Abstract
Bottlenose dolphins can have iron overload (that is, hemochromatosis), and managed populations of dolphins may be more susceptible to this disease than are wild dolphins. Serum iron, total iron-binding capacity (TIBC), transferrin saturation, and ferritin were measured in 181 samples from 141 dolphins in 2 managed collections and 2 free-ranging populations. Although no iron indices increased with age among free-ranging dolphins, ferritin increased with age in managed collections. Dolphins from managed collections had higher iron, ferritin, and transferrin saturation values than did free-ranging dolphins. Dolphins with high serum iron (exceeding 300 μg/dL) were more likely to have elevated ferritin but not ceruloplasmin or haptoglobin, demonstrating that high serum levels of iron are due to a true increase in total body iron. A time-series study of 4 dolphins with hemochromatosis that were treated with phlebotomy demonstrated significant decreases in serum ferritin, iron, and TIBC between pre- and posttreatment samples; transferrin saturation initially fell but returned to prephlebotomy levels by 6 mo after treatment. Compared with those in managed collections, wild dolphins were 15 times more likely to have low serum iron (100 μg/dL or less), and this measure was associated with lower haptoglobin. In conclusion, bottlenose dolphins in managed collections are more likely to have greater iron stores than are free-ranging dolphins. Determining why this situation occurs among some dolphin populations and not others may improve the treatment of hemochromatosis in dolphins and provide clues to causes of nonhereditary hemochromatosis in humans.
Abbreviations: TIBC, total iron-binding capacity
Bottlenose dolphins (Tursiops truncatus) are susceptible to iron overload (that is, hemochromatosis).19,32,33,35 Dolphins with hemochromatosis have high serum iron levels that progress over years, have high transferrin saturation (85% and greater), and are responsive to phlebotomy therapy.19 Left untreated, dolphins with hemochromatosis are more likely to have increased liver aminotransferases, chronic inflammation, and hyperlipidemia than are healthy controls.35 A 25-y retrospective study of one population demonstrated that 67% of dolphins had excessive hepatic hemosiderin deposition at time of death, 92% of which had hemosiderin deposition in Kupffer cells; hemolytic anemia, anemia of chronic disease, and viral infections were not associated with hemosiderin deposition, and the primary hypothesis is that dolphins in managed collections may be susceptible to iron storage disease.32
The etiology of dolphin iron overload is unknown. The most common cause in humans is hereditary hemochromatosis, which is most often attributed to a point mutation of the HFE gene.12 Other causes of iron overload include primary liver disease, excessive iron intake, and insulin resistance.3,36 Comparisons of the HFE gene among dolphins with and without hemochromatosis showed no significant differences (unpublished data), and iron deposition in Kupffer cells (compared with hepatocytes) is not supportive of a hereditary cause.3,32 Liver enzymes decrease in response to the removal of body iron through phlebotomy; this effect indicates that iron overload leads to liver disease rather than that primary liver disease causes iron overload.19 At least one dolphin population from which cases of hemochromatosis have been reported have not received iron supplementation for more than 10 y, although the iron content in their frozen–thawed fish diet has not been assessed fully.19 Dolphins with hemochromatosis have significantly higher 2-h postprandial insulin levels than do controls, suggesting that insulin resistance plays a role in this disease, as occurs in humans.33
In addition to dolphins and humans, iron overload occurs in diverse captive birds, callitrichids, black rhinos, Egyptian fruit bats, lemurs, northern fur seals, California sea lions, tapirs, and Saler cattle.5,11,15,18,20,21,23,29,31 Similar to that in other exotic species, iron overload in dolphins is thought to be a disease that is associated with managed care, but comparisons of iron levels among managed collections and free-ranging dolphin populations have been lacking.19 Further, measurements of serum ferritin, ceruloplasmin, and haptoglobin have not typically been included in iron overload evaluations in dolphins. Although serum ferritin levels can be an indicator of total iron body stores, ferritin also increases in response to inflammation or hemolytic anemia.4 In humans, ceruloplasmin is a plasma protein that increases with acute and chronic inflammation and plays a role in iron metabolism. Haptoglobin is a protein that, in humans, decreases with intravascular hemolytic anemia, among other conditions.2 As such, concurrent measurements of iron, ferritin, ceruloplasmin, and haptoglobin provide insight into the true cause of changing iron and ferritin levels.
Low serum iron is used as a general indicator of inflammation in marine mammals, and return of serum iron to normal levels during illness is seen as a positive indicator for recovery.13,24 The dynamics of iron, erythrocyte sedimentation rate, and fibrinogen in inflammation have remained important as indicators of dolphin health for decades, yet little has been published about dolphin iron stores and associated acute-phase proteins in the past 30 y.24 One barrier to the use of acute-phase proteins, including C-reactive protein, has been the need for species-specific assays; development of tests that better detect early phases of inflammation and infection could lead to earlier treatment and better outcomes. Other barriers include the need for consecutive samples from dolphins with known health status to validate indicators for inflammation, including ceruloplasmin.
To better understand the prevalence of high serum iron and potential risks for hemochromatosis among various dolphin populations, indices of serum iron, ferritin, total iron-binding capacity (TIBC), percentage transferrin saturation, ceruloplasmin, and haptoglobin concentrations were measured in 2 populations of free-ranging dolphins and 2 managed collections, including the cohort of Navy dolphins. These variables were compared by age, sex, and population. Serum ferritin was measured by using a newly developed dolphin-specific assay.
Materials and Methods
Animal care and use.
