Abstract
B virus, a natural pathogen of macaques, can cause a fatal zoonotic disease in humans. Serologic screening of macaques by titration ELISA (tELISA, screening test) and by Western blot analysis (WBA, confirmatory test) is one of the principle measures to prevent human infection. Here we slightly modified these 2 tests and reevaluated their correlation. We developed a high-throughput tELISA and used it to screen 278 sera simultaneously against the homologous BV antigen and the heterologous antigens of Papiine herpesvirus 2 and Human herpesvirus 1. More sera (35.6%) were positive by the BV-ELISA than by the HVP2-ELISA (21.6%) or HSV1-ELISA (19.8%). The superiority of the homologous tELISA over the heterologous tELISA was prominent in low-titer sera. WBA confirmed only 21% of the tELISA-positive sera with low or intermediate antibody titers. These sera might have contained antibodies to conformational epitopes that could not be detected by WBA, in which denatured antigens are used, but that could be detected by tELISA, which detects both linear and conformational epitopes. WBA confirmed 82% of the tELISA high-titer sera. However, WBA defined the remaining 18% of sera, which were negative by tELISA, as nonnegative. This finding can be attributed to the difficulties encountered with the subjective interpretation of results by WBA. Together, the current results indicate the inadequacy of WBA as a confirmatory assay for sera with low antibody titers.
Abbreviations: BV, B virus (Macacine herpesvirus 1); CLIA, Clinical Laboratory Improvement Amendments; EU, ELISA units; HSV, Human herpesvirus; HVL, langur herpesvirus; HVM, Herpesvirus managabey; HVP, Papiine herpesvirus; SA8, simian agent 8; tELISA, titration ELISA; UN, uninfected; WBA, Western blot analysis
B virus (BV; Macacine herpesvirus 1), which is endemic in all species of macaques (natural hosts), is a member of the genus Simplexvirus, subfamily Alphaherpesvirinae and family Herpesviridae. Alphaherpesviruses are characterized by the ability to establish a neurotropic, generally asymptomatic, infection in their natural hosts. Macaques spread BV within a group by contact with macaques that are shedding virus during an acute or intermittently reactivated infection. BV is closely related to 2 well-characterized human alphaherpesviruses, Human herpesvirus (HSV) types 1 and 2, to simian agent 8 (SA8; Cercopithecine herpesvirus 2), which is endemic in African green monkeys (Cercopithecus aethiops), and to 2 recently identified simian alphaherpesviruses, HVP2 (Papiine herpesvirus 2) in baboons (Papio spp.) and langur herpesvirus (HVL), which is endemic in langur monkeys (Presbytis spp.)5,6,7 and which has not been officially classified or named.9 Recently, sera from a group of sooty mangabey monkeys (Cercocebus atys) were found to crossreact serologically with other simian simplexviruses.6,8,11 It was assumed that crossreactive antibodies were induced by a putative alphaherpesvirus that is endemic in mangabeys. This virus was provisionally named as Herpesvirus managabey (HVM) and is pending taxonomic evaluation.
Each of the simplex viruses has remarkable host specificity in nature. However, cross-species infections with BV have been reported. BV is the only nonhuman primate alphaherpesvirus that infects humans. When it does so, BV causes an often-fatal zoonotic disease in 80% of untreated humans.7,10,21,23,32,33
BV is transmitted through bites, scratches, or contact with infected oral or genital body fluids. In addition, the virus can be transmitted via fomites and from human to human through contact with contaminated wounds. Virus replication occurs in epithelial or fibroblast cells at the epidermal or dermal site of virus entry; however, BV also enters the peripheral nervous system via axons without replicating locally in surrounding epithelial cells, as has been reported for other simplex viruses.20,23 Once BV enters peripheral nerves, life-long latency is established in the dorsal root of spinal ganglia or cranial ganglia of infected hosts. BV undergoes periodic reactivation in macaques as well as in humans that survive this zoonosis. In both macaques and humans, BV can be reactivated in the ganglia, generally resulting in anterograde travel of the virus and replication at the original site of infection.10,33 This event results in virus shedding from infected cells, an event that can be detected by PCR or virus isolation if samples are collected during this event. However, because virus shedding is unpredictable and sporadic, identification of BV infection by means of PCR or virus isolation is rare. A more practical approach to identifying infected macaques or humans is the use of serologic methods for identifying antibodies specific for BV, although the shortcomings of this approach are appreciated when screening sera from subjects that are in the midst of a primary infection but have not yet produced detectable levels of antibodies or from BV-infected subjects that lack detectable antibody because of waning levels or anergy.
Because of the high lethality of BV to humans and life-long infection in survivors that lack effective strategies to clear this virus, BV antigen is produced under BSL4 conditions according to federal guidelines and under strict biosecurity regulations.3 Many laboratories in the United States, Europe, and Asia cannot produce BV antigens because of these restrictions and therefore use alternative crossreacting (heterologous) herpesvirus antigens such as HVP2 and HSV1 for the detection of antibodies to BV.10,19,26,29,34 Our previous studies16 indicated that using heterologous viruses in serologic assays for detecting BV antibodies contributes to increased false-negative results.
Serologic diagnosis of BV infection in macaques at the National B Virus Resource Laboratory has been based on 2 principal tests that meet standards proscribed by the Clinical Laboratory Improvement Amendments:4 the titration ELISA (tELISA) and Western blot analysis (WBA).15,31 tELISA detects antibody in sera from most BV-infected macaques by using the complex mixture of BV antigens that is present in lysates from infected cells and adsorbed onto polystyrene microtiter plates. These infected-cell lysates are prepared by using nondenaturing detergents. Quality-control assessment of each antigen lot is performed, including determinations of protein concentration, antigen mass, immunoreactivity with macaque serum pools, and reactivity in WBA assays.
WBA was chosen as the confirmatory test for the BV-screening tELISA; in addition, WBA serves as the primary screening assay for detecting antibodies to BV, HSV1, and HSV2 in human sera collected after exposure in the workplace.27,31 The relationship between ELISA and WBA is complex, and therefore the agreement between the tests may be lower than expected given that WBA detects only conformationally independent epitopes, whereas ELISA identifies both linear and conformational epitopes (unless the epitopes are cryptic).28 In addition, the different antibodies detected by each test might be induced at distinctly different time points after infection, so that what one assay detects, the other may not.2,8,9 Despite the difficulties encountered and due to the lack of a better alternative, both assays are required to maximize the likelihood of correctly identifying whether BV antibodies are present in a sample.
Because the identification of BV-infected macaques in any colony, especially SPF colonies, is of great medical and economic significance, assays should be designed to identify low levels of antibody as soon as possible after infection.12,31 For BV, as for other alphaherpesviruses, the presence of serum antibodies likely indicates the presence of virus that is either in an active lytic state (and actively replicating) or in an inactive, latent state. It is therefore important to have both a sensitive assay, to prevent missing low-responders, and a highly specific assay, to avoid obtaining false-negative results that can lead to costly veterinary and medical decisions.
In our continuing efforts to improve the accuracy of the tELISA for BV, we recently modified and automated it.18 This process included validation experiments that enabled us to reexamine some features of the BV serologic diagnosis.
