Abstract
Synergistic killing was achieved when Small Cell Lung Cancer (SCLC) cell lines were incubated with ABT-263 and an immunotoxin directed to the transferrin receptor. SCLC lines are variably sensitive to the BH-3 only peptide mimetic, ABT-263. To determine their sensitivity to toxin-based reagents, we incubated four representative SCLC lines with a model Pseudomonas exotoxin-based immunotoxin directed to the transferrin receptor. Remarkably in 4-of-4 lines, there was little evidence of immunotoxin-mediated cytotoxicity despite near complete inhibition of protein synthesis. However, when combinations of ABT-263 and immunotoxin were added to the ABT-263-resistant cell lines (H196 and H69AR), there was synergistic killing as evidenced by increased activation of caspase 3/7, annexin V staining and loss of cell integrity. Synergistic killing was evident at 6 hr and correlated with loss of Mcl-1. This synergy was also noted when the closely related compound ABT-737 was combined with the same immunotoxin. To establish that the synergy seen in tissue culture could be achieved in vivo, H69AR cells were grown as tumors in nude mice and shown to be susceptible to the killing action of an immunotoxin-ABT-737 combination but not to either agent alone. When immunotoxin-ABT combinations were added to ABT-263-sensitive lines (H146 and H1417), killing was additive. Our data support combination approaches for treating ABT-263-resistant SCLC with ABT-263 and a second agent that provides synergistic killing action.
Keywords: SCLC, ABT-263, ABT-737, Immunotoxin and Mcl-1
Introduction
Immunotherapy for cancer represents an important opportunity to achieve positive therapeutic outcomes with minimal damage to normal cells 1, 2. Approaches include the use of monoclonal antibodies 3 and their derivatives 4 and the administration of cell-based agents such as activated T-cells or T-cells transduced with chimeric antigen receptor (CAR) vectors 5.
Immunotoxins are antibody-based therapeutic proteins targeted to kill cancer cells expressing specific surface antigens or receptors 4, 6. Typically, the immunotoxin includes an Fv fragment, directing surface binding, which is joined with a bacterial or plant toxin responsible for cytotoxic action. Toxins are chosen because of their potency; resulting from their enzymatic activity. The protein synthetic machinery of eukaryotic cells is a frequent target of many protein toxins. Diphtheria toxin and Pseudomonas exotoxin (PE) each ADP-ribosylate EF2 and inhibit protein synthesis at the 'elongation step'. While ricin-related plant toxins inhibit protein synthesis via N-glycosidase action on ribosomal RNA 7, 8. Inhibition of protein synthesis is lethal for certain cancer cells, and this has been reflected in immunotoxin-mediated apoptotic responses in tissue culture experiments 9, 10, antitumor action in xenograft models of human cancer and the achievement of a high rate of complete remissions in patients with Hairy Cell Leukemia treated with immunotoxins directed to CD22 11, 12. Similarly, patients with B-ALL have benefited from combination immunotoxins to CD22 and CD19 7. Inhibition of protein synthesis frequently results in the loss of Mcl-1, a short-lived prosurvival Bcl-2 protein, and this may contribute to the potency of these protein toxins 13, 14. Other prosurvival proteins such as Bcl-2 and Bcl-xl are longer lived and when cancer cells depend on one of these, toxin-mediated killing may be more difficult to achieve. Further, Bcl-2 and Bcl-xl are frequently associated with resistance to chemotherapy 15.
Antibody-based therapies for cancer treatment have progressed significantly over the past 20 years 3. There are now more than 25 approved agents and many more in development 3. However, the treatment of Small Cell Lung Cancer (SCLC) has received relatively little attention compared to other cancers. CD56 was reported as a possible surface target and at least one clinical trial, using blocked ricin targeted to this antigen, reported a 25% objective response rate in SCLC with one PR and 3 stabilizations 16. Before constructing PE-based immunotoxins targeted to antigens expressed specifically on SCLC, we decided to evaluate a model immunotoxin directed to the transferrin receptor. Transferrin receptors are universally expressed on the surface of nucleated mammalian cells including cancer cells. Four representative SCLC cell lines, chosen for their variable sensitivity to ABT-263 (see below), were assayed for their sensitivity to this model PE-based immunotoxin, termed HB21-PE40.
SCLC is typically very difficult to cure with existing therapies and new approaches are clearly needed 17. One approach, recently evaluated, is the use of ABT-737 and it clinical analog, ABT-263 17. These two very closely related compounds kill cells via binding to and neutralization of the prosurvival proteins Bcl-2 and Bcl-xl. ABT-263 was evaluated as a single agent in Phase I and II trials for treating SCLC and results indicate that only minor objective responses can be achieved 18, 19. SCLCs that depend on Bcl-2 or Bcl-xl are most likely to respond to treatment 20. However, when survival is due to high levels of Mcl-1, a prosurvial protein that is unaffected by ABT-263/737, responses are typically poorer. Because PE based immunotoxins lead to loss of Mcl-1 14, 21, we decided to investigate responses to HB21-PE40, either alone or in combination with ABT-263/737. Four representative SCLC cell lines were chosen based on their variable sensitivity to ABT-263: the ABT-sensitive cell lines were H146 and H1417 and the resistant lines were H196 and H69AR.
Results indicated that ABT-resistant cells (which were also resistant to immunotoxin killing) were very sensitive to the combination of both compounds, indicating synergy. Apoptosis was evident as early as 6 hr after the addition of the combination. Neither agent alone was toxic. In ABT-sensitive lines, the action of ABT-263 and immunotoxin was additive.
