table 1 summarizes the baseline patient demographics. 35 (55%) patients were CP-A and 28 (45%) were CP-B7. Median age was 65 years. Most patients were performance status 0 (48%), male (75%), treatment naïve (90%), exhibited bilobar (63%) and multifocal disease (87%); median size of the index lesion was 7.8 cm. All patients were UNOS T4b and BCLC C. 34 and 29 patients exhibited lobar and main PVT respectively.
Table 1.
Baseline Patient Characteristics
| Characteristic N (%) | Patients (N=63) | |
|---|---|---|
| Age (years) | Median | 65 |
| Range | 34 – 89 | |
| Ethnic Group | Caucasian | 43 (68) |
| Asian | 7 (11) | |
| Hispanic | 4 (6) | |
| African-American | 9 (15) | |
| Gender | Male | 47 (75) |
| Female | 16 (25) | |
| Etiology | Alcohol | 12 (19) |
| HCV | 25 (39) | |
| HCV + Alcohol | 7 (11) | |
| HBV | 4 (6) | |
| NASH | 1 (2) | |
| HCV+HBV | 1 (2) | |
| Cryptogenic | 13 (21) | |
| Method of Diagnosis | Imaging | 30 (48) |
| Biopsy | 33 (52) | |
| Prior Failed Therapies | None | 57 (90) |
| Chemoembolization | 3 (5) | |
| Resection | 3 (5) | |
| Portal Hypertension | Present | 38 (60) |
| Absent | 25 (40) | |
| Ascites | Present | 2 (3) |
| Absent | 61 (97) | |
| Lobar Distribution | Unilobar | 23 (37) |
| Bilobar | 40 (63) | |
| Distribution | Solitary | 8 (13) |
| Multifocal | 55 (87) | |
| Largest Tumor Size (cm) | Median (Range) | 7.8 (1.5 – 21.2) |
| Tumor Burden | ≤25% | 43 (68) |
| 26–50% | 13 (21) | |
| >50% | 7 (11) | |
| AFP (ng/mL) | >200 | 34 (54) |
| ≤200 | 26 (41) | |
| N/A | 3 (5) | |
| ECOG | 0 | 30 (48) |
| 1 | 27 (43) | |
| 2 | 6 (9) | |
| Child-Pugh | A | 35 (55) |
| B7 | 28 (45) | |
Abbreviations: AFP: Alpha-fetoprotein; ECOG: Eastern Cooperative Oncology Group; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NASH: Nonalcoholic Steatohepatitis
Analysis of Progression
A total of 269 scans were analyzed (4.3/patient). The earliest manifestations of progression in the 35 CP-A patients were 26%, 23%, 29%, 31%, 26% and 26% by WHO PD, EASL PD, new lesions, metastases, and increase in PVT length and width, respectively. For the 28 CP-B7 patients, 14%, 14%, 21%, 14%, 36% and 36% demonstrated WHO PD, EASL PD, new lesions, metastases, and increase in PVT length and width, respectively (Table 2).
Table 2.
Analysis of Progression
| Pattern of Progression (first observation of progression) | ||
|---|---|---|
| N (%) | N (%) | |
| CP-A | CP-B7 | |
| WHO PD | 9 (26) | 4 (14) |
| EASL PD | 8 (23) | 4 (14) |
| New Lesions | 10 (29) | 6 (21) |
| Metastases | 11 (31) | 4 (14) |
| PVT Length | 9 (26) | 10 (36) |
| PVT Width | 9 (26) | 10 (36) |
| Pattern of Progression (progression observed at anytime) | ||
| N (%) | N (%) | |
| CP-A | CP-B7 | |
| WHO PD | 11 (31) | 6 (21) |
| EASL PD | 12 (34) | 6 (21) |
| New Lesions | 13 (37) | 9 (32) |
| Metastases | 13 (37) | 4 (14) |
| PVT Length | 13 (37) | 12 (43) |
| PVT Width | 13 (37) | 12 (43) |
Abbreviations: EASL: European Association for the Study of the Liver; PD: Progressive disease; WHO: World Health Organization, PVT: portal vein thrombosis
When patterns of progression were analyzed without any timeline, CP-A patients demonstrated WHO PD, EASL PD, new lesions, metastases, and increase in PVT length-width with frequencies of 31%, 34%, 37%, 37%, 37% and 37% respectively. Overall, 29 of 35 (83%) CP-A patients progressed after 90Y; 17% did not exhibit progression at the end of follow-up/death. Similarly, CP-B7 patients demonstrated WHO PD, EASL PD, new lesions, metastases, and increase in PVT length-width in 21%, 21%, 32%, 14%, 43% and 43%, respectively (Table 2). Overall, 15 of 28 (54%) CP-B7 patients progressed after 90Y; 46% did not exhibit progression at the end of follow-up/death.