Here we describe additional modifications of the tELISA and its adaptation to an automated, high-throughput 384-well format. This format enabled us to assess the sensitivities of tELISA using homologous compared with heterologous antigens in the same run. In addition, we evaluated the correlation between tELISA and WBA and discuss the effectiveness of WBA as a confirmatory test for tELISA. All assays were developed and performed inhouse in accordance with CLIA regulations,4 because each test either was used or could be used for identification of BV antibodies in human sera as well as macaque sera. Although CLIA does not regulate veterinary assays, we feel strongly that the tests described are superior because of the strict quality control that must be maintained for testing human samples.
Materials and Methods
Antigens.
BV antigen was prepared by infecting Vero cells (catalog no. CC L8, American Type Culture Collection, Manassas, VA) with BV (strain E2490, a generous gift from the late Dr. RN Hull, Eli Lilly, Indianapolis IN) at a multiplicity of infection of 2.0. Cells were collected by using a plastic scraper within 18 to 24 h after the observation of nearly 100% cytopathic effect. After centrifugation, cell pellets were resuspended in water containing protease inhibitor cocktail. Sodium deoxycholate and Tween 40 (Sigma Chemical, St Louis, Mo) then were added to a final concentration of 1% each. Lysates were stored in –80 °C. Before use in immunologic tests, infected cells were thawed to release virus, and the resulting lysate antigen was assayed for protein concentration and assessed against previous antigen and antibody lots according to standard quality assessment methodology for antigens used in both human and macaque testing. Polyclonal rabbit antibodies prepared against selected BV recombinant proteins25 were used to validate the presence of all major viral glycoproteins. Propagation and harvesting of BV was performed in the Viral Immunology Center's BSL4 Laboratory in accordance with the fifth edition of BMBL and the federal regulations.3 Other alphaherpesvirus antigens (HVP2, HVL, HVM, SA8, HSV1, and HSV2) were prepared similarly under standard BSL2 conditions. Control antigen was prepared from lysates of passage-matched uninfected cells (UN antigen)11,15,16 and validated as described for BV.
Sample collection.
Nonhuman primate sera were submitted to the National B Virus Resource Center (Atlanta, GA) for diagnostic evaluation for the presence of BV-specific antibodies. These samples were collected as part of routine surveillance or as a result of a human injury associated with a specific macaque. Macaque sera were obtained from the National B Virus Resource Center after being analyzed by using the robotic 96-well tELISA, as previously described.17 For the purpose of the current study, no distinctions were made between subspecies or sexes. The number of samples used differed for each experiment.
Preparation of standard BV-positive and -negative pooled macaque sera.
BV-negative macaque serum pools were prepared from at least 100 macaque sera that were determined as negative by tELISA. BV-positive macaque serum pools were prepared from at least 100 macaque sera that were determined as positive by tELISA. Sera were considered to be positive for antibodies to BV if the positive:negative (P:N) ratio was 3.5 or greater; negative sera fell below this threshold. To determine the P:N ratio of a sample, the OD405 of the 1:5-diluted sample in the BV-coated well was divided by the OD405 of the UN-coated well.
The titer, expressed in ELISA units (EU), of each new positive standard pool of sera was predetermined in a quality-control experiment in which at least 12 replicate dilution series were tested in wells coated with the whole-BV lysate and in control wells coated with lysates from uninfected cells. We defined 1 EU as the reciprocal serum dilution of the standard positive serum at a cutoff value. The cutoff value was arbitrarily defined as 2 times the average OD405 values obtained from UN-coated wells that reacted with the first positive serum pool dilution that generated an OD405 of 1.0 or greater in BV-coated wells.
Rabbit antibodies.
Antibodies used in this study were produced in New Zealand white rabbits (Myrtle's Rabbitry, Thompson Station, TN) that were acclimated and then immunized by intradermally injecting 0.2 mL Freund complete adjuvant containing 50 μg/mL antigen at multiple sites (1 mL total), with subsequent booster injections comprising Freund incomplete adjuvant containing 50 μg/mL antigen delivered intramuscularly in each of 2 flanks (0.5 mL per flank) at 1-mo intervals for 3 mo. Antibodies were tittered before each booster injection. All animal work followed an IACUC-approved protocol that was prepared in accordance with the Guide for Care and Use of Laboratory Animals.13
ELISA for detecting BV antibodies in macaques.
All ELISA tests used BV polypeptides and glycoproteins derived from detergent-solubilized lysates of infected cells. ELISA procedures were similar to those previously described,15,31 except that the blocking and dilution buffers (2% liquid gelatin and 10% normal goat serum, respectively) were replaced by a buffer composed of 2.5% nonfat milk and 2.5% liquid gelatin in borate-buffered saline. In addition, the cutoff value for ELISA-positive samples was modified to a P:N value of 3.5.
The 96- and 384-well automated tELISA were performed by using the same robotic equipment that had been previously described.18 Programs for automated procedures were written by using SAMI NT (Beckman Coulter, Fullerton, CA) and Gemini (TECAN, San Jose, CA) software programs.18 Adjustments were made to enable handling of 384-well microtiter plates and for the corresponding data processing and retrieval. All procedures for the automated 96-well tELISA format remained the same for the 384-well format, including those for coating, blocking, and washing wells; serum incubation in antigen-coated wells; and incubations with conjugate and substrate. The 384-well tELISA used 384-well microplates (Nunc Polystyrene Plates, catalog no. 242757, Thermo Fisher Scientific, Rochester, NY). The primary changes necessary to scale up from the 96-well format to the 384-well format involved reducing reagent volumes from 50 µL per well to 35 µL per well and increasing the number of wash cycles from 4 to 6.
The 384-well ELISA enabled sera to be tested against multiple antigens simultaneously, with a single antigen source per well. In this mode of testing, each serum sample was distributed by the liquid handler to an array of wells that had been coated with different antigens. The cutoff value of a P:N ratio of 3.5 (or greater) for positive samples was applied to the 384-well format.
Each tELISA plate included a standard antibody-positive macaque serum pool with a predetermined titer value (expressed in EU), a standard antibody-negative serum, and high-titer, moderate-titer, and negative BV antibody control pooled sera. The remaining wells were used for testing unknown sera. Each control and unknown sample was tested at 1:5 and 1:100 dilutions in both BV- and UN-coated wells. Results (in OD405) were analyzed and recorded as both qualitative and quantitative data.15,18 A serum sample was defined qualitatively as positive when its P:N value was at least 3.5, providing that OD405 values from the BV-coated wells were higher than 0.05. That is, when the P:N value was at least 3.5 at the 1:5 dilution or less than 3.5 at the 1:5 dilution and at least 3.5 at the 1:100 dilution, the serum was considered as positive for the presence of BV antibodies. Some sera were defined as having high background; the 1:5 dilution of these samples yielded high (≥0.5) OD405 values from the UN wells and comparable values from the BV-coated wells, thus preventing the calculation of a meaningful P:N ratio.
For quantitative results, the net OD405 values of the diluted antibody-positive serum samples were compared against a standard curve generated from a sample with a predetermined titer expressed in EU. The test serum dilution (1:5 or 1:100) that resulted in OD values within the linear range of the standard curve was used for calculating the test serum's titer in EU.15,18
Sera positive by tELISA also were tested by WBA, for confirmation of their tELISA-determined status as positive or negative for the presence of antibodies to BV.
WBA.
