Abstract
Cardiovascular diseases involve the heart or blood vessels and remain a leading cause of morbidity and mortality in developed countries. A variety of animal models have been used to study cardiovascular diseases and have contributed to our understanding of their pathophysiology and treatment. However, mutations or abnormal expression of specific genes play important roles in the pathophysiology of some heart diseases, for which a closely similar animal model often is not naturally available. With the advent of techniques for specific genomic modification, several transgenic and knockout mouse models have been developed for cardiovascular conditions that result from spontaneous mutations. However, mouse and human heart show marked electrophysiologic differences. In addition, cardiac studies in mouse models are extremely difficult because of their small heart size and fast heart rate. Therefore, larger genetically engineered animal models are needed to overcome the limitations of the mouse models. This review summarizes the transgenic rabbit models that have been developed to study cardiovascular diseases.
Abbreviations: APD, action potential duration; apo, apolipoprotein; LCAT, lecithin-cholesterol acyltransferase; LPL, lipoprotein lipase; LQT, long QT; MHC, myosin heavy chain; MMP, matrix metalloproteinase
Since the generation of the first transgenic rabbit was reported in 1985,25,64 transgenic rabbit lines have been established and used as animal models for a variety of human diseases, especially cardiovascular diseases. Although transgenic and knockout mice are predominantly used as models for many human diseases, the causative mutations of some human diseases do not lead to corresponding pathologic changes in mice.7 In addition, whereas the major myosin heavy chain (MHC) in rodents is α-MHC, β-MHC is predominantly expressed in larger mammals, including rabbits and humans.15,43 Moreover, rats and mice are considered inappropriate for modeling cardiac ion channel disorders such as long QT (LQT) syndrome because the ionic mechanisms of repolarization in adult rats and mice differ from those in larger species, including humans.52 In addition, various techniques—such as those for studies of the LQT syndrome and hypertrophic cardiomyopathy—are difficult to apply to mice due to their small size and phylogenetic features.10,40,48,52 Alternatively, because of their intermediate size between rodents and farm animals, rabbits (Oryctolagus cuniculus) are well-suited to various physiologic manipulations; these attributes have made rabbits appealing models of diverse human diseases. Since the generation of transgenic rabbits that overexpressed hepatic lipase,21 transgenic rabbits have often been generated for use as models of cardiovascular diseases, including atherosclerosis, hypertrophic cardiomyopathy, and LQT syndrome. The current review focuses on recent publications involving transgenic rabbit models of human cardiovascular diseases.
Transgenic Rabbit Models for Atherosclerosis
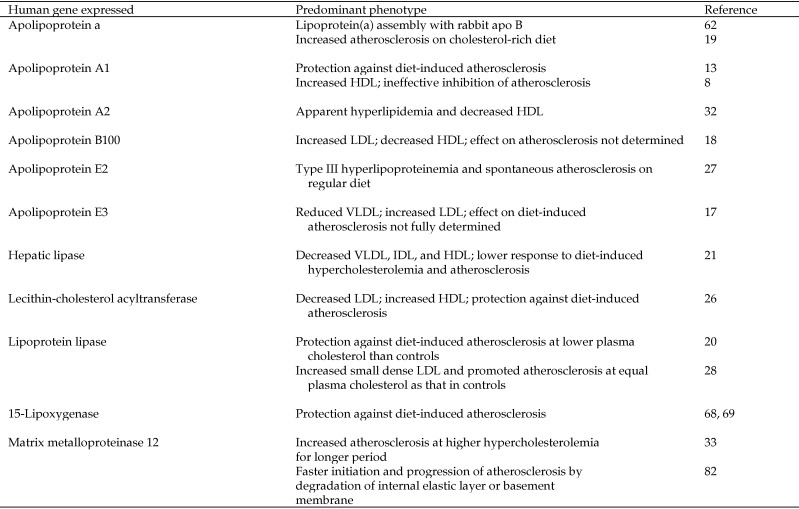
Atherosclerosis, which is characterized by thickened artery walls due to the accumulation of fatty materials such as cholesterol, remains a leading cause of death and disability in developed countries. High plasma concentrations of cholesterol (hypercholesterolemia), especially LDL cholesterol, are an important risk factor in the formation of atherosclerotic lesions. Because of their intermediate size between rodents and the large-animal species commonly used for research, ease of manipulation, quick response to dietary cholesterol, and similar lipoprotein metabolism to that of humans, laboratory rabbits have been used widely in studies of atherosclerosis. Hypercholesterolemia can be induced rapidly in rabbits by administration of a high-cholesterol diet for only a few days.6 Cholesterol-fed rabbits have become the classic model for studying lipoproteins and their roles in atherosclerosis. The unique characteristics of this rabbit model include low plasma concentrations of total cholesterol, high activity of cholesterol ester transfer protein, low hepatic lipase activity, and the lack of an analog of human apolipoprotein (apo) A2.9 The generation and use of transgenic rabbits have attracted great interest in the study of the pathogenesis of atherosclerosis during the last decade. The number of applications of transgenic rabbit models for the studies of lipid metabolism and atherosclerosis has gradually increased since 1994, when the first report of a transgenic rabbit model was published.21 To date, transgenic rabbit lines expressing nearly a dozen proteins involved in atherogenesis have been established, including those for human apo a, apoA1, apoA2, apoB, apoE2, apoE3, hepatic lipase, lecithin–cholesterol acyltransferase (LCAT), lipoprotein lipase (LPL), 15-lipoxygenase, matrix metalloproteinase (MMP) 12, and macrophage metalloelastase (Figure 1). Most of these transgenic rabbit models have been well characterized and are discussed in other review articles.9,23 Studies of these transgenic rabbits have helped to elucidate the metabolic roles of diverse proteins in atherogenesis.
Figure 1.
Transgenic rabbit models for lipid metabolism and atherosclerosis.
apo a.