The Navy Marine Mammal Program (San Diego, CA) is AAALAC-accredited and adheres to the national standards of the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals and the Animal Welfare Act.1,25 As required by the Department of Defense, the Program's animal care and use program is routinely reviewed by an IACUC and the Department of Defense Bureau of Medicine. Sampling of wild dolphins was approved by the IACUC at the Harbor Branch Oceanographic Institution (Fort Pierce, FL). Blood samples collected from wild dolphins were approved under National Marine Fisheries Service Permit Number 998-1678 (issued to Dr Greg Bossart). All samples used in this study were archived samples from previous routine health assessments, and no samples were collected prospectively from live dolphins specifically for this study.
Study population.
A total of 181 serum samples from 2 managed collections and 2 free-ranging bottlenose dolphin populations (Table 1) were assayed for serum iron analytes. Dolphins in the Navy program live in netted enclosures in the San Diego Bay, and those at Six Flags Discovery Kingdom (Vallejo, CA) live in closed systems in an aquarium. Both of these populations are fed high-quality, frozen–thawed fish, consisting of primarily capelin (Mallotus villosus) and herring (Clupea harengus) but also including smaller amounts of mackerel (Scomber scombrus) and squid (Loligo brevis). Both populations receive daily vitamin supplements that do not include iron. Although Navy dolphins have access to fish in San Diego Bay, trainer observations confirm that little of their diet comes from live fish. The samples from free-ranging dolphins were collected from animals at 2 different sites, Indian River Lagoon, FL, and Charleston, SC, in 2004 and 2005.
Table 1.
Characteristics of the 4 populations of bottlenose dolphins in the current study
| Population | No. of dolphins | Age range (y) | No. of samples |
| Navy Marine Mammal Program | 70 | 1 to 44 | 110 |
| Six Flags Discovery Kingdom | 13 | 4 to 46 | 13 |
| Charleston, SC | 18 | 2 to 24 | 18 |
| Indian River Lagoon, FL | 40 | 2 to 27 | 40 |
Some dolphins in the Navy population were sampled more than once as they aged, and these were included as independent data points; most Navy dolphins had 2 blood samples included in the study. In addition, banked serial samples from 4 animals in the managed collection undergoing phlebotomy treatment for iron storage disease were analyzed (time 1, prephlebotomy; time 2, mid-treatment; time 3, end of treatment; time 4, 6 mo after treatment; and time 5, 1 y after treatment) and evaluated for changes in analyte concentrations over time.
The age of the dolphins in the current study ranged from 1 to 45 y. The ages of animals in managed collections were either known (that is, captive-born) or estimated from the age at the time of collection (more than 25 y ago). The ages of the free-ranging dolphins were determined by counting postnatal dentine layers in an extracted tooth.17 Male dolphins were divided into 3 age groups: juveniles (younger than 10 y), adults (10 to 24 y), and older adults (25 y and older). Slightly different age groups (juvenile, younger than 7 y; adult, 7 to 24 y; and older adults, 25 y and older) were established for female dolphins, in light of their earlier age of sexual maturity. Samples from dolphins in managed collections were collected during routine physical examinations, illness, or treatment or as part of other research projects.
Sample collection.
Blood samples were originally collected from managed collection dolphins by venipuncture of either the periarterial venous rete in the caudal peduncle or a fluke blade. These methods for blood collection have been used by the Navy for more than 30 y, including for establishing normal reference intervals of CBC counts and serum chemistries. Although blood collection from the periarterial venous rete has the potential for mixed arterial–venous sampling, none of the blood values reported in the current study has been observed by the authors to demonstrate significant differences by sampling site. Dolphins were trained to present their flukes for sampling, or behavioral conditioning was used to facilitate the collection of samples by having the dolphins rest on a foam mat out of the water during a routine physical exam. Samples were collected by using either a 20- or 21-gauge, 3.8-cm vacuum phlebotomy tubes with needles (Becton Dickinson Vacutainer Systems, Rutherford, NJ) or a 21-gauge, 1.9-cm butterfly needle attached to a vacuum phlebotomy tube holder. Blood was collected into a serum separator tube. Samples were chilled for 30 min and centrifuged (1006 × g at 21 °C for 10 min) within 2 h. Fibrin clots were removed, and serum was transferred to plastic tubes for archived storage at −80 °C.
Dolphins with hemochromatosis that were treated with phlebotomy according to described methodology.19 In general, each phlebotomy treatment removed 7% to 17% (1 to 3 L) of the estimated total blood volume. Treatment consisted of an induction phase of weekly phlebotomy procedures for 22 to 30 wk; this phase was complete when serum iron concentration and aminotransferase activities were within reference ranges and serum transferrin saturation was less than 20% or hematocrit was less than 30%. The total amount of iron removed from each dolphin was 53 to 111 mg/kg (24.1 to 50.5 mg/lb) of body weight.
Samples from free-ranging animals were collected as part of the Health and Risk Assessment (HERA) dolphin project.10 The collection sites, methods, and evaluation of health status of the dolphins have been described previously.10 All samples were stored at −80 °C and shipped on dry ice for analysis.
Sample analyses.
Serum iron and TIBC were measured by using an automated chemistry analyzer (Cobas Mira Chemistry Analyzer, Roche, Indianapolis, IN) with the Raichem Iron TIBC Reagent with Standard Kit (Cliniqa, San Marcos, CA). The spectrophotometric measurement of serum iron is accomplished by releasing the protein-bound iron from its carrier protein transferrin and complexing the released iron with a suitable chromogen. Four serum controls were used for each assay; 2 controls were purchased analyzed controls, and 2 controls were pooled serums. Percentage transferrin saturation was calculated as (serum iron ÷ TIBC) × 100%. Serum haptoglobin was measured spectrophotometrically.30 Serum ceruloplasmin was measured spectrophotometrically by using p-phenylenediamine dihydrochloride as the substrate.6
Development and validation of serum ferritin assay.
