Abstract
Background
Oral rapid HIV testing has been reported to have a lower sensitivity and specificity than rapid HIV testing with whole-blood and has been associated with clusters of false positive results. Patient preference for oral rapid HIV testing compared to more invasive whole-blood fingerstick may influence the acceptance of rapid HIV testing.
Objective
To compare HIV test acceptance rates among patients routinely offered fingerstick compared to those routinely offered oral fluid screening in an urban hospital emergency department (ED).
Methods
USHER-Phase II was a single-center, prospective, randomized controlled trial that randomized subjects to either fingerstick or oral rapid HIV screening in an urban academic ED. From May 5, 2009 to January 4, 2010, eligible patients aged 18 to 75 years were invited to participate in the trial. The primary outcome measure was HIV test acceptance rate.
Results
2,012 eligible patients were approached, of whom 1,651 (82%) consented to trial participation and enrolled. Among those enrolled 830 and 821 were randomized to the fingerstick and oral fluid arms, respectively. Acceptance of rapid HIV testing was similar in both arms; 67% (553/830) of subjects accepted fingerstick testing compared to 69% (565/821) who accepted oral (p=0.34).
Conclusions
Although fingerstick rapid HIV testing is more invasive than oral fluid testing, test acceptance rates did not differ. Given the option, preference should therefore be given to fingerstick testing because of its slightly superior test characteristics. System factors such as ease of staff use, necessary CLIA waivers, laboratory capacity, and HIV prevalence should also be considered.
Keywords: HIV Screening, Randomized trial, OraQuick, Emergency Department
INTRODUCTION
In 2006, the Centers for Disease Control and Prevention (CDC) recommended expanded Human Immunodeficiency Virus (HIV) screening in U.S. emergency departments.1 Successful execution of the CDC recommendation requires two important components, minimal testing barriers (e.g. low personnel and financial costs) and high test acceptance rates. The expansion of rapid HIV testing to clinical and non-clinic settings plays an important role in the implementation of the CDC guidelines by increasing test acceptance, facilitating receipt of test results, and promoting linkage to care.2-6 Yet, the uptake of rapid tests may vary based on the testing modality offered - fingerstick versus oral swab.
Early studies demonstrate high rates of oral HIV test acceptability among patients and providers due to the noninvasiveness of the test and speed of specimen collection.7-9 These favorable test attributes have been used to maximize rates of HIV testing.7,10 However, this increase in rapid oral HIV test acceptance has been also accompanied by several reported clusters of false positive test results.11-13 A recent meta-analysis by Pai et al. examining the accuracy OraQuick rapid HIV-antibody-based point-of-care tests found that oral testing had a lower sensitivity than fingerstick testing (98.0% vs 99.7%) and a lower positive predictive value (PPV) in low-prevalence settings (88.6% vs 97.7%).14 Such reports have raised public concern among health care providers and consumers, and numerous testing clinics have replaced oral fluid testing for fingerstick due to such occurrences.
Rapid fingerstick HIV testing has limitations as well. Patients consistently demonstrate a preference for noninvasive, painless oral testing methods 7,9 which may compromise fingerstick test acceptance. Given the distinct limitations of the two testing methods – slightly poorer performance of oral fluid versus potentially lower test acceptance of fingerstick – it is not clear whether the frequency of rapid HIV test acceptance would differ between oral fluid and fingerstick tests. To address this question, we conducted a randomized controlled trial of routine rapid HIV screening in an urban hospital ED to directly evaluate the frequency of HIV test acceptance as well as test completion using oral fluid and fingerstick testing modalities.
METHODS
Ethics Statement
The study was approved by the Partners Human Research Committee (2006P-000136) and was overseen by a Data Safety and Monitoring Board.
Trial setting
The Universal Screening for HIV in the Emergency Room (USHER)-Phase II study was conducted in the emergency department at Brigham and Women's Hospital (BWH), a tertiary academic medical center in Boston, MA. The BWH Emergency Department (ED) is a Level 1 trauma center that treats more than 56,000 patients annually and serves a demographically diverse patient population of whom 48% are white, 25% black, and 20% Hispanic. In this ED, approximately 60% of presenting patients are women, and the median age is 44 years. Prior to the implementation of the USHER study, HIV testing was not performed in the BWH ED.
