Abstract
Objective
To examine the hypothesis that glial activation would regulate the expression of the NR1 subunit of the N-methyl-D-aspartate receptor in the trigeminal subnucleus caudalis (Sp5C) after temporomandibular joint (TMJ) inflammation.
Methods
Inflammation of temporomandibular joint (TMJ) was produced in rats by injecting 50μl complete Freund's adjuvant (CFA) into unilateral TMJ space. Sham control rats received incomplete Freund's adjuvant (IFA) injection. Mechanical nociception in the affected and non-affected TMJ site was tested by using a digital algometer. Fractalkine, fluorocitrate, and/or MK801 were intracisternally administrated to examine the relationship between astroglial activation and NR1 upregulation.
Results
CFA TMJ injection resulted in persistent ipsilateral mechanical hyperalgesia 1, 3 and 5 days after CFA injection. The inflammation also induced significant upregulation of CX3CR1 and GFAP beginning on day 1, and of NR1 beginning on day 3, within the ipsilateral Sp5C. Intracisternal administration of fluorocitrate for 5 days blocked the development of mechanical hyperalgesia as well as the upregulation of GFAP and NR1 in the Sp5C. Conversely, intracisternal injection of fractalkine for 5 days exacerbated the expression of NR1 in Sp5C and mechanical hyperalgesia induced by TMJ inflammation. Moreover, once daily intracisternal fractalkine administration for five days in naïve rats induced the upregulation of NR1 and mechanical hyperalgesia.
Conclusions
These results suggest that astroglial activation contributes to the mechanism of TMJ pain through the regulation of NR1 expression in Sp5C.
Keywords: Trigeminal pain, NMDA receptor, NR1, Astroglia, GFAP, Sp5C
Introduction
Temporomandibular disorder (TMD) is a heterogeneous group of clinical conditions of the temporomandibular joint (TMJ) region including increased pain sensitivity, limited jaw movement, and referred pain beyond the affected TMJ. While the exact mechanism of TMJ pain remains unclear, there is a growing body of evidence indicating that persistent TMJ pain may be mediated by both central and peripheral mechanisms [1,2]. Central glial activation has been considered to be an important component in the development, facilitation and maintenance of allodynia and hyperalgesia in various models of nerve injury, peripheral inflammation, or visceral insults [3], resulting in the consequent release of proinflammatory cytokines due to activation of microglia [4]. Astrocytes are a sub-type of glial cells in the central nervous system (CNS). Gliosis is a proliferation of astrocytes in response to CNS injury. In response to tissue injury and inflammation, astrocytes often undergo morphological changes, increase synthesis of glial fibrillary acidic protein (GFAP, an astrocyte marker), and release cytokines.
Cytokines have been shown to regulate nociception. For example, intracerebroventricular injection of interleukin-1β (IL-1β) induced hyperalgesia in rats, which was blocked by pretreatment with IL-1 receptor antagonist [5,6]. Electrical stimulation of primary afferents of the sciatic nerve in a rat model of chronic constriction nerve injury evoked a dramatic increase in the spinal IL-1β and IL-6 content [7]. IL-6 and tumor necrosis factorα (TNFα) induced hyperalgesia through a prostanoid-dependent mechanism [8]. Treatment with the glial inhibitor propentofylline significantly reduced the spinal IL-1β, IL-6 and TNFα level, prevented the afferent stimulation-evoked release of both IL-1β and IL-6, and normalized allodynia following nerve injury [7]. Collectively, these results indicate that glial activation may be critical in the development of hyperalgesia through the regulation of the spinal cytokine content including IL-1β, IL-6 and TNFα.
Activation of spinal N-methyl-D-aspartate receptors (NMDAR) has been shown to play a role in the mechanisms of pathological pain [9-13]. On-going afferent discharges in a persistent pain state may maintain activation of NMDAR [13]. NMDAR antagonists, such as dextromethorphan, have been shown to be as least partially effective in pain relief in both clinical [14] and preclinical studies [15]. Genetic knockdown [16] or conditional deletion [17] of the NR1 subunit of NMDAR, which is necessary for the function of NMDAR, reduced the hypersensitivity in rodent models of neuropathic and inflammatory pain. In addition, it has been reported that phosphorylated NR1 is upregulated after trigeminal nerve injury [18] or masseter muscle tendon ligation [19], which was attenuated by the astrocyte inhibitor fluorocitrate [20].
Recent studies have shown that spinal neuroimmune activation (e.g., enhanced cytokine expression and glial activation) and neuroinflammation (e.g., infiltration of leukocytes into the central loci) are present in rodent models of neuropathy [21-24]. Astroglial activation within the trigeminal Vi/Vc transition zone following masseter inflammation modulated the phosphorylation of NMDAR [25]. To date, however, it remains unclear with regard to the regulatory mechanism of the NR1 expression within trigeminal nuclei. In the present study, we tested the hypothesis that glial activation would regulate the expression of the NR1 subunit of NMDAR in the trigeminal subnucleus caudalis (Sp5C) induced by TMJ inflammation.