Serum ferritin concentration was measured by using an ELISA that was developed and validated specifically for bottlenose dolphins in the current study. Ferritin was isolated from liver and spleen of a bottlenose dolphin.22 The purity of the isolated protein was determined by SDS-PAGE and compared with an equine ferritin standard. Ferritin standards and immunoglobulin concentrations were determined by using the bicinchonic acid assay (Pierce Chemical, Rockford, IL) with BSA as a standard.
Antiequine ferritin (affinity-isolated) antibody (F6146, Sigma Chemical, St Louis, MO) was diluted to 3 µg/mL by using 10 mM sodium carbonate buffer (pH 9.6), and 100 µL was added to each well of 96-well high-binding plate (no. 9018 EIA/RIA, Costar, Corning, Corning, NY). Plates were sealed, incubated for 2 h at 37 °C, and stored at −20 °C until needed. PBS (pH 7.4) containing 0.1% Tween 80 and 0.5% BSA was used as a blocking buffer to fill unoccupied protein-binding sites on the plates, for diluting sera and dolphin ferritin stock, and diluting antiequine ferritin horseradish peroxidase conjugate. PBS containing 0.1% Tween 80 was used to wash plates.
Ferritin standards of 10, 20, 30, 40, and 50 µg/L were prepared from purified dolphin ferritin stock. Premix ABTS Microwell Peroxidase Substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was used for color development. Wash solution, dilution solution, and substrate solutions were warmed to 37 °C before use.
Frozen plates were thawed at 37 °C for 1 h and washed 5 times with wash solution. Plates were blocked for 30 min and washed again 5 times. Diluted sera, standards, and controls were added to individual wells (100 µL/well), and the plates were sealed and incubated for 2 h. Plates were washed 5 times with wash solution. Antiequine ferritin conjugated to horseradish peroxidase and diluted to 1.5 µg/mL in blocking solution was added to each well (100 µL per well); plates were sealed, incubated for 2 h at 37 °C, and washed 5 times. Premix ABTS substrate solution (Kirkegaard and Perry Laboratories; 100 µL) was added to each well, and kinetic absorbance change at 410 nm was recorded in an automated 96-well plate reader (Versa Max, Molecular Devices, Sunnyvale, CA).
Linearity for standards and diluted sera was determined by using a computer program (Versamax, Molecular Devices). In all analytic and statistical evaluations, the mean of 3 wells was used as the ferritin concentration for that particular sample. Recovery of ferritin from sera was determined by comparing ferritin concentration in diluted dolphin sera containing added ferritin with measurements from the same sera without added ferritin. Within-assay variability was determined by measuring ferritin concentration in one serum sample 9 times on the same assay plate on the same day. Assay-to-assay variability was determined by measuring ferritin concentration in 3 different samples on 20 separate days.
Statistical analyses.
The analytical software programs Statistix 8 (Tallahassee, FL) and SAS (release 9.2, SAS, Cary, NC) were used for analyses. Descriptive analyses (mean, standard deviation minimum and maximum values) were conducted on the entire data set. Differences in age and sex among the 4 populations were assessed by using one-way ANOVA with a general linear model and χ2 tests, respectively. All comparisons of iron indices were conducted by using nonparametric Kruskal–Wallis tests. Iron indices were compared by sex among all populations. Due to the significant differences in age between free-ranging and Navy populations, the effects of age categories on iron indices were assessed separately for these 2 groups. Dolphins in the Six Flags population were omitted from this analysis because of low numbers. In addition, iron indices were compared by age among the 4 study populations; the oldest age category was excluded due to low numbers among free-ranging dolphins. High serum iron was defined as levels exceeding 300 μg/dL, and low serum iron was defined as levels less than 100 μg/dL; these definitions were based on 1100 routine blood samples collected over 10 y from healthy Navy marine mammals; that dataset excluded dolphins with known chronic diseases, including iron overload.34 χ2 tests (n = 42) were conducted to compare the prevalence of high and low serum iron in managed collection and free-ranging populations.
Linear associations among iron and ferritin, iron and ceruloplasmin, and iron and haptoglobin serum levels were tested among samples with high or low iron. In addition, associations were tested among iron and ferritin levels against WBC count and erythrocyte sedimentation rate. Repeated-measures ANOVA was used to examine changes in dolphins undergoing phlebotomy treatment. Significance among all analyses was defined as a P value less than 0.05.
Results
Dolphin ferritin assay.
Ferritin standards were linear from 0 to 50 µg/L. The relationship between serum dilution and serum ferritin concentration was linear. When 10, 20, 30, 40, and 50 µg/L of purified ferritin standard was added to diluted serum containing 12 µg/L ferritin, the recoveries were respectively, 90%, 88%, 86%, 83%, and 87%. Within-assay variability for the test serum was 9%. The assay-to-assay variabilities ranged from 17% to 19% for the 3 serum samples.
Overall values and population comparisons.
The values (mean ± 1 SD [range]) for iron, TIBC, percentage transferrin saturation, and ferritin among all dolphins were 184 ± 132 (15 to 649) μg/dL, 304 ± 108 (145 to 744) μg/dL, 56% ± 24% (6% to 99%), and 6090 ± 16,744 (2 to 177,465) ng/dL, respectively. Those for ceruloplasmin and haptoglobin were 22 ± 12 (3 to 74) mg/dL and 26 ± 26 (2 to 249) mg/dL, respectively. There were no significant differences in sex distribution by population; percentage female samples for the Charleston, Indian River Lagoon, Navy, and Six Flags populations were 39%, 40%, 49%, and 38%, respectively (P = 0.48). Among all populations, female dolphins were more likely to have higher iron, TIBC, and transferrin saturation than were male dolphins (Table 2).