WBA was performed according to standardized diagnostic lab protocols essentially as previously described.9 Briefly, lysates of BV-infected cells were diluted in SDS disruption buffer (4% SDS, 4% 2-mercaptoethanol, and10% glycerol in 50 mM Tris-HCl, pH 6.8), boiled for 3 min, and loaded on discontinuous gradient gels (8% to 16%). Proteins were transferred from the gel to nitrocellulose membranes (0.45 µm). After transfer, membranes were cut into 3 × 95-mm strips, which were blocked with milk–gelatin buffer and incubated with test sera diluted in the same buffer (1 h at 37 °C). Antibodies were detected by incubating strips in biotinylated goat antihuman IgG (1 h at 37 °C), followed by treatment with avidin–alkaline phosphatase (30 min at room temperature) and an appropriate alkaline phosphatase substrate (incubated until sufficient color developed). This protocol is the standard assay approved by CLIA and is used for both human and macaque samples.
Macaque sera were tested against controls that consisted of BV-positive and -negative pooled macaque sera and a pooled rabbit antiserum to recombinant BV antigens.28 The protein banding patterns after WBA of test samples were visually compared with those of each control membrane strip and with baseline strips from samples obtained during baseline or convalescent conditions, when available. Results are reported as negative when the test strip had no bands that matched those on the BV-positive control strip, indeterminate when 1 to 3 bands matched those on the positive control, and positive for antibodies to BV when at least 4 bands in the test sample match those from the BV-positive control.
Definition of indeterminate results from the 96-well tELISA.
Indeterminate results, specifically sample values that fell between negative and positive values and around the P:N cutoff, were defined as described, with some modifications.25 Pooled macaque sera with different levels of antibodies to BV were prepared and tested at a dilution of 1:5 by using the 96-well tELISA. Each pool was tested 10 to 16 times during the same run. The number of positive results for each group of replicates was determined based on a P:N cutoff of 2.5. The percentage of positive results in each replicate was calculated and plotted against the average P:N value of each serum pool. This graph was used to define the indeterminate zone around the cutoff value.
Comparison of homologous and heterologous antigens in tELISA for detection of BV antibodies.
The 384-well microtiter plate (wells A1 to P24) was designed for testing 40 serum samples (S1 to S40). For coating, the plate was divided in 6 sections containing 4 columns per section. Each column (16 wells) was coated with 1 of the 4 antigens: control antigen (UN), BV (homologous) antigen, and HVP2 and HSV1 (heterologous) antigens. Microplate sections 1 and 2 were assigned for testing controls (wells A1 through H8) and 8 serum samples (samples S1 through S8; wells I1 through I8). Samples S9 through S24 were tested in sections 3 and 4 (wells A9 through P16). The remaining 16 samples (S25 through S40) were tested in the other 2 sections (wells A17 through P24). Each of the serum samples (n = 40) was tested at 1:5 and 1:100 dilutions. Well A1 was assigned as the blank control well. Wells D1 through D7 accommodated seven 3-fold dilutions of the positive standard serum pool and were tested against each of the 4 antigens at 3-fold dilutions starting at 1:100 and ending at 1:71,600. The high-, moderate-, and low-titer and negative antibody controls were tested against each of the 4 antigens at 1:5 and 1:100 dilutions.
The antibody crossreactivity test.
BV antibodies that were tested by ELISA against a panel of alphaherpesviruses resulted in a typical pattern of crossreactivity, the strength of which response decreased in the order of BV, HVP2, HVL, SA8, and HSV1 and HSV2.16 This observation was used in the current study for validation of samples with low titers of antibodies that could not be confirmed by WBA. The antibody crossreactivity test was performed as previously described.16 By using the modified tELISA (that is, incorporating a buffer composed of 2.5% nonfat milk and 2.5% liquid gelatin in borate-buffered saline), each of the sera was tested in wells coated individually with 6 different alphaherpesvirus antigens (BV, HVP2, HVL, SA8, HSV1, and HSV2). The percentage crossreactivity of each of the sera on the heterologous viral antigens relative to the reactivity to the homologous BV antigen was calculated and plotted as bar graphs. Sera that resulted in the typical crossreactivity pattern were considered to be true positives in regard to BV antibodies.
Statistics.
Data were analyzed for proportion of negative or positive reactivity according to the described serologic methods or for the proportion of sera in a certain tELISA-determined titer group, with 95% confidence intervals calculated as described.24 The noninferiority test was performed by comparing probability estimates (percentage of positive sera by each assay in each of the tELISA-determined titer groups) and their 95% confidence intervals. When the 95% confidence interval of the first probability falls within the 95% confidence interval of the second probability, the first probability is not inferior to the second. The method for calculating the 95% confidence intervals used a normal approximation to the binomial probability distribution that requires at least 5 observations in each arm of the distribution. When the sample size was too small, the probability was estimated by adding 2 successes and 2 failures, that is, the adjusted Wald interval.1
The t test statistics were determined by using GraphPad Prism 5.0 for Macintosh (http://www.mackiev.com). Coefficients of variation were calculated by determining the percentage of the standard deviations of the mean.
Results
Defining the ‘gray zone’ for the 96-well automated tELISA.
Decades of testing macaque sera by using WBA and tELISA have revealed that numerous tELISA low-titer positive results are not confirmed by WBA and therefore are regarded by definition as false positives.12,31 During the implementation of automated immunoassays for the identification of serum BV antibodies, we reexamined the tELISA procedures to maximize the performance of the assay and reduce the number of false-positive (unconfirmed tELISA-positive) samples.18 Variables investigated included dilution and blocking buffers. Preliminary results (data not shown) indicated that changing the blocking and dilution buffer from 2% gelatin and 10% normal goat serum to a buffer composed of 2.5% nonfat milk and 2.5% liquid gelatin in borate-buffered saline improved the agreement between WBA and the modified tELISA. However, WBA failed to confirm as nonnegative approximately 50% to 60% of tELISA-positive values from samples with low levels of serum BV antibodies. Because the low correlation between these WBA and ELISA data could have been due to an excessively low tELISA cutoff value, we reexamined the establishment of the cutoff value by defining the criteria for indeterminate results (that is, by establishing the gray zone) of the assay.
