Abstract
We previously reported strain-specific susceptibility to dexamethasone-induced osteonecrosis in mice. Here we report that BALB/cJ and BALB/cAnNHsd mice display substrain-specific differences in dexamethasone-induced adverse effects. As compared with BALB/cJ mice, BALB/cAnNHsd weighed more (16.6 g compared with 13.7 g) at the beginning of dexamethasone administration on postnatal day 28 and fewer died during the dexamethasone regimen (10% compared with 50%). Although the 2 substrains had similar plasma concentrations of dexamethasone, BALB/cJ mice were more susceptible to developing dexamethasone-induced osteonecrosis. A higher dose of dexamethasone (8 mg/L) throughout the treatment period compared with a lower dose (8 mg/L loading dose during week 1 followed by 4 mg/L for the remainder of the treatment period) and earlier start of treatment (postnatal day 24 compared with postnatal day 28) was required to induce osteonecrosis with a similar frequency in BALB/cAnNHsd mice as in BALB/cJ mice. Our results show, for the first time, substrain-specific differences in the development of osteonecrosis in mice.
Abbreviations: P, postnatal day
Osteonecrosis is a severe and relatively common dexamethasone-induced dose-limiting toxicity.6 We previously screened 14 mouse strains and found that only BALB/cJ and C57BL/6J developed dexamethasone-induced osteonecrosis.13 Strain-specific differences in drug disposition and development of phenotypes are well documented and attributed to the different genetic backgrounds of these strains.1,5,7,9 Furthermore, substrains, which differ by only minor genetic differences,2,4,8,11,12 and even identical strains from different vendors, can also differ significantly with respect to some phenotypes.3 Because we observed unexpectedly high mortality due to steroid-induced toxicity in the BALB/cJ substrain, we tested for dexamethasone tolerance and osteonecrosis in the BALB/cAnNHsd substrain. The 2 substrains showed striking differences; the BALB/cAnNHsd substrain had lower toxicity and better survival and was more resistant to developing glucocorticoid-induced osteonecrosis.
Materials and Methods
Diet and reagents.
Because folate insufficiency can contribute to glucocorticoid-induced osteonecrosis,13 we continued to use an irradiated folic-acid–deficient diet that contained less than 0.05 ppm folic acid (TestDiet, Richmond, IN). Dexamethasone sodium phosphate solution was obtained from American Pharmaceutical Partners (Schaumburg, IL). Sulfamethoxazole (200 mg) and trimethoprim (40 mg) per 5 mL oral suspension was obtained from Hi-Tech Pharmacal, (Amityville, NY) and tetracycline was purchased from Sigma (St Louis, MO).
Animals.
BALB/cJ mice (Mus musculus) were obtained from the maximum barrier facility of Jackson Laboratories (age, 24 d; stock number 000651, Sacramento, CA), and BALB/cAnNHsd were obtained from Harlan Laboratories (age, 21 and 24 d; catalog number 4704M BALB/cAnNHsd, Houston, TX). Mice that were 21 d old were shipped with foster mothers during transport due to the young age of the mice. Because male mice were observed to be more susceptible to development of dexamethasone-induced osteonecrosis than were female mice,13 only male mice were included in the current study. All experiments were conducted in accordance with a protocol approved by the IACUC of St Jude Children's Research Hospital (Memphis, TN). Mice were checked for health daily initially and twice daily after they started showing any early possible symptoms of toxicity. Mice that became moribund were immediately euthanized according to IACUC-approved procedures.
Mice were SPF in our facility for fur mites, pinworms, mouse parvovirus type 1, mouse parvovirus type 2, minute virus of mice, parvovirus generic assay, mouse hepatitis virus, Theiler murine encephalomyelitis virus, epizootic diarrhea of infant mice, Sendai virus, pneumonia virus of mice, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, and ectromelia virus. Indirect health surveillance was accomplished with the use of traditional outbred (ICR) sentinels and dirty-bedding exchange. The sentinels were tested quarterly for the organisms listed earlier. Mice were housed 3 to 5 mice per cage (Micro Vent System 75 JAG, Allentown, Allentown, NJ) with corncob bedding (Andersons Bed-O'cobs, Pharmaserv, Framingham, MA). Relative humidity was maintained on average at 50%, and the animal room had a 12:12-h light:dark cycle in an AAALAC-accredited facility. Folic-acid–deficient diet (Test Diet) and water were provided ad libitum. Medications (dexamethasone, tetracycline, and sulfamethoxazole–trimethoprim oral suspension) were added to drinking water, which was changed twice weekly. Mice were observed daily and weighed weekly, and all animal handling was performed in biosafety cabinets.