Methodology
Materials and Methods
ABT-737 was purchased from Selleck Chemicals LLC, dissolved in DMSO at 10 mmol/L stock concentration, and stored frozen at −20°C. ABT-263 was obtained from Selleck Chemicals LLC, Inc., dissolved in 70% DMSO at 3 mmol/L, and stored frozen at −20°C. HB21-PE40 was produced recombinantly in Escherichia coli as described previously 22. Staurosporine was purchased from Sigma. PE Annexin V Apoptosis Detection Kit I was purchased from BD Biosciences. Propidium iodide for cell cycle analysis was purchased from Invitrogen. RNase was purchased from sigma.
Cell lines
The following SCLC lines were obtained from ATCC: H196, H146, H69AR and H1417. RPMI-1640 medium containing 2mM L-glutamine, 4.5g/L glucose, 10mM HEPES, 1mM sodium pyruvate and 1.5g/L sodium bicarbonate was purchased from ATCC.
Assays
WST-1 (Roche) and Caspase-Glo (Promega) measured cytotoxic activity and were used according to the directions supplied by the manufacturers. Routinely, cells were incubated for 48 hr prior to the addition of WST-1 or overnight when measuring caspase activity. To stain treated cells, methylene blue (0.5% w/v) in 50% (v/v) methanol/water was added for approximately 15 min. Treated cells were assayed for inhibition of protein synthesis by the addition of 3H-leucine (2 µCi/ml) for 4 hr in 96-well plates. Cells were collected on filter mats and samples counted using a Wallac Beta plate reader.
Tumors
H69AR were grown in Balb/c nude mice. 8×106 cells were mixed in serum free RPMI + Matrigel (4mg/ml) and injected subcutaneously in the flank of 5–6 week old athymic nude mice weighing approximately 20g. After tumor growth to a volume of 80mm3, treatment was initiated with vehicle, ABT-737 alone (50mg/kg), immunotoxin alone (0.4mg/kg) or immunotoxin plus ABT-737. For injections ABT-737 was first mixed with 30% propylene glycol, 5% Tween-80, and 3.3% D5W (pH 1.0), and 1% DMSO then sonicated, and pH adjusted 4–5. Immunotoxin, HB21-PE40 was prepared in 1× PBS+0.2% human serum albumin. Treatments of 200 ul were given via IP injection. Compounds or vehicle were administered daily for 8 days with ABT-737 given in the morning followed by HB21-PE40 4–5hrs later. The animal protocol was approved by the National Cancer Institute (NCI) Animal Care and Use Committee. According to protocol, mice were sacrificed routinely when tumors reached 1000mm3 in size. Statistical analyses of treatment responses were conducted for days 24–32 (while all animals were alive) using the Student’s two tailed t-test to determine if responses were significantly different from the 'vehicle' control. P values of less than 0.05 were considered statistically significant. GraphPad Prism software was used to calculate values.
Western blot analysis
immunotoxin-treated cells in the presence or absence of ABT-737, were washed with PBS and then solubilized with RIPA buffer containing both protease and phosphatase inhibitors. Precast NU-PAGE 8–16% gels were used to separate cell lysates. Lysates transferred to PVDF membranes were probed with rabbit anti-Mcl-1 (Cell Signaling Technology, Cat# 4572) to monitor changes in the level of this prosurvival protein. The primary antibody was detected with goat anti-rabbit-HRP (Jackson Immunoresearch).
Cell cycle analysis by flow cytometry
cells were incubated with HB21-PE40 (10ng/ml), ABT-263 (1uM), a combination of the two or staurosporine (1uM) for 8hrs. Following the treatment cells were washed in ice-cold PBS and fixed in 70% ethanol. Subsequently, the cells were treated with 0.1% RNase followed by staining with propidium iodide for 30 minutes at room temperature.
Apoptosis analysis by flow cytometry
Apoptosis was detected using PE-annexin-V staining and 7-Amino-Actinomycin (7-AAD) exclusion using the BD Pharmagen PE Annexin V Apoptosis Detection Kit I. Briefly, 105 cells were washed in PBS and resuspended in 1× binding buffer with 5µl of PE annexin V solution and 5 µl of 7-AAD. After incubating for 15 min at room temperature, the cells were evaluated using a FACScan flow cytometer with CellQuest software (BD Bioscience).