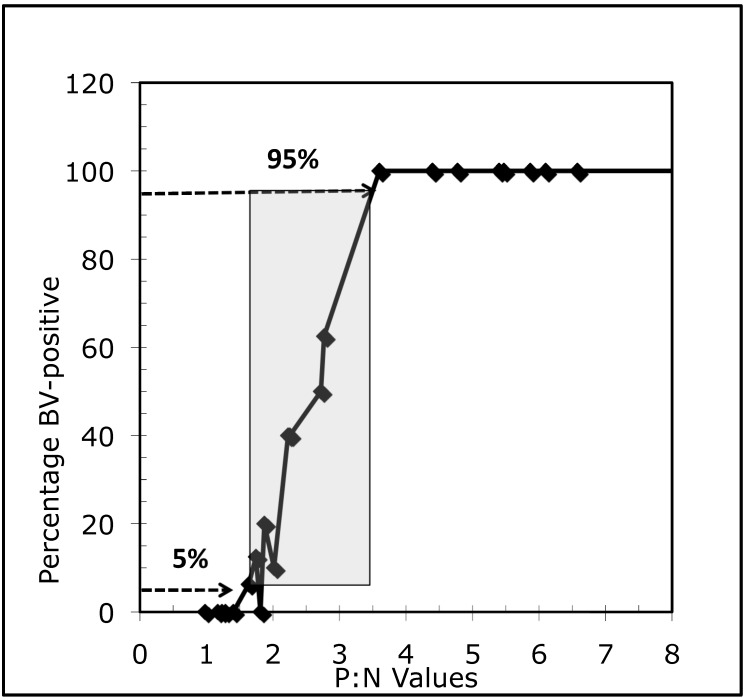
The gray zone around an assay's cutoff is defined as the range between which test results are 0% to 100% positive or any other more relaxed criteria that may narrow the gray zone (for example, 5% to 95% positive).25 Ideally, to determine the gray zone for an assay, numerous sera must be selected that represent span diverse antibody concentrations from 0 (negative) to high (positive). Each serum must be tested 10 to 20 times in the same run.25 Because numerous sera of sufficiently large volumes for the desired number of replicates were unavailable, we selected and grouped sera according to their P:N values as predetermined by the diagnostic laboratory. Sera in each titer-defined group then were pooled to provide sufficient volume for 10 to 20 replicate tests for each pool. We used the 96-well tELISA to test these pools (n = 28) in 2 independent experiments, which comprised either 10 or 16 replicates for each pool. The number of positive results for each group of repetitions was determined based on a P:N cutoff of 2.5. The percentage of positive results in each of the repetitions were calculated and plotted against the average P:N value of each serum pool. As demonstrated in Figure 1, the range of P:N values was 1.4 to 3.6 for which results were 0% and 100% positive by tELISA and 1.8 to 3.5 for which results were 5% and 95% positive. As expected, the P:N midpoint of the calculated gray zones was 2.5. According to these results, we decided to use the upper limit of the gray zone, a P:N value of 3.5, as the cutoff value for defining samples as being negative or positive for antibodies to BV.
Figure 1.
The gray zone of BV-indeterminate samples for a P:N range (1.8 to 3.5) around a cutoff P:N value of 2.5 that includes 5% to 95% of positive sera as determined by the automated 96-well tELISA for macaque sera tested at the dilution of 1:5.
Distribution of P:N values in macaque sera tested for BV antibodies by using the 96-well tELISA.
To evaluate the specificity and sensitivity of the 96-well tELISA, we determined the P:N values for a group of 468 macaque sera that were diluted 1:5 and screened for BV antibodies. Samples were defined as being positive or negative for BV antibodies according to the P:N cutoff value of 3.5, as defined by the gray-zone criteria. As expected, we observed 2 main populations, which were represented by 2 P:N ranges, one of less than 1 to 8.0 and the other of 9 to greater than 10. A gap was present between these 2 populations and comprised P:N values greater than 8.0 to less than 9. For the sake of evaluating the sensitivity and specificity of the assay, we assumed that the sera within the lower P:N range represent true negative samples, such that all sera with a P:N value of 3.5 to 8.0 should be considered as false positives. We calculated that the percentage of sera within this P:N range (3.5 to 8.0) was 5.6% of the 468 samples tested. The estimated specificity therefore is 94.4% (that is, 100% – 5.6%). Similarly, the group of sera within with P:N values of 9 to >10 were assumed to be positive in light of the frequency distribution. The sensitivity of tELISA using the 3.5 cutoff is 100% (no false negatives), because all sera in this group of 468 samples had P:N values higher than 3.5.
Optimization and validation of the automated 384-well tELISA.
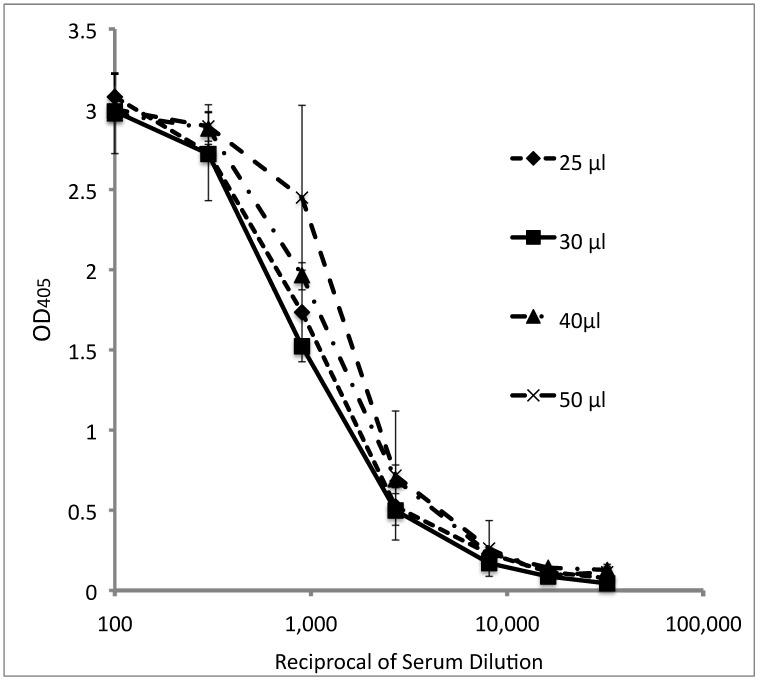
To define the optimal reagent volume that can be used in the automated 384-well tELISA and to validate resulting assay, we compared data obtained by using the 384-well format with those from the 96-well format. The optimal reagent volume for performing the 384-well ELISA was determined by comparing 4 volumes (25, 30, 40, and 50 µL) in two 384-well microtiter plates. Dilutions of a standard BV-positive serum were tested (in quadruplicate) by using wells coated with lysate from BV-infected cells (Figure 2). No significant differences between volumes were observed, as demonstrated by the overlapping standard deviations. However, experimental errors were higher when 25- and 50-µL reagents volumes were used (average coefficient of variation: 18% and 35%, respectively) as compared with the 30- and 40-µL reagent volumes (average coefficient of variation: 10% and 9%, respectively). Similar results were obtained after testing 8 repetitions of pooled macaque sera defined as high-, moderate-, and low-titer and negative controls that were tested at dilutions of 1:5 and 1:100 dilutions on BV-coated wells. Therefore we chose a reagent volume of 35 µL as the optimal reagent volume for performing tELISA in 384-well plates.
Figure 2.
The effect of reagent volume (25, 30, 40, or 50 µL) on the titration curves of a standard BV-positive macaque serum pool tested by the automated 384-well tELISA. Data are given as OD405 units (mean ± 1 SD; n = 4).
To compare the 96- and 384-well tELISAs formats, we used both formats to test 120 macaque serum samples in BV- and UN-coated wells. High-, moderate-, and low-titer and negative standards were included on each plate. Fifty-two of the 120 sera had been tested at 1:5 and 1:100 dilutions by using the 96-well tELISA, and the remaining 68 sera were tested at the 1:5 dilution only. All 120 sera tested by the 384-well tELISA format were tested in duplicates at dilutions of 1:5 and 1:100. A single 384-well plate accommodated a total of 40 samples in addition to the controls; therefore 3 plates were required to test all 120 sera. In addition, various dilutions (1:100, 1:300, 1:900, 1:2700, 1:8100, 1:16,200, and 1:32,400) of the standard positive serum were run in duplicate wells in 2 different areas of the plate. The standard curves from the three 384-well plates practically overlapped, indicating excellent repeatability of the 384-well tELISA (results not shown).