Apolipoproteins are a group of proteins that bind with lipids to form lipoproteins and transport the lipids through the lymphatic and circulatory systems. apo a is a component of lipoprotein a and occurs naturally only in humans and Old World nonhuman primates. apo a can be bound to apoB100 through a disulfide linkage to form the lipoprotein a complex.12 Increased plasma levels of lipoprotein a are associated with increased incidence of cardiovascular disease, stroke, and restenosis.41,74 To further investigate the assembly of the lipoprotein a complex and its role in atherosclerosis, transgenic rabbit lines expressing human apo a were established by 2 independent groups.16,62 In the plasma of the transgenic rabbits, human apo a associated with rabbit endogenous apoB to form a chimeric lipoprotein a complex. In contrast, human apo a cannot bind to murine apoB in the plasma of transgenic mice. Therefore, results from studies in the transgenic rabbits provide new insights into the expression of apo a and the assembly of the lipoprotein a complex. In addition, these transgenic rabbits are a potential model for studying the lipoprotein a complex and its role in promoting atherosclerosis. When fed a diet containing 0.3% cholesterol for 16 wk, rabbits transgenic for human apo a showed more extensive atherosclerotic lesions than did nontransgenic controls, although both groups showed similarly increased plasma cholesterol levels19 Results from the cited study suggest that lipoprotein a may have proatherogenic effects in rabbits transgenic for apo a that were fed a cholesterol-rich diet.
apoA.
apoA1 is the major protein component of HDL in plasma. Prospective epidemiologic studies suggest that high levels of HDL and apoA1 protect against the progression of atherosclerosis.24,44 To study the effect of apoA1 in the inhibition of atherosclerosis, transgenic rabbits expressing human apoA1 were fed a cholesterol-rich diet (0.48 g cholesterol per 120 g of the diet).14 The plasma level of HDL cholesterol in the transgenic group was twice that of the control group. Correspondingly, the atherosclerotic lesions in the thoracic aorta were reduced by 50% in transgenic rabbits compared with nontransgenic controls. Results from the cited study14 indicated that the protective effects of human apoA1 on cholesterol-rich diet-induced atherosclerosis were associated with increased HDL levels, possibly through the mechanism of reverse cholesterol transport.13 However, a later study showed that human apoA1 transgenic rabbits with high HDL cholesterol levels were not protected against the development of atherosclerosis when the rabbits were fed a cholesterol-rich diet, which induced dramatic hypercholesterolemia.8 Therefore, the role of human apoA1 in atherogenesis needs to be further characterized.
A recent study reported the production of transgenic rabbits expressing human apoA232 Data from preliminary analyses of apoA2 expression in these rabbits indicate that apoA2 plays an important role in the metabolism of both VLDL and HDL by promoting apparent hyperlipidemia and decreasing HDL levels. Although the precise mechanism remains to be elucidated, rabbits transgenic for apoA2 may provide a new model for studying human familial combined hyperlipidemia.
apoB100.
The development of transgenic rabbits expressing human apoB100 was reported in 1995.18 Analysis of plasma lipid concentrations demonstrated that total plasma cholesterol and triglyceride levels were 2 to 3 times higher in rabbits transgenic for human apoB100 than in nontransgenic controls. In addition, cholesterol and human apoB100 were found predominantly in the LDL fraction and were accompanied by significant enrichment of the triglyceride content.18 Further study indicated that the LDL of the apoB100-transgenic rabbits contained large amounts of apoC3 and apoE, suggesting that overexpression of apoB100 in liver might lead to the assembly in these animals of nascent triglyceride-rich particles that had the size and density of LDL rather than of VLDL9 However, the susceptibility of apoB100-transgenic rabbits to atherosclerosis has not been determined.
apoE2.
The apoE2 variant is associated with the human genetic disorder type III hyperlipoproteinemia.59 The generation of transgenic rabbits that overexpressed human apoE2 in their plasma was reported in 1997.27 Overexpression (30 to 70 mg/dL) of human apoE2 in these transgenic rabbits led to marked accumulation of β-migrating VLDL and significantly increased concentrations of VLDL and IDL, suggesting type III hyperlipoproteinemia. In addition, susceptibility to atherosclerosis was assessed in both male and female transgenic rabbits and nontransgenic controls, all of which received a normal rabbit diet. The study27 demonstrated that both male and female transgenic rabbits, but not nontransgenic controls, developed spontaneous atherosclerosis in the aortic arch and proximal abdominal aorta. However, male transgenic rabbits showed more extensive atherosclerosis than did female transgenic rabbits, suggesting that sex hormones may play a role in modulating type III hyperlipoproteinemia.27
Hepatic lipase.
Hepatic lipase is expressed in the liver and adrenal glands, and one of its principal functions is to convert IDL to LDL.63 Transgenic rabbits that overexpressed human hepatic lipase were generated for studying the effects of this enzyme in development of atherosclerosis.21 Overexpression of human hepatic lipase showed significant effects on plasma lipid and lipoprotein levels in the transgenic rabbits. Total cholesterol and triglyceride levels were reduced by 42% and 58%, respectively, in the transgenic rabbits in comparison to wildtype controls. Lipoprotein analysis revealed a significant reduction of HDL, VLDL, and IDL in the transgenic rabbits. When rabbits were fed a diet with 0.3% cholesterol, attenuated hypercholesterolemia developed in the transgenic rabbits but not in nontransgenic littermates.21 Data from a subsequent study suggest that the attenuated hypercholesterolemia in hepatic lipase transgenic rabbits might be associated with a diminished extent of aortic atherosclerosis.73
LCAT.
The enzyme LCAT is involved in cholesterol and HDL metabolism. The study of transgenic rabbits revealed that overexpression of human LCAT substantially changed plasma lipid and lipoprotein concentrations.26 Plasma total, free, and esterified cholesterol concentrations as well as phospholipid concentrations were significantly increased in transgenic rabbits in comparison to their wildtype littermates.26 The elevation in plasma total cholesterol contents was associated with a marked increase in HDL concentration. Analyses of other lipoproteins also revealed markedly increased apoA1 and apoE concentrations but reduced levels of apoB in the plasma of the LCAT transgenic rabbits.26 In addition, the LCAT transgenic rabbits showed significantly reduced atherosclerosis after they had been fed a diet containing 0.3% cholesterol for 17 d.26
Lipoprotein lipase.