Study Design
The USHER trial is an NIH-funded, single-center, randomized controlled trial. Details of the original USHER trial have been published elsewhere.15 Between May 5, 2009 and January 4, 2010, USHER-Phase II consented eligible patients for the opportunity to be offered routine opt-in, rapid HIV screening. Enrolled subjects were randomized to fingerstick whole-blood or oral fluid specimen collection. Details regarding the informed consent process have been previously reported.15 Per Massachusetts law, all subjects provided separate written informed consent for rapid HIV testing in addition to providing written informed consent for trial participation. Participants were also asked to complete a questionnaire which gathered data on age, race, ethnicity, income and high-risk behavior. Patients who were interested in HIV testing but refused trial participation were provided with a hard copy list – kept in the ED – of all locally available and Department of Public Health affiliated free HIV counseling, testing and referral sites.
Eligibility Criteria
Patient eligibility was assessed using the ED charts and the BWH computerized patient tracking system. Eligible patients met the following criteria: 1) 18-74 years old; 2) fluent in English or Spanish; 3) not engaged in pre-natal care; 4) not self-reportedly known to be HIV-infected; 5) not enrolled in the USHER trial in the previous three months; and 6) had an Emergency Severity Index (ESI) score of 3-5 (indicating lower clinical severity)16-18 or an ESI score of 1 or 2 (potentially higher clinical severity), with signed approval from the ED attending physician indicating participant's clinical stability and clear mental status. During trial enrollment hours, HIV counselors (trained research assistants) assessed ED patients who had been registered, triaged, and escorted to their rooms to determine if these patients were eligible for USHER-Phase II. Enrollment times spanned from 8 a.m. to 12 a.m. and encompassed a minimum of 60 hours per week, including weekends. No financial incentives were provided to patients for trial participation.
Randomization
After providing informed consent for trial participation, subjects were randomized to one of the two test modality arms: 1) fingerstick whole-blood HIV testing, or 2) oral fluid HIV testing. The fingerstick consent form stated the manufacturer-reported accuracy of the test and discussed the required blood collection methods 19 while the consent form for the rapid oral test stated the risk of false positive results associated with this test as identified in USHER-Phase I (e.g. “3 out of 4 patients with a ‘reactive’ test do not have HIV infection”).13 Since HIV test acceptance has been found to vary with sex and age groups,20 USHER-Phase II participants were randomized using computer-generated block randomization within four strata (i.e., men <40 years old; men ≥40 years old; women <40 years old; and women ≥40 years old). Neither subjects nor counselors were blinded to the assigned arms.
Staff Training
Counselors were trained by the Massachusetts Department of Public Health 21 in both methods of specimen collection for the OraQuick®ADVANCE™ Rapid HIV 1/2 Antibody Test (OraSure Technologies, Inc. Bethlehem, PA).
Primary and secondary outcome measures and statistical methods
The primary outcome measure was HIV test acceptance rate defined by the proportion of participants whom accepted HIV testing among those randomized within each trial arm (fingerstick or oral fluid). The secondary outcome measure was frequency of HIV test completion as defined by the proportion of participants who completed HIV testing among those randomized within each trial arm.
Data from the original cohort of the USHER trial (Phase I) were used to inform the sample size estimation for this study.15 Sample size was chosen to detect a 10% difference in acceptance rates between two testing modality arms (90% power, 0.05 level of significance) and was estimated at 992 subjects tested, 496 per arm.
We used the intention-to-treat principle in which data were analyzed according to the arm to which they were randomly allocated, irrespective of whether they actually received the assigned test. To illustrate the balance between arms achieved by randomization, we present baseline demographic information stratified by study arm. Means and standard deviations are provided for continuous variables (age) while frequencies are presented for categorical variables (gender, race/ethnicity, primary language, and education). The difference in the acceptance rates was estimated along with 95% confidence intervals and tested using the chi-square test. All analyses were performed using SAS statistical software Version 9.2 (Cary, NC).