Table 2.
Effects of sex on iron indices (mean ± 1 SD) in bottlenose dolphins
| Female dolphins (n = 81) | Male dolphins (n = 92) | Kruskal–Wallis P | |
| Iron (μg/dL) | 225 ± 155 | 147 ± 95 | <0.001 |
| Total iron-binding capacity (μg/dL) | 337 ± 128 | 275 ± 78 | 0.002 |
| Ferritin (ng/dL) | 5884 ± 13344 | 6264 ± 19224 | 0.84 |
| Transferrin saturation (%) | 61 ± 24 | 50 ± 23 | 0.003 |
| Haptoglobin (mg/dL) | 28 ± 32 | 24 ± 19 | 0.16 |
| Ceruloplasmin (mg/dL) | 22 ± 10 | 22 ± 13 | 0.24 |
Navy dolphins were older than those in the Charleston and Indian River Lagoon populations (Navy, 20.1 ± 10.4 y; Charleston, 12.6 ± 8.0 y; Indian River Lagoon, 10.2 ± 5.3 y; and Six Flags, 18.1 ± 12.1 y; P < 0.001). There were no significant trends in increasing iron indices with age among free-ranging dolphins. However, adult and older adult Navy dolphins (female, 7 y and older; male, 10 y and older) had higher ferritin concentrations than did juvenile dolphins (female, younger than 7 y; male, younger than 10 y); and adult dolphins older than 25 y had lower transferrin saturation than juveniles and adults (25 y or younger; Table 3). Among juvenile and adult dolphins, animals from managed collections had higher iron, ferritin, and transferrin saturation values than did wild dolphin populations (Table 4).
Table 3.
Effects of age on iron indices (mean ± 1 SD) in bottlenose dolphins by population
| Juveniles (female, < 7 y; male, < 10 y) | Adults (female, 7–24 y; male, 10-24 y) | Adults 25 y and older | Kruskal–Wallis P | |
| Charleston and Indian River Lagoon | ||||
| Number of dolphins | 24 | 33 | — | |
| Iron (μg/dL) | 99 ± 46 | 84 ± 29 | — | 0.43 |
| Total iron-binding capacity (μg/dL) | 268 ± 75a | 209 ± 27 | — | <0.001 |
| Ferritin (ng/dL) | 343 ± 483 | 222 ± 197 | — | 0.19 |
| Transferrin saturation (%) | 38 ± 15 | 41± 15 | — | 0.80 |
| Haptoglobin (mg/dL) | 23 ± 23 | 18 ± 18 | — | 0.13 |
| Ceruloplasmin (mg/dL) | 13 ± 12 | 17 ± 12 | — | 0.09 |
| Navy Marine Mammal Program | ||||
| Number of dolphins | 15 | 60 | 25 | |
| Iron (μg/dL) | 227 ± 86 | 249 ± 136 | 193 ± 173 | 0.06 |
| Total iron-binding capacity (μg/dL) | 389 ± 96 | 346 ± 118 | 323 ± 109 | 0.12 |
| Ferritin (ng/dL) | 2000 ± 3936b | 7535 ± 12184 | 7045 ± 16268 | 0.001 |
| Transferrin saturation (%) | 60 ± 19 | 68 ± 22 | 51 ± 28b | 0.02 |
| Haptoglobin (mg/dL) | 24 ± 23 | 29 ± 25 | 41 ± 47 | 0.23 |
| Ceruloplasmin (mg/dL) | 27 ± 9 | 27 ± 11 | 22 ± 7b | 0.03 |
The superscripted letters indicate values that are significantly higher (a) or lower (b) than others in the same row.
Table 4.
Comparisons of iron indices (mean ± 1 SD) in bottlenose dolphins by population and age category
| Charleston | Indian River | Navy | Six Flags | Kruskal–Wallis P | |
| Juveniles (female, < 7 y; male, < 10 y) | |||||
| Number of dolphins | 7 | 17 | 15 | 3 | — |
| Iron (μg/dL) | 112 ± 67 | 94 ± 35 | 227 ± 94a | 185 ± 21a | <0.001 |
| Total iron-binding capacity (μg/dL) | 255 ± 62 | 274 ± 81 | 389 ± 98a | 292 ± 29 | 0.002 |
| Ferritin (ng/dL) | 195 ± 230 | 404 ± 550 | 2000 ± 4343a | 1564 ± 1107a | 0.012 |
| Transferrin saturation (%) | 45 ± 23 | 35 ± 9 | 60 ± 20a | 64 ± 12a | 0.002 |
| Haptoglobin (mg/dL) | 25 ± 9 | 22 ± 12 | 24 ± 17 | 20 ± 16 | 0.59 |
| Ceruloplasmin (mg/dL) | 7 ± 1 | 16 ± 13 | 27 ± 8a | 18 ± 8 | 0.003 |
| Adults (female, 7-24 y; male, 10-24 y) | |||||
| Number of dolphins | 11 | 22 | 60 | 6 | — |
| Iron (μg/dL) | 95 ± 28 | 79 ± 28 | 249 ± 142a | 214 ± 46a | <0.001 |
| Total iron-binding capacity (μg/dL) | 197 ± 23 | 216 ± 27 | 346 ± 120a | 253 ± 29 | <0.001 |
| Ferritin (ng/dL) | 212 ± 100 | 227 ± 234 | 7535 ± 12,616a | 10,940 ± 6416a | <0.001 |
| Transferrin saturation (%) | 49 ± 19 | 36 ± 10 | 68 ± 22a | 84 ± 13* | <0.001 |
| Haptoglobin (mg/dL) | 20 ± 11 | 17 ± 6 | 29 ± 25a | 13 ± 6 | 0.002 |
| Ceruloplasmin (mg/dL) | 14 ± 67 | 18 ± 6 | 27 ± 12a | 17 ± 2 | <0.001 |
Value significantly higher than others in the same row.