Results
Immunotoxin inhibits protein synthesis in SCLC lines
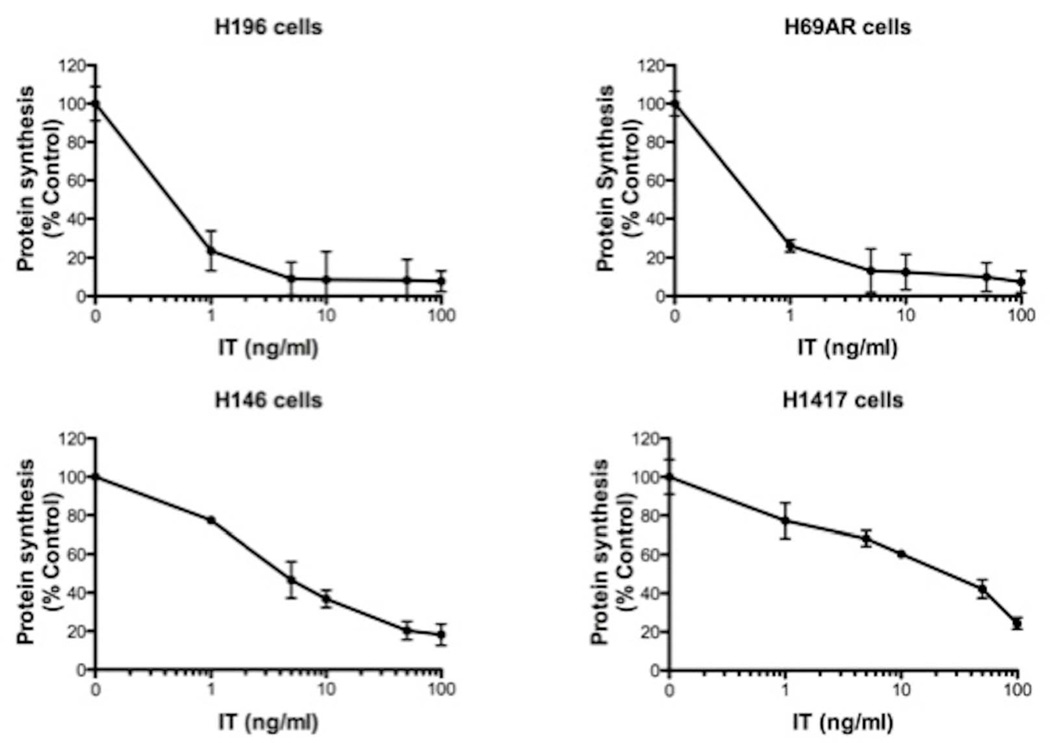
To assess the potential of immunotoxin therapy for treating SCLC, a model immunotoxin (using a range of concentrations from 1–100 ng/ml) directed to the human transferrin receptor (huTFR) was added to four representative cell lines. There was a dose dependent inhibition of protein synthesis in H196, H69AR, H146 and H1417 cells (Fig 1). At 24 hr post treatment, IC50 values were less than 1 ng/ml for H196 and H69AR cells, 3 ng/ml for H146 cells, and 25 ng/ml for H1417 cells. From these data we conclude that three (H146, H196, H69AR) of these lines are 'very sensitive', possessing the cellular 'machinery' to internalize and process sufficient amounts of immunotoxin to inhibit protein synthesis at external immunotoxin concentrations of less than 100 pM (62ng/ml of immunotoxin = 1 nM). H1417 cells are 'moderately sensitive' with an IC50 of approximately 400 pM.
Fig 1. Protein synthesis levels in four SCLC cell lines (H196, H69AR, H146 and H1417) treated with immunotoxin.
The immunotoxin, HB21-PE40, was added at various concentrations (0–100 ng/ml) as indicated and protein synthesis was determined by measuring the incorporation of 3H-leucine into cells 24h post treatment. To calculate 'percent control', treated wells were compared with control wells that did not receive immunotoxin.
Immunotoxin is poorly cytotoxic as a single agent
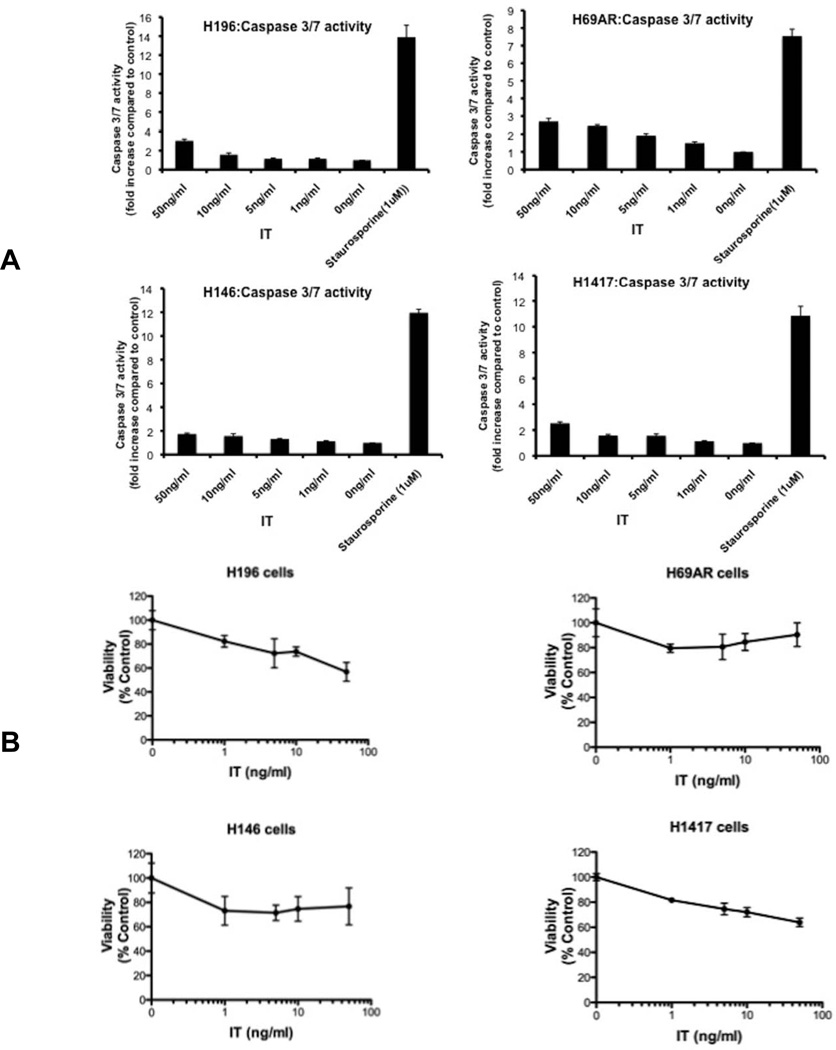
While inhibition of protein synthesis confirmed toxin action, it did not predict the fate of these cells. In many cell lines, toxin-mediated inhibition of protein synthesis results in the loss of viability and cell death via apoptosis 9. To assess immunotoxin-mediated apoptosis, we measured caspase activation after an overnight incubation with immunotoxin (1–50 ng/ml) and noted, paradoxically, that even at concentrations 10-fold higher that the IC50 value for inhibition of protein synthesis, there was little activation of caspase in any of the four cell lines tested (Fig 2A). However, these cells were not intrinsically resistance to apoptosis. For each cell line, staurosporine (1 uM) produced a 7–14 fold increase in caspase activity, confirming that the pathway for apoptosis was functional albeit with another agent (Fig 2A). By comparison, immunotoxin treatments even at 50 ng/ml generated no more than 2–3 fold increases in caspase activity. Caspase assays are frequently used to detect early events in the apoptotic pathway. We also assessed immunotoxin action at 48 hr using the WST-1 agent to measure viability. Using this assay, there was no more than a 40% reduction in viability (compared to untreated controls) for immunotoxin treatments up to 50 ng/ml, further confirming that SCLC cells exhibit resistance to immunotoxin-mediated cytotoxicity and apoptosis (Fig 2B).