Methods
A rat model of unilateral TMJ inflammation
Adult male Sprague-Dawley rats (S.D., Charles River Lab, Wilmington, MA, 200-250g) were used. The experimental protocol was approved by our Institutional Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were housed under controlled temperature (21°C±2°C), relative humidity (50%±10%) and artificial lighting (12/12 h light/dark cycle, lights on at 7 A.M.). All animals had ad libitum access to distilled water and a standard rat diet.
Sub-acute inflammation of the TMJ region was induced by the injection of pre-prepared CFA (Sigma, St Louis) into the right TMJ space [26]. A volume of 50μl (25μg heat-killed mycobacterium) was injected for each rat under pentobarbital sodium (50mg/kg, i.p.) anesthesia. A 22-gauge needle was percutaneously advanced into the TMJ immediately inferior to the posterior border of the zygomatic arch until it reached the mandibular condyle. CFA was injected into TMJ and surrounding tissues but not restricted to a TMJ capsule. For sham injection, incomplete Freund's adjuvant (IFA, Sigma, St Louis) was injected into the right TMJ using the same technique and injection volume. The needle was withdrawn after the injection. Both CFA and IFA injection groups were exposed to the same anesthesia and handling during the experiment.
Experimental design
To examine the role of glial activation in the expression of NR1 within Sp5C and the role of NMDAR in mechanical hyperalgesia following TMJ inflammation, nine groups of rat (n=6-7) were used, including 1) CFA/TMJ injection alone, 2) sham(IFA)/TMJ injection alone, 3) CFA/TMJ injection plus 0.5nmol fluorocitrate (intracisternal, in 10μl saline, Sigma, St Louis), 4) CFA/TMJ injection plus 2nmol fluorocitrate (intracisternal, in 10μl saline), 5) CFA/TMJ injection plus 25 ng fractalkine (intracisternal, in 10μl saline, Sigma, St Louis), 6) naïve rats plus 25ng fractalkine daily in 10μl saline, 7) CFA/TMJ injection plus 10nmol MK801, 8) Naïve rats plus 10nmol MK801 daily, and 9) CFA/TMJ injection plus 10μl saline (intracisternal). All intracisternal injections were given once daily for five consecutive days except for fluorocitrate, which was divided into two equal doses and injected twice daily, beginning immediately after the TMJ injection. Behavioral tests were performed on day 0 (before operation, baseline), 1, 3, and 5 of the TMJ inflammation and before daily intracisternal administration.
Behavioral testing
Behavioral testing was performed between 9:00 and 11:00 am. Habituation sessions (60min) in three consecutive days were conducted before the baseline test. To test mechanical nociceptive response, a rat's head was positioned against the experimenter's hand during the test (the experimenter switched hand when testing opposite TMJ). A linearly increasing force generated by a digital algometer (Wagner Instruments, Greenwich, CT) was delivered through a level plastic tip of 0.5cm2 against the ipsilateral or contralateral (to CFA or IFA) TMJ. In order to minimize variations among all groups, the site where the algometer tip was placed was in the middle of a TMJ immediately at the edge of the zygomatic arch. A threshold force was defined as the force (in gram) that induced vocalization or clear withdrawal of the rat's head from the experimenter's hand.
Intracisternal drug delivery
For implanting a catheter cephalically into the intracisternal/fourth ventricle space, a rat was anesthetized with pentobarbital (50mg/kg, i.p.) and mounted onto a stereotaxic apparatus. The atlantooccipital membrane was exposed and a small incision was made. A PE-10 tube was inserted cephalically and placed just dorsal to the obex. The tube was secured by 3-0 silk sutures. Skin cut was closed with wound clips. Rats exhibiting neurological deficits and distress (e.g., poor eating, grooming, and paralysis) were excluded from the experiment. Intracisternal injection was made slowly using a microsyringe (50μl) with 10-μl volume followed by a 10μl saline flush.
Immunohistochemistry
Rats from each group were anesthetized with sodium pentobarbital (50mg/kg, i.p.) and transcardiacally perfused with saline followed by cold 4% paraformaldehyde in a phosphate buffer solution (PBS, 0.1M, pH 7.35). Brain stem and cervical spinal cord blocks were harvested and post-fixed overnight in the same fixative for 4 hours and then cryoprotected in 30% sucrose in 0.1M PBS until the samples sank to the bottom. Sections were cut on a cryostat (Leica, 30-μm thickness). Coronal sections were floated in PBS and processed for immunostaining. The rat's brain atlas by Paxinos and Watson [27] was used to identify the trigeminal area of interest for the data collection under microscopic examination.
Immunostaining was used to detect GFAP (astroglial marker, 1:2000, chicken polyclonal; Abcam Inc., Cambridge, MA), CX3CR1 (a chemokine receptor also known as the fractalkine receptor, 1:500, rabbit polyclonal; Abcam Inc., Cambridge, MA), NeuN (neuronal marker, 1:1000, mouse monoclonal; Abcam Inc., Cambridge, MA), and NR1 (1:500, mouse monoclonal; United States Biological, Swampscott, MA) within Sp5C. Tissue sections were blocked with 1% BAS, 3% normal donkey serum, and 3% normal goat serum in 0.3% Triton X-100 for 30min at room temperature and incubated overnight at 4°C with a primary antibody. For controls, a primary antibody was omitted. Sections were then washed and incubated for one hour at room temperature with a corresponding DyLight 488- or DyLight 594-conjugated secondary antibody (1:200; JacksonImmuno Research, West Grove, PA). For double staining, a second primary antibody was added after incubation with the first primary antibody following the same procedure as described above. Sections were randomly selected, analyzed using a fluorescence microscope (Olympus, Tokyo, Japan), recorded with a digital camera, and processed using Adobe Photoshop.