Response, Survival, TTP and Imaging Follow-up
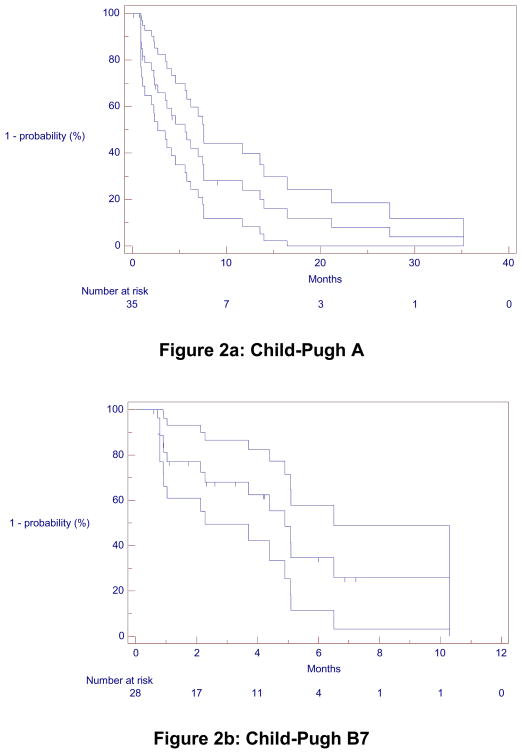
Table 3 summarizes response, survival, TTP and TTHD. For CP-A patients, response rate was 49%, 49% and 57% by WHO, EASL guidelines and PVT response, respectively. For CP-B7 patients, response rate was 25%, 32% and 36% by WHO, EASL guidelines and PVT response, respectively. For CP-A patients, survival was 13.8 (95% confidence interval [CI]: 9.0–18.8) months from treatment and 6.2 (CI: 4.0–11.5) months from date of progression. For CP-B7 patients, survival was 6.5 (CI: 5.0–7.5) months from treatment and 3.0 (CI: 1.8–4.8) months from date of progression. Survival was significantly higher for CP-A patients (p=0.0013).
Table 3.
Response, Survival, Time-to-progression and Time-to-hepatic decompensation
| CP-A | CP-B7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Response | |||||||||
| Category | CR | PR | SD | PD | CR | PR | SD | PD | |
| WHO | 0 (0) | 17 (49) | 13 (37) | 5 (14) | 0 (0) | 7 (25) | 18 (64) | 3 (11) | |
| EASL | 4 (12) | 13 (37) | 13 (37) | 5 (14) | 1 (3) | 8 (29) | 16 (57) | 3 (11) | |
| PVT | 1 (3) | 19 (54) | 9 (26) | 6 (17) | 0 (0) | 10 (36) | 11 (39) | 7 (25) | |
| Variable | N | Months (95% CI) | N | Months (95% CI) | |||||
| Overall Survival | |||||||||
| Median Survival from first treatment | 35 | 13.8 (9.0 – 18.8) | 28 | 6.5 (5.0 – 7.5) | |||||
| Median Survival from date of progression | 29 | 6.2 (4.0 – 11.5) | 15 | 3.6 (1.8 – 4.8) | |||||
| Survival Stratified by PVT Location | Lobar PVT | 19 | 15.7 (9.0 – 20.0) | 15 | 6.5 (5.2 – 8.0) | ||||
| Main PVT | 16 | 9.0 (5.0 – 14.0) | 13 | 5.8 (4.2 – 10.0) | |||||
| Time-to-Progression | |||||||||
| TTP from first treatment | 35 | 5.6 (3.5 – 7.5) | 28 | 4.9 (2.2 – 6.3) | |||||
| Time-to-Hepatic Decompensation | |||||||||
| CP-A (n=35) | 35.2 (6.0, not calculable) | ||||||||
| CP-B7 (n=28) | 4.2 (2.3 – 5.0) | ||||||||
| All (n=63) | 5.8 (4.8 – 11.0) | ||||||||
Abbreviations: CI: Confidence Interval; CP: Child-Pugh, CR: Complete Response; EASL: European Association for Study of the Liver Disease, PD: Progressive Disease, PR: Partial Response, PVT: Portal Venous Thrombosis, SD: Stable Disease, TTP: Time-to-progression, WHO: World Health Organization
When stratified by lobar or main PVT, survival for CP-A patients was 15.7 (CI: 9.0–20.0) and 9.0 (CI: 5.0–14.0) months, respectively. For CP-B7, it was 6.5 (CI: 5.2–8.0) and 5.8 (CI: 4.2–10.0) months, respectively.