LPL is a key enzyme involved in hydrolysis of triglyceride-rich lipoproteins in lipid and lipoprotein metabolism. To further study its physiologic role, transgenic rabbits expressing human LPL were established.1 Compared with levels in nontrangenic controls, LPL activity was 4 times higher in the transgenic rabbits, but plasma triglyceride was decreased by 80% and HDL by 59%. Analysis of the lipoprotein-dense fractions from transgenic rabbits revealed that overexpression of human LPL led to remarkably decreased levels of VLDL and IDL. An initial study for susceptibility to atherosclerosis in the transgenic rabbits showed marked protection against diet-induced hypercholesterolemia and development of aortic atherosclerosis.20 However, plasma cholesterol levels in the transgenic rabbits were significantly lower than those in control rabbits.28 The antiatherogenicity may have been due to the increased LPL activity itself or the antiatherogenic effect of LPL may have been dependent on the LPL lipid-lowering effect. To clarify these possibilities, LPL transgenic rabbits and control littermates were fed diets with different amounts of cholesterol (0.3% to 0.6%) to adjust and maintain their plasma cholesterol concentrations to similarly high levels for 16 wk.28 This subsequent study demonstrated that the aortic atherosclerosis in the transgenic rabbits was nearly twice as extensive as that in control rabbits, even though both lines had the same level of high hypercholesterolemia. In addition, overexpression of human LPL in transgenic rabbits decreased remnant lipoproteins (that is, β-VLDL) but concomitantly significantly increased large and small LDL compared with the levels in nontransgenic controls.28 The results of these studies led to the conclusion that “LPL exerts a dual function in terms of its atherogenicity, namely antiatherogenicity, through enhancing receptor-mediated remnant lipoprotein catabolism, and proatherogenicity, by the generation of a large amount of small-sized LDL.”28
MMP12.
MMP are zinc-dependent endopeptidases, belonging to a large family of proteases known as the metzincin superfamily.5 MMP12 was first identified as a potent elastolytic metalloproteinase specifically secreted by macrophages.67 The activity of MMP12 subsequently was noted to be increased in many inflammatory processes, including atherosclerosis, and expression of MMP12 was prominently upregulated in atherosclerotic lesions in cholesterol-fed rabbits.2,42,77 To further study the pathophysiologic roles of MMP12 in atherosclerosis, expression of human MMP12 gene was targeted in macrophages of transgenic rabbits.22,33 A preliminary study showed no significant difference in either aortic atherosclerotic lesion size or quality between transgenic and nontransgenic rabbits with lower hypercholesterolemia.33 However, transgenic rabbits with higher hypercholesterolemia for longer periods developed more extensive atherosclerosis in the aortas and coronary arteries than did nontransgenic controls.33 Moreover, increased expression of MMP12 derived from macrophages was associated with elevated expression of MMP3. Results from these studies suggested that macrophage-derived MMP12 plays a pivotal role in the cascade action of other MMP, which may be involved in exacerbating degradation of the extracellular matrix during the progression of atherosclerosis.33 In a subsequent study to investigate the relationship between macrophage migration and elastolysis to fatty streaks, MMP12 transgenic rabbits fed a 1% cholesterol diet for 6 wk developed more pronounced fatty streaks, associated with more significant degradation of the internal elastic layer, than did nontransgenic controls.81 In addition, transgenic rabbits had more infiltrating macrophages and smooth muscle cells in the lesions than did control rabbits, suggesting that MMP12 may be critical to both initiation and progression of atherosclerosis by degradation of the elastic layers or basement membrane and indicating that these transgenic rabbits may provide a potential system to test specific MMP12 inhibitors for the treatment of progressive atherosclerosis.81
Other factors involved in atherogenesis.
Other transgenic rabbit lines that have been reported for studying atherosclerosis include those involving apoE3, apoB mRNA editing enzyme catalytic polypeptide 1, and 15-lipoxygenase.17,68,69,82 In addition, the generation of double-transgenic rabbits through the crossbreeding of 2 different single-transgenic lines and of Watanabe transgenic rabbits is well addressed elsewhere.9,23
Transgenic Rabbit Models for LQT Syndrome
LQT syndrome is an inherited disorder of cardiac rhythm that usually is associated with an autosomal dominant or autosomal recessive mutation.46 The condition is characterized by prolongation of the QT interval, as detected by electrocardiography, due to delayed ventricular repolarization and concomitant prolonged action potential duration (APD), which usually lead to spontaneous polymorphic ventricular tachycardia and sudden cardiac death in young patients.45 At least 9 different forms of the LQT syndrome have been identified so far. Among genotyped patients, mutations in genes encoding repolarizing K+ channels (LQT1:KCNQ1; LQT2:KCNH2) are found in 90% of affected persons, given that the underlying causes and many of the mutations show a dominant negative mechanism.61 Ventricular arrhythmias and sudden death in general are often triggered by emotional stress or physical exercise.46 In addition, an increase in the incidence of arrhythmias has been associated with menses and during early postpartum months in female patients with LQT syndrome.66
Transgenic mouse models of LQT syndrome have been instrumental in improving our understanding of the assembly and role of potassium channels in regulating heart repolarization.52 However, the small heart size, rapid heart rates (more than 300 per minute at rest), and very short APD of mice have limited their uses in cardiac studies. In addition, the main repolarizing currents in mice are carried by channels that differ from those in humans, thereby hindering the translation of findings from mouse models to human medicine.51 In contrast to that in mice, the rabbit heart is much larger and beats much more slowly (130 beats per minute at rest). Furthermore, compared with the mouse heart, the rabbit heart is more similar to the human heart in terms of the contractile proteins and the ion channels important for repolarization.75 In addition, the rabbit heart has the same K+ current—the rapidly activating component (Ikr) and the slowly activating component (Iks)—which determine the repolarization of action potential. Therefore, the rabbit represents a more physiologically relevant model system to study human diseases involving these channels.
