Abstract
The sucrose breath test (SBT) is a simple noninvasive technique used currently to determine intestinal absorptive function in humans and rodents. However, to date, the test has not been adapted for use in swine. During weaning, intestinal sucrase activity in piglets temporarily declines in response to stressors and is commonly used as a marker of the intestinal response to weaning. Here we assessed the sucrose dose needed for using the SBT in piglets. Six randomly allocated piglets were orogastrically gavaged with 13C-labeled sucrose at a dose of 2 g/kg; breath samples were collected for measurement of 13CO2 on days 0 (approximately 17 h after weaning), 5, and 10 after weaning. The resultant SBT value (cumulative dose at 90 min) was decreased by 46% on day 5 after weaning relative to baseline levels, consistent with temporal changes in gastrointestinal sucrase activity associated with weaning. We conclude that a sucrose dose of 2 g/kg is satisfactory to conduct SBT studies in piglets. With further development, the SBT may provide a new tool to noninvasively monitor digestive function in weaned piglets, to assess the effects of nutritional strategies on intestinal health, and as an indicator of gut integrity and function in swine models of human gastrointestinal disease.
Abbreviations: SBT, sucrose breath test
The activity of the digestive brush border enzymes, specifically sucrase, develops gradually in the small intestine as piglets mature.3,9 Sucrase activity is absent in the newborn piglet, with levels increasing slowly during the first 4 d postnatally.3,7,9 Sucrase activity is measurable in all segments of the small intestine by 7 d of age9,16 and continues to increase thereafter, reaching a plateau at approximately 14 d of age.7 However, this pattern of sucrase activity is adversely affected by the weaning process, with the abrupt weaning typical of commercial pig production resulting in decreased villus height, increased crypt depth, and significantly reduced intestinal sucrase activity.13
Studies in rats1,2,6,11,22,23 and humans21 have demonstrated that total intestinal sucrase activity can be determined accurately and noninvasively by using the recently developed sucrose breath test (SBT). The release of 13CO2 from 13C-labeled substrates forms the basis for several noninvasive gut function tests, including those to assess gastric emptying,17 and gastrointestinal transit.8 The SBT relies on the detection of 13CO2 in expired breath after oral ingestion of an appropriate substrate. For the SBT, the substrate used is sucrose, which is metabolized to glucose and fructose by sucrase. After transit through the hepatic and respiratory systems, 13CO2 is released. The expression of sucrase decreases in a proximal-to-distal gradient along the small intestine.15 Therefore, the SBT provides an integrated index of the total activity of sucrase throughout the small intestine.
The SBT quantifies 13CO2 in the expired breath after oral ingestion of 13C-sucrose to provide a marker of total intestinal sucrase activity.2,20 This technique has demonstrated diagnostic application in humans to monitor the development of chemotherapy-induced intestinal mucositis.21 Moreover, the SBT has been used in rats to determine the potential of diverse nutraceutical agents1,22,23 and probiotics19 to modify small intestinal absorption. Pigs are an excellent omnivore model on which to base relevant nutritional models to investigate the mechanisms underlying chronic human gastrointestinal diseases.14 However, to date, the SBT has not been adapted for use in pigs, in which it might become a valuable tool to assess longitudinal changes in intestinal sucrase activity in response to weaning, dietary manipulations, and disease processes. This technique therefore might be applied in the biomedical field in addition to the animal production research environment. In the current study, we sought to establish the experimental conditions required to conduct the SBT in piglets and noninvasively determine total intestinal sucrase activity at 3 time points after weaning.
Materials and Methods
The study was conducted at the Roseworthy piggery (University of Adelaide, South Australia), with approval from the Animal Ethics Committee of The University of Adelaide. The piggery is maintained as an SPF unit by entry controls and a program of regular screening in accordance with pig industry standards. The facility is free of Pasteurella multocidia (atrophic rhinitis), Mycoplasma hyopneumoniae (enzootic pneumonia), Actinobacillus pleuropneumoniae, and Brachyspira hyodysenteriae (swine dysentery). All experiments were conducted in accordance with the National Health and Medical Research Council (NHMRC) Australian code of practice for the care and use of animals for scientific purposes (seventh edition 2004).10
Animals, housing, and feeding.