High iron and managed collection dolphins.
A total of 25% (28 of 115) of samples from managed collections had high serum iron (exceeding 300 μg/dL). None of the 58 free-ranging dolphins tested had high serum iron. When mean iron indices among managed-collection dolphins with and without high iron levels were compared, there were no statistical differences in mean ceruloplasmin, haptoglobin, or WBC count (Table 5). However, dolphins with high iron had higher ferritin, percentage transferrin saturation, TIBC, and 60-min erythrocyte sedimentation rate than did dolphins that did not have high iron.
Table 5.
Comparison of means, standard deviations, and ranges of iron analytes among bottlenose dolphins with and without high serum iron (greater than 300 μg/dL) in managed collections
| High serum iron (n = 28) |
Not high serum iron (n = 87) |
||||
| Mean | 1 SD | Mean | 1 SD | Kruskal–Wallis P | |
| Total iron-binding capacity (μg/dL) | 488 | 116 | 296 | 57 | <0.001 |
| Transferrin saturation (%) | 87 | 11 | 57 | 22 | <0.001 |
| Ferritin (ng/dL) | 14,810 | 20,562 | 5081 | 6731 | 0.001 |
| Ceruloplasmin (mg/dL) | 25 | 9 | 25 | 9 | 0.82 |
| Haptoglobin (mg/dL) | 24 | 15 | 29 | 34 | 0.83 |
| WBC count (×103 cells/μL) | 9000 | 3000 | 9000 | 4000 | 0.49 |
| Erythrocyte sedimentation rate (mm/h) | 22 | 2 | 11 | 3 | 0.02 |
Response to phlebotomy.
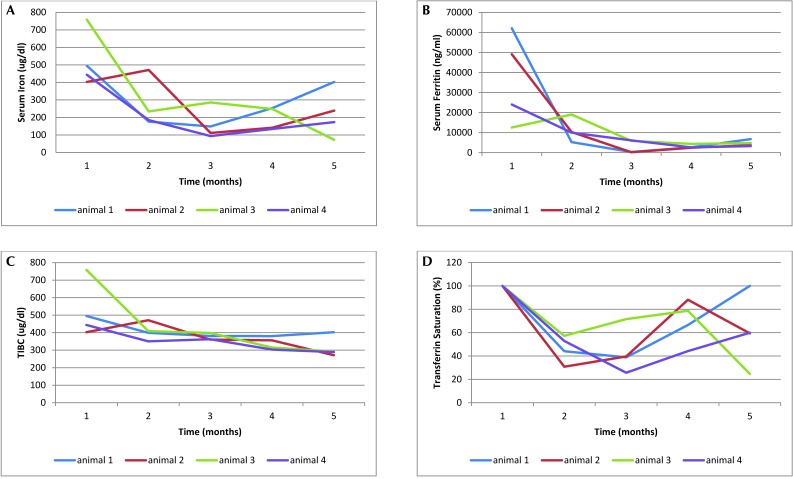
Changes in iron analytes were assessed among the 4 dolphins that were treated for iron storage disease by using phlebotomy. The baseline (time 1) samples were significantly (P < 0.05) higher than one or more of the phlebotomy or postphlebotomy samples for serum ferritin (times 3 through 5), serum iron (times 2 through 5), TIBC (times 4 and 5), and percentage transferrin saturation (times 2 and 3; Figure 1 A through D). Percentage transferrin saturation returned to prephlebotomy levels by 6 mo after treatment.
Figure 1.
Changes in (A) iron, (B) ferritin, (C) total iron-binding capacity, and (D) % transferring saturation in 4 dolphins undergoing phlebotomy treatment. Time 1, prephlebotomy; time 2, halfway through treatment; time 3, end of treatment phase; time 4, 6 mo after treatment; time 5, 1 y after treatment.
Low iron and free-ranging dolphins.
Dolphins from free-ranging populations were 15 times more likely to have low serum iron than were dolphins in managed collections (37 of 58 [64%] and 9 of 87 [10%], respectively). When free-ranging dolphins with and without low iron were compared, those with low iron were more likely to have lower TIBC, transferrin saturation, and haptoglobin (Table 6). Compared with Charleston dolphins, Indian River Lagoon dolphins had significantly (P < 0.05) lower serum iron (Charleston, 102 ± 46 μg/dL; Indian River Lagoon, 86 ± 32 μg/dL) and percent transferrin saturation (Charleston, 48% ± 20%; Indian River Lagoon, 35% ± 10%) but ceruloplasmin was significantly (P < 0.05) higher (Charleston, 11 ± 16 mg/dL; Indian River Lagoon, 17 ±10 mg/dL).
Table 6.