Transgenic rabbits expressing pore mutants of the human genes KCNQ1 (KvLQT1-y315S) and KCNH2 (HERG-G628S), which respectively are the causes of the LQT1 and LQT2 phenotypes, have successfully been created and characterized.10 Overexpression of these dominant-negative mutant genes is targeted to the cardiomyocytes of rabbits by the β-myosin heavy chain (β-MHC) promoter. The KvLQT1-y315S and HERG-G628S transgenic rabbits have been characterized as having prolongation of the QT interval and APD at 90% repolarization (APD90) due to the elimination of Iks and IKr currents, respectively.10 Clinical studies have revealed that the elimination of Iks and IKr has different effects. LQT1 rabbits show QT prolongation but neither spontaneous arrhythmias nor an increase in sudden death.10 In contrast, LQT2 rabbits show a more pronounced QT prolongation at slow heart rates as well as spontaneous arrhythmias and a significant increase in sudden death with the end of puberty at the age of about 6 mo. In addition, spatial dispersion of APD in LQT2 rabbits is increased across the epicardial surface, and this increased APD dispersion is linked to their arrhythmogenesis.10 Furthermore, electrophysiologic studies in LQT1 and LQT2 rabbits revealed genotype-specific differences in ventricular refractoriness and His conduction.56 These studies demonstrated the occurrence of infraHis blocks in LQT2 rabbits under isoflurane anesthesia and intraHis block in LQT1 rabbits after dofetilide, suggesting differential regional sensitivities of the rabbit His–Purkinje system to drugs blocking Iks and IKr.
Data from studies of drug-induced animal models for LQT syndrome suggest that discordant alternans may be involved in LQT-related arrhythmias.11 The mechanisms underlying discordant alternans in LQT syndrome and its relationship to LQT-related ventricular arrhythmias was further investigated in LQT2 rabbits by using optical mapping techniques.83 Results from that study indicate that tissue spatial heterogeneity plays a significant role in the formation of discordant alternans and vulnerability to reentrant arrhythmias in LQT2 rabbits.83 In addition, female LQT2 rabbits exhibit a high incidence of sudden death during lactation, similar to the increased incidence of arrhythmias in women with LQT2 syndrome postpartum.66 Sex hormones may modulate cardiac repolarization and arrhythmogenesis in female humans and rabbits with LQT2. Data from a recent study using ovariectomized LQT2 rabbits revealed that treatment with high-dose estradiol resulted in a higher degree of arrhythmias and sudden death than did treatment with progesterone or dihydrotesterone.54,57 These studies further indicated that the underlying mechanisms are an increased Iks current that contributes to the steepening of the QT and RR intervals by shortening QT at fast heart rates and a substantially increased ICa,L that contributes to both prolonged refractoriness and an increased propensity to depolarize the membrane in response to Ca2+ oscillations.54,57 In contrast, progesterone exerts an antiarrhythmic effect by preventing early after depolarization.
Rabbit models for LQT syndrome have also been used to test potential side effects of anesthetic agents in subjects genetically predisposed to sudden cardiac death.55 The study investigated 5 common anesthetic agents: isoflurane, thiopental, midazolam, propofol, and ketamine. Isoflurane resulted in a prolonged QT interval in LQT2 but not in LQT1 rabbits; thiopental prolonged the QT interval in both LQT1 and LQT2 models but was less pronounced in LQT1 rabbits; midazolam prolonged the QT duration in both LQT1 and LQT2 transgenic but not control rabbits; and propofol significantly increased the QT interval in both LQT1 and LQT2 rabbits as well as controls.55 In addition, signs for indication of altered repolarization and arrhythmias were monitored only in LQT2 rabbits under anesthesia. Multiple premature ventricular contractions, which can have a marked effect in humans, were noted in many LQT2 rabbits that had been anesthetized with midazolam, ketamine, or thiopental. Furthermore, isoflurane and propofol were especially proarrhythmic in LQT2 rabbits and led to a 30% sudden death.55 These findings indicate the importance of careful evaluation of electrocardiograms by anesthesiologists prior to surgery, to facilitate the selection of appropriate anesthetic agents for individual patients. LQT transgenic rabbits may play a key role in studying the mechanisms of sudden cardiac death and may be useful in screening for drugs that interact with the HERG potassium channel, thereby causing QT prolongation and potentially fatal arrhythmias.
In 2 recent studies,3,4 LQT1 rabbits were used to evaluate candidate therapeutic compounds for the treatment or prevention of LQT syndrome. One study showed that nicorandil, an opener of ATP-sensitive potassium channels, ameliorated repolarization abnormalities and heterogeneities in LQT1 rabbits.4 In the other study, the effect of NS1643, a Kv11.1 (HERG) activator, on repolarization was investigated in LQT1 rabbits.3 The data indicate that NS1643 shortens cardiac APD in LQT1 rabbits via Kv11.1 channel activation. However, administration of NS1643 in the ex vivo study was associated with increased risk of spontaneous and induced arrhythmias. The study suggested that “indiscriminate Kv11.1 activation could be detrimental, and Kv11.1 channel activation might have the highest propensity as a successful treatment in situations with severely impaired repolarization.”3
Transgenic Rabbit Models for Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is a genetic disease characterized by cardiac hypertrophy, myocyte disarray, interstitial fibrosis, and left ventricular dysfunction.80 Mutation in various genes encoding sarcomeric proteins has been identified as causes of hypertrophic cardiomyopathy.39 The most common gene linked to hypertrophic cardiomyopathy is β- MHC; a point mutation in this gene occurs in 35% to 50% of hypertrophic cardiomyopathy cases.78 The mechanism and molecular pathogenesis of the mutant sarcomeric proteins, including mutant β-MHC, in hypertrophic cardiomyopathy has been well studied.38 However, an attractive small animal model for identifying new therapeutic targets and diagnostic methods for hypertrophic cardiomyopathy had not been available until a transgenic rabbit model for hypertrophic cardiomyopathy was established in 1999.40 Although transgenic mice expressing a variety of mutant sarcomeric proteins show various disease phenotypes, including cardiac myocyte disarray, interstitial fibrosis, systolic and diastolic dysfunction, and premature death, they did not develop left ventricular hypertrophy, the hallmark of hypertrophic cardiomyopathy in human patients.47,53,76 In addition, the composition of cardiac sarcomeric proteins differs greatly between humans and mice, in that β-MHC is predominant in human ventricles but α-MHC is predominant in mouse ventricles.72 Similar to the situation in humans, β-MHC is the major myosin isoform in rabbit myocardium.31 In addition, rabbit β-MHC is approximately 98% homologous to the human β-MyHC protein.29 Given that various MHC isoforms show significantly different actin-activated myosin–ATPase activity and cross-bridge kinetics,70 differences in cardiac sarcomeric protein composition likely can affect the phenotypic response of the heart to a mutant protein. Therefore, rabbits provide an excellent model for studying hypertrophic cardiomyopathy.