Six male Large White piglets, born to parity 2 or 3 sows, were selected randomly from a subset that comprised animals from 3 litters with a median weight of 7.82 ± 0.45 kg at 27 d of age. Piglets had access to solid food from 21 d of age onward. At 27 d of age, piglets were weaned and relocated into solid-floored group-housing pens with subjects of comparable age. In this housing scenario, piglets had ad libitum access to water and a commercial starter diet (17 MJ digestible energy per kilogram of dry matter; 21.9% [w/w] protein; 1.53% total lysine). Piglets were weighed on days 0, 4, and 9 after weaning.
13C SBT.
On days 0 (17 h after weaning), 5, and 10 after weaning, piglets received 13C-labeled sucrose (dose, 2 g/kg; BDH, Merck, Victoria, Australia) completely dissolved in 50 mL water by orogastric gavage, and breath was collected every 15 min for 3 h. The sucrose dose was determined on the basis of previous SBT studies in humans21 and rats,2 scaled according to body weight. The SBT on day 0 required removal of the piglets from the sow on the previous day (age, 27 d) at 1600 for fasting. Piglets were weighed on the afternoon prior to testing, at which point food was removed; piglets continued to have unrestricted access to water. Baseline (time 0) breath samples were taken prior to administration of the radiolabeled sucrose solution at 0845 on the day of testing. Subsequent breath samples were collected from each piglet every 15 min after sucrose administration for a total of 180 min. Food was returned at the conclusion of testing. A single batch of sucrose was used throughout the experiment.
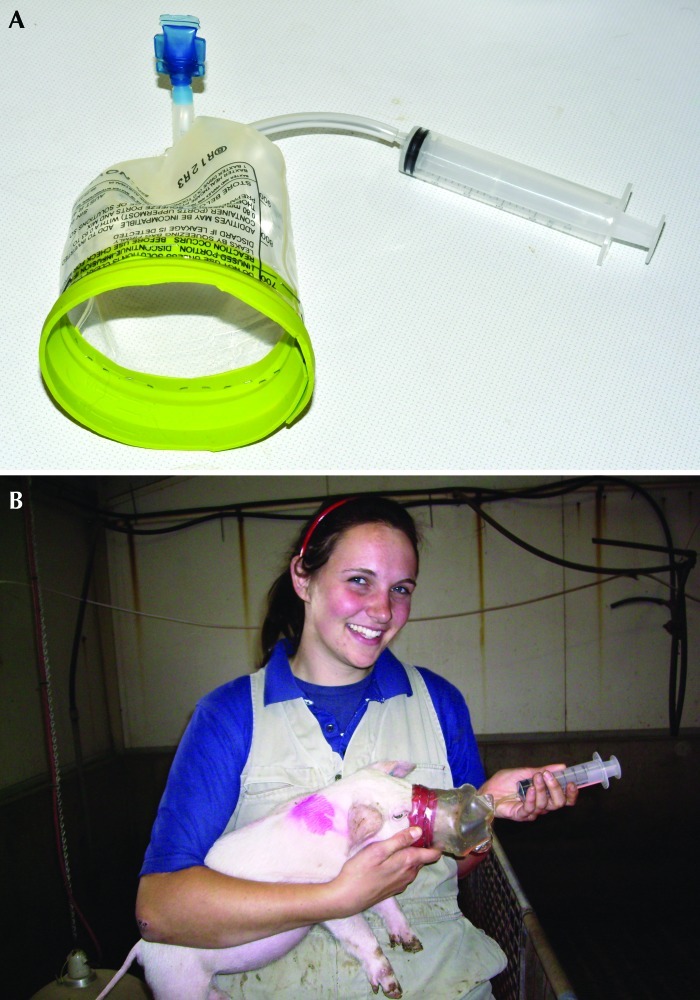
Breath samples were collected by using a custom mask designed to fit the snouts of conscious piglets (Figure 1). The breath mask was made of an empty intravenous solution bag, with a rubber latex seal fixed to the inside rim. A small rubber tube was inserted into the bottom of the bag to attach it to a syringe for breath collection. The breath sample was transferred from the syringe into an evacuated glass tube (Exetainer, Labco Limited, High Wycombe, England). Breath samples were analyzed for 13C by isotope ratio mass spectrometry (ABCA 20/20, Europa Scientific, Crewe, United Kingdom) as described previously.11 This analysis produces a δ value representing the 13C:12C ratio in the sample relative to the internal standard of calcium carbonate. Raw data are expressed as parts per thousand. The current convention in reporting SBT data is to express the 13CO2 data as the percentage of the 13C dose recovered hourly (that is, the percentage cumulative dose of 13C).2,20
Figure 1.