Western Blot
Rats from each group were anesthetized using isoflurane in a 100% oxygen flow before decapitation. Trigeminal tissue samples (ipsilateral versus contralateral Sp5C, dorsal lateral portions of the oblongata and the rostral region of the cervical spinal cord) were removed and immediately placed into tubes on dry ice and stored at -80°C until use. Samples were homogenized in a SDS sample buffer containing a mixture of protease inhibitors (Sigma). Protein samples were separated on SDS-PAGE gels (4-10% gradient gel) and transferred to polyvinylidene difluoride filters (Millipore, Bedford, MA). The filters were blocked with 5% milk for one hour at room temperature and incubated overnight at 4 °C with GFAP (Chicken anti-GFAP, 55kD, 1:50000, Abcam Inc., Cambridge, MA), NR1 (mouse monoclonal, 89kD, 1:500, United States Biological), or CX3CR1 (rabbit polyclonal, 37kD, 1:500, Abcam Inc., Cambridge, MA) antibodies followed by HRP-conjugated secondary antibody(1:10000; Amersham Biosciences, Arlington Heights, IL) incubated for one hour at room temperature. The blots were visualized in ECL solution (NEN, Boston, MA) for one minute and exposed onto hyperfilms (Amersham Biosciences) for 1-10 minutes. The blots were then incubated in a stripping buffer (67.5mM Tris, pH 6.8, 2% SDS, and 0.7% β-mercaptoethanol) for 20min at 50°C and re-probed with mouse anti-β-actin antibody (1:20,000; Abcam Inc., Cambridge, MA) as loading control. All Western analysis was made in triplicates.
Statistical Analysis
For the behavioral data analysis, repeated measure two-way ANOVA was used followed by the post-hoc Tukey test using SPSS 16.0 for Windows to examine interactions between time points and treatment effects. For Western blot, the band density was measured with Photoshop and pixels were used for the statistic analysis. Differences were compared using one-way ANOVA (SPSS 16.0). The statistical significance was set at the level of α=0.05.
Results
Mechanical hyperalgesia and astroglial expression in Sp5C
Injection of CFA into unilateral TMJ region induced mechanical hyperalgesia on the ipsilateral site on day 1, 3, and 5 after the injection, as measured by an algometer and expressed as escape threshold in grams (Fig.1A, P<0.05; n=6). In contrast, there were no changes from the baseline in escape threshold over time on the contralateral TMJ site of rats receiving the CFA injection (Fig. 1A, P>0.05; n=6). Injection of IFA into unilateral TMJ region also did not induce mechanical hyperalgesia as compared with CFA-injected rats (Fig. 1A, P>0.05; n=6).
Fig. 1.
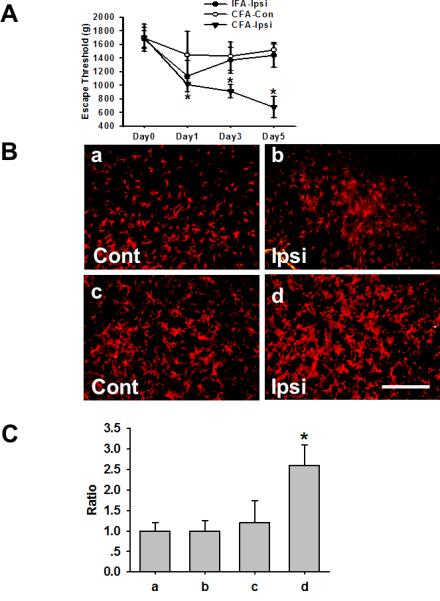
A. Time course of mechanical hyperalgesia after CFA-induced inflammation: CFA induced TMJ inflammation and lowered escape threshold in rats. CFA-Ipsi: TMJ ipsilateral to the CFA injection; CFA-Con: TMJ contralateral to the CFA injection; IFA-Ipsi: TMJ ipsilateral to the IFA (control) injection. * P<0.05 as compared with CFA-Con. B. Upregulation of GFAP within Sp5C: GFAP immunoreactivity in the Sp5C of sham rats (a & b) and rats with TMJ inflammatory (c & d) contralateral or ipsilateral to TMJ IFA or CFA injection. Samples were taken at 5 days after the CFA or IFA injection. Bar, 100μm. C. Quantification of GFAP immunoreactivity in B. * P<0.05 as compared with a, b, and c.
Injection of CFA also upregulated the expression of GFAP (an astroglial marker) within the ipsilateral Sp5C, as compared with the IFA injection (Fig.1B). Moreover, Western blot results showed that the expression of CX3CR1 (37kD), GFAP (55kD), and NR1 (89kD) was all time-dependently upregulated beginning on day 1 (CX3CR1 and GFAP) or day 3 (NR1) after the CFA injection (Fig.2).