Transgenic rabbits with targeted cardiac expression of the mutant β-MHC-Q403, which is associated with familial hypertrophic cardiomyopathy in humans, have been generated.79 The phenotype of transgenic rabbits in which β-MHC-Q403 protein was expressed was identical to that of the hypertrophic cardiomyopathy caused by the same mutant gene in humans; disease signs included cardiac hypertrophy, myocyte and myofibrillar disarray, excess interstitial collagen, and high incidence of premature death.79 In light of its comprehensive phenotype of hypertrophic cardiomyopathy and the feasibility of high-resolution serial echocardiography, the β-MyHC-Q403 transgenic rabbit has become an ideal model for pathogenic and therapeutic studies of hypertrophic cardiomyopathy.
In a series of studies,35,58,65 β-MyHC-Q403 transgenic rabbits were used to test the effects of several drugs on hypertrophic cardiomyopathy. Results from these studies indicated that atorvastatin prevented the development of hypertrophic cardiomyopathy in young transgenic rabbits,65 and simvastatin induced regression of cardiac hypertrophy and fibrosis and improvement of cardiac function.58 A subsequent study demonstrated that treatment with N-acetylcysteine resulted in reversion of established cardiac hypertrophy and fibrosis and prevented systolic dysfunction in the transgenic rabbits.35 Moreover, this transgenic rabbit model has been used to explore novel methods with increased sensitivity and accuracy for early diagnosis of hypertrophic cardiomyopathy. Results from one study48 indicated that tissue Doppler imaging consistently detected myocardial contraction and relaxation abnormalities, irrespective of cardiac hypertrophy in the transgenic rabbits and was more sensitive than was conventional echocardiography for screening for hypertrophic cardiomyopathy.48 A preclinical trial in human patients with hypertrophic cardiomyopathy confirmed that Doppler imaging is an accurate and sensitive method for early diagnosis before and independent of hypertrophy.49
Transgenic Rabbit Models for Tachycardia-Induced Cardiomyopathy
The mammalian heart expresses 2 cardiac myosin isoforms, β-MHC and α-MHC. In larger adult mammals, including humans and rabbits, β-MHC is the isoform that is expressed predominantly, whereas α-MHC is expressed at high levels in rodents.15,31,72 The relative expression of these 2 isoforms is species-dependent and is regulated developmentally and hormonally.15,36 Isoform composition varies during development and can be shifted by various stimuli, including thyroid hormone and pressure overload.34 Significant downregulation of α-MHC mRNA expression may indeed be of some consequence to the failing human heart.37,50 Furthermore reduced expression of α-MHC, from 5% to 7% of total MHC to virtually undetectable levels, was reported to occur in failing hearts.45,60 These results suggest that even modest shifts in the relative amounts of these isoforms can be functionally significant.
To study the effects and mechanism of cardiac myosin isoform variation in human heart failure, transgenic rabbits were produced that expressed different α-MHC contents in the β-MHC background.71 In transgenic rabbits that expressed 40% and 15% α-MHC, echocardiograms during the resting state did not show differences in cardiac dimensions or shortening, compared with those in control rabbits. However, the presence of 40% α-MHC partially protected the rabbits from pacing-tachycardia–induced cardiomyopathy.30 The studies further revealed that the expression of 40% α-MHC in the rabbits increased myofilament power production and hastened crossbridge cycling. These changes facilitated ejection and relengthening during short cycle intervals and thus protected against tachycardia-induced cardiomyopathy in the rabbits.71 Results from these studies help us to understand and identify a myofilament-based mechanism underlying the tachycardia-induced cardiomyopathy protection and to extrapolate the effect of MHC isoform variation on myofilament function in human hearts. However, these results also suggest that, even compared with the virtual absence of α-MHC in the failing heart, the 5% to 7% α-MHC content of the normal human heart has little, if any, functional significance.71
Because of their intermediate size, similar ion channels and lipoprotein metabolism as in humans, and quick response to dietary cholesterol to develop hypercholesterolemia, laboratory rabbits have been used widely as models for human cardiovascular diseases. These features, combined with transgenesis for specific human genes, have made rabbits even more powerful tools to gain insight into pathogenic mechanisms of diverse human cardiovascular diseases. With the advent of novel genetic technologies, such as zinc-finger nuclease-mediated gene deletion, new genetically modified rabbits likely soon will be generated to model still other human diseases.
Acknowledgment
This work was supported in part by the Department of Comparative Medicine of the Pennsylvania State University College of Medicine.