Treatment regimens.
Treatment generally started at postnatal day 28 (P28), except where indicated that treatment started at P24. Mice in control groups were treated only with prophylactic antibiotics, while mice in dexamethasone treatment groups were treated with low- or high-dose dexamethasone regimen and prophylactic antibiotics. The low-dose dexamethasone treatment regimen consisted of an initial loading dose of 8 mg/L during the first week of therapy and a maintenance dose of 4 mg/L for the remaining weeks, whereas the high-dose dexamethasone treatment regimen consisted of 8 mg/L of dexamethasone throughout the treatment period. All BALB/cJ and most BALB/cAnNHsd mice were treated with dexamethasone for 6 wk; some cohorts of BALB/cAnNHsd mice were treated for 8.5 to 9 wk in an effort to increase the frequency of osteonecrosis in these mice. Prophylactic antibiotics to prevent dexamethasone-induced opportunistic infections included tetracycline (1 g/L) constantly and sulfamethoxazole (600 mg/L) and trimethoprim (120 mg/L) given 3.5 d of every week; where noted, mice were treated with amoxicillin (4 mg/L) instead of tetracycline (1 g/L), and these antibiotics have no effect on the development of osteonecrosis.13 In a few cases, amoxicillin was used instead of tetracycline to prevent terminal loss of mice due to opportunistic infections caused by dexamethasone treatment, but it had no effect on overall survival or the development of osteonecrosis.
Mice were treated with dexamethasone in the drinking water because it is palatable and can easily be administered in that manner for the long time periods (6 to 9 wk) needed to induce the phenotype of glucocorticoid-induced osteonecrosis.13 Moreover, intermouse pharmacokinetic variability is low,13 and the low trauma and convenience of administration in drinking water (compared with oral gavage or intraperitoneal injection) avoids the possibility of loss of mice due to trauma that would be incurred by thrice-daily dosing (to mimic dexamethasone dosage in pediatric patients6). At dexamethasone concentrations in the drinking water of 4 to 8 mg/L, dosing is equivalent to 1.33 to 2.66 mg/kg daily, assuming that the average weight of a mouse is 15 g and that the water consumption rate is 5 mL daily. Daily consumption of 5 mL/day of drinking water was confirmed in preliminary experiments.
Estimation of plasma concentrations of dexamethasone and corticosterone.
At the end of dexamethasone treatment (6 or 9 wk) between 0900 and 1200, mice were anesthetized with 2% isoflurane, and blood (500 to 1000 µL) was collected via cardiocentesis. Plasma was stored at −80 °C until further analysis. Dexamethasone and corticosterone were extracted from plasma and quantified by HPLC, as described previously.6,13
Aerobic enteric cultures.
Fecal pellets from a single mouse per cage were placed in tryptic soy broth and incubated at 37 °C for 24 h, after which the broth culture was used to inoculate a blood agar plate and a MacConkey agar plate by using a sterile cotton swab; these plates were incubated at 37 °C for 24 h. When no growth was observed, the plates were incubated for an additional 48 h to confirm lack of growth. No growth from fecal pellets at the midpoint or end of treatment might reflect a decreased microbial load in the gastrointestinal tract or feces due to prophylactic antibiotic treatment. All bacterial colonies observed on the blood agar or MacConkey agar plates were transferred to fresh blood agar plates to obtain pure cultures. Gram staining and analysis (Vitek II Compact System, BioMerieux, Durham, NC) were used to identify the organisms. Antibiotic sensitivity of bacterial isolates was determined by using the zone of inhibition assay.
Detection of osteonecrosis.
Osteonecrosis was detected by histologic examination, as described earlier.13 Briefly, bone samples were fixed in 10% formalin overnight, followed by decalcification in TBD2 (Thermo Fisher Scientific, Waltham, MA). Specimens were processed routinely, paraffin-embedded, cut at 4 µm, stained with hematoxylin and eosin, and analyzed by light microcopy. The analysis focused on the distal femur based on the results of preliminary screening of multiple appendages.13 Bone samples were analyzed by one of the authors (KB), who was blinded to the treatment arm. Osteonecrotic lesions were defined by the presence of all of the following criteria: empty lacunae, pyknotic nuclei of ghost osteocytes in the bone trabeculae, and necrosis of the neighboring marrow and stromal elements. Mice having an osteonecrotic lesion in at least one stifle joint (distal femur or proximal tibia) were considered positive for osteonecrosis.