Linear regression analysis of the OD values from the 384- and 96-well tELISA yielded a correlation coefficient (r) of 0.8826, indicating good correlation between the assays.
The P:N values obtained with the 384-well format were lower than those obtained by using the 96-well tELISA, indicating that the 96-well version was more sensitive than the 384-well. One reason for the lower P:N values was the significantly (P < 0.0001, t test) higher nonspecific reactivity obtained for the 384-well UN controls as compared with the 96-well UN controls. The increased nonspecific reactivity in the 384-well plates occurred with both the 1:5 and 1:100 serum dilutions. To decrease the background in the 384-well tELISA, we increased the number of wash cycles from 4 to 6 in all future experiments. This change in procedure slightly reduced background values in the 384-well tELISA but did not significantly increase its sensitivity relative to that of the 96-well assay.
Among the 132 sera evaluated (120 test sera and 3 sets of high-, moderate-, and low-titer and negative controls), 17 were positive and 109 were negative (total, 126) by both assays. Therefore the agreement between the 2 tELISA procedures was 95%. The remaining 6 samples were positive by the 96-well tELISA but negative by the 384-well version, and all 6 samples had exceptionally high nonspecific reactivity (leading to negative P:N ratios) in the UN control wells of the 384-well tELISA.
Coefficients of variation were determined from the average OD values of the duplicate wells of the three 384-well plates. There were no significant differences between the 3 plates. The mean coefficients of variation for the 1:5 serum dilution were 12.6% for BV antigen and 17.1% for the UN control. As expected, the lower OD values of the 1:100 serum dilution yielded higher coefficients of variation (32% for BV-coated wells and 28% for UN-coated wells). These relatively modest coefficients of variation and the repeatability of the titration curves indicate that, for practical and economic reasons, it is sufficient to screen samples in single (rather than duplicate) wells.
Comparison of homologous and heterologous antigens in tELISA for detecting antibodies to BV in macaque sera.
Several laboratories that test samples for BV-specific antibodies use heterologous herpesvirus (HSV1 or HVP2) antigens instead of the homologous BV antigen. Our past studies indicated that the signals obtained from tELISA using heterologous antigens were significantly lower in certain antibody-titer groups than were the signals due to homologous antigen.15,16 To compare the sensitivities of the assays, we tested 278 sera against homologous and heterologous antigens in a total of seven 384-well microtiter plates. We grouped the results into 7 categories according to their P:N values at the 1:5 and 1:100 dilutions and OD cutoff values as defined in Materials and Methods (Table 1); 4 of the 7 categories were interpreted as positive and 3 as negative. Of the 278 sera tested, 99 (35.6%) sera were positive for BV antibodies by the BV-based ELISA, 60 (21.6%) were positive by the HVP2-based ELISA, and 55 (19.8%) by the HSV1-based ELISA. The 95% confidence intervals for the proportion of positives by each ELISA were calculated (Table 1). The range of confidence intervals for the BV-based tELISA does not overlap with those of the assays using HVP2 and HSV1, underscoring the significant differences between the homologous and heterologous tELISA. The observed differences between the homologous and heterologous tELISA were larger for sera with low antibody titers. The positive sera (n = 99) identified by the BV-based tELISA were arranged in 3 titer-based groups (50 EU or less, 500 EU or less, and 5000 EU or less). The percentages of positive sera in the 2 low-titer groups were lower for the heterologous-antigen tELISA than the BV-based tELISA (Figure 3).
Table 1.
Diagnostic criteria for interpreting tELISA results based on OD405values and a P:N cutoff value of 3.5 for determination of BV infection (positive) or lack of infection (negative)
| Result | Category | Sample characteristics | Diagnostic criteria | BV | HVP2 | HSV1 | |
| Positive | 1 | Low titer of BV antibodies | OD(BV) ≥0.05 at 1:5; OD(UN) <0.5 at 1:5 | 16 | 13 | 4 | |
| P:N ≥3.5 at 1:5, <3.5 at 1:100 | |||||||
| 2 | High titer of BV antibodies | OD(BV) ≥0.05; OD(UN) <0.5 | 65 | 31 | 37 | ||
| P:N ≥3.5 at 1:5, ≥3.5 at 1:100 | |||||||
| 3 | High background; positive at 1:100 dilution | OD(UN) ≥0.5 at 1:5 | 12 | 10 | 9 | ||
| P:N <3.5 at 1:5, ≥3.5 at 1:100 | |||||||
| 4 | High background; positive at 1:5 and 1:100 dilutions | OD(UN) ≥0.5 at 1:5 | 6 | 6 | 5 | ||
| P:N ≥3.5 at 1:5, >3.5 at 1:100 | |||||||
| Negative | 5 | Antibody negative | OD(UN) <0.5 | 8 | 9 | 5 | |
| P:N <3.5 at 1:5, >3.5 at 1:100 | |||||||
| 6 | Weakly antibody negative | OD(UN) <0.5 at 1:5 | 119 | 155 | 162 | ||
| P:N <3.5 at 1:5, <3.5 at 1:100 | |||||||
| 7 | High background and antibody negative | OD(UN) ≥0.5 at 1:5 | 52 | 54 | 56 | ||
| P:N <3.5 at 1:5, <3.5 at 1:100 | |||||||
| Total no. (%) of positive sera (278 sera tested) | 99 (35.6) | 60 (21.6) | 55 (19.8) | ||||
| 95% Confide nce interval | 29.9 –41.2 | 16.7 –26.4 | 15.1 –24.5 | ||||
| Total no. of negative sera | 179 | 218 | 223 |
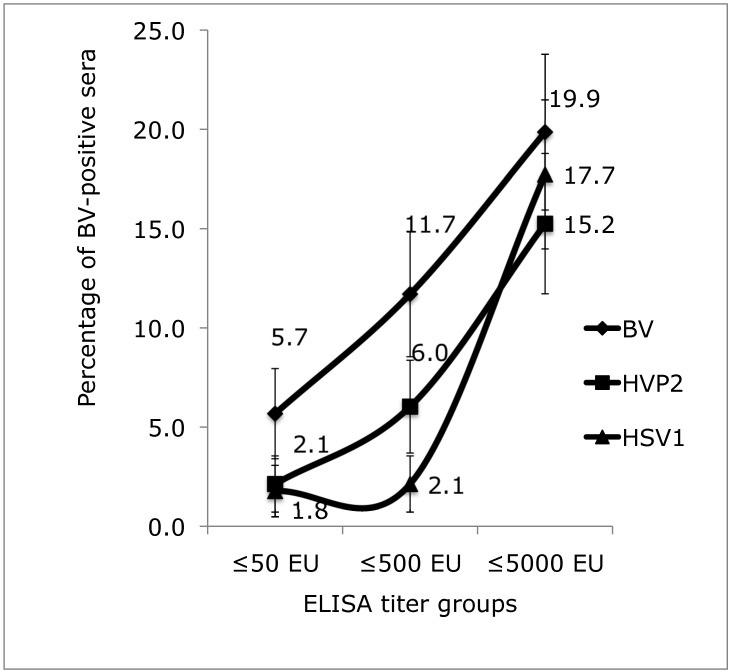
Figure 3.