Comparison of means, standard deviations, and ranges of iron analytes among free-ranging bottlenose dolphins with and without low serum iron (less than 100 μg/dL)
| Low serum iron (n = 37) |
Not low serum iron (n = 21) |
||||
| Mean | 1 SD | Mean | 1 SD | Kruskal–Wallis P | |
| Total iron-binding capacity (μg/dL) | 220 | 40 | 258 | 79 | 0.009 |
| Transferrin saturation (%) | 32 | 9 | 52 | 14 | <0.001 |
| Ferritin (ng/dL) | 344 | 407 | 231 | 414 | 0.08 |
| Ceruloplasmin (mg/dL) | 15 | 9 | 17 | 16 | 0.69 |
| Haptoglobin (mg/dL) | 18 | 9 | 24 | 10 | 0.03 |
Discussion
We found a 25% prevalence of high serum iron (greater than 300 μg/dL) among 83 managed-collection bottlenose dolphins, including animals from a population known to be susceptible to hemochromatosis and excessive hepatic hemosiderin deposition.19,32,33,35 None of the 58 free-ranging dolphins we evaluated had high iron. Mean iron and ferritin levels both were higher among managed-collection than free-ranging dolphins.
In the current study, 21% of Navy dolphins had high serum iron, and 24% had transferrin saturation greater than 90%. Hemochromatosis in Navy dolphins has been associated with phasic increases in liver aminotransferases, hyperglobulinemia, and hyperlipidemia.35 Although liver biopsies were not conducted on all dolphins to confirm iron storage disease, hemochromatosis had been confirmed in many of the evaluated dolphins as chronically high elevated serum iron and hepatic hemosiderin deposition at the time of this study.19,35Although the true prevalence of hemochromatosis in wild dolphins remains unknown, the current study demonstrates a higher prevalence of high serum iron, total iron body stores, and transferrin saturation among managed-collection than wild dolphins. This finding is consistent with comparisons of iron stores among other managed collections of exotic species, including birds and white rhinos.21,29
Iron overload has been described in dolphins, but ferritin, perhaps the best indicator of total body iron stores, has not previously been studied in this species. When iron is absorbed in plasma, it binds with transferrin, and the normal percentage of transferrin saturated with iron in other mammals is 20% to 50%.16 Once in the plasma, iron can either be used to generate hemoglobin or be stored in tissues as ferritin, which is reconverted to iron as needed by the body. Serum ferritin, therefore, is a direct indicator of total body iron stores. If tissue levels of ferritin are high, iron can be stored secondarily as hemosiderin, which is much more difficult to convert back to free iron. Excessive iron deposition in tissues, in turn, can lead to pathologic changes.
In addition to increasing due to true increases in total body iron stores, serum ferritin can increase due to either inflammation or hemolytic anemia. To rule out inflammation and hemolytic anemia as causes of increased serum ferritin, ceruloplasmin and haptoglobin often are measured concurrently with ferritin. In humans, ceruloplasmin is an acute-phase protein, and haptoglobin decreases in response to hemolytic anemia.6,26 In the current study, dolphins with high serum iron had higher transferrin saturation, ferritin, and TIBC than did dolphins without high serum iron. There were, however, no differences in haptoglobin and ceruloplasmin values between these 2 groups. Although these findings likely demonstrate that dolphins with high serum iron have a true increase in total body iron stores, the ability of ceruloplasmin and haptoglobin to detect inflammation and hemolytic anemia in dolphins has not been determined.
Phlebotomy in dolphins successfully returns serum iron and other clinical chemistries to normal values.19 Several of these previously reported dolphins, however, have required periodic maintenance phlebotomy treatments to maintain these improved analyte values. In the current study, transferrin saturation among dolphins with hemochromatosis decreased to normal levels during the first 2 to 3 mo after phlebotomy treatment but returned to prephlebotomy measurements within 6 mo of treatment. These findings are consistent with those reported in humans. Although iron overload is ameliorated by phlebotomy, there is still a need to find the underlying cause of iron overload to successfully treat and prevent this disease in dolphins.
In the current study, dolphins from free-ranging collections were 15 times more likely to have low serum iron than were managed-collection dolphins (37 of 58 [64%] and 9 of 87 [10%], respectively). Low serum iron in dolphins is used as a general indicator of inflammation, and return of serum iron to normal levels during illness is seen as a positive indicator for recovery.13,24 The roles of iron and acute-phase proteins in inflammation have been used as indicators of dolphin health for decades, yet little has been published during the past 30 y about dolphin iron stores and associated acute-phase proteins.24 One barrier has been the need for species-specific assays, including C-reactive protein; development of tests that better detect early phases of inflammation and infection could lead to earlier treatment and better outcomes. Other barriers include the need for consecutive samples from dolphins with known health status to validate indicators for inflammation, including ceruloplasmin. Further, there is a need to better understand how dolphin iron metabolism compares with that of other species, including the presence and role of hepcidin.
When free-ranging dolphins with and without low iron were compared in the current study, dolphins with low iron were more likely to have lower TIBC, transferrin saturation, and haptoglobin. These findings may indicate that the high prevalence of low iron among this study's free-ranging dolphins was due to hemolytic anemia. Without RBC data, however, this hypothesis could not be tested. Further, haptoglobin has not been validated in dolphins as a valid measurement of hemolytic anemia.
The health of free-ranging dolphins living near Charleston, SC, and in the Indian River Lagoon, FL, is well documented.7-10,14,27,28 Although the overall percentages of dolphins that were considered healthy were comparable between these populations, these groups exhibit differences in disease and environmental contaminant exposures. For instance, only dolphins from Indian River Lagoon had lobomycosis and detectable concentrations of organic chemicals, including perfluroinated, polybrominated diphenyl ethers and polychlorinated biphenyls; pesticide levels were higher in Charleston dolphins.9,28 Previously reported mean and median values for iron among apparently healthy dolphins were 110 ± 32 μg/dL (range, 33 to 224 μg/dL) for Charleston dolphins and 99 ± 35 μg/dL (range, 32 to 206 μg/dL) for those from Indian River Lagoon.10,14 Compared with Charleston dolphins, Indian River Lagoon dolphins in the current study had lower serum iron and percentage transferrin saturation, where ceruloplasmin was higher. Further studies are needed to determine the clinical significance of these differences between the 2 populations.