Statistical analysis.
The log-rank test was used to evaluate the difference in survival between different substrains of BALB/c mice and treatment groups. Comparisons of body weight between substrains were performed by using the Kruskal–Wallis test and repeated-measures ANOVA. Differences in the frequency of osteonecrosis were evaluated using a χ2 test. All statistical analyses were performed by using Statistica software (version 10; StatSoft, Tulsa, OK). A P value of less than 0.05 was used to define statistical significance.
Results
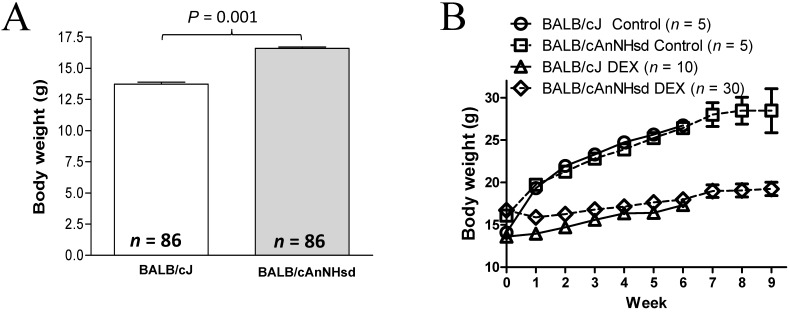
On P28, BALB/cAnNHsd mice were about 2.5 g (21%) heavier (P = 0.001) than BALB/cJ mice (Figure 1 A). The untreated BALB/cJ mice gained weight and reached weights similar (P = 0.15) to those of untreated BALB/cAnNHsd mice within 1 wk. Body weight was lower (P = 1 × 10−5) in dexamethasone-treated mice than in saline-treated mice. Dexamethasone-treated BALB/cJ mice required about 4 wk to achieve body weights similar to those of dexamethasone-treated BALB/cAnNHsd mice (Figure 1 B).
Figure 1.
(A) Body weight (g) of BALB/cJ and BALB/cAnNHsd at the start of treatment (postnatal day 28). (B) Body weight (g) in dexamethasone-treated (DEX; low dose: 8 mg/L during week 1 followed by 4 mg/L thereafter) and untreated (Control) mice throughout the experiment.
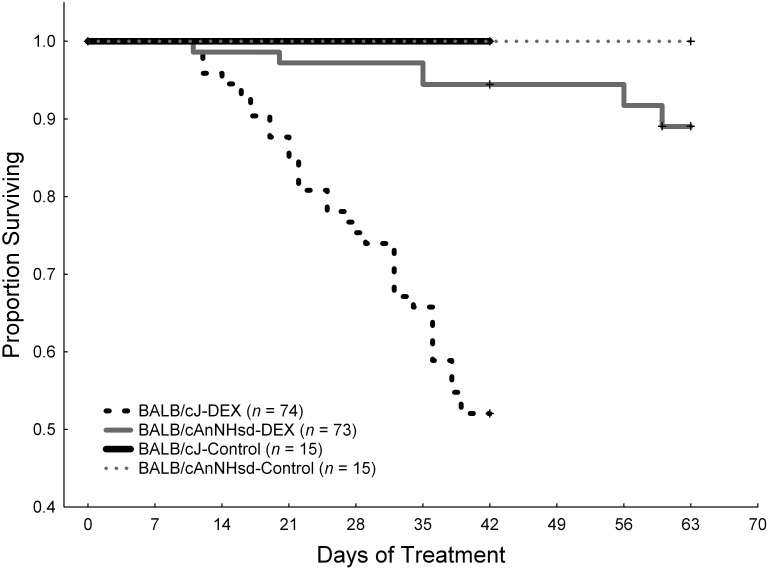
BALB/cJ mice on P28 treated with the low-dose dexamethasone regimen (8 mg/L during week 1 and 4 mg/L thereafter) had 50% survival at the end of 6 wk of therapy compared with 95% survival (P = 1 × 10−5) in BALB/cAnNHsd mice treated with either the low-dose or high-dose (8 mg/L throughout the experiment) dexamethasone regimen that began treatment on either P24 or P28 (Figure 2). Death of mice in the dexamethasone-treated group was attributed to sepsis, because blood cultures tested positive for pathogens (Enterococcus gallinarum in 5 BALB/cJ mice and E. faecalis in 1 BALB/cAnNHsd mouse) in all 6 of the moribund mice from which blood cultures could be obtained.