RESULTS
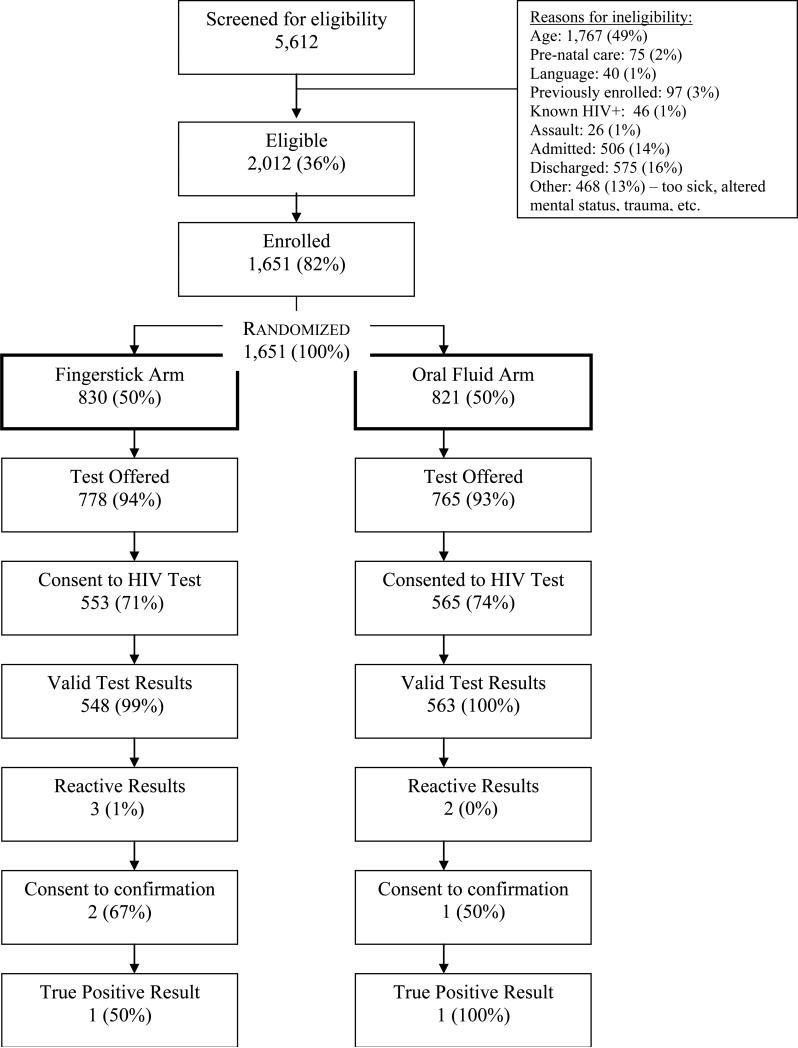
From May 5, 2009 through January 4, 2010, 5,612 patients were screened for USHER-Phase II trial eligibility, and 2,012 (36%) were eligible for enrollment. The most frequently documented reason for ineligibility was age (n=1,767; 49% of all ineligible). Among the 2,012 eligible patients approached, 1,651 (82%) agreed to study participation (Figure 1). The 361 eligible patients who refused USHER-Phase II trial enrollment were older than study participants (40 versus 34 years of age; p<0.0001) yet similar in other demographic features and ESI scores.
Figure 1.
USHER-Phase II Trial enrollment schema. Percentages are calculated using the number stated in the cell above as the denominator.
Among those 1,651 patients who agreed to enrollment, 830 were randomized to the fingerstick arm and 821 to the oral fluid arm. Trial arms were balanced in their demographic distribution; mean age was 33 years (SD 13), 65% were female, 24% were white, 24% African-American, and 38% were Hispanic (Table 1).
Table 1.