Percentage of positive sera in each titer group (50 EU or less, 500 EU or less, and 5000 EU or less) from among 278 sera tested by using homologous (BV) and heterologous (HVP2, HSV1) automated 384-well tELISA. Bars represent 90% confidence intervals.
These results were analyzed further by using the noninferiority test.1 In the less than 50 EU titer group, 5.7% of sera were positive by BV-based tELISA, compared with 2.1% and 1.8% by the HVP2- and HSV1-based tELISA, respectively. Similarly, in the less than 500 EU titer group, 11.7% of the sera were positive by BV-based tELISA compared with 6.0% and 2.1% by the HVP2- and HSV1-based tELISA, respectively. The 95% confidence intervals of these 2 titer groups overlapped only minimally between the BV- and HVP2-based tELISA (results not shown), and the 90% confidence intervals did not overlap between these 2 groups (Figure 3). These data demonstrate the superiority of the BV-based tELISA for detecting low-titer BV antibodies in macaque sera. Furthermore, the 95% confidence intervals for the less than or equal to 50 and less than or equal to 500 EU titer groups did not overlap between the BV- and HSV1-based tELISA. These data clearly demonstrate the inferiority of the HSV1-based assay. In the group of high-titer sera, 19.4% of sera were positive for BV antibodies by the BV-based tELISA, 14.7% by the HVP2-based assay, and 17.3% by the HSV1-based test. These values were not significantly different, as evidenced by the remarkable overlap of the 90% and 95% confidence intervals.
Of the 278 sera tested, 70 were identified as having high background (Table 1 categories 3, 4, and 7). The UN control wells of the 1:5 dilutions of these sera yielded OD values of 0.5 or greater (mean OD, 0.9; range, 0.5 to 1.9). Nevertheless, 6 of these sera were identified as BV-positive, with P:N values of 3.5 or greater. Further dilution (1:100) of these 70 high-background samples decreased the nonspecific signal, such that the mean OD value was 0.24 (range, 0.2 to 1.3). Of the 70 sera tested at the 1:100 dilution, only 7 had OD values of 0.5 or greater when tested against the UN control. All sera that were positive (that is, yielded a P:N value of 3.5 or greater) at the 1:5 dilution were similarly positive at the 1:100 dilution.
In this group of 70 high-background sera, 18 were positive by the BV-based tELISA, 16 by the HVP2-based assay, and 14 by the HSV1-based test (Table 1). The somewhat higher correlation between the homologous and heterologous tELISA results for this group of sera (categories 3 and 4) compared with those of the positive sera that had less background (categories 1 and 2) appeared to be due to the high BV-antibody titer of most high-background samples. Among the 18 sera that were positive, 14 had titers of 5000 EU or greater, 3 had titers of 500 EU or less, and only 1 had a titer of 50 EU or less.
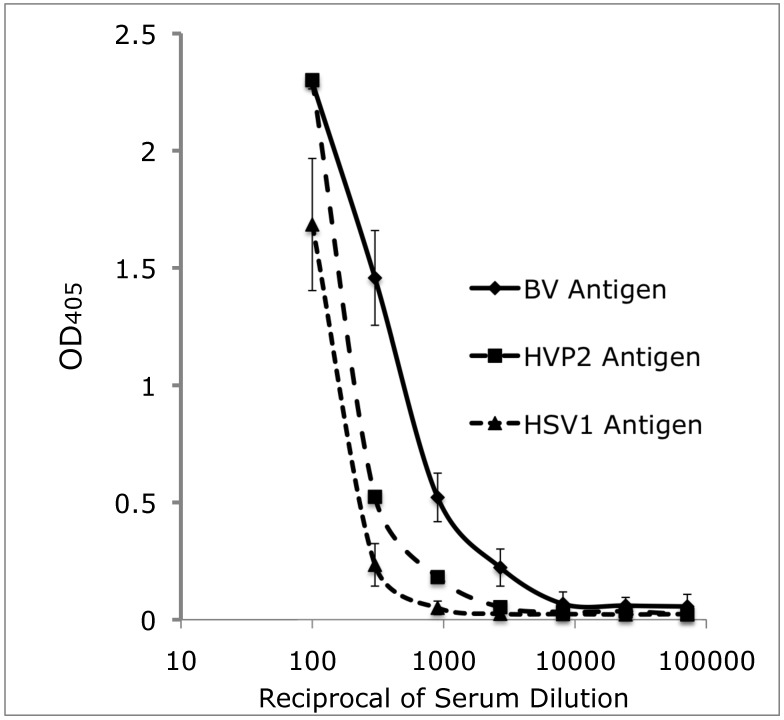
The mean OD values and standard deviations of each of the 7 titration curves obtained from the seven 384-well tELISA are shown in Figure 4. In general, mean OD values were higher for the homologous BV antigen than for the heterologous antigens. Differences between the mean OD values obtained by the HVP2-based tELISA compared with the HSV1-based were not significant. Differences between the mean OD values obtained by the BV-based tELISA as compared with either of the heterologous antigens were statistically significant (P ≤ 0.05, t test) at the serum dilutions of 1:300, 1:900 and 1:2700 but not 1:100. These data support our preliminary observations that the differences between the homologous and heterologous tELISA were lower for samples containing high titers of BV antibody and higher in sera that contain low titers or poorly specific BV antibodies. In addition, early low-avidity antibodies may react with preferentially homologous antigens to a significantly greater extent than with heterologous antigens.
Figure 4.
Antibody titration of a positive-control standard macaque serum pool in automated 384-well tELISA by using BV, HVP2, and HSV1 antigens. Data are given as OD405 units (mean ± 1 SD; n = 7).
We also analyzed the mean OD values and standard deviations of the 7 sets of control sera with high, moderate, and low titers of BV antibody (results not shown). As expected, tELISA yielded high OD values with the BV antigen, low OD values with the HVP2 antigen, and the lowest OD values with the HSV1 antigen. These differences between mean OD values were all statistically significant as analyzed by paired t tests that were not corrected for multiple comparisons, because all P values were lower than 0.009. These findings further support our observations that the homologous BV-based tELISA is superior to heterologous ELISA for detecting BV antibodies in macaque sera.
Correlation of ELISA with WBA.
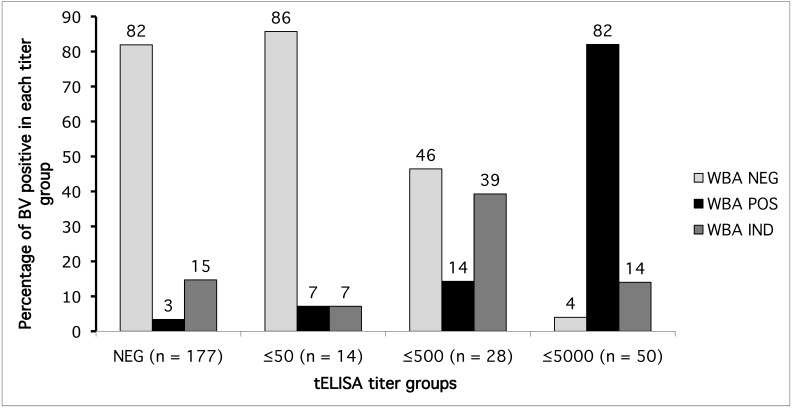
Of the 278 macaque sera that were tested by tELISA, 269 also were tested by WBA. Furthermore, 177 of the 269 sera were negative and the remaining 92 were positive for BV antibodies by tELISA. We allocated the 92 positive sera into 3 groups according to their titers as follows: 50 EU or less, 500 EU or less, and 5000 EU or less. The negative group and each of the 3 positive groups (as determined by tELISA) were analyzed for their correlation with the results from WBA (Figure 5).