Figure 2.
Kaplan–Meier survival curves of dexamethosone-treated (DEX) or untreated (Control) BALB/cJ and BALB/cAnNHsd (A) from 4 experiments combined in which BALB/cAnNHsd were treated beginning on postnatal day (PD) 24 or 28 with either the low- or high-dose dexamethasone regimen, and BALB/cJ mice were treated with the low-dose dexamethasone regimen starting on PD28. Censoring at 42 d (6 wk) indicates the euthanasia of a cohort of mice that had completed therapy and their analysis for osteonecrosis. Survival differed significantly (P = 1 × 10−5; log-rank test) between BALB/cJ DEX and BALB/cAnNHsd DEX groups. Note that subsets of BALB/cJ (n = 22) and BALB/cAnNHsd (n = 20) mice received amoxicillin for antimicrobial prophylaxis and that this treatment did not affect survival.
To investigate whether aerobic gastrointestinal flora differed between BALB/cAnNHsd and BALB/cJ substrains and whether changes in microbial sensitivity (according to the zone of inhibition assay) to prophylactic antibiotics might explain the difference in the frequency of death of mice due to sepsis between the 2 substrains, we characterized enteric (fecal) aerobic culture isolates at the start of therapy, at the midpoint (3 wk after the start of therapy), and at the end of treatment (6 wk after the start of therapy). BALB/cAnNHsd mice had more types of microbial isolates at the start, midpoint, and end of treatment than did BALB/cJ mice (Table 1). Furthermore, more of the isolates cultured before treatment of BALB/cAnNHsd mice were sensitive to the prophylactic antibiotics compared with those from BALB/cJ mice. Isolates from both BALB/cAnNHsd and BALB/cJ mice developed antibiotic-resistant Enterococcus faecalis after exposure to prophylactic antibiotics and dexamethasone; however, both substrains of mice also displayed new resistant microbes at the end of therapy that had not been present prior to therapy (Table 1).
Table 1.
Results of enteric culture isolates from BALB/cAnNHsd and BALB/cJ mice
| BALB/cAnNHsd |
BALB/cJ |
|||||
| Isolate | Tetracycline sensitivity | Trimethoprim sensitivity | Isolate | Tetracycline sensitivity | Trimethoprim sensitivity | |
| Before the start of treatment | ||||||
| Enterococcus faecalis | sensitive | sensitive | Enterococcus faecalis | resistant | sensitive | |
| Escherichia coli | sensitive | sensitive | Staphylococcus xylosus | sensitive | sensitive | |
| Enterococcus durans | sensitive | sensitive | Staphylococcus epidermidis | sensitive | resistant | |
| Lactobacillus spp. | sensitive | sensitive | ||||
| Staphylococcus sciuri | sensitive | intermediate | ||||
| Midpoint (3 wk after the start of treatment) | ||||||
| Enterococcus faecalis | resistant | sensitive | Enterococcus faecalis | resistant | resistant | |
| Staphylococcus aureus | resistant | resistant | Enterococcus gallinarum | resistant | sensitive | |