(A) The mask (created from the bottom of an intravenous fluid bag) and 60-mL syringe (attached via tubing inserted into the mask) that was used to perform the SBT. (B) Restraint of a piglet while taking a breath sample. Note that the technician's attire reflects standard practice for an agricultural research facility but may not be appropriate in a biomedical research arena.
For determining percentage cumulative dose, CO2 production (mL/h) was estimated according to the following equation, which was adapted from a previous study that determined CO2 production after intraperitoneal administration of doubly labeled water to pigs.18
The complete 13CO2 analysis therefore considers animal body weight, proportion of dose of 13C recovered at a specific time point, and CO2 production rate for the species of interest. Typically the percentage of cumulative dose plateaus at 90 min, indicating that substrate transit through the small intestinal is complete; the cumulative breath 13CO2 analysis before 90 min therefore is taken as the standard SBT reading.2,21 However because the current study was a pilot study, we sampled multiple time points to ascertain 13CO2 kinetics.
All statistical analyses were performed by using Genstat (10th edition, Committee of the Statistics Department, Rothamsted Experimental Station, Harpenden, United Kingdom). SBT data were analyzed by using the unbalanced treatment structure model, with the Y-variate as an individual time point of the percentage of cumulative dose.
Results
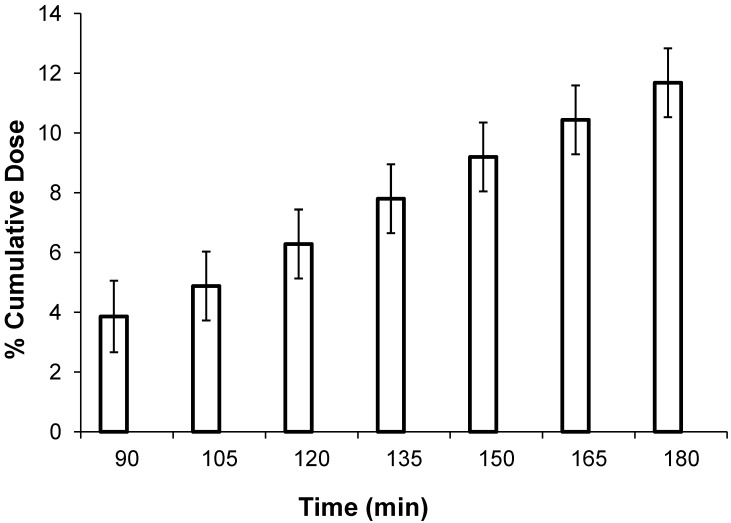
The mean body weight of piglets increased by 1.73 kg between days 0 and 10 after weaning (Table 1). Compared with that on day 0, the mean percentage of cumulative dose was lower on days 5 (46% decrease) and 10 (27% decrease; P < 0.001; Table 1). Regardless of the day of measure, the percentage of cumulative dose increased (P < 0.001) over time, although a plateau was not reached (Figure 2).
Table 1.
Percentage of cumulative dose and body weight on days 0, 5, and 10 after weaning of piglets that received sucrose at 2 g/kg
| Day 0 | Day 5 | Day 10 | Pooled across day of measure | |
| % Cumulative dose | 10.19 ± 0.81b | 5.52 ± 0.81a | 7.45 ± 0.67a | 7.77 ± 0.44 |
| Weight (kg) | 7.82 ± 0.57 | 8.40 ± 0.70 | 9.55 ± 0.85 | 8.19 ± 0.92 |
Data are expressed as mean % cumulative dose ± SEM (n = 6).
Different lowercase letters indicate a significant (P < 0.05) between values.
Figure 2.
Percentage of cumulative dose (mean ± SEM; combined over measurement days) over time after dosage with sucrose at 2 g/kg body weight. The graph starts at t = 90 min, because no appreciable 13CO2 was detected over the first 60 min of collection. This lag may reflect delays due to orogastric transit and gastric emptying.
Discussion
To our knowledge, this study is the first to apply the SBT as a method to determine the effects of weaning on small intestinal function in piglets. The low standard errors in percentage of cumulative dose suggest the SBT was sufficiently sensitive to detect changes in digestive function in normal piglets in response to weaning.