For CP-A and CP-B, TTP was 5.6 (CI: 3.5–7.5) and 4.9 (CI: 2.2–6.3) months (Figure 2). Median interval between last scan and death/last follow-up for CP-A and CP-B7 patients was 3.7 (Inter-quartile range [IQR]: 1.8–11.6) and 3.2 (IQR: 1.4–4.3) months, respectively.
Figure 2.
Kaplan-Meier Time-to-Progression
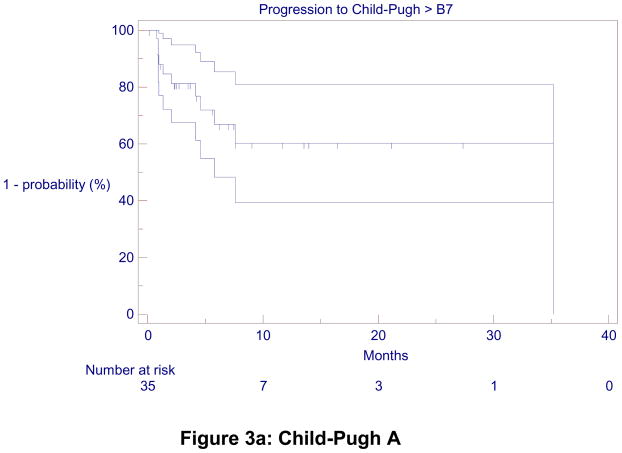
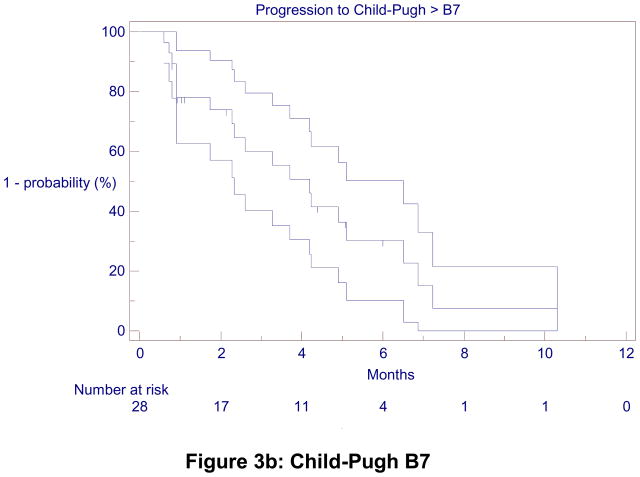
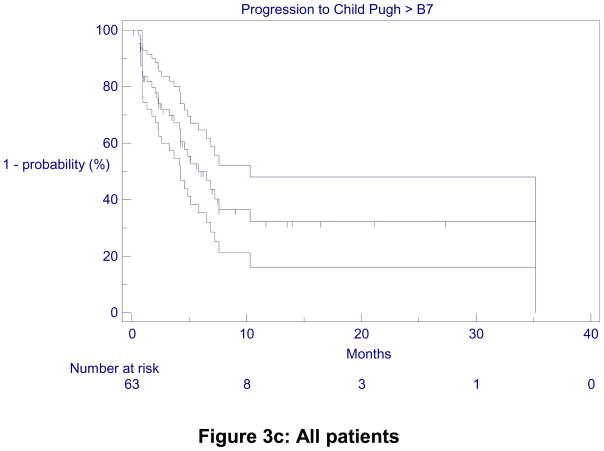
TTHD was 5.8 months (4.8 – 11.0) for the entire cohort. It was 35.2 (6.0, not calculable) months for CP-A patients, with a 1 and 2-year hepatic decompensation-free rate of 60%. TTHD was 4.2 (2.3 – 5.0) months for CP-B7 patients (Figure 3).