| Staphylococcus epidermidis | sensitive | resistant | ||||
| End of treatment (6 wk after the start of treatment) | ||||||
| Enterococcus faecalis | resistant | resistant | Enterococcus faecalis | resistant | resistant | |
| Staphylococcus aureus | resistant | resistant | Enterococcus gallinarum | resistant | sensitive | |
| Enterococcus avium | resistant | sensitive | ||||
| Bacillus spp. | sensitive | sensitive | ||||
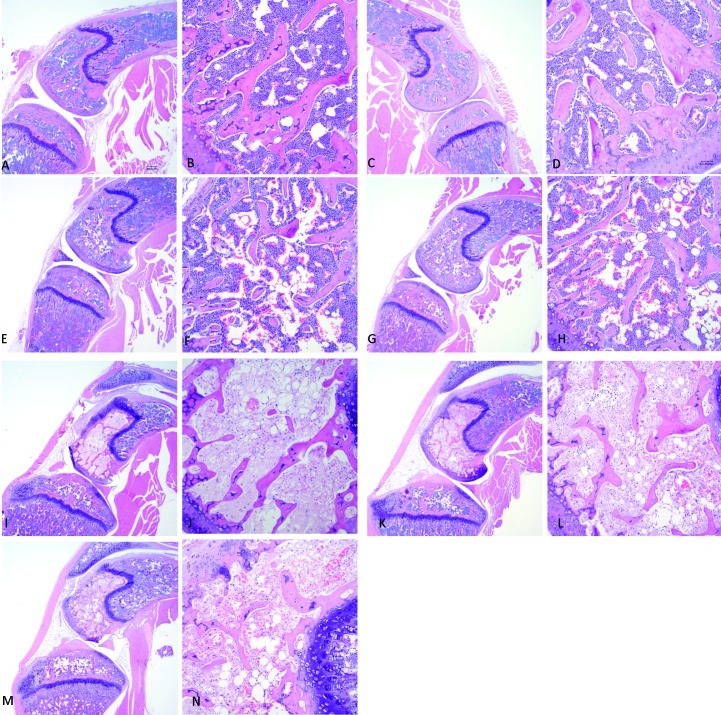
When BALB/cAnNHsd mice started treatment with the low-dose dexamethasone regimen at P28, they did not develop significant osteonecrosis (one positive for osteonecrosis among a total of 20 mice), whereas this regimen induced osteonecrosis in P28 BALB/cJ mice (9 positive for osteonecrosis among a total of 34 mice; P = 0.027). Therefore, we tested whether starting treatment earlier (P24 compared with P28) and at a higher dose (8 mg/L throughout the 6-wk treatment period) increased the frequency of osteonecrosis in BALB/cAnNHsd mice. Whereas 50% of P24 BALB/cAnNHsd mice treated with the 8-mg/L dexamethasone regimen for 6 wk (3 of 6) or 8.5 wk (2 of 4) developed osteonecrosis, none (n = 14) of the P24 BALB/cAnNHsd mice treated with the low-dose regimen (8 mg/L during week 1 followed by 4 mg/L thereafter) developed osteonecrosis, regardless of treatment duration (P = 0.01). Representative osteonecrotic lesions are shown in Figure 3. The high-dose dexamethasone regimen tended to be more toxic, with 95% probability of survival for P24 low-dose BALB/cAnNHsd mice at the end of 8.5 wk compared with 55% for high-dose mice (P = 0.08, log-rank test). Among mice that developed osteonecrosis, the extent of lesions was similar in the 2 substrains, usually involving greater than 50% of the area through the femoral condyles in each slide.
Figure 3.
Histology of stifle joints in untreated control BALB/cJ mice on postnatal day 28 (P28) at magnifications of (A) 4× and (B) 20×, in untreated P24 BALB/cAnNHsd mice at magnifications of (C) 4× and (D) 20×; in P24 BALB/cAnNHsd mice treated with the low-dose dexamethasone regimen (8 mg/L during week 1 and then 4 mg/L throughout weeks 2 through 6 wk) at magnifications of (E) 4× and (F) 20×; and in dexamethasone-treated P24 BALB/cAnNHsd (8 mg/L during week 1 and 4 mg/L for weeks 2 through 9) at magnifications of (G) 4× and (H) 20×. Osteonecrotic lesions are evident in P24 BALB/cAnNHsd mice treated with the high-dose dexamethasone regimen (8 mg/L for 6 wk) at magnifications of (I) 4× and (J) 20×; in P24 BALB/cAnNHsd treated with dexamethasone (8 mg/L for 8.5 wk) at magnifications of (K) 4× and (L) 20×; and in P28 BALB/cJ mice on the low-dose dexamethasone regimen (8 mg/L during week 1 and 4 mg/L for weeks 2 through 6) at magnifications of (M) 4× and (N) 20×.