Fig. 2. Upregulation of CX3CR1, GFAP, and NR1 after TMJ inflammation.
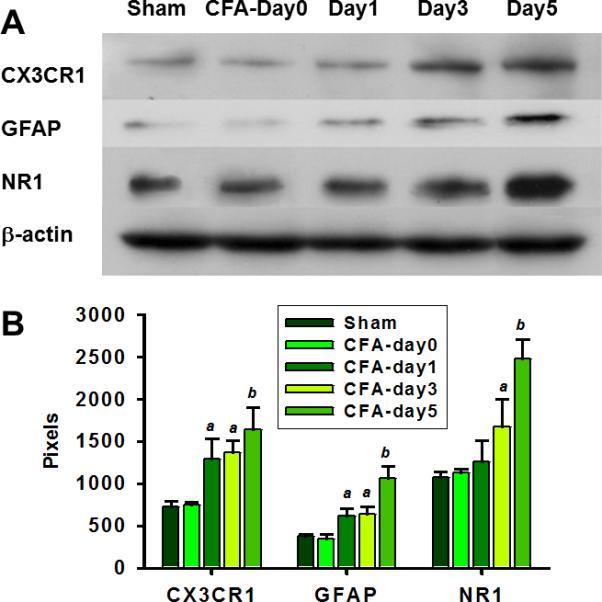
A. Western blots showing the CX3CR1, GFAP, and NR1 expression in the ipsilateral Sp5C before and following TMJ inflammation. B, Analysis of Western blot band density (Pixels) in A. a, P<0.05, b, P<0.01, as compared with the sham group and the CFA injection group on day 0, respectively.
Co-expression of CX3CR1 or NR1 with NeuN and GFAP
Immunostaining results showed that CX3CR1 was co-expressed with NeuN and GFAP (Fig. 3), indicating that this chemokine receptor was present in both neuronal and glial cells. In addition, NR1 was co-expressed with NeuN and CX3CR1 in the same cells (Fig.3). These results demonstrate that CR3CR1 and NR1 are expressed in Sp5C in response to TMJ inflammation with a similar cellular association.
Fig. 3. Co-expression of NeuN, CX3CR1, GFAP or NR1 within Sp5C.
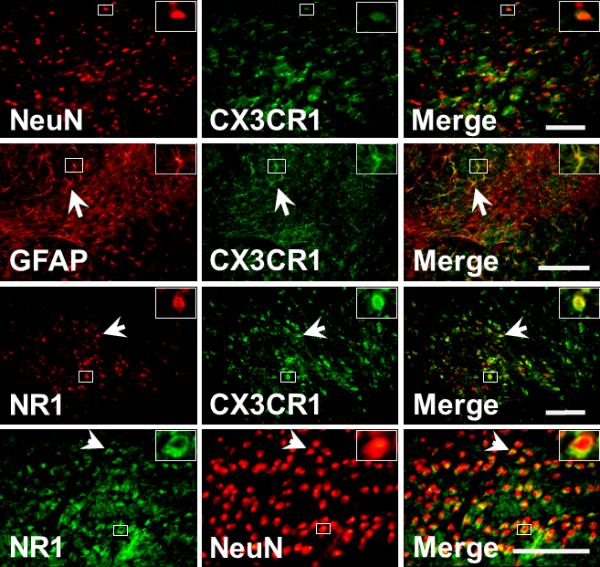
The expression of CX3CR1, NeuN, GFAP, and NR1 in the Sp5C ipsilateral to TMJ inflammation. Arrows indicated the colocalization of immunoreactivity. Bar, 100μm. Inserts: magnified cell profiles in frame.
Effect of fractalkine on mechanical hyperalgesia, glial activation, and NR1 expression
Since CX3CR1 was upregulated within the ipsilateral Sp5C in response to TMJ inflammation, we examined whether fractalkine (a ligand to CX3CR1) would enhance mechanical hyperalgesia following TMJ inflammation. Fractalkine (25ng) was intracisternally administered once daily for five consecutive days. This treatment regimen exacerbated TMJ mechanical hyperalgesia when examined on day 5 as compared with control rats receiving intracisternal vehicle administration (Fig. 4A, n=6-7, P< 0.05).
Fig.4.