Fig 2. Immunotoxin resistance.
(A). Caspase 3/7 activation in SCLC lines following immunotoxin treatment. SCLC cell lines were incubated with various concentrations of immunotoxin (HB21-PE40) or staurosporine (1uM) for 18h as indicated. Caspase 3/7 assays were performed as described in the materials and methods. Caspase activity is represented as fold change of luminescence units compared to control. (B). Viability of SCLC lines after immunotoxin treatment. HB21-PE40 was added to the four SCLC cell lines at various concentrations (0–50 ng/ml) as indicated. Viability, using the WST-1 reagent, was determined after 48h. The viability of cells is represented as 'percent control' compared to cells not treated with immunotoxin.
Synergistic killing via combinations of ABT-263/737 and immunotoxin
SCLC cell lines are reported to exhibit a range of sensitivities to ABT-26317, 23, 24. Thus, we thought it would be instructive to examine combinations of immunotoxin and ABT-263 in cells that exhibited either sensitivity or resistance to ABT-263. We confirmed that cell lines H196 and H69AR were resistant to apoptotic killing by ABT-263. (IC50 values for HI96, H69AR, H146 and H1417 cell lines treated with ABT-263 alone are supplied in Supplementary Figure 1). ABT-263 at a concentration of 1 uM produced little or no caspase activation when added to either of these lines (Fig 3, top panels). By contrast, H146 and H1417 were relatively sensitive to ABT-263 exhibiting a 6–8 fold increase in caspase activity (Fig 3, bottom panels). The ABT-resistant cell lines, H196 and H69AR, were killed readily with combinations of immunotoxin (10 ng/ml) and ABT-263 (1 uM) producing a 7–10 fold increase in caspase activity. Because neither compound caused apoptotic death when added as a single agent, and the combination did, this result constitutes clear evidence of synergy. When H196 cells were used to titrate the amount of ABT-263 that could enhance immunotoxin action, we detected reproducible synergy down to 50 nM ABT-263 (Supplementary Fig 2). Interpreting the responses of ABT-sensitive H146 and H1417 cell lines was more complex. For H146 and H1417 cells, a modest action of immunotoxin alone (10 ng/ml) plus the action of ABT-263 (1 uM) produced an additive cytotoxic response (Fig 3).
Fig 3. Caspase 3/7 activity following immunotoxin, ABT-263 or a combination of both agents.
Immunotoxin, HB21-PE40 (10ng/ml) or ABT 263 (1uM) were added to the four SCLC cell lines individually or in combination for 18h. Caspase 3/7 activity is represented as fold change of luminescence units compared to control.
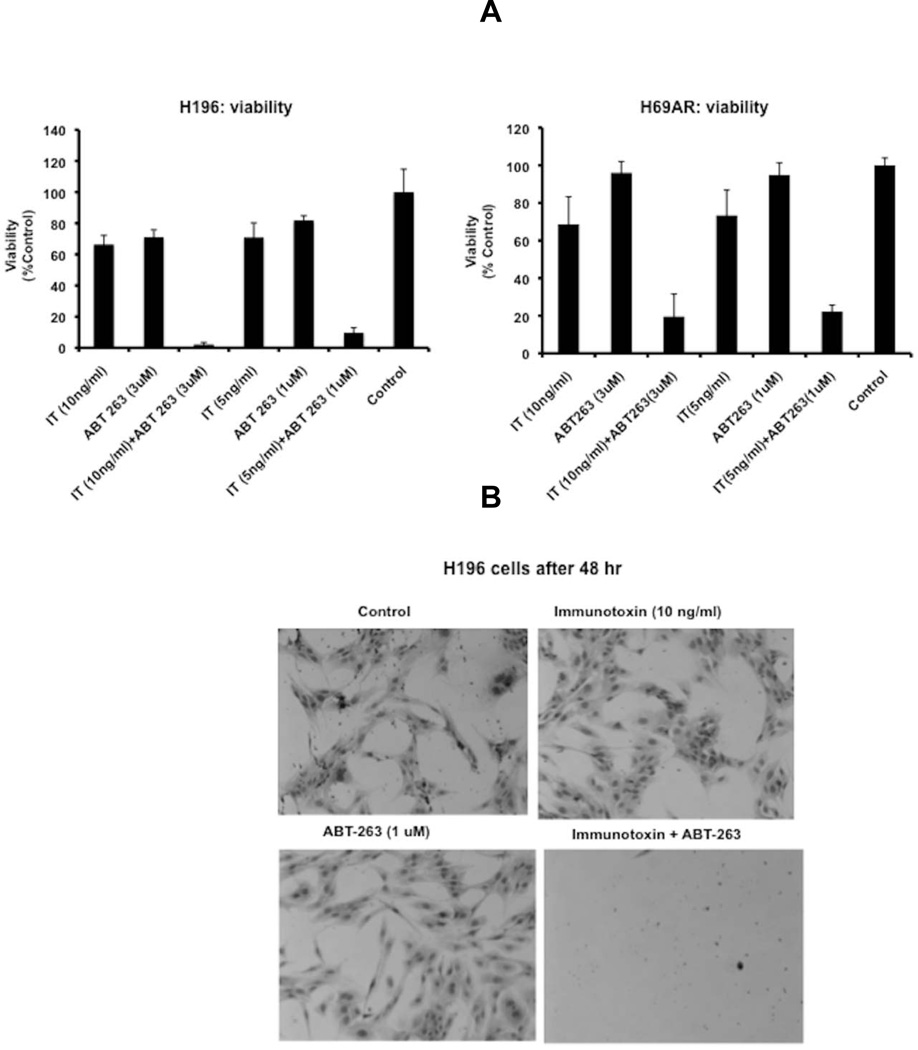
In viability assays, there was also clear evidence of synergy when immunotoxin and ABT were added to H196 and H69AR cells in the range of 5–10 ng/ml of immunotoxin and 1–3 uM ABT-263 (Fig 4 A and B). For H196 cells, this range of combination treatments resulted in complete loss of viability (Fig 4A and 4B). In H69AR cells there was an 80% loss of viability with the same concentrations of agents (Fig 4A). H196 cells were particularly sensitive to combinations of immunotoxin and ABT-263 and killing was clearly evident 'visually'. A representative micrograph of four treatments is presented in Fig 4B. Cells received: control, immunotoxin alone (10ng/ml), ABT-263 alone (1uM) or a combination treatment at the same concentrations for 48 hr. As can be seen from the photomicrograph of stained cells, the combination treatment showed no surviving cells while incubations with either the immunotoxin or ABT-263 alone revealed intact and apparently healthy cells (Fig 4B).