Figure 3.
Kaplan-Meier Time-to-Hepatic Decompensation
Clinical Stage at progression
Figure 1 (and Supplementary Table 2) summarizes the CP scores (and class) of patients at progression. For the 29 progressing patients CP-A at baseline, 45% maintained their CP stage and 55% decompensated to CP-B. For the 15 progressing patients that were CP-B7 at baseline, 20% improved to CP-A, 20% remained CP-B7; 13% and 20% decompensated to CP-B8 and CP-B9, respectively. 13%, 7% and 7% decompensated to CP-C10, CP-C11 and CP-C12, respectively. Thus, of all patients progressing after 90Y (29+15=44), 16 (36%) maintained their baseline CP class, 3 (7%) improved and 25 (57%) worsened. Moreover, of the 44 patients exhibiting imaging progression, 16 (36%) were CP-A, 24 were CP-B (55%) and 4 patients (9%) were CP-C at the time of progression.
Figure 1 (and Supplementary Table 2) also summarizes the CP status of patients who did not demonstrate progression at last follow-up. For 6 patients who were baseline CP-A, 4 remained in the same class and 1 each progressed to CP-B and C, respectively. For 13 patients who were CP-B7 at baseline, 2 remained CP-B7 while the remainder decompensated to higher CP scores. Overall, of the 19 patients who did not progress at last follow-up, 32% maintained their CP status while 68% worsened.
Table 4 also lists the performance status at progression as compared to baseline.
Clinical follow-up
Of the 44 patients who progressed, 25 (57%) did not receive any therapy after progression. 19 patients received alternate treatments including: repeat 90Y N=9; 90Y/RFA N=2; 90Y/bland embolization/RFA N=1; 90Y/bland embolization N=1; bland embolization N=1; chemotherapy N=1; clinical trial N=1; liver transplant N=1; Trans-arterial chemoembolization (TACE)/chemotherapy N=1; 90Y/bland embolization/TACE/sorafenib N=1.
DISCUSSION
HCC is a unique malignancy where the prognosis is not only affected by the tumor itself but also by underlying liver cirrhosis. Hence, the competing risks of death in HCC translate into a natural history of disease where patients succumb to either cirrhosis or the unique pattern of cancer progression. In this already complicated scenario, the development of PVT further increases risks and potentially reduces treatment options. The presence of PVT is a hallmark of advanced BCLC disease where systemic therapy is proposed for such patients. In recent years, a niche application for 90Y in this specific context has been investigated by many experienced centers; the idea has been to consider local 90Y radiotherapy given the intense cytotoxic effect and, at the time of progression, consider the use of cytostatic agents. Outcomes have been encouraging and have sparked the initiation of combinatorial clinical trials. However, one aspect of this approach that is under-studied (and hence under-reported) is the assumption that patients will have preserved liver functions and CP class permitting the use of systemic agents at progression. CP-A is a well-accepted and necessary liver function criterion for systemic treatments that was recommended in the 2008 research, 2011 American and 2012 European HCC guidelines.[16, 18, 19] The purpose of this study was therefore to assess the candidacy (by liver functions and CP class) of patients with PVT and HCC for systemic treatment and/or clinical trials at disease progression following 90Y, as well as to assess the patterns of progression in PVT patients.
Some interesting observations were derived from this study. First, a majority of patients (64%) with progressive disease by imaging following 90Y were not candidates for further clinical trials or systemic therapy by CP. Second, the survival difference was significantly different in this cohort when comparing CP-A and CP-B7. Third, the majority of patients (57%) had a worsening CP class at progression when compared to baseline. Finally, progressive disease could be more thoroughly documented in CP-A compared to CP-B7 patients; this is due to the inherently longer survival (and therefore follow-up) of CP-A patients.
In this cohort, 64% of patients at progression following radioembolization exhibited CP class B or C, precluding them from systemic agents/clinical trials. As a result, only a minority (36%) maintained CP-A status and could be offered systemic agents. These findings shed light on the effect of LRT and ongoing cirrhosis in HCC patients with PVT; the maintenance of liver functions does not appear to be indefinite. This suggests a time-limited “therapeutic-window” where systemic agents could be considered after LRTs but prior to disease progression.