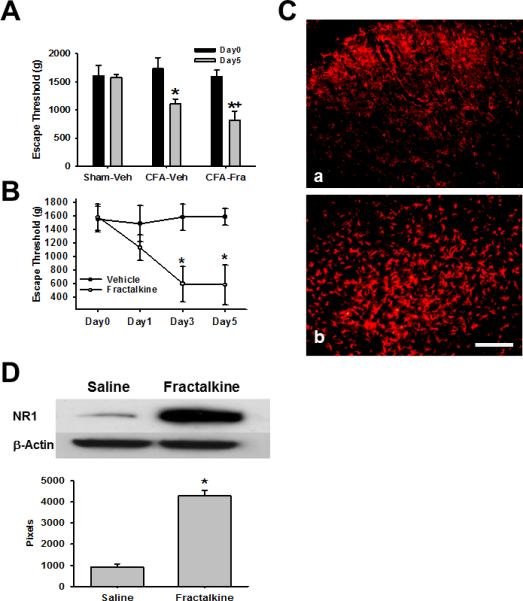
A. Effect of fractalkine on mechanical hyperalgesia Fractalkine (25ng) but vehicle (Saline) given intracisternally (in 10μl) for 5 consecutive days exacerbated mechanical hyperalgesia following TMJ inflammation. *P<0.05, as compared with Day0 of the sham (Sham-Veh) or CFA (CFA-Veh or CFA-Fra) groups; + P<0.05 as compared with Day5 of the vehicle group with TMJ inflammation (CFA-Veh). B. Effect of fractalkine in naïve rats TMJ mechanical hyperalgesia was induced by daily intracisternal fractalkine (25ng in 10μl saline) administration for 5 days. * P<0.05, as compared with Day0. C. Upregulation of GFAP within Sp5C by fractalkine in naïve rats GFAP expression in the Sp5C of naïve rats 5 days after intracisternal administration of saline (a) or 25ng fractalkine (b). Bar, 100μm. D. Upregulation of NR1 by intracisternal administration of fractalkine in naïve rats Western blot results showing that NR1 expression was upregulated in Sp5C by intracisternal administration of fractalkine (25ng) for 5 days in naïve rats. * P< 0.05, as compared with the vehicle group.
To examine whether fractalkine would induce mechanical hyperalgesia in naïve rats without TMJ inflammation, the same fractalkine dose (25ng) was intracisternally administered once daily for five consecutive days. This treatment regimen also induced TMJ mechanical hyperalgesia when examined on day 3 and 5 as compared with naïve rats injected with vehicle (Fig. 4B, n=6-7, p<0.05). The time course of fractalkine-induced mechanical hyperalgesia in naïve rats was similar to that induced by TMJ inflammation in CFA-treated rats. Moreover, immunostaining and Western blot results showed that the expression of GFAP (Fig. 4C) and NR1 (Fig. 4D) was upregulated within Sp5C of naïve rats when tested on day 5 of the intracisternal administration of fractalkine. These results indicate that activation of CX3CR1 induced TMJ mechanical hyperalgesia in the absence of TMJ inflammation and further exacerbated TMJ mechanical hyperalgesia following TMJ inflammation.
Effect of fluorocitrate on mechanical hyperalgesia, glial activation, and NR1 expression
To further examine the role of glial activation in TMJ mechanical hyperalgesia and the NR1 expression, two doses of fluorocitrate (0.25, 1nmol), a glial metabolism inhibitor with the effect as an astrocyte inhibitor, or vehicle were intracisternally administered twice daily for five consecutive days beginning immediately after the CFA injection into the right TMJ. The lower dose (0.25nmol) fluorocitrate treatment significantly attenuated ipsilateral TMJ mechanical hyperalgesia when examined on day 3 and 5 (Fig. 5A, n=6, P< 0.05), whereas the higher dose (1nmol) fluorocitrate treatment prevented the development of TMJ mechanical hyperalgesia (Fig. 5A, n=7, P< 0.05) as compared with the vehicle group. Western blot results showed that the expression of GFAP and NR1 in the ipsilateral Sp5C was downregulated when tested on day 5 of the intracisternal fluorocitrate administration (Fig.5B, C, P< 0.05). Collectively, these results indicate that inhibition of glial activation dose-dependently prevented the development of TMJ mechanical hyperalgesia.
Fig. 5.
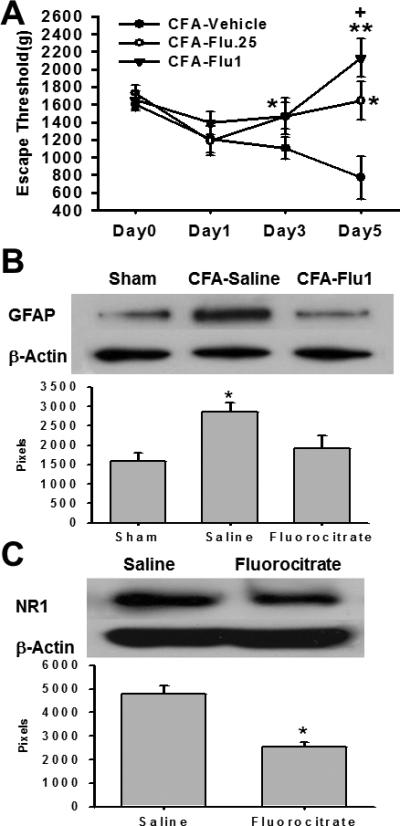
A. Effect of fluorocitrate on mechanical hyperalgesia Fluorocitrate (0.25 or 1nmol, twice daily) given intracisternally for 5 days attenuated the development of TMJ mechanical hyperalgesia. * P<0.05, ** P<0.01, as compared with the vehicle group; + P<0.05, as compared with the CFA-Flu (0.25nmol) group. B. Regulation of GFAP expression by fluorocitrate Western blot results showing that the GFAP upregulation in the ipsilateral Sp5C on day 5 was attenuated by intracisternal administration of 1nmol fluorocitrate for 5 days. * P<0.05, as compared with other groups. C. Prevention of NR1 upregulation by fluorocitrate Western blot results showing that the NR1 upregulation in the ipsilateral Sp5C was attenuated by intracisternal administration of fluorocitrate (1nmol) for 5 days. * P<0.05, as compared with the vehicle group.