Fig 4. Cell viability following incubations with immunotoxin, ABT-263 or a combination of both.
(A). Immunotoxin, HB21-PE40 (10ng/ml) or ABT 263 (1uM) was added to H196 or H69AR cells individually or in combination for 48h. Cell viability was determined using the WST-1 reagent. The viability of cells is represented as % absorbance of cells at 450nm vs. control (cells not treated with immunotoxin). (B). H196 cells, cultured in 6-well plates, were treated with immunotoxin, (HB21-PE40 at 10ng/ml) or ABT 263 (3uM) individually or in combination for 48h. Surviving cells were visualized using methylene blue as the stain.
Two very similar agents have been developed to neutralize Bcl-2/xl/w, ABT-263 and ABT-737 25, 26. While ABT-737 was described first and has been studied most extensively 26, ABT-263 was developed for the clinic because of its bioavailability as an oral medicine 25 To determine if similar effects could be achieved using a combination of ABT-737 and immunotoxin, we repeated viability experiments comparing the activity of the two compounds on H196 cells. Results are reported in supplementary Fig 3 and reveal that ABT-737 and ABT-263 have similar actions both qualitatively and quantitatively (compare supplementary Fig 3 with Fig 4A).
Early onset of apoptosis correlates with loss of Mcl-1
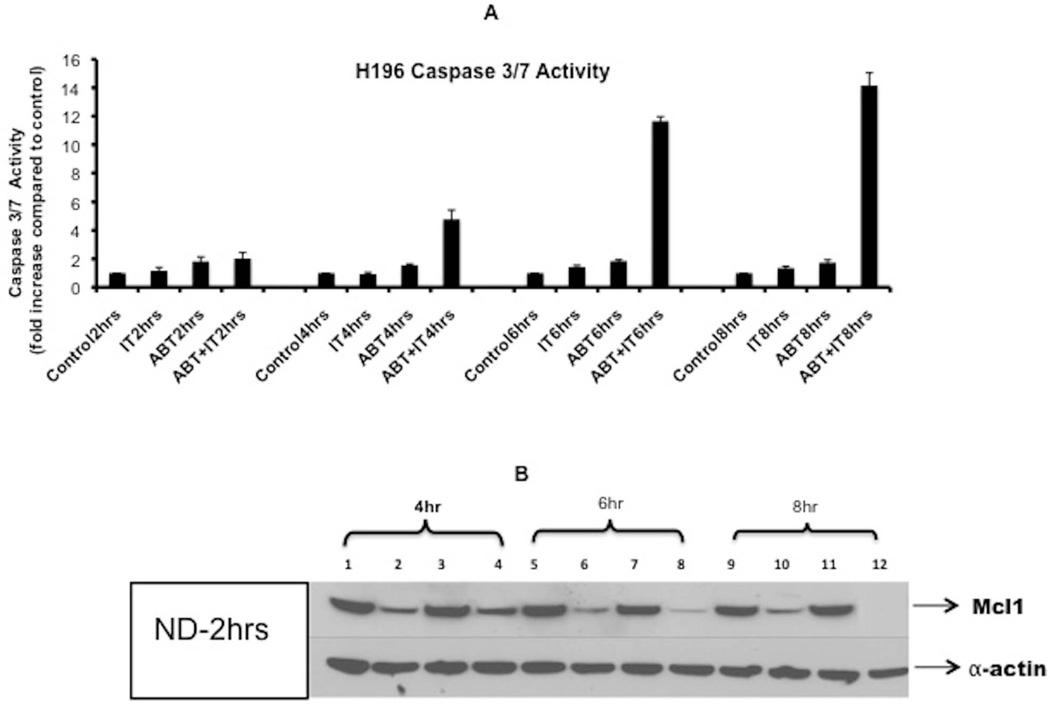
Visually, combination treatments added to either H196 or H69AR cells showed evidence of cytotoxic damage as early as 6–8 hrs after the addition of immunotoxin plus ABT-263 (data not shown). Therefore we conducted additional experiments at early time points to monitor the onset of apoptosis via caspase activation (Fig 5) and cell surface Annexin V staining (Supplementary Figure 5). We also probed cells for loss of Mcl-1 (Fig 5). Previously, Mcl-1 was identified as a marker of insensitivity in the preclinical development of ABT-737 and ABT-263 for the treatment of SCLC 25, 26. Therefore it was important to determine if our combination treatments were accompanied by a loss of Mcl-1. Analyses confirmed that as early as 6hr post treatment with a combination of ABT-263 and immunotoxin, extensive (greater than 10-fold) caspase activation was evident while most Mcl-1 was lost from the cells (Fig 5). At eight hours there was a complete loss of Mcl-1 (Fig 5, lane 12) and slightly more (14-fold) activation of caspase. Thus the loss of Mcl-1 was correlated with restoration of ABT-263 sensitivity and cell death. Further, in H196 cells treated with immunotoxin alone, there was a loss of Mcl-1, presumably representing inhibition of protein synthesis and degradation of this short-lived prosurvival protein. However, loss of Mcl-1 alone was not sufficient to cause apoptosis.