In conclusion, managed-collection dolphins may be at higher risk of iron storage disease, as evidenced by a higher prevalence of high serum iron (greater than 300 μg/dL) and elevated transferrin saturation among dolphins in managed collections as compared with wild dolphins. Comparisons of postmortem tissue hemosiderin deposition between these 2 populations may help confirm this hypothesis. Further, causes of low iron among wild dolphins living in the southeastern United States should be assessed.
Acknowledgments
We thank the animal care and management staff of the Navy Marine Mammal Program and Six Flags Discovery Kingdom, and the free-ranging dolphin health assessment teams for Charleston, South Carolina, and the Indian River Lagoon, Florida. We thank Wayne McFee for age analysis on the free-ranging dolphin populations. We also thank the reviewers of this manuscript for their valued time and contributions. This work was supported by the Navy Marine Mammal Program at the Space and Naval Warfare Systems Center Pacific and the Office of Naval Research Grant No. N000141210294. The free-ranging dolphin samples used in this study were collected under NMFS Permit no. 998-1678, issued to Gregory Bossart, VMD. This constitutes scientific contribution no. 214 from the Sea Research Foundation.
References
- 1.Animal Welfare Act as Amended. 2008. 7 USC §2131-2159.
- 2.Asleh R, Levy AP.2005. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag 1: 19–28.
- 3.Batts KP. 2007. Iron overload syndromes and the liver. Mod Pathol 20:S31–S39 [DOI] [PubMed] [Google Scholar]
- 4.Beutler E, Felitti V, Ho NJ, Gelbart T. 2002. Relationships of body stores of serum ferritin, serum iron, unsaturated iron-binding capacity, and transferrin saturation in patients with iron storage disease. Acta Haematol 107:145–149 [DOI] [PubMed] [Google Scholar]
- 5.Bonar CJ, Trupkiewicz JG, Toddes B, Lewandowski AH. 2006. Iron storage disease in tapirs. J Zoo Wildl Med 37:49–52 [DOI] [PubMed] [Google Scholar]
- 6.Demetriou JA, Dreses PA, Gin JB.1974. Ceruloplasmin, p 857–886. In: Henry RJ, Cannon DC, Winkleman JW. Clinical chemistry principles and techniques. New York (NY): Harper and Row.
- 7.Fair PA, Adams JD, Mitchum G, Hulsey TC, Reif JS, Houde M, Muir D, Wirth E, Wetzel D, Zolman E, McFee W, Bossart GD. 2010. Contaminant blubber burdens in Atlantic bottlenose dolphins (Tursiops truncatus) from 2 southeastern US estuarine areas: concentrations and patterns of PCBs, pesticides, PBDEs, PFCs, and PAHs. Sci Total Environ 408:1577–1597 [DOI] [PubMed] [Google Scholar]
- 8.Fair PA, Adams JD, Zolman E, McCulloch SD, Goldstein JD, Murdoch MEM, Varela R, Hansen L, Townsend F, Kucklick J, Bryan C, Christopher S, Pugh R, Bossart GD.2006a. Protocols for conducting dolphin capture–release health assessment studies. NOAA Technical Memorandum 49, p 83. Rockville (MD): National Oceanic and Atmospheric Administration.
- 9.Fair PA, Houde M, Hulsey T, Bossart G, Adams J, Balthis L, Muir D. 2012. Assessment of perfluorinated compounds (PFCs) in plasma of bottlenose dolphins from 2 southeast US estuarine areas: relationship with age, sex, and geographic locations. Mar Pollut Bull 64:66–74 [DOI] [PubMed] [Google Scholar]
- 10.Fair PA, Hulsey T, Varela R, Goldstein J, Adams J, Zolman E, Bossart G. 2006b. Hematology, serum chemistry, and cytology findings from apparently healthy Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the estuarine waters of Charleston, SC. Aquat Mamm 32:182–195 [Google Scholar]
- 11.Farina LL, Heard DJ, LeBlanc DM, Hall JO, Stevens G, Wellehan JF, Detrisac CJ. 2005. Iron storage disease in captive Egyptian fruit bats (Rousettus aegyptiacus): relationship of blood iron parameters to hepatic iron concentrations and hepatic histopathology. J Zoo Wildl Med 36:212–221 [DOI] [PubMed] [Google Scholar]
- 12.Feder JN, Gnrike A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo RJ, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Less VK, Loeb DB, Mapa FA, McClellandv E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quinatant L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon JJ, Wolff RK. 1996. A novel MHC class I-like gene is mutated in patients with hereditary hemochromatosis. Nat Genet 13:399–408 [DOI] [PubMed] [Google Scholar]
- 13.Fenwick BW, Schroeder JP. 1989. Acute-phase response kinetics of the dolphin: diagnosis and therapeutic considerations. International Association for Aquatic Animal Medicine, 20th Annual Meeting Proceedings 20:84.
- 14.Goldstein JD, Reese E, Reif JS, Varela RA, McCulloch SD, Defran RH, Fair PA, Bossart GD, Hansen L. 2006. Hematologic, biochemical, and cytologic findings from apparently healthy Atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the Indian River Lagoon, Florida, USA. J Wldl Dis 42:447–454 [DOI] [PubMed] [Google Scholar]
- 15.Gonzales J, Benirschke K, Saltman P, Roberts J, Robinson PT. 1984. Hemosiderosis in lemurs. Zoo Biol 3:255–265 [Google Scholar]
- 16.Guyton AC, Hall JE. 1996. Textbook of medical physiology, 9th ed. Philadelphia (PA): WB Saunders.