Demographic characteristics of patients randomized in the USHER-Phase II Trial
| Fingerstick (N=830) | Oral Fluid (N=821) | |
|---|---|---|
| Mean Age (SD) | 34 (13) | 33 (13) |
| Sex | ||
| Male | 283 (35%) | 288 (35%) |
| Female | 536 (65%) | 529 (65%) |
| Race/Ethnicity | ||
| Non-Hispanic White | 198 (24%) | 201 (25%) |
| Non-Hispanic Black | 188 (23%) | 200 (25%) |
| Hispanic | 329 (40%) | 297 (36%) |
| Asian/Asian-American | 22 (3%) | 22 (3%) |
| Native American/Alaskan Native | 1 (0%) | 4 (0%) |
| Multi-racial/Other | 85 (10%) | 92 (11%) |
| Primary Language | ||
| English | 643 (78%) | 644 (79%) |
| Spanish | 148 (18%) | 141 (17%) |
| Other | 34 (4%) | 29 (4%) |
| Education | ||
| Less than High School | 116 (14%) | 96 (12%) |
| High School/General Education Diploma | 255 (32%) | 261 (32%) |
| Some College | 198 (24%) | 222 (28%) |
| College Degree | 173 (21%) | 144 (18%) |
| Some Post-College/Graduate Degree | 67 (8%) | 82 (10%) |
Test acceptance rates
Among subjects randomized to rapid HIV testing, the test acceptance did not differ meaningfully between arms, 67% (553/830) in the fingerstick arm compared to 69% (565/821) in the oral fluid arm (p=0.34). Frequencies of test acceptance did not differ by race, gender or education. The proportion of HIV tests completed – the proportion of subjects who were tested among those who were randomized – was 66% (549/830) in the fingerstick arm and 69% (563/821) in the oral fluid arm (p=0.29). More than 99% of those who accepted an HIV test received the test in both arms.
Among the 1,111 study participants who had a valid rapid HIV test result, five tests were reactive. Three of these subjects consented to confirmatory testing. Two new cases of HIV infection were identified (one fingerstick and one oral) – a yield of new case identification of 0.2% (95% CI: 0.0-0.6%). One fingerstick test was a false positive. No harm was reported in this trial.
DISCUSSION
In a randomized controlled trial of routine ED-based HIV screening, we found no meaningful difference in the acceptability of an HIV test when comparing fingerstick versus oral fluid rapid HIV test collection modalities.
Our randomized trial corroborates the 2009 study result of White and colleagues.22 White et al. allocated fingerstick vs. oral HIV testing based upon the day of the week and showed that testing modality had minimal effect on testing rates.22 Reasons for declining screening in that study were generally similar for both screening modalities and seldom related to testing method - the most common being “having recently been tested for HIV” (50%) and “lack of perceived HIV risk” (31%).22
This study is subject to several limitations. Because the USHER-Phase II trial was a single-site study with findings that are applicable to rapid HIV screening using fingerstick or oral collection modality, some of our results may not be generalizable to other settings or test kits. Failure to enroll overnight, in addition to the lengthy consent process required to conduct an IRB-approved randomized trial in Massachusetts (one for trial, one for testing per Massachusetts state law, and one for confirmation of reactive results, if necessary) may have led to selection bias. We did not collect preference data and were therefore not able to fully characterize trade-offs considered in the decision for oral testing given its ease of administration versus the decision for fingerstick testing given its reported superior test characteristics. Furthermore, the frequency of test offers as well as test acceptance may be lower in EDs that do not utilize ancillary testing personal, as we used in our trial. Finally, several important factors may also influence acceptance of HIV testing and were not measured in our study. These include system-level factors such as location convenience, confidentiality, consent processes, cost, counseling opportunities, and results disclosure.2,10
In the development of HIV screening protocols in the ED, urgent care, and primary care settings, it is critical to tailor optimal screening approaches to enhance test acceptability by decreasing testing barriers, particularly among those less approachable for the test offer and/or those less willing to accept testing. Many EDs have recently started to favor HIV screening using specimens collected for clinical purposes.23 However, this streamlined testing process fails to account for patients who refuse or do not require phlebotomy or who do not present to an ED setting, making our findings still very relevant.