Fig 5. Early activation of caspase was coincident with loss of Mcl-1.
(A) Immunotoxin, HB21-PE40 (10ng/ml) or ABT 263 (1uM) , were added to the H196 cell line, individually or in combination. Caspase 3/7 activity was measured at time points from 2–8h. Caspase 3/7 activity is represented as fold change of luminescence units compared to control. (B) H196 cells were treated with HB21-PE40 (10ng/ml) or ABT 263 (1uM), individually and in combination for indicated time periods. Immunoblot for MCL1 was done as indicated in materials and methods. No sample was taken at 2 h. However, for each of the other time points, the sample order was the same: control (lanes 1,5,9) immunotoxin alone (lanes 2,6,10) ABT-263 alone (lanes 3,7,11) and the combination (lanes 4,8,12). Alpha actin was used as the loading control.
Frequently, cytotoxic treatments are preceded or accompanied by cell cycle arrest 27,28. To investigate the effect of immunotoxin-ABT combinations on cell cycle status we exposed asynchronous H69AR or H196 cells to immunotoxin (10ng/ml), ABT-263 (1uM) or a combination of both for 8 hr and then analyzed DNA content (Supplementary Figs 4A and 4B) or apoptotic status by Annexin V and 7-AAD staining (Supplementary Figure 5). The treatment of these lines for 8 hrs with immunotoxin-ABT combinations demonstrated no consistent changes in cell cycle status (data are presented in supplementary results section), even though the cells clearly showed signs of apoptosis (Supplementary Figure 5). The addition of staurosporine at 1 uM produced 42% cell death in H196 and 70% in H69AR (Supplementary Figure 5). To achieve similar levels of death, immunotoxin-ABT combinations were necessary as neither agent alone showed evidence of cell death (Supplementary Figure 5).
Combination treatments in mice bearing H69AR xenografts
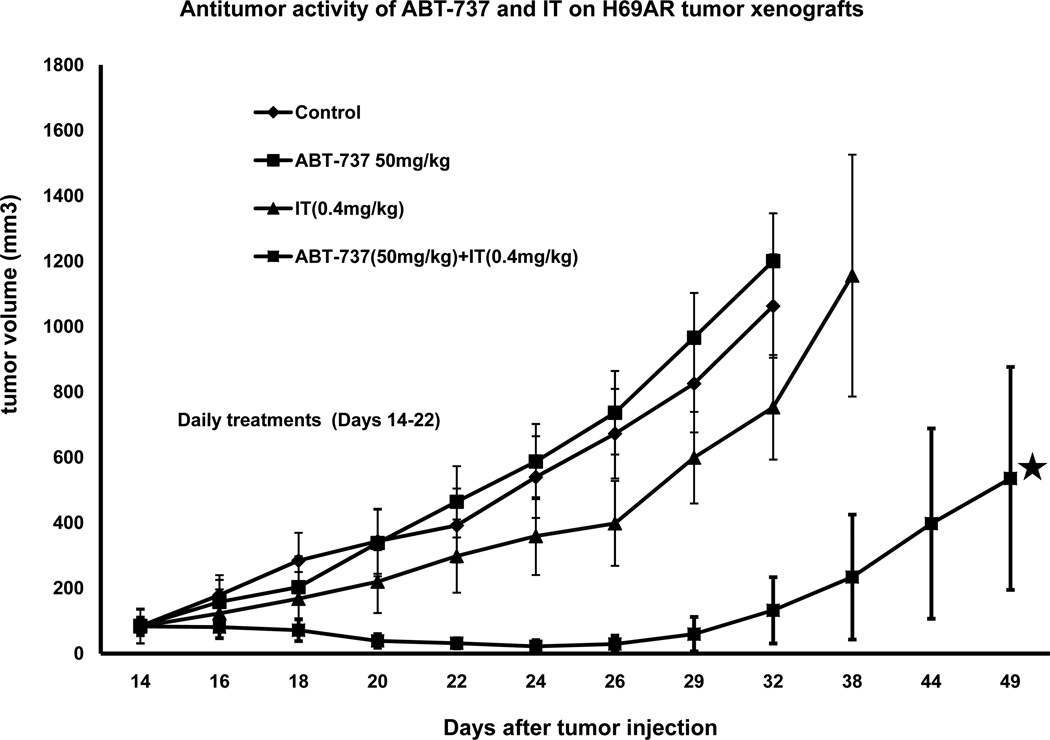
To assess immunotoxin-ABT combination treatments for treating tumors in vivo, we injected athymic nude mice with 8 × 10^6 H69AR cells. While H196 cells were most sensitive to immunotoxin-ABT combinations in cell culture, H69AR cells were chosen for in vivo experiments because, in contrast to H196 cells, they produced reproducible subcutaneous tumors within 2 weeks of injection. H69AR cells were injected in the presence of Matrigel (4 mg/ml). After tumors had grown to 80–100 mm3 (approximately 14 days post injection) treatments began with either 'vehicle', ABT-737 alone, immunotoxin alone or a combination of both agents (Fig 6 & Supplementary Figure 6). ABT-737 was chosen because its formulation allows parenteral administration at concentrations high enough to achieve therapeutic levels. ABT-263 is usually administered orally and we had no method to assess absorption levels following administration via this route (see discussion). Treatments consisted of daily intraperitoneal injections of ABT-737 (50 mg/kg) or vehicle followed 4–5 hr later with an IP injection of immunotoxin (0.4 mg/kg) or vehicle. Daily treatments were for 8 days as indicated in Fig 6. Mice were also weighed on a daily basis. Results indicated that only the combination of ABT-737 and immunotoxin produced a clear antitumor effect. There was a modest retardation of growth with the immunotoxin alone but this was not statistically significant. The entire experiment was repeated with a second set of mice and produced an identical result. Further, a third experiment using 25 mg/kg ABT-737 in combination with immunotoxin (0.4 mg/kg) produced an antitumor response. Treatment outcomes on days 24, 26, 29 and 32 (when all mice were alive) were analyzed statistically. Using a student's t-test only the group receiving immunotoxin plus ABT-737 was statistically different from the vehicle group with p-values of 0.038 (on day 24) to 0.024 (on day 32) (see supplementary Table 1 for additional information). Combination treatments resulted in ~15% reduction in body weight (by day 8 of treatment). Body weight was regained rapidly two days after the treatment period ended. We conclude that combination treatments of immunotoxin plus ABT-737 produce synergistic antitumor activity and could be used to treat tumors resistant to either agent.