Effect of MK801 on TMJ mechanical hyperalgesia
If the role of glial activation on TMJ mechanical hyperalgesia was related to NMDAR, as suggested by the above results, inhibition of NMDAR would be expected to attenuate mechanical hyperalgesia with or without the administration of fractalkine. To examine this possibility, we used the non-competitive NMDAR antagonist MK801 in either a repeated or single treatment regimen. Repeated intracisternal administration of MK801 (10nmol), but not saline, prevented the development of mechanical hyperalgesia when tested on day 3 and 5 after the CFA injection (Fig.6A, P<0.05; n=5-7) without changing the baseline escape threshold in naive rats (Fig. 6A, n=6).
Fig.6. Effect of MK801 on mechanical hyperalgesia.
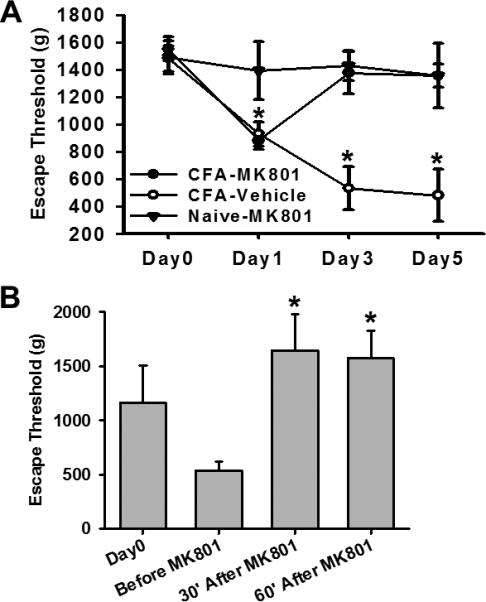
A. Repeated intracisternal MK801 treatment (10nmol, once daily for 5 days), beginning immediately after the CFA injection, prevented the development of mechanical hyperalgesia. MK801 had no effect on the escape threshold in naïve rats. * P<0.05, as compared with the naïve group at the same time point. B. A single intracisternal MK801 treatment (10nmol) on day 5 reversed mechanical hyperalgesia exacerbated by the intracisternal fractalkine (25ng) administration for 5 days. Behavioral tests were made at the baseline (day 0), day 5 before MK801, as well as at 30 and 60min after MK801. *P< 0.05, as compared with “Before MK801”.
A single intracisternal administration of MK801 (10nmol) on day 5 also effectively reversed CFA-induced TMJ mechanical hyperalgesia that was exacerbated by the fractalkine administration. Mechanical hyperalgesia was assessed at 30 and 60min after MK801 (Fig. 6B, p< 0.05, n=5-7). Moreover, the same single dose MK801 treatment reversed mechanical hyperalgesia induced by repeated intracisternal administration of 25ng fractalkine in naïve rats (n=5, data not shown). These results indicate that the expression of NMDAR, possibly mediated by glial activation, was critically involved in the development and maintenance of TMJ mechanical hyperalgesia.
Discussion
In this study, we demonstrated that 1) unilateral TMJ inflammation significantly increased the expression of the NR1 subunit of NMDAR, CX3CR1 (the chemokine receptor for fractalkine), and astroglial marker (GFAP) within the ipsilateral Sp5C; 2) intracisternal injection of a broad glial inhibitor (fluorocitrate) for 5 days prevented astroglial activation and NR1 upregulation within the ipsilateral Sp5C as well as attenuated TMJ mechanical hyperalgesia; 3) intracisternal injection of fractalkine (a ligand for the CX3CR1 chemokine receptor) further upregulated GFAP and NR1 expression within the ipsilateral Sp5C and exacerbated TMJ mechanical hyperalgesia; 4) intracisternal injection of fractalkine for 5 days also induced the upregulation of GFAP and NR1 within Sp5C and resulted in mechanical hyperalgesia similar to that seen after TMJ inflammation; and 5) intracisternal administration of MK801 both prevented and reversed mechanical hyperalgesia exacerbated by fractalkine. These results indicate that astroglial activation, via CX3CR1, regulated the expression of NR1 within the ipsilateral Sp5C after TMJ inflammation, which contributed to the development and maintenance of TMJ mechanical hyperalgesia in rats.
Chemokines are a class of small proteins and act as inflammatory mediators of leukocyte chemotaxis through cell-surface G protein-coupled receptors [28]. In the central nervous system, chemokines are produced by activated astrocytes [29], microglia [30], and neurons [31]. Moreover, chemokine mRNAs were constitutively expressed, albeit at a low level, in the spinal cord without tissue inflammation [16]. Fractalkine (also known as CX3CL1) is a member of the chemokine family and exists as a membrane-bound and soluble protein. Both fractalkine and its sole receptor CX3CR1 are expressed primarily in the central nervous system and the soluble form of fractalkine has multiple functions. For example, the increased release of fractalkine has been shown to contribute to pathological pain via microglial activation [32]. However, a markedly diminished soluble fractalkine level may ultimately affect neuronal survival through inhibition of alpha-secretase activity [33] and that the deficiency of CX3CR1 could dysregulate microglial responses resulting in neurotoxicity and cell death [34]. In this study, we showed that astroglial activation is also influenced by fractalkine, which occurred during the early stage of an inflammatory condition and contributed to the development of TMJ mechanical hyperalgesia.