SBT data in rats are highly correlated (r2 = 0.85) with in vitro sucrase activity, allowing it to be used as a reliable marker of small intestinal health in rats.11,20 The activities of the brush border enzymes sucrase and lactase are widely accepted as useful markers of piglet intestinal digestive function.13 Early weaning causes changes to the histology and biochemistry of the small intestine,12 including villous atrophy, crypt hyperplasia, and altered activity of lactase and sucrase. The specific activity of sucrase in weaned piglets decreases rapidly after weaning, reaching minimal values by days 5 to 7 after weaning.5,13 The decreased sucrase activity in weaned piglets that we noted by using the SBT is, therefore, consistent with the literature.4,5
Given that the gastrointestinal tracts of all mammals contain complex microbiota, CO2 will be released due to the fermentation of sucrose that has not been digested or absorbed. This outcome occurs in both humans and rodents, in which an extended experimental time course produces 2 peaks of 13CO2 excretion, the first being representative of sucrase activity, and the second indicating colonic fermentation. It therefore is important to conduct an extended time-course of 13CO2 release after 13C-sucrose gavage to discriminate between specific sucrase activity in the small intestine and nonspecific fermentation in the colon. Those studies would be most informative if conducted in parallel with determinations of intestinal transit time.
Although a preliminary study, the current findings suggest that future studies of the SBT in piglets should use a sucrose dose 2 g/kg body weight. However, because the percentage of cumulative dose had not reached a plateau by 180 min (the duration predicted on the basis of results from rats),2 follow-up studies are required to fully characterize the kinetics of 13C-sucrose metabolism in pigs. Having thus established proof-of-concept for the SBT in piglets, we propose that additional SBT studies in piglets should be extended to 6 h to determine whether 90 min is the optimal time point for estimating intestinal sucrase activity. Furthermore, future studies of the SBT in piglets should define the degree of correlation between the SBT and biochemically assessed sucrase.
In conclusion, the current pilot study demonstrated that the SBT has the requisite sensitivity to detect changes in sucrase activity in response to weaning and showed that this technique can be performed relatively easily in conscious young swine. The SBT therefore may become a valuable technique to noninvasively assess absorptive function in weaned piglets, to facilitate early diagnosis of intestinal disease, and to assess the effect of novel dietary supplements on intestinal function.
Acknowledgments
We thank the staff of the Roseworthy campus piggery (University of Adelaide) for their assistance with care, feeding, and treatment of experimental animals. We acknowledge Professor Ross Butler and Stamatiki Kritas for their valuable advice. Professor Gordon Howarth is supported by the Sally Birch Cancer Council Australia Senior Research Fellowship in Cancer Control. This study was funded by the Cooperative Research Centre for an Internationally Competitive Pork Industry (The Pork CRC). There are no conflicts of interest.
References
- 1.Cheah KY, Howarth GS, Yazbek R, Wright T, Whitford E, Payne C, Butler RN, Bastian S. 2009. An extract from grape seed improves parameters of small intestinal mucositis in rats with mucositis induced by 5-fluorouracil. Cancer Biol Ther 8:382–390 [DOI] [PubMed] [Google Scholar]
- 2.Clarke JM, Pelton NS, Bajka BH, Howarth GS, Read LC, Butler RN. 2006. Use of the 13C-sucrose breath test to assess chemotherapy-induced small intestinal mucositis in the rat. Cancer Biol Ther 5:34–38 [DOI] [PubMed] [Google Scholar]
- 3.Dahlqvist A. 1961. Intestinal carbohydrases of a newborn pig. Nature 190:31–32 [DOI] [PubMed] [Google Scholar]
- 4.Hampson DJ. 1986. Alterations in piglet small intestinal structure at weaning. Res Vet Sci 40:32–40 [PubMed] [Google Scholar]
- 5.Hampson DJ, Kidder DE. 1986. Influence of creep feeding and weaning on brush border enzyme activities in the piglet small intestine. Res Vet Sci 40:24–31 [PubMed] [Google Scholar]
- 6.Howarth GS, Tooley KL, Davidson GP, Butler RN. 2006. A noninvasive method for detection of intestinal mucositis induced by different classes of chemotherapy drugs in the rat. Cancer Biol Ther 5:1189–1195 [DOI] [PubMed] [Google Scholar]
- 7.James PS, Smith MW, Tivey DR, Wilson TJG. 1987. Epidermal growth factor selectively increases maltase and sucrase activities in neonatal piglet intestine. J Physiol 393:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HC, Prather C, Fisher RS, Meyer JH, Summers RW, Pimentel M, McCallum RW, Akkermans LM, Loening-Baucke V; AMS Task Force Committee on Gastrointestinal Transit 2005. Measurement of gastrointestinal transit. Dig Dis Sci 50:989–1004 [DOI] [PubMed] [Google Scholar]
- 9.Manners MJ, Stevens JA. 1972. Changes from birth to maturity in the pattern of distribution of lactase and sucrase activity in the mucosa of the small intestine of pigs. Br J Nutr 28:113–127 [DOI] [PubMed] [Google Scholar]
- 10.National Health and Medical Research Council. 2004. Australian code of practice for the care and use of animals for scientific purposes, 7th ed. Canberra (Australia): National Health and Medical Research Council.