Figure 5.
Percentages of sera positive (POS), negative (NEG), and indeterminate (IND) by WBA in different tELISA titer groups.
WBA did not confirm the tELISA-positive results from some sera regardless of the antibody-titer groups into which the sample was classified (Figure 5). However, the percentage of sera confirmed appeared to depend on the tELISA-determined titer. Specifically, 82% of the sera in the less than or equal to 5000 EU titer group were also positive by WBA, and another 14% were classified as indeterminate (overall, 96% nonnegative). In the group of sera with titers of 500 EU or less, WBA classified 14% of tELISA-positive sera as positive and 39% as indeterminate (total, 53% nonnegative). WBA found few sera in the lowest-titer group to be positive (7.0%) or indeterminate (7.0%) for BV antibodies. These results indicate that WBA may misclassify many low-titer sera as false negatives. Furthermore, 6 (3%) of the 177 tELISA-negative sera were positive according to WBA, and 26 (15%) were classified as indeterminate.
Examination of tELISA-determined high- and low-titer sera by the crossreactivity test.
To further validate our tELISA procedure, we performed crossreactivity tests using HVP2, HVL, SA8, HSV1, and HSV2 antigens.16 tELISA results from high-titer sera were compared with results from low-titer sera (Table 2). Our hypothesis was that sera tested by tELISA and classified as positive but low-titer for BV antibodies will demonstrate similar patterns of crossreactivity against the panel of closely related simplexvirus antigens as do high-titer sera. As previously shown,16 a typical crossreactivity pattern for a serum that contains BV antibodies shows higher reactivity with BV, HVP2, and HVL antigens and lower reactivity to SA8 and the 2 human viruses, HSV1 and HSV2.
Table 2.
Evaluation of BV antibodies in macaque sera by alphaherpesvirus crossreactivity analysis
| Sample | WBA result | 96-well tELISA (EU) | Crossreactivity analysis | |
| Negative by tELISA; negative by WBA | NRhS pool | NEG | NEG | NEG |
| Low titer by tELISA; negative by WBA | MMU33404 | NEG | ≤500 | POS |
| X2109 | NEG | ≤500 | POS | |
| MMU24796 | NEG | ≤500 | POS | |
| MMU24840 | NEG | ≤50 | POS | |
| BV36 | NEG | ≤50 | NEG | |
| CG64 | NEG | ≤50 | NEG | |
| Low titer by tELISA; indeterminate by WBA | H314 | IND | ≤50 | NEG |
| High titer by tELISA; nonnegative by WBA | F20759M | IND | ≤5000 | POS |
| 60-88 | POS | ≤5000 | POS | |
| RQ3700 | POS | ≤5000 | POS | |
| 8642M | POS | ≤5000 | POS | |
| MMU-34162 | POS | ≤5000 | POS | |
| 02-14 | IND | ≤5000 | POS |
IND, indeterminate; NEG, negative; NrhS pool, pool of rhesus macaque sera that were negative for BV antibodies; POS, positive
We selected 6 WBA-negative sera from the low-titer tELISA groups (50 EU or less and 500 EU or less) and 6 sera (4 positive and 2 indeterminate by WBA) from the high-titer group (5000 EU or less) to be tested in the crossreactivity test. We also included a WBA-indeterminate, low-titer serum (H314) and a tELISA-negative rhesus serum pool for comparison (Table 2).
All 6 high-titer sera yielded typical patterns of crossreactivity for BV-positive sera.16 The negative serum pool and 2 of the low-titer sera (BV36 and CG64) showed low reactivity to each virus, as is typical for tELISA-negative sera. Of the remaining 5 low-titer sera, 4 showed typical crossreactivity with heterologous viral antigens. The fifth serum, H314, showed an atypical crossreactivity pattern, in which most of the reactivity was toward BV with very little reactivity against the other alphaherpesviruses. These data indicate that at least 4 of the low-titer tELISA sera that were negative by WBA likely were true positives for BV antibodies.
Discussion
The goal of diagnostic medicine is to develop and use assays capable of detecting the earliest signals of infection or disease, the value of which is underscored when dealing with agents that cause significant morbidity and mortality, as is the case with BV zoonosis. In the testing of human samples, CLIA regulates laboratories regardless of whether they use assays licensed by the Food and Drug Administration or developed inhouse. However, laboratories testing animals are not required to validate assays in terms of limits of detection, accuracy, precision, trending, and so forth. However, because our laboratory is CLIA-regulated, we continually perform studies and analyses to assess assay performance and reliability.
Assay sensitivity is of utmost importance, but this sensitivity must be coupled with specificity. For BV diagnosis by serology, the most commonly used primary screening assay is tELISA, and the confirmatory assay is WBA. Confirmatory assays are designed to provide rigorous verification of the primary assay. Concordance between the screening assay (high sensitivity) and confirmatory assay (high specificity) is ideal, from a diagnostic point of view. However, we here noted that WBA results in animals with low levels of tELISA-detectable antibodies sometimes are misclassified as seronegative, thereby allowing these animals to threaten the biointegrity of BV-negative captive colonies and endanger humans that assume that the animals are not infected with BV. In addition, we report for the first time that use of WBA may have led to misclassification of tELISA-negative sera as positive or indeterminate, underscoring the subjectivity in analyses of WBA data. Of the 177 tELISA-negative sera tested, 6 were positive and 26 were indeterminate according to results from WBA. In addition, 72% of these 32 sera had high background signal (> 0.5 OD or more on UN-coated wells at the 1:5 serum dilution). This high background signal may have obscured the tELISA results or, alternatively, caused the false-nonnegative results according to WBA. Several factors may underlie the lack of correlation between WBA and tELISA. One reason may be a poor performance of either one or both tests at a given time; this possibility is minimized in our laboratory through the use of stringent quality-control measures. However, no assay is completely free of occasional technical issues. A second possible explanation for the lack of correlation between tELISA and WBA is the inherent differences in the ways that peptide, polypeptides, and glycoprotein antigens are displayed in these 2 tests. Because of the associated antigen-denaturation steps, WBA is restricted to the detection of linear epitopes of viral polypeptides in the lysates of infected cells, whereas antigen in our described tELISA is prepared under nondenaturing conditions, thereby enabling the detection of most linear and conformational epitopes.2,8,9,14,17,27,30
In general, the agreement between WBA and tELISA was greater with high-titer than low-titer sera. Manz and colleagues22 summarized numerous studies suggesting that most antibodies in low-titer sera are induced by conformationally dependent epitopes and that only later in infection are antibodies induced against numerous linear as well as conformational epitopes. Interestingly, we found that WBA even fails to confirm a few high-titer sera; however, if the scenario presented by Manz and colleagues19 applies to our observations, these high-titer sera that fail to be confirmed by WBA may reflect early antibodies to large antigenic loads. It is possible that, during the course of BV infection, these macaques produced high-titer antibodies to linear epitopes at a time when conformationally dependent antibodies had waned. It is widely recognized that antibody specificities and antibody classes and subclasses change during the course of viral infections. In addition, the types of antibodies induced are determined by where the infecting agent resides for any period of time, as well as by the antigenic loads at any given time, so that particularly those agents that are life-long and cycling intermittently from acute to latent stages are only intermittently capable of antibody induction. Early antibodies are characterized by low-affinity interactions with cognate antigens and these mature over time increasing in affinity and concentration. Because of the uncertainty involved with tELISA-positive results that cannot initially be confirmed as positive by WBA, we recommend that subsequent serum samples are followed for observation of change in antibody titers or conversion to positive status by WBA.