Activation of NMDA receptor is considered to initiate intracellular cascades through calcium influx and activation of protein kinases, which in turn modulates cell membrane excitability and enhances nociceptive transmission [35, 36]. Several recent studies have demonstrated the interaction between cytokines and glutamate receptors including NMDAR. For example, it has been reported that astrocytes actively participate in synaptic integration via releasing glutamate, a calcium-regulated process mediated by chemokine receptor CXCR4 [37], which is further amplified by microglial activation [24]. CXCR2, another chemokine receptor, has been shown to regulate the AMPA-type glutamate receptor GluR1 [38]. In behavioral experiments, knockout of certain chemokine receptors such as CXCR2 decreased hypersensitivity induced by tissue inflammation and nerve injury [39]. Moreover, mice with the deficient NR1 function (NR1+/-) had decreased mechanical allodynia [16]. It is of interest to note that regulation of NR1 is likely to be downstream to the cascade of chemokine effects since fractalkine upregulated the expression of NR1 and fluorocitrate inhibited the expression of NR1. In addition, nerve injury did not induce a significant spinal increase in the chemokine mRNA expression in NR1+/- mice [16].
The present data support a cascade of events involving a complex of chemokine-glia-NMDAR communications in trigeminal pain states. Specifically, TMJ inflammation may initially increase the expression of CX3CR1 within the trigeminal system leading to activation of glial cells (e.g., astrocytes) and subsequently NR1 expression. This hypothesis is supported by several findings in this study. First, the time course of the CX3CR1, GFAP, and NR1 expression differed after tissue inflammation such that the CX3CR1 and GFAP upregulation was present on day 1 after TMJ inflammation while the upregulation of NR1 was first detected on day 3 by Western blot. Early CX3CR1 upregulation was also reported in a rat model of selective nerve ligation [40]. Second, intracisternal injection of fluorocitrate, a broad glial metabolic inhibitor, prevented the NR1 upregulation within the ipsilateral Sp5C and diminished mechanical hyperalgesia after TMJ inflammation. These findings are in agreement with a previous report showing that fluorocitrate injected into the ventral subnuclei interpolaris/caudalis transition zone attenuated hyperalgesia in the masseter region [25]. Third, inhibition of NMDAR by daily MK801 administration prevented mechanical hyperalgesia exacerbated by the intracisternal administration of fractalkine, indicating that the effect of fractalkine on TMJ hyperalgesia is mediated by NMDA receptors. The NR1 subunit in astrocytes could be a target for MK801 as well, given that the NR1 subunit has been located in astrocytes [41].
In summary, these findings support the notion that glial activation regulates persistent nociception via the action of chemokines and suggest that pharmacologically targeting the action of glial cells and chemokines might be effective in preventing trigeminal pain conditions. It should be pointed out that although the current study focused on examining astroglial cells, it does not rule out the possible involvement of microglial cells in this process. It will be of interest in future studies to clarify a temporal relationship between microglial and astroglial cells under similar experimental conditions. Future studies should further explore the mechanism of the neuroimmune system in the regulation of TMJ pain conditions.
Acknowledgments
This study is supported by NIH RO1 grants NIH grants R01DE18214, R01DE18538, and P20DA26002.
Footnotes
Disclosures:
There is no Conflict of Interest (COI) in this project.