References
- 1.Araki M, Fan J, Challah M, Bensadoun A, Yamada N, Honda K, Watanabe T. 2000. Transgenic rabbits expressing human lipoprotein lipase. Cytotechnology 33:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaudeux JL, Giral P, Bruckert E, Foglietti MJ, Chapman MJ. 2004. Matrix metalloproteinase, inflammation, and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med 42:121–131 [DOI] [PubMed] [Google Scholar]
- 3.Bentzen BH, Bahrke S, Wu K, Larsen AP, Odening KE, Franke G, vans Gravesande KS, Biermann J, Peng X, Koren G, Zehender M, Bode C, Grunnet M, Brunner M. 2011. Pharmacological activation of Kv11.1 in transgenic long QT1 rabbits. J Cardiovasc Pharmacol 57:223–230 [DOI] [PubMed] [Google Scholar]
- 4.Biermann J, Wu K, Odening KE, Asbach S, Koren G, Peng X, Zehender M, Bode C, Brunner M. 2011. Nicorandil normalizes prolonged repolarisation in the first transgenic rabbit model with long QT syndrome 1 both in vitro and in vivo. Eur J Pharmacol 650:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. 1993. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4:197–250 [DOI] [PubMed] [Google Scholar]
- 6.Bocan TM, Mueller SB, Mazur MJ, Uhlendorf PD, Brown EQ, Kieft KA. 1993. The relationship between the degrees of dietary-induced hypercholesterolemia in the rabbit and atherosclerotic lesion formation. Atherosclerosis 102:9–22 [DOI] [PubMed] [Google Scholar]
- 7.Bosze Zs, Hiripi L, Carnwath JW, Hiemann H. 2003. The transgenic rabbit as model for human diseases and as a source of biologically active recombinant proteins. Transgenic Res 12:541–553 [DOI] [PubMed] [Google Scholar]
- 8.Boullier A, Hennuyer N, Tailleux A, Fuman C, Duverger N, Caillaud JM, Castro G, Fievet C, Fruchart JC, Duriez P. 2001. Increased levels of high-density lipoprotein cholesterol are ineffective in inhibiting the development of immune responses to oxidized low-density lipoprotein and atherosclerosis in transgenic rabbits expressing human apolipoprotein (apo) A1 with severe hypercholesterolaemia. Clin Sci (Lond) 100:343–355 [PubMed] [Google Scholar]
- 9.Brousseau ME, Hoeg JM. 1999. Transgenic rabbits as models for atherosclerosis research. J Lipid Res 40:365–375 [PubMed] [Google Scholar]
- 10.Brunner M, Peng X, Liu GX, Ren X-Q, Ziv O, Choi B-R, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mttchell GF, Poppas A, Zehender M, Koren G. 2008. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest 118:2246–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinushi M, Kozhevnikov D, Caref EB, Restivo M, El-Sherif N. 2003. Mechanism of discordant T wave alternans in the in vivo heart. J Cardiovasc Electrophysiol 14:632–638 [DOI] [PubMed] [Google Scholar]
- 12.Danesh J, Collins R, Peto R. 2000. Lipoprotein (a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 102:1082–1085 [DOI] [PubMed] [Google Scholar]
- 13.Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P. 1996. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein AI transgenic rabbits. Circulation 94:713–717 [DOI] [PubMed] [Google Scholar]
- 14.Duverger N, Viglietta C, Berthou L, Emmanuel F, Tailleux A, Parmentier-Nihoul L, Laine B, Fievet C, Castro G, Fruchart JC, Houbebine LM, Denefie P. 1996. Transgenic rabbits expressing human apolipoprotein A1 in the liver. Arterioscler Thromb Vasc Biol 16:1424–1429 [DOI] [PubMed] [Google Scholar]
- 15.Everett AW, Sinha AM, Umeda PK, Jakoxcic S, Rabinowitz M, Zak R. 1984. Regulation of myosin synthesis by thyroid hormone: relative change in the α- and β-myosin heavy-chain mRNA levels in rabbit heart. Biochemistry 23:1596–1599 [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Araki M, Wu L, Chalah M, Shimoyamada H, Lawn MR, Kakuta H, Shikama H, Watanabe T. 1999. Assembly of lipoprotein (a) in transgenic rabbits expressing human apolipoprotein (a). Biochem Biophys Res Commun 255:639–644 [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Ji ZS, Huang Y, de Silva H, Sanan D, Mahley R, Innerarity T, Taylor J. 1998. Increased expression of apolipoprotein E in transgenic rabbits results in reduced levels of very-low–density lipoproteins and an accumulation of low density lipoproteins in plasma. J Clin Invest 101:2151–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan J, McCormick SP, Krauss RM, Taylor S, Quan R, Taylor JM, Young SG. 1995. Overexpression of human apolipoprotein B100 in transgenic rabbits results in increased levels of LDL and decreased levels of HDL. Arterioscler Thromb Vasc Biol 15:1889–1899 [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Shimoyamada H, Sun H, Marcovina S, Honda K, Watanabe T. 2001. Transgenic rabbits expressing human apolipoprotein (a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arterioscler Thromb Vasc Biol 21:88–94 [DOI] [PubMed] [Google Scholar]
- 20.Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T. 2001. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 276:40071–40079 [DOI] [PubMed] [Google Scholar]
- 21.Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. 1994. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high-density lipoproteins and intermediate-density lipoproteins. Proc Natl Acad Sci USA 91:8724–8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan J, Wang X, Wu L, Matsumoto SI, Liang J, Koike T, Ichikawa T, Sun H, Shikama H, Sasaguri Y, Watanabe T. 2004. Macrophage-specific overexpression of human matrix metalloproteinase 12 in transgenic rabbits. Transgenic Res 13:261–269 [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Watanabe T. 2003. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther 99:261–282 [DOI] [PubMed] [Google Scholar]
- 24.Gordon DJ, Knoke J, Probsfield JL, Superko R, Tyroler HA. 1986. High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation 74:1217–1225 [DOI] [PubMed] [Google Scholar]
- 25.Hammer RE, Pursel VG, Rexroad CE, Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. 1985. Production of transgenic rabbits, sheep, and pigs by microinjection. Nature 315:680–683 [DOI] [PubMed] [Google Scholar]