In an era when public health efforts are emphasizing prevention, routine HIV screening, and early entry to care, the use of testing methods that can expand acceptability of HIV testing is essential. Our study is among the first to demonstrate the application of two rapid HIV test modalities in a randomized trial where the frequencies of test offer and acceptability are compared within the context of routine, voluntary counseling and screening in an emergency department. We find that test modality was not an important factor in test offer and acceptance rates among patients. Given the option, preference should therefore be given to fingerstick rapid HIV testing because of its slightly superior test characteristics compared to that of the oral rapid HIV test. In low prevalence settings, rapid oral testing should remain an operational solution for a subset of patients who refuse whole blood HIV testing. In addition to test characteristics, however, we believe that system factors such as ease of staff use, necessary CLIA waivers, laboratory capacity, and HIV prevalence in the testing setting should also be heavily considered.
Table 2.
Summary of Intent-to-Treat analysis by trial arm
| Fingerstick (N=830) | Oral Fluid (N=821) | Difference (95% CI) | P value | |
|---|---|---|---|---|
| HIV test offered | 778 (94%) | 765 (93%) | 0.6% (-1.8%, 2.9%) | 0.65 |
| HIV test accepted among those randomized | 553 (67%) | 565 (69%) | -2.2% (-6.7%, 2.3%) | 0.34 |
| HIV test completed among those randomized | 549* (66%) | 563 (69%) | -2.4% (-7.0%, 2.1%) | 0.29 |
Includes one invalid test result
Acknowledgements
The authors would like to acknowledge the co-investigators and staff of the USHER-Phase II Trial including: Carrie Braverman; Kenneth A. Freedberg, MD, MSc; Susan Larrabee; A. David Paltiel, PhD; Mariesa Ricks; Paul Sax, MD; and Ron Walls, MD. We would like to thank the faculty and staff of the Brigham and Women's Hospital Emergency Department for their participation in the USHER-Phase II Trial and for their dedication to the identification of undiagnosed HIV infection. Dr. Walensky affirms that she has listed everyone who contributed significantly to the work in this Acknowledgment section.
Source of Funding: This research was funded by the National Institute of Mental Health (R01 MH073445, R01 MH65869) and the Doris Duke Charitable Foundation, Clinical Scientist Development Award to Rochelle P. Walensky.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Abstract presented at the International AIDS Society Conference, July 2011, Rome, Italy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22;55(RR-14):1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 2.Peralta L, Deeds BG, Hipszer S, Ghalib K. Barriers and facilitators to adolescent HIV testing. AIDS Patient Care STDS. 2007 Jun;21(6):400–408. doi: 10.1089/apc.2006.0112. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006 Mar;8(2):125–131. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 4.San Antonio-Gaddy M, Richardson-Moore A, Burstein GR, Newman DR, Branson BM, Birkhead GS. Rapid HIV antibody testing in the New York State Anonymous HIV Counseling and Testing Program: experience from the field. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):446–450. doi: 10.1097/01.qai.0000243055.65698.51. [DOI] [PubMed] [Google Scholar]
- 5.Spielberg F, Branson BM, Goldbaum GM, et al. Choosing HIV counseling and testing strategies for outreach settings: a randomized trial. J Acquir Immune Defic Syndr. 2005 Mar 1;38(3):348–355. [PubMed] [Google Scholar]
- 6.Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, del Rio C. Understanding the patient's perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev. 2004 Apr;16(2):101–114. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- 7.Peralta L, Constantine N, Griffin Deeds B, Martin L, Ghalib K. Evaluation of youth preferences for rapid and innovative human immunodeficiency virus antibody tests. Arch Pediatr Adolesc Med. 2001 Jul;155(7):838–843. doi: 10.1001/archpedi.155.7.838. [DOI] [PubMed] [Google Scholar]