- 17.Hohn AA, Scott MD, Wells RS, Sweeney JC, Irvine AB. 1989. Growth layers in teeth from known-age, free-ranging bottlenose dolphins. Mar Mamm Sci 5:315–342 [Google Scholar]
- 18.House JK, Smith BP, Maas J, Lane VM, Anderson BC, Graham TW, Pino MV. 1994. Hemochromatosis in Salers cattle. J Vet Intern Med 8:105–111 [DOI] [PubMed] [Google Scholar]
- 19.Johnson SP, Venn-Watson S, Cassle SE, Jensen ED, Smith CR, Ridgway SH. 2009. Use of phlebotomy treatment in Atlantic bottlenose dolphins with iron overload. J Am Vet Med Assoc 235:194–200 [DOI] [PubMed] [Google Scholar]
- 20.Juan-Salles C, Marco A, Ramos-Vara JA, Resendes A, Verges J, Valls X, Montesinos A. 2002. Islet hyperplasia in callitrichids. Primates 43:179–190 [DOI] [PubMed] [Google Scholar]
- 21.Kock N, Foggin C, Kock MD, Kock R. 1992. Hemosiderosis in the black rhinoceros (Diceros bicornis): a comparison of free-ranging and recently captured with translocated and captive animals. J Zoo Wildl Med 23:230–234 [Google Scholar]
- 22.Linder MC, Munro HN. 1972. Assay of tissue ferritin. Anal Biochem 48:266–278 [DOI] [PubMed] [Google Scholar]
- 23.Mazzaro LM, Dunn JL, St. Aubin DJ, Andrews GA, Chavey PS. 2004. Serum indices of body stores of iron in northern fur seals (Callorhinus ursinus) and their relationship to hemochromatosis. Zoo Biol 23:205–218 [Google Scholar]
- 24.Medway W, Geraci JR.1986. Clinical pathology of marine mammals, p 791–797. In: Fowler ME, editor. Zoo and wild animal medicine, 2nd ed. Philadelphia (PA): WB Saunders.
- 25.Office of Laboratory Animal Welfare 2002. Public health service policy on humane care and use of laboratory animals. Bethesda (MD): Department of Health and Human Services [Google Scholar]
- 26.Ong DS, Wang L, Zhu Y, Ho B, Ding J. 2005. The response of ferritin to LPS and acute phase of Pseudomonas infection. J Endotoxin Res 11:267–280 [DOI] [PubMed] [Google Scholar]
- 27.Reif JS, Fair PA, Adams J, Joseph B, Kilpatrick DK, Sanchez R, Goldstein JD, Townsend FI, McCulloch SD, Mazzoil M, Zolman E, Hansen LJ, Bossart GD. 2008. Health status of Atlantic bottlenose dolphins (Tursiops truncatus) from the Indian River Lagoon, FL, and Charleston, SC. J Am Vet Med Assoc 233:299–307 [DOI] [PubMed] [Google Scholar]
- 28.Reif JS, Mazzoil MM, McCulloch SD, Varela RA, Fair PA, Bossart GD. 2006. Lobomycosis in Atlantic bottlenose dolphins (Tursiops truncatus) from the Indian River Lagoon, Florida. J Am Vet Med Assoc 228:104–108 [DOI] [PubMed] [Google Scholar]
- 29.Sheppard C, Dierenfeld E. 2002. Iron storage disease in birds: speculation on etiology and implications for captive husbandry. J Avian Med Surg 16:192–197 [Google Scholar]
- 30.Smith JE, Chavey PS, Andrews GA. 1998. Semiautomatic and robotic methods for determining serum haptoglobin levels. Vet Clin Pathol 27:11–14 [DOI] [PubMed] [Google Scholar]
- 31.Smith KM, McAloose D, Torregrossa AM, Raphael BL, Calle PL, Moore RP, James SB. 2008. Hematologic iron analyte values as an indicator of hepatic hemosiderosis in callitrichidae. Am J Primatol 70:629–633 [DOI] [PubMed] [Google Scholar]
- 32.Venn-Watson S, Benham C, Carlin K, St. Leger J. 2012. Hemochromatosis and fatty change: building evidence for insulin resistance in bottlenose dolphins (Tursiops truncatus). J Zoo Wildl Med 43:S35-S47 [DOI] [PubMed] [Google Scholar]
- 33.Venn-Watson S, Carlin K, Ridgway S. 2011. Dolphins as animal models for type 2 diabetes: sustained, postprandial hyperglycemia and hyperinsulinemia. Gen Comp Endocrinol 170:193–199 [DOI] [PubMed] [Google Scholar]
- 34.Venn-Watson S, Jensen ED, Ridgway SH. 2007. Effects of age and sex on clinicopathologic reference ranges in a healthy managed Atlantic bottlenose dolphin population. J Am Vet Med Assoc 231:596–601 [DOI] [PubMed] [Google Scholar]
- 35.Venn-Watson S, Smith CR, Jensen E. 2008. Clinical relevance of elevated transaminases in a bottlenose dolphin (Tursiops truncatus) population. J Wildl Dis 44:318–330 [DOI] [PubMed] [Google Scholar]
- 36.Wrede CE, Buettner R, Bollheimer LC, Scholmerich J, Palitzsch KD, Hellerbrand C. 2006. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol 154:333–340 [DOI] [PubMed] [Google Scholar]