- 26.Hoeg JM, Santamarina-Fojo S, Berard AM, Cornhill JF, Herderick EE, Feldman SH, Haudenschild CC, Vaisman BL, Hoyt RF, Jr, Demosky SJ, Jr, Kauffman RD, Hazel CM, Marcovina SM, Brewer HB., Jr 1996. Overexpression of lecithin:cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc Natl Acad Sci USA 93:11448–11453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Schwendner SW, Rall SC, Sanan D, Mahley RW. 1997. Apolipoprotein E2 transgenic rabbits. J Biol Chem 272:22685–22694 [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa T, Kitajima S, Liang J, Koike T, Wang X, Sun H, Okazaki M, Morimoto M, Shikama H, Watanabe T, Yamada N, Fan J. 2004. Overexpression of lipoprotein lipase in transgenic rabbits leads to increased small dense LDL in plasma and promotes atherosclerosis. Lab Invest 84:715–726 [DOI] [PubMed] [Google Scholar]
- 29.Jaenicke T, Diederich KW, Haas W, Schleich J, Lichter P, Pfordt M, Bach A, Vosberg HP. 1990. The complete sequence of the human β-myosin heavy chain gene and a comparative analysis of its product. Genomics 8:194–206 [DOI] [PubMed] [Google Scholar]
- 30.James J, Martin L, Krenz M, Ouatman C, Jones F, Klevitsky R, Gulick J, Robbins J. 2005. Forced expression of α-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation 111:2339–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavinsky CJ, Umeda PK, Levin JE, Sinha AM, Nigro JM, Jakovcic S, Rabinowitz M. 1984. Analysis of cloned mRNA sequences encoding subfragment 2 and part of subfragment 1 of α- and β-myosin heavy chains of rabbit heart. J Biol Chem 259:2775–2781 [PubMed] [Google Scholar]
- 32.Koike T, Kitajima S, Yu Y, Li Y, Nishijima K, Liu E, Sun H, Waqar AB, Shibata N, Inoue T, Wang Y, Zhang B, Kobayashi J, Morimoto M, Saku K, Watanabe T, Fan J. 2009. Expression of human apoA2 in transgenic rabbits leads to dyslipidemia: a new model for combined hyperlipidemia. Arterioscler Thromb Vasc Biol 29:2047–2053 [DOI] [PubMed] [Google Scholar]
- 33.Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Watanabe S, Fan J. 2006. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation 113:1993–2001 [DOI] [PubMed] [Google Scholar]
- 34.Litten RZ, Martin BJ, Low RB, Alpert RL. 1982. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res 50:856–864 [DOI] [PubMed] [Google Scholar]
- 35.Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, Willerson JT, Betocchi S, Wickline SA, Efimo IR, Marian AJ. 2009. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation 119:1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lompre AM, Nadal-Ginard B, Mahdavi V. 1984. Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259:6437–6446 [PubMed] [Google Scholar]
- 37.Lowes BD, Minoee W, Araham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR. 1997. Changes in gene expression in the intact human heart: downregulation of α-myosin heavy chain in hypertrophied failing ventricular myocardium. J Clin Invest 100:2315–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marian AJ, Roberts R. 1995. Recent advances in the molecular genetics of hypertrophic cardiopathy. Circulation 92:1336–1347 [DOI] [PubMed] [Google Scholar]
- 39.Marian AJ, Roberts R. 1998. Molecular genetic basis of hypertrophic cardiomyopathy: genetic markers for sudden cardiac death. J Cardiovasc Electrophysiol 9:88–99 [DOI] [PubMed] [Google Scholar]
- 40.Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu Q-t, Brugada R, DeMayo F, Quinone M, Roberts R. 1999. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest 104:1683–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher VM, Brown BG. 1995. Lipoprotein (a) and coronary heart disease. Curr Opin Lipidol 6:229–235 [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T. 1998. Expression and localization of matrix metalloproteinase 12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol 153:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNally EM, Kraft R, Bravo-Zehnder M, Taylor DA, Leinwand LA. 1989. Full-length rat α and β cardiac myosin heavy chain sequences: comparisons suggest a molecular basis for functional differences. J Mol Biol 210:665–671 [DOI] [PubMed] [Google Scholar]
- 44.Miller NE. 1987. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J 113:589–597 [DOI] [PubMed] [Google Scholar]
- 45.Miyata S, Minobe W, Bristow MR, Leinwand LA. 2000. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86:386–390 [DOI] [PubMed] [Google Scholar]
- 46.Moss AJ. 2003. Long QT syndrome. J Am Med Assoc 289:2041–2044 [DOI] [PubMed] [Google Scholar]
- 47.Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, Wolska B, Evans C, Solaro RJ, Wieczorek DF. 1999. Mouse model of a familial hypertrophic cardiomyopathy mutation in α-tropomyosin manifests cardiac dysfunction. Circ Res 85:47–56 [DOI] [PubMed] [Google Scholar]
- 48.Nagueh SF, Kopelen HA, Lim DS, Zoghbi WA, Quinones MA, Roberts R, Marian AJ. 2000. Tissue Doppler imaging consistently detects myocardial contraction and relaxation abnormalities, irrespective of cardiac hypertrophy, in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 102:1346–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, Quinones MA, Roberts R, Marian AJ. 2001. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 104:128–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. 1997. Myosin heavy-chain gene expression in human heart failure. J Clin Invest 100:2362–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nerbonne JM. 2004. Studying cardiac arrhythmias in the mouse—a reasonable model for probing mechanisms? Trends Cardiovasc Med 14:83–93 [DOI] [PubMed] [Google Scholar]
- 52.Nerbonne JM, Nichols CG, Schwarz TL, Escande D. 2001. Genetic manipulation of cardiac K+ channel function in mice: what have we learned and where do we go from here? Circ Res 89:944–956 [DOI] [PubMed] [Google Scholar]