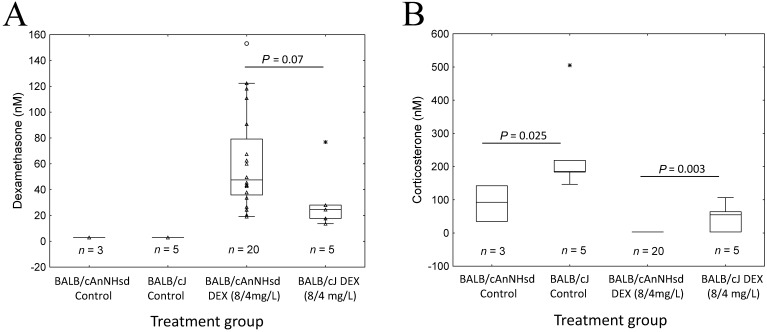
To investigate whether the substrain differences in frequency of osteonecrosis and toxicity were due to different plasma levels of dexamethasone, we measured plasma concentrations of dexamethasone and corticosterone (a marker of dexamethasone pharmacodynamic effect) at the end of the experiment (Figure 4 A). Dexamethasone levels did not differ significantly between BALB/cAnNHsd and BALB/cJ mice and tended to be higher in BALB/cAnNHsd mice (P = 0.07). As expected, corticosterone levels inversely reflected dexamethasone exposure, with lower levels in BALB/cAnNHsd compared with BALB/cJ mice in control (P = 0.025) and in dexamethasone-treated (P = 0.003, Figure 4 B) groups.
Figure 4.
Plasma concentrations of (A) dexamethasone and (B) corticosterone at the time of euthanasia in BALB/cJ and BALB/cAnNHsd mice treated for 6 wk with the low-dose (8 mg/L during week 1 followed by 4 mg/L thereafter) dexamethasone regimen (DEX) and their untreated controls.
Discussion
Strain-specific differences in phenotypes due to variation in genetic background are well documented.1,5,7,9 We previously reported that only 2 (BALB/cJ and C57BL/6J) of 14 murine strains tested were susceptible to developing dexamethasone-induced osteonecrosis, with the BALB/cJ strain having the higher frequency of osteonecrosis.13 Over time, we observed an unacceptable increase in loss of mice due to sepsis among dexamethasone-treated BALB/cJ mice. Because substrains can show significant differences in phenotype,2,4,8,11,12 we investigated whether the BALB/cAnNHsd substrain would have acceptable toxicity and still be susceptible to dexamethasone-induced osteonecrosis. We found that, compared with BALB/cJ mice, the BALB/cAnNHsd substrain had less toxicity and better survival in response to dexamethasone treatment, but they also were less susceptible to developing dexamethasone-induced osteonecrosis. By starting treatment at a younger age (P24 compared with P28) and increasing the dexamethasone dose, we were able to induce osteonecrosis in BALB/cAnNHsd mice at a frequency similar to that in BALB/cJ mice.
At P28, BALB/cAnNHsd mice weighed more than did BALB/cJ mice (Figure 1). The difference in body weight could be due to differences in the diet used in each vendor facility or to faster pubertal development in BALB/cAnNHsd mice. However, starting dexamethasone treatment earlier (P24) in the BALB/cAnNHsd substrain did not overcome their relative resistance to dexamethasone-induced osteonecrosis, but doubling the dose combined with earlier treatment (P24) resulted in osteonecrosis in these mice.
Because blood cultures revealed that aerobic bacteria were responsible for sepsis in some of the treated mice, we cultured aerobic bacteria of the gastrointestinal tract. The antibiotic sensitivity and composition of aerobic bacteria differed among the various groups of mice prior to treatment. By 3 wk after the start of treatment, E. faecalis isolated from BALB/cJ mice developed resistance to both tetracycline and trimethoprim, but the isolate from BALB/cAnNHsd mice was susceptible to trimethoprim. However, aerobic enteric cultures at the end of the experiment indicated that the E. faecalis isolates from both BALB/cAnNHsd and BALB/cJ mice were resistant to trimethoprim. In addition, E. gallinarum was isolated only from enteric cultures of moribund BALB/cJ mice. Overall, the differences in aerobic gastrointestinal flora and sensitivity to prophylactic antibiotics may explain why BALB/cAnNHsd mice had better survival in response to dexamethasone treatment than did BALB/cJ mice. The composition of anaerobic microflora may affect body weight and overall susceptibility to infection in the substrains of mice. Obligate anaerobes comprise most of the colonic microbiota, with facultative anaerobes being about approximately 1000-fold fewer.10 Additional analysis of the gastrointestinal microflora in these mice may provide insight into the underlying causes of these differences; however, this evaluation was beyond the scope of the current study.