- 8.Liang TS, Erbelding E, Jacob CA, et al. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care STDS. 2005 Apr;19(4):253–257. doi: 10.1089/apc.2005.19.253. [DOI] [PubMed] [Google Scholar]
- 9.Kowalczyk Mullins TL, Braverman PK, Dorn LD, Kollar LM, Kahn JA. Adolescent preferences for human immunodeficiency virus testing methods and impact of rapid tests on receipt of results. J Adolesc Health. Feb;46(2):162–168. doi: 10.1016/j.jadohealth.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Spielberg F, Kurth A, Gorbach PM, Goldbaum G. Moving from apprehension to action: HIV counseling and testing preferences in three at-risk populations. AIDS Educ Prev. 2001 Dec;13(6):524–540. doi: 10.1521/aeap.13.6.524.21436. [DOI] [PubMed] [Google Scholar]
- 11.Delaney KP, Branson BM, Uniyal A, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS. 2006 Aug 1;20(12):1655–1660. doi: 10.1097/01.aids.0000238412.75324.82. [DOI] [PubMed] [Google Scholar]
- 12.False-positive oral fluid rapid HIV tests--New York City, 2005-2008. MMWR Morb Mortal Wkly Rep. 2008 Jun 20;57(24):660–665. [PubMed] [Google Scholar]
- 13.Walensky RP, Arbelaez C, Reichmann WM, et al. Revising expectations from rapid HIV tests in the emergency department. Ann Intern Med. 2008 Aug 5;149(3):153–160. doi: 10.7326/0003-4819-149-3-200808050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai NP, Balram B, Shivkumar S, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis. Jan 23; doi: 10.1016/S1473-3099(11)70368-1. [DOI] [PubMed] [Google Scholar]
- 15.Walensky RP, Reichmann WM, Arbelaez C, et al. Counselor- versus provider-based HIV screening in the emergency department: results from the Universal Screening for HIV Infection in the Emergency Room (USHER) randomized controlled trial. Annals of Emergency Medicine. 2011 Jul;58(1):S126–S132.e124. doi: 10.1016/j.annemergmed.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuerz RC, Travers D, Gilboy N, Eitel DR, Rosenau A, Yazhari R. Implementation and refinement of the emergency severity index. Acad Emerg Med. 2001 Feb;8(2):170–176. doi: 10.1111/j.1553-2712.2001.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 17.Wuerz RC, Milne LW, Eitel DR, Travers D, Gilboy N. Reliability and validity of a new five-level triage instrument. Acad Emerg Med. 2000 Mar;7(3):236–242. doi: 10.1111/j.1553-2712.2000.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 18.Eitel DR, Travers DA, Rosenau AM, Gilboy N, Wuerz RC. The emergency severity index triage algorithm version 2 is reliable and valid. Acad Emerg Med. 2003 Oct;10(10):1070–1080. doi: 10.1111/j.1553-2712.2003.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 19.OraSure Technologies [27 January 2012];OraQuick ADVANCE Rapid HIV-1/2 Antibody Test [Customer letter] Accessed at www.orasure.com/products-infectious/products-infectious-oraquick.asp.
- 20.Liddicoat RV, Losina E, Kang M, Freedberg KA, Walensky RP. Refusing HIV testing in an urgent care setting: results from the “Think HIV” program. AIDS Patient Care STDS. 2006 Feb;20(2):84–92. doi: 10.1089/apc.2006.20.84. [DOI] [PubMed] [Google Scholar]
- 21.JRI Health HCoSM, Inc, AIDS Housing Corporation, AdCare Educational Institute HIV/AIDS provider training calendar September-December 2008.
- 22.White DA, Scribner AN, Huang JV. A comparison of patient acceptance of fingerstick whole blood and oral fluid rapid HIV screening in an emergency department. J Acquir Immune Defic Syndr. 2009 Sep 1;52(1):75–78. doi: 10.1097/QAI.0b013e3181afd33d. [DOI] [PubMed] [Google Scholar]
- 23.Hoxhaj S, Davila JA, Modi P, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Annals of Emergency Medicine. 2011 Jul;58(1):S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]