Fig 6. Antitumor effects of immunotoxin-ABT-737 combinations.
H69AR cells (8×106 ), injected in matrigel (4mg/ml), were implanted in nude mice (3 per group). Treatments began when the tumor size reached approximately 80 mm3. Mice received either 'vehicle', ABT −737 alone (50mg/kg/i.p.), immunotoxin alone (0.4mg/kg/i.p.) or a combination of both where the immunotoxin was administered ~4hr after the injection of ABT-737. Routinely, mice were sacrificed when the tumor size reached 1000mm3. ⋆ Mice in the combination group that developed ulcers were sacrificed on day 49.
Discussion
Combination therapy has been a mainstay of cancer chemotherapy for several decades. And ABT-263 has only modest activity in treating patients with SCLC in early clinical trials where the compound was administered as a single agent 18, 19. Here we describe a novel combination of agents that may guide the development of therapies for ABT-263-resistant SCLC. SCLC cells are variably sensitive to ABT-263 and for those cancers that are resistant to ABT-263 we propose the addition of second agent, such as an immunotoxin, that can greatly enhance tumor killing. Rarely, do two agents act in such a clearly synergistic way. Neither compound was cytotoxic yet the combination was profoundly lethal. Further, since the compounds are inactive individually, a fold-enhancement calculation cannot be made. And while the ABT-263 compound is likely to be present systemically, the use of an immunotoxin to target only cancer cells can provide this combination with tumor specificity. Here we show that two ABT-263 resistant lines, H196 and H69AR are killed by the addition of two agents that, individually, are non-toxic. Tissue culture results were recapitulated in vivo with anti-tumor action.
For those cancer cells that exhibit susceptibility to ABT-263, the addition of a second treatment agent can add to the cytotoxic potential. Again, the second agent, when targeted only to the cancer cells, can add specificity to the combination treatment. We noted no synergy with ABT-263 sensitive cells. However, for H146 and H1417 cells there was evidence of additivity, especially in caspase assays, reflecting enhanced apoptotic death.
Our in vivo results also indicated synergistic responses but tumor eradication was not complete. In our experience, immunotoxin delivery to every cell in a xenograft tumor model is rarely achieved. In the case of H69AR tumors, responses likely reflected only those cells that received adequate amounts of both compounds. Improved access to tumor cells has been reported with agents that disrupt tumor architecture suggesting that 'access' is a rate-limiting step 29, 30. Further, H196 cells, which showed the most dramatic killing in tissue culture, when treated with combinations of immunotoxin and ABT-263/737, did not grow well as tumors in athymic nude mice. Therefore it is noteworthy that we achieved synergistic tumor killing even with a less than optimal choice of cells.
Several commentaries on the use of ABT-263 have suggested the need for additional cytotoxic compounds that can reduce Mcl-1 levels. Generally these will be non-targeted. Here we use an immunotoxin approach where the immunotoxin can be targeted to the tumor cells. As antigen expression becomes better defined for SCLC, either CD56 or another surface target could be the focus of immunotoxin development.
Antitumor results were achieved with ABT-737 rather than ABT-263. ABT-263 is given to patients as an oral medicine. While we considered a gavage approach for treating tumor-bearing mice, we had no assays available to measure absorption and blood levels. Further, ABT-263 could not be formulated to inject 1 mg of drug in 0.2 ml (standard dosing for parenteral injection). Therefore, we administered ABT-737 via injection where formulations allowed systemic delivery. Results indicated in triplicate experiments that only the combination treatment produced statistically significant antitumor activity, thus lending support to this approach for treating ABT-263-resistant SCLC.