Analysis of progression, TTP and survival did yield interesting findings. 37% of CP-A patients with HCC and PVT developed metastatic disease; this is consistent with recent reports of PVT representing a significant risk factor for the development of metastases.[13] The rate of development of metastatic disease was lower for CP-B7; this may be explained by shorter survival not permitting sufficient time for the identification of metastases by imaging. Although CP-A patient lived longer than CP-B, the stratification by lobar and main PVT was important. The location of PVT was more relevant in the CP-A patients, where lobar PVT patients demonstrated longer survival than main PVT. This was not the case with CP-B7, where both lobar and main PVT patients did poorly; survival in this case was likely mostly driven by liver (dys) function.
Another observation from our experience was the combined effect of progressive disease and 90Y radioembolization on the status of liver function. In patients who demonstrated progression, a majority (57%) had a worsening of their CP status, while 43% either maintained or improved their CP status. In patients who did not show disease progression, 32% maintained their CP status while 68% worsened. It is possible that the improvement in CP status may have resulted from treatment response and tumor size reduction; conversely, worsening CP status may also have resulted from tumor progression and/or toxicity from 90Y. The fisher’s exact test was performed in order to investigate the relationship between progression and maintenance/worsening of CP; this was not found to be significant (p=0.17). We do recognize that worsening of hepatic function is multifactorial and not singularly affected by tumor progression. While worsening hepatic function may not necessarily affect patients without tumor progression (no treatment needed), it may significantly limit treatment options for those patients who simultaneously demonstrate tumor progression. Furthermore, since BCLC guidelines support the use of LRTs in CP-A or B in intermediate disease, the feasibility of LRTs appears less dependent on liver function as compared to systemic therapies, where CP-A is mandated (or strongly encouraged).[16, 18, 19]
This study has helped further elucidate the current challenges in treatment of patients with advanced HCC. The general agreement is that when feasible, patients who progress on one form of therapy should be offered a different treatment option. In the case of disease progression after LRTs, patients should be offered systemic therapy (clinically or on-study). However, in our assessment, many patients progressing after 90Y also decompensate in terms of liver function are not eligible for systemic therapy. This challenges the concept of sequential treatment of LRTs following by systemic agents at progression and suggests alternate possibilities. First, combining a systemic therapy with LRTs at the time of initial LRT may be supported by potential synergies, such as compensating for growth factor stimulation following LRTs (such as chemoembolization). However, recent data (Sorafenib or Placebo in Combination with Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma: SPACE) presented did not seem to provide compelling support for this rationale. Alternatively, an adjuvant approach may be considered, where all planned cytotoxic LRTs are first completed to the entire treatment field and subsequently followed with systemic agents (cytostatic effect). Our data seem to support investigating this approach of using systemic agents at a time when liver functions are maintained as opposed to waiting for disease progression, where liver functions have decompensated. Current ongoing trials have in fact selected this approach when combining 90Y with sorafenib (sorafenib +/− 90Y: STOP-HCC [NCT01556490], SORAMIC [NCT01126645]). Finally, although crossover to alternate therapies is a common problem in HCC studies, our findings also support the feasibility of head-to-head studies comparing 90Y and systemic agents (in HCC with PVT) with minimal crossover, since, at the time of progression following 90Y, CP-A liver function is not often maintained, thereby limiting the ability to crossover to systemic therapy (sorafenib versus 90Y: SIRveNIB [NCT01135056], YES-p [NCT pending]).
There are strengths to the study. First, the analysis is novel and the concept of sequential LRTs and systemic agents is of relevance and a clinical reality, particularly as it relates to new trials. Second, the imaging analysis was comprehensive and mature, with acceptable time from last scan to death. Third, the study suggest that CP-B7 patients demonstrate lower survival following LRTs using this treatment option, supporting HCC research guidelines mandating CP-A status to minimize the confounding effect of liver function.[16, 18, 19] Fourth, a correlation between TTP and survival was not observed; this is of relevance since it challenges the concept of a TTP/survival correlation in HCC that has recently emerged in expert panel discussions. Factors other than preventing tumor progression affected survival; these potentially included background cirrhosis and/or treatment toxicity. Finally, in keeping with recent response assessment guidelines, we did incorporate worsening PVT in TTP; this was deemed essential in order to capture a TTP endpoint that would parallel clinical deterioration.[27] There are limitations. Although sample size was limited, this clinical condition is not common; further confirmatory reports will be required. Another limitation of this type of analysis involves the different clinical implications of “disease progression” when referring to LRTs versus systemic agents. Although a treated (and responding) lesion demonstrating new enhancement months/years after LRT may be deemed progressing, repeat LRT is rational in the proper clinical context (no new mets, acceptable liver functions). For the same clinical scenario where new tumoral enhancement is seen in a responding lesion while on systemic therapy, progression implies the need for a change in treatment. Hence, the term “disease progression” may have different clinical implications depending on the type of therapy received. Furthermore, the data suggest that treatment of HCC with PVT in CP-B stage patients should be prospectively investigated at centers with significant clinical experience in radioembolization. Finally, although it is recognized that multiple factors determine clinical trial/systemic drug eligibility (performance status [see Figure 1 ECOG scores], comorbidities, platelet count), our intent was to focus more on objective parameters limiting HCC clinical trial enrollment, such as liver function and CP class.