The lower frequency of osteonecrosis and toxicity in BALB/cAnNHsd mice compared with BALB/cJ mice was not due to higher dexamethasone plasma clearance; in fact, dexamethasone plasma levels tended to be higher in BALB/cAnNHsd than in BALB/cJ mice (Figure 4 A). Corticosterone levels were higher in untreated and dexamethasone-treated BALB/cJ mice compared with BALB/cAnNHsd mice (Figure 4 B), indicating physiologic and pharmacodynamic differences between these substrains.
In summary, we report significant differences between BALB/cJ and BALB/cAnNHsd mice with regard to their initial body weight, aerobic gastrointestinal flora, and survival and incidence of osteonecrosis after dexamethasone treatment. Considerable modification of the dexamethasone dose and age of mice at start of therapy was required to induce similar frequencies of osteonecrosis in the 2 substrains.
Acknowledgments
Supported by NCI grants CA 142665 and CA 21765 and by the American Lebanese Syrian Associated Charities (ALSAC). We thank Mr C Mike Straign for helping us to characterize the enteric and blood cultures from mice and their antibiotic sensitivity and Sean Savage, Lourie West, Pamela Johnson, and other members of the St Jude Veterinary Pathology Core lab for their assistance in preparing the tissues and slides and histology to analyze the stifle joints for osteonecrosis. We also thank Xiangjun Cai for measuring the plasma concentrations of dexamethasone and corticosterone.
References
- 1.Eisenstein TK, Meissler JJ, Jr, Rogers TJ, Geller EB, Adler MW. 1995. Mouse strain differences in immunosuppression by opioids in vitro. J Pharmacol Exp Ther 275:1484–1489 [PubMed] [Google Scholar]
- 2.Glant TT, Bardos T, Vermes C, Chandrasekaran R, Valdez JC, Otto JM, Gerard D, Velins S, Lovasz G, Zhang J, Mikecz K, Finnegan A. 2001. Variations in susceptibility to proteoglycan-induced arthritis and spondylitis among C3H substrains of mice: evidence of genetically acquired resistance to autoimmune disease. Arthritis Rheum 44:682–692 [DOI] [PubMed] [Google Scholar]
- 3.Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. 2011. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med 61:356–360 [PMC free article] [PubMed] [Google Scholar]
- 4.Henricks KK, Miner LL, Marley RJ. 1997. Differential cocaine sensitivity between 2 closely related substrains of C57BL mice. Psychopharmacology (Berl) 132:161–168 [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Takahashi M, Sudo K, Sugiyama Y. 2007. Marked strain differences in the pharmacokinetics of an α4β1 integrin antagonist, 4-[1-[3-chloro-4-[N-(2-methylphenyl)-ureido]phenylacetyl]-(4S)-fluoro-(2S)-pyrrolidine-2-yl]-methoxybenzoic acid (D01-4582), in Sprague–Dawley rats are associated with albumin genetic polymorphism. J Pharmacol Exp Ther 320:124–132 [DOI] [PubMed] [Google Scholar]
- 6.Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, Neale G, Howard SC, Evans WE, Pui CH, Relling MV. 2011. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood 117:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marks MJ, Romm E, Gaffney DK, Collins AC. 1986. Nicotine-induced tolerance and receptor changes in 4 mouse strains. J Pharmacol Exp Ther 237:809–819 [PubMed] [Google Scholar]
- 8.Nicholson SM, Peterson JD, Miller SD, Wang K, Dal Canto MC, Melvold RW. 1994. BALB/c substrain differences in susceptibility to Theiler murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol 52:19–24 [DOI] [PubMed] [Google Scholar]
- 9.Ralph RJ, Paulus MP, Geyer MA. 2001. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J Pharmacol Exp Ther 298:148–155 [PubMed] [Google Scholar]
- 10.Sekirov I, Finlay BB. 2009. The role of the intestinal microbiota in enteric infection. J Physiol 587:4159–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvia OJ, Urosevic N. 1999. Variations in LPS responsiveness among different mouse substrains of C3H lineage and their congenic derivative sublines. Immunogenetics 50:354–357 [DOI] [PubMed] [Google Scholar]
- 12.Teuscher C, Hickey WF, Korngold R. 1990. Experimental allergic orchitis in mice. V. Resistance to actively induced disease in BALB/cJ substrain mice is mediated by CD4+ T cells. Immunogenetics 32:34–40 [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Boyd K, Kaste SC, Kamdem Kamdem L, Rahija RJ, Relling MV. 2009. A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday. J Orthop Res 27:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]