- 11.Pelton NS, Tivey DR, Howarth GS, Davidson GP, Butler RN. 2004. A novel breath test for the noninvasive assessment of small intestinal mucosal injury following methotrexate administration in the rat. Scand J Gastroenterol 39:1015–1016 [DOI] [PubMed] [Google Scholar]
- 12.Pluske JR, Kerton DJ, Cranwell PD, Campbell RG, Mullan BP, King RH, Power GN, Pierzynowski SG, Westrom B, Rippe C, Peulen O, Dunshea FR. 2003. Age, sex, and weight at weaning influence organ weight and gastrointestinal development of weanling pigs. Aust J Agric Res 54:515–527 [Google Scholar]
- 13.Pluske JR, Williams IH, Aherne FX. 1996. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim Sci 62:131–144 [Google Scholar]
- 14.Schook L, Beattie C, Beever J, Donovan S, Jamison R, Zuckermann F, Niemi S, Rothschild M, Rutherford M, Smith D. 2005. Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol 16:183–190 [DOI] [PubMed] [Google Scholar]
- 15.Semenza G, Brunner J, Wacker H. 1983. Biosynthesis and assembly of the largest and major intrinsic polypeptide of the small intestinal brush borders. Ciba Found Symp 95:92–112 [DOI] [PubMed] [Google Scholar]
- 16.Shulman RJ, Henning SJ, Nichols BL. 1988. The miniature pig as an animal model for the study of intestinal enzyme development. Pediatr Res 23:311–315 [DOI] [PubMed] [Google Scholar]
- 17.Symonds EL, Butler RN, Omari TI. 2000. Assessment of gastric emptying in the mouse using the 13C-octanoic acid breath test. Clin Exp Pharmacol Physiol 27:671–675 [DOI] [PubMed] [Google Scholar]
- 18.Theil PK, Kristensen NB, Jorgenson H, Labouriau R, Jakobsen K. 2007. Milk intake and carbon dioxide production of piglets determined with the doubly labelled water technique. Animal 1:881–888 [DOI] [PubMed] [Google Scholar]
- 19.Tooley KL, Howarth GS, Davidson GP, Butler RN. 2006. Oral ingestion of Streptococcus thermophilus diminishes severity of small intestinal mucositis in methotrexate-treated rats. Cancer Biol Ther 5:593–600 [DOI] [PubMed] [Google Scholar]
- 20.Tooley KL, Howarth GS, Lymn KA, Butler RN. 2010. Optimization of the noninvasive 13C-sucrose breath test in a rat model of methotrexate-induced mucositis. Cancer Chemother Pharmacol 65:913–921 [DOI] [PubMed] [Google Scholar]
- 21.Tooley KL, Saxon BR, Webster J, Zacharakis B, McNeil Y, Davidson GP, Butler RN. 2006. A novel noninvasive biomarker for assessment of small intestinal mucositis in children with cancer undergoing chemotherapy. Cancer Biol Ther 5:1275–1281 [DOI] [PubMed] [Google Scholar]
- 22.Whitford EJ, Cummins AG, Butler RN, Prisciandaro LD, Fauser JK, Yazbeck R, Lawrence A, Cheah K, Wright TH, Lymn KA, Howarth GS. 2009. Effects of Streptococcus thermophilus TH-4 in a rat model of 5-fluorouracil–induced mucositis. Cancer Biol Ther 8:505–511 [PubMed] [Google Scholar]
- 23.Wright TH, Yazbek R, Lymn KA, Whitford EJ, Cheah K, Butler RN, Feinle-Bisset C, Pilichiewicz A, Mashtoub S, Howarth GS. 2009. The herbal extract iberogast partially improves small intestinal integrity in rats with mucositis induced by 5-fluorouracil. Cancer Biol Ther 8:923–929 [DOI] [PubMed] [Google Scholar]