CONCLUSION
The majority of patients (64%) with HCC and PVT that progressed after 90Y were not deemed suitable for systemic treatments or clinical trials on the basis of liver function. The findings challenge the validity/feasibility of sequentially treating patients with LRTs following by systemic agents (clinically or on-study) at progression. In the scenario where LRTs combined with systemic agents are being considered, an adjuvant approach may be feasible, with the systemic treatment administered after LRT but before disease progression, when liver function is still maintained. This concept requires further investigation.
Supplementary Material
Acknowledgments
Role of Funding: There was no funding provided for this study. RS and RAO are supported in part by NIH grant CA126809.
Abbreviations
- BCLC
Barcelona Clinic Liver Cancer
- CI
95% confidence interval
- CP
Child-Pugh
- CT
Computed Tomography
- EASL
European Association for the Study of the Liver
- HCC
Hepatocellular Carcinoma
- LRT
Locoregional therapy
- MRI
Magnetic Resonance Imaging
- PD
Progressive Disease
- PVT
Portal venous thrombosis
- SIRveNIB
Phase III Multicenter Open-label Randomized Trial of Selective Internal Radiation Therapy versus Sorafenib in Locally Advanced Hepatocellular Carcinoma
- SORAMIC
Evaluation of Sorafenib in combination with local micro-therapy guided by Gd-EOB-DTPA enhanced MRI in patients with inoperable hepatocellular carcinoma
- SPACE
Sorafenib or Placebo in Combination with Transarterial Chemoembolization for Intermediate-Stage Hepatocellular Carcinoma
- STOP-HCC
Phase III Clinical Trial of Intra-arterial TheraSphere in the Treatment of Patients with Unresectable Hepatocellular Carcinoma
- TACE
Trans-arterial chemoembolization
- TTP
time-to-progression
- UNOS
United Network for Organ Sharing
- WHO
World Health Organization
- 90Y
Yttrium-90 radioembolization
- YES-p
a prospective randomized clinical trial of 90Y radioembolization vs sorafenib for the treatment of advanced HCC with portal vein thrombosis
Footnotes
Conflict of Interest: LK, RJL, MFM, ABB and RS are advisors to MDS Nordion. LK, ABB and RS are advisors to Onyx/Bayer. None of the other authors have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DMBF, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194–205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2011;140:497–507. e492. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 7.Riaz A, Miller FH, Kulik LM, Nikolaidis P, Yaghmai V, Lewandowski RJ, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303:1062–1069. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 9.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 10.Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792–800. doi: 10.1016/j.ijrobp.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 11.Davidson BR, Gibson M, Dick R, Burroughs A, Rolles K. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation. 1994;57:1174–1177. doi: 10.1097/00007890-199404270-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 13.Senthilnathan S, Memon K, Lewandowski RJ, Kulik L, Mulcahy MF, Riaz A, et al. Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: analyzing patterns of progression in 285 patients. Hepatology. 2012;55:1432–1442. doi: 10.1002/hep.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association For The Study Of The L European Organisation For R Treatment Of C. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem R, Thurston KG. Radioembolization with 90Yttrium Microspheres: A State-of-the-Art Brachytherapy Treatment for Primary and Secondary Liver Malignancies: Part 1: Technical and Methodologic Considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 22.Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 25.Riaz A, Memon K, Miller FH, Nikolaidis P, Kulik LM, Lewandowski RJ, et al. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: Radiologic–pathologic correlation. Journal of Hepatology. 2011;54:695–704. doi: 10.1016/j.jhep.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.