References
- 1.Dubner R. The neurobiology of persistent pain and its clinical implications. Suppl Clin Neurophysiol. 2004;57:3–7. doi: 10.1016/s1567-424x(09)70337-x. [DOI] [PubMed] [Google Scholar]
- 2.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 3.Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 2010;22:499–511. doi: 10.1111/j.1365-2982.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain Intensity and Duration Can Be Enhanced by Prior Challenge: Initial Evidence Suggestive of a Role of Microglial Priming. J Pain. 2010;11:1004–14. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1 beta induces hyperalgesia in rats. Brain Res. 1993;624:61–8. doi: 10.1016/0006-8993(93)90060-z. [DOI] [PubMed] [Google Scholar]
- 6.Oka T, Aou S, Hori T. Intracerebroventricular injection of prostaglandin E2 induces thermal hyperalgesia in rats: the possible involvement of EP3 receptors. Brain Res. 1994;663:287–92. doi: 10.1016/0006-8993(94)91275-0. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead KJ, Smith CG, Delaney SA, Curnow SJ, Salmon M, Hughes JP, Chessell IP. Dynamic regulation of spinal pro-inflammatory cytokine release in the rat in vivo following peripheral nerve injury. Brain Behav Immun. 2010;24:569–76. doi: 10.1016/j.bbi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Oka T, Oka K, Hosoi M, Hori T. Intracerebroventricular injection of interleukin-6 induces thermal hyperalgesia in rats. Brain Res. 1995;692:123–8. doi: 10.1016/0006-8993(95)00691-i. [DOI] [PubMed] [Google Scholar]
- 9.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Mabuchi T, Matsumura S, Okuda-Ashitaka E, Kitano T, Kojima H, Nagano T, Minami T, Ito S. Attenuation of neuropathic pain by the nociceptin/orphanin FQ antagonist JTC-801 is mediated by inhibition of nitric oxide production. Eur J Neurosci. 2003;17:1384–92. doi: 10.1046/j.1460-9568.2003.02575.x. [DOI] [PubMed] [Google Scholar]
- 11.Meller ST, Dykstra C, Gebhart GF. Production of endogenous nitric oxide and activation of soluble guanylate cyclase are required for N-methyl-d-aspartate-produced facilitation of the nociceptive tail-flick reflex. Eur J Pharmacol. 1992a;214:93–6. doi: 10.1016/0014-2999(92)90102-a. [DOI] [PubMed] [Google Scholar]
- 12.Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992b;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- 13.Pitcher GM, Henry JL. Second phase of formalin-induced excitation of spinal dorsal horn neurons in spinalized rats is reversed by sciatic nerve block. Eur J Neurosci. 2002;15:1509–15. doi: 10.1046/j.1460-9568.2002.01984.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SP, Wang S, Chen L, Kurihara C, McKnight G, Marcuson M, Mao J. An intravenous ketamine test as a predictive response tool in opioid-exposed patients with persistent pain. J Pain Symptom Manage. 2009;37:698–708. doi: 10.1016/j.jpainsymman.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Zhang L, Lim G, Sung B, Tian Y, Chou CW, Hernstadt H, Rusanescu G, Ma Y, Mao J. A combined effect of dextromethorphan and melatonin on neuropathic pain behavior in rats. Brain Res. 2009;1288:42–9. doi: 10.1016/j.brainres.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bursztajn S, Rutkowski MD, Deleo JA. The role of the N-methyl-D-aspartate receptor NR1 subunit in peripheral nerve injury-induced mechanical allodynia, glial activation and chemokine expression in the mouse. Neuroscience. 2004;125:269–75. doi: 10.1016/j.neuroscience.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 17.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–40. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K, Watanabe M, Suekawa Y, Ito G, Inubushi T, Hirose N, Murasaki K, Hiyama S, Uchida T, Tanne K. IL-1beta in the trigeminal subnucleus caudalis contributes to extra-territorial allodynia/hyperalgesia following a trigeminal nerve injury. Eur J Pain. 2011;15:467, e1–14. doi: 10.1016/j.ejpain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Wang H, Zou S, Wei F, Dubner R, Ren K. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: a model of myogenic orofacial pain. Mol Pain. 2010;6:40. doi: 10.1186/1744-8069-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–18. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski M, Winkelstein BA, Hickey WF, Pahl J, DeLeo JA. Lumbar root injury induces CNS neuroinflammation and neuroimmune activation in the rat: Relationship to painful radiculopathy. Spine. 2002;27:1604–13. doi: 10.1097/00007632-200208010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Sweitzer S, Hickey WF, DeLeo JA. Focal nerve injury induces leukocyte trafficking into the CNS: Relationship to neuropathic pain. Pain. 2002;100:163–70. doi: 10.1016/s0304-3959(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 24.Winkelstein BA, Rutkowski M, Sweitzer S, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacological treatment. J Comp Neurol. 2001;439:127–39. [PubMed] [Google Scholar]
- 25.Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;5:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic Press; New York: 1997. [Google Scholar]
- 28.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 29.Glabinski AR, Balasingam V, Tani M, Kunkel SL, Streiter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–8. [PubMed] [Google Scholar]
- 30.Gourmala NG, Buttini M, Limonta S, Sauter A, Boddeke HW. Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: effects of ischemia and peripheral lipopolysaccharide administration. J Neuroimmunol. 1997;74:35–44. doi: 10.1016/s0165-5728(96)00203-2. [DOI] [PubMed] [Google Scholar]
- 31.Coughlan C, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VMY, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokines receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 32.Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: a role for fractalkine. J Neuroimmunol. 2008;198:113–20. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook A, Hippensteel R, Shimizu S, Nicolai J, Fatatis A, Meucci O. Interactions between chemokines: regulation of fractalkine/CX3CL1 homeostasis by SDF/CXCL12 in cortical neurons. J Biol Chem. 2010;285:10563–71. doi: 10.1074/jbc.M109.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 35.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–64. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 36.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 37.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFa: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 38.Lax P, Limatola C, Fucile S, Trettel F, Di Bartolomeo S, Renzi M, Ragozzino D, Eusebi F. Chemokine receptor CXCR2 regulates the functional properties of AMPA-type glutamate receptor GluR1 in HEK cells. J Neuroimmunol. 2002;129:66–73. doi: 10.1016/s0165-5728(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 39.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino AJ, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100:7947–52. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX(3)CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–51. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palygin O, Lalo U, Pankratov Y. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br J Pharmacol. 2011;63:1755–66. doi: 10.1111/j.1476-5381.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