Previously, we reported on the activity of immunotoxins targeted to epithelial cancers and the fact that they could be enhanced by ABT-737 21. These results were extended in a recent report from Andersson's group showing a similar result could be achieved using immunotoxins directed to a high MW antigen expressed on melanoma 31. In these published reports, 'moderate' immunotoxin activity was enhanced by the addition of ABT-737. Here we report on a remarkable level of synergy In the case of ABT-resistant SCLC lines, two non-cytotoxic treatments, when combined, resulted in rapid and complete cell death.
The development of immunotoxins specifically targeted to CD56 on SCLC has been reported. Blocked ricin was used as the toxin 16. Regarding the future development of PE-based immunotoxins targeted to SCLC, it would seem prudent to consider combination treatment rather than administer immunotoxins as a single agent especially if the cell line data reflect human tumor responses. Based on our results, ABT-263 would be a suitable combination agent but others agents may also be discovered via screens of chemical libraries.
Supplementary Material
Acknowledgements
We thank Craig Thomas, National Center for Advancing Translational Sciences, National Institutes of Health for advice on the formulation of ABT-737 for injection. Also, Robert Sarnvosky, LMB, CCR, NCI and Richard Beers LMB, CCR, NCI for providing key immunotoxin agents.
Grant Support:
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of conflicts of interest: No potential conflicts of interest are disclosed.
References
- 1.Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012 doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future direction. J Gene Med. 2012 doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weldon JE, Pastan I. A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–4700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindler J, Gajavelli S, Ravandi F, Shen Y, Parekh S, Braunchweig I, Barta S, Ghetie V, Vitetta E, Verma A. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol. 2011;154:471–476. doi: 10.1111/j.1365-2141.2011.08762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Keppler-Hafkemeyer A, Brinkmann U, Pastan I. Role of caspases in immunotoxin-induced apoptosis of cancer cells. Biochemistry. 1998;37:16934–16942. doi: 10.1021/bi980995m. [DOI] [PubMed] [Google Scholar]
- 10.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–334. [PubMed] [Google Scholar]
- 11.Kreitman RJ, Arons E, Stetler-Stevenson M, Fitzgerald DJ, Wilson WH, Pastan I. Recombinant immunotoxins and other therapies for relapsed/refractory hairy cell leukemia. Leuk Lymphoma. 2011;52 Suppl 2:82–86. doi: 10.3109/10428194.2011.565843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, Fitzgerald DJ, Wilson WH, Pastan I. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogner C, Dechow T, Ringshausen I, Wagner M, Oelsner M, Lutzny G, Licht T, Peschel C, Pastan I, Kreitman RJ, Decker T. Immunotoxin BL22 induces apoptosis in mantle cell lymphoma (MCL) cells dependent on Bcl-2 expression. Br J Haematol. 2010;148:99–109. doi: 10.1111/j.1365-2141.2009.07939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson Y, Juell S, Fodstad O. Downregulation of the antiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor-Pseudomonas exotoxin a immunotoxin. Int J Cancer. 2004;112:475–483. doi: 10.1002/ijc.20371. [DOI] [PubMed] [Google Scholar]
- 15.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 16.Lynch TJ, Jr, Lambert JM, Coral F, Shefner J, Wen P, Blattler WA, Collinson AR, Ariniello PD, Braman G, Cook S, Esseltine D, Elias A, et al. Immunotoxin therapy of small-cell lung cancer: a phase I study of N901-blocked ricin. J Clin Oncol. 1997;15:723–734. doi: 10.1200/JCO.1997.15.2.723. [DOI] [PubMed] [Google Scholar]
- 17.Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, Oleksijew A, O'Connor JM, Wang B, Frost DJ, Bauch J, Marsh K, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–3277. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, Chu Q, Giaccone G, Khaira D, Ramalingam SS, Ranson MR, Dive C, et al. Phase 2 Study of Single Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H, Elmore SW, Kroeger PE, Tse C, Rosenberg SH, Anderson MG. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther. 2010;9:545–557. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]
- 21.Traini R, Ben-Josef G, Pastrana DV, Moskatel E, Sharma AK, Antignani A, Fitzgerald DJ. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther. 2010;9:2007–2015. doi: 10.1158/1535-7163.MCT-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batra JK, Fitzgerald DJ, Chaudhary VK, Pastan I. Single-chain immunotoxins directed at the human transferrin receptor containing Pseudomonas exotoxin A or diphtheria toxin: anti-TFRFv)-PE40 and DT388-anti-TFRFv) Mol Cell Biol. 1991;11:2200–2205. doi: 10.1128/mcb.11.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, Lin X, Hierman JS, Wilburn DL, Watkins DN, Rudin CM. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, Rosenberg SH, Tse C. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 25.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 26.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 27.Meikrantz W, Schlegel R. Apoptosis and the cell cycle. J Cell Biochem. 1995;58:160–174. doi: 10.1002/jcb.240580205. [DOI] [PubMed] [Google Scholar]
- 28.Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 29.Zhang Y, Xiang L, Hassan R, Paik CH, Carrasquillo JA, Jang BS, Le N, Ho M, Pastan I. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci U S A. 2007;104:17099–17104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risberg K, Fodstad O, Andersson Y. Synergistic anticancer effects of the 9.2.27PE immunotoxin and ABT-737 in melanoma. PLoS One. 2011;6:e24012. doi: 10.1371/journal.pone.0024012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.