- 53.Oberst L, Zhao G, Park JT, Brugada R, Michael LH, Entman ML, Roberts R, Marian AJ. 1998. Dominant-negative effect of a mutant cardiac troponin T on cardiac structure and function in transgenic mice. J Clin Invest 102:1498–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odening KE, Choi BR, Liu GX, Hartmann K, Ziv O, Chaves L, Schofield L, Centracchio J, Zehender M, Peng X, Brunner M, Koren G. 2012. Estradiol promotes sudden cardiac death in transgenic long QT type 2 rabbits while progesterone is protective. Heart Rhythm 9:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odening KE, Hyder O, Chaves L, Schofield L, Brunner M, Kirk M, Zehender M, Peng X, Koren G. 2008. Pharmacogenomics of anesthetic drugs in transgenic LQT1 and LQT2 rabbits reveal genotype-specific differential effects on cardiac repolarization. Am J Physiol Heart Circ Physiol 295:H2264–H2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odening KE, Kirk M, Brunner M, Ziv O, Lorvidhaya P, Liu GX, Schofield L, Chaves L, Peng X, Zehender M, Choi BR, Koren G. 2010. Electrophysiological studies of transgenic long QT type 1 and type 2 rabbits reveal genotype-specific differences in ventricular refractoriness and His conduction. Am J Physiol Heart Circ Physiol 299:H643–H655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odening KE, Peng X, Choi B-R, Brunner M, Chaves L, Schofield L, Zehender M, Koren G. 2009. Estradiol and progesterone exert opposite effects on cardiac repolarization and arrhythmogenesis in transgenic long QT syndrome 2 rabbits. Biophys J 96:563a [Google Scholar]
- 58.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, Quinones MA, Zoghbi WA, Entman ML, Roberts R, Marian AJ. 2001. Simvastatin induces repression of cardiac hypertrophy and fibrosis and improves cardiac function in a transenic rabbit model of human hypertrophic cardiomyopathy. Circulation 104:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rall SC, Jr, Mahley RW. 1992. The role of apolipoprotein E genetic variants in lipoprotein disorders. J Intern Med 231:653–659 [DOI] [PubMed] [Google Scholar]
- 60.Reiser PJ, Portman MA, Ning XH, Schomisch MC. 2001. Human cardiac myosin heavy-chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol 280:H1814–H1820 [DOI] [PubMed] [Google Scholar]
- 61.Roden DM. 2008. Long QT syndrome. N Engl J Med 358:169–176 [DOI] [PubMed] [Google Scholar]
- 62.Rouy D, Duverger N, Lin SD, Emmanuel F, Houdebine LM, Denefle P, Viglietta C, Gong E, Rubin EM, Hughes SD. 1998. Apolipoprotein (a) yeast artificial chromosome transgenic rabbits. Lipoprotein(a) assembly with human and rabbit apolipoprotein B. J Biol Chem 273:1247–1251 [DOI] [PubMed] [Google Scholar]
- 63.Santamarina-Fojo S, Haudenschild C, Amar M. 1998. The role of hepatic lipase in lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol 19:211–219 [DOI] [PubMed] [Google Scholar]
- 64.Selden RC, Springman K, Hondele J, Meyer J, Winnacker EL, Kräußlich H, Brem G, Brenig B, Goodman HM, Graf F, Kruff B. 1985. Production of transgenic mice, rabbits, and pigs by microinjection into pronuclei. Zuchthygiene 20:251–252 [Google Scholar]
- 65.Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, Roberts R, Marian AJ. 2005. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res 97:285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seth R, Moss AJ, McNitt S, Zareba W, Andrews ML, Qi M, Robinson JL, Goldenberg I, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. 2007. Long QT syndrome and pregnancy. J Am Coll Cardiol 49:1092–1098 [DOI] [PubMed] [Google Scholar]
- 67.Shapiro SD, Kobayashi DK, Ley TJ. 1993. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 268:23824–23829 [PubMed] [Google Scholar]
- 68.Shen J, Kuhn H, Petho-Shramm A, Chan L. 1995. Transgenic rabbits with the integrated human 15-lipoxygenase gene driven by a lysozyme promoter: macrophage-specific expression and variable positional specificity of the transgenic enzyme. FASEB J 9:1623–1631 [DOI] [PubMed] [Google Scholar]
- 69.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NY, Chan L. 1996. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest 98:2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugiura S, Kobayakawa N, Fujita H, Yamashita H, Momomura S-i, Chaen S, Omata M, Sugi H. 1998. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by optical trap technique: molecular basis for cardiac adaptation. Circ Res 82:1029–1034 [DOI] [PubMed] [Google Scholar]
- 71.Suzuki T, Palmer BM, James J, Wang Y, Chen Z, VanBuren P, Maughan DW, Robbins J, LeWinter MM. 2009. Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ Heart Fail 2:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swynghedauw B. 1986. Developmental and functional adaptation on contractile proteins in cardiac and skeletal muscles. Physiol Rev 66:710–771 [DOI] [PubMed] [Google Scholar]
- 73.Taylor JM. 1997. Transgenic rabbit models for the study of atherosclerosis. Ann NY Acad 811:146–152 [DOI] [PubMed] [Google Scholar]
- 74.Utermann G, Hoppichler F, Dieplinger H, Seed M, Thompson G, Boerwinkle E. 1989. Defects in the low density lipoprotein receptor gene affect lipoprotein (a) levels: multiplicative interaction of 2 gene loci associated with premature atherosclerosis. Proc Natl Acad Sci USA 86:4171–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermeulen JT, McGuire MA, Opthof T, Coronel R, de Bakker JM, Klopping C, Janse MJ. 1994. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc Res 28:1547–1554 [DOI] [PubMed] [Google Scholar]
- 76.Vikstrom KL, Factor SM, Leinwand LA. 1996. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med 2:556–567 [PMC free article] [PubMed] [Google Scholar]
- 77.Watanabe N, Ikeda U. 2004. Matrix metalloproteinase and atherosclerosis. Curr Atheroscler Rep 6:112–120 [DOI] [PubMed] [Google Scholar]
- 78.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. 1995. Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiopathy. N Engl J Med 332:1058–1064 [DOI] [PubMed] [Google Scholar]
- 79.Watkins H, Rosenzweig A, Hwang DS, Levi T, McKenna W, Seidman CE, Seidman JG. 1992. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med 326:1108–1114 [DOI] [PubMed] [Google Scholar]
- 80.Wigle ED, Rakowski H, Kimball BP, Williams WG. 1995. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92:1680–1692 [DOI] [PubMed] [Google Scholar]
- 81.Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M, Watanabe T, Yasumoto K, Sasaguri Y. 2008. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol 172:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamanaka S, Balestra ME, Ferrell LD, Fan J, Armold KS, Taylor S, Taylor JM, Innerarity TL. 1995. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci USA 92:8483–8487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ziv O, Morales E, Song Y-k, Peng X, Odening KE, Buxton AE, Karma A, Koren G, Choi B-R. 2009. Origin of complex behavior of spatially discordant alternans in a transgenic rabbit model of type 2 long QT syndrome. J Physiol 587:4661–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]