One of the goals of the current study was to revisit standard assays and reestablish the limits of the tELISA used for the diagnosis of BV antibodies in macaques. This goal was accomplished by the determination of the gray zone between negative and positive sera according to the method of O'Fegan.25 As shown in the Results, by using a P:N cutoff value of 2.5, the gray zone was defined as being between P:N ratios of 1.8 and 3.5. By this definition, any P:N value equal to or higher than 3.5 has a 95% probability of being a true positive result by tELISA, and any P:N value below 1.8 has only a 5% probability of being a false-positive result by tELISA. Therefore we chose the P:N cutoff value of 3.5 as the standard tELISA cutoff. Increasing the cutoff value from a P:N ratio of 2.5 to 3.5 increased the correlation with WBA, but because the degree of correlation was still low, we elected to explore alternative approaches for confirming the results of screening assays performed in our laboratory.17,27 In the current study, approximately 30% of the 92 sera that were positive by tELISA were negative by WBA. Using the described crossreactivity pattern test, we were able to demonstrate that low-titer, tELISA-positive sera that were negative by WBA showed a typical pattern of interaction with BV antigens and those of 5 other heterologous alphaherpesviruses. In this way, we established that these sera likely represented macaques that had experienced a true BV infection. These data support that conclusion that the automated tELISA correctly classified sera from BV-infected macaques, whereas WBA was not sufficiently sensitive and therefore did not confirm the findings from tELISA.
Further confounding factors include the fact that the interpretation of WBA data is more subjective than that for tELISA and sometimes is challenging due to nonspecific antibody interactions with proteins from infected cells. These nonspecific interactions can lead to the classification of sera as positive or, more frequently, indeterminate with respect to the identification of virus-specific antibodies. With viruses as complex as simplexviruses, standardizing WBA interpretation can prove challenging. Alternative assays are being investigated. A recent study indicated that tELISA based on an array of recombinant BV antigens can effectively replace WBA as a confirmatory assay for tELISA that uses lysates from BV-infected cells.17
Determination of the specificity or sensitivity of tELISA is difficult because of the lack of a reliable ‘gold standard.’ Because clinical symptoms and virus shedding are not consistent hallmarks of BV infection, a standard that is based on typical symptoms and virus culture is inappropriate. As a result, antibody detection is currently the only diagnostic option until unequivocal biomarkers that are consistently present in infected animals are identified.
In the assays described here, we calculated the sensitivity and specificity of the tELISA simply by observing the distribution of negative (P:N < 3.5) and positive (P:N ≥ 3.5) samples in a population of 468 macaques. By doing so, sensitivity was estimated as 100% and specificity as 95%.
We have established that the previously described automated high-throughput tELISA for detecting BV antibodies, the 96-well tELISA,18 can be performed in 384-well microplates (384-well tELISA) with a single antigen source per well, allowing rapid processing of a greater number of serum samples.14 The 384-well tELISA was optimized and compared with the previously developed 96-well tELISA.17 Although these 2 assays showed good correlation, the 384-well assay was associated with higher background values. We surmise that differences in the wells’ shape and size contributed to the increased background in the 384-well microplates. The ratio of the surface area of the bottom of the well to the surface area of the walls is smaller in the wells of a 384-well plate than in those of a 96-well plate. In addition, the wells of 384-well plates are rectangular with rounded corners whereas the wells of 96-well plates are round. These differences might have increased the adhesion of liquid to the surface of the 384-well plates, leading to less efficient washing of the wells and higher background values. Additional wash cycles are used for the 384-well plates to reduce this background.
Regardless of the tELISA format used, some serum samples have high values of reactivity with mock-infected control antigens, so an additional factor to be considered in serodiagnosis is the presence of contaminants in sera that react with cellular proteins in the negative (UN) control wells. In the current study, we identified 70 (25%) of the 278 sera tested as such high-background samples, according to the criteria shown in Table 1 (categories 3, 4, and 7). Despite their high background, many of the 70 samples could be classified as BV-positive, because their P:N values were greater than or equal to 3.5. Diluting these sera by a factor of 1:100 decreased the background signal. Among the 70 sera tested at the 1:100 dilution, only 7 (10%) still had high background (OD405 of 0.5 or greater) in the UN control wells. The drop in background signal due to the 1:100 dilution of the sample enabled identification of additional BV-positive (P:N ≥ 3.5) sera.
In the singleplex mode of tELISA, multiple antigens are distributed to different wells (1 antigen per well) and arranged in an array. Each serum sample then is delivered automatically to the array of antigens. The result is a pattern of reactivity of each serum against multiple antigens, very similar to the results obtained from multiplex assays, in which each well contains multiple antigens. The flexibility of automation that is based on liquid handling and workstations enables fast and simple development of singleplex immunoassays that can include antigens optimized for the detection of a wide variety antibodies induced by multiple infectious agents. We adapted the singleplex mode for antibody testing against as many as 8 antigens per array. When an array of 8 antigens was used, it was possible to test 32 sera and all internal controls in a single 132-well plate, and a total of 10 plates could be processed in 6 h.
We used the singleplex 384-well tELISA to examine various controversial issues related to the use of heterologous antigens for detecting BV antibodies in macaques. In light of our previous results, we estimated that using heterologous herpesvirus antigens in tELISA for the detection of BV antibodies would result in less sensitive assays as compared with assays in which the homologous BV antigen is used.16 Immunologic crossreactivity can have different manifestations depending on the assay used. BV antibodies indeed can neutralize HSV infection of cell cultures most efficiently; however, most of the BV-induced antibodies react weakly, if at all, with HSV antigens in both ELISA and WBA. There is still no reasonable explanation for this phenomenon.
In summary, we used singleplex ELISA in 384-well microplates to simultaneously test 278 macaque sera against BV antigen and the 2 heterologous antigens, HVP2 and HSV1. Compared with the homologous BV tELISA, the HVP2-based ELISA identified 14.2% fewer positive sera and the HSV1-based assay detected 16% fewer positive sera (Table 1). Other authors were satisfied with the use of heterologous ELISA using HVP2 and HSV1 as antigens for the detection of BV antibodies;19,26,34 however, the ideal immunodiagnostic test uses homologous antigens to capture cognate antibodies induced during infection. One major difference distinguishes all assays performed by using heterologous antigens as compared with our use of the homologous BV-based tELISA: in the current study, serum samples were tested at 2 dilutions, 1:5 and 1:100. We found that many low-titer sera were positive at 1:5 and negative at 1:100. Other authors have tested samples at dilutions of 1: 50,19 1:100,19,26 and 1:20034 in heterologous assays. By doing so, they eliminated low-titer sera from their study and thereby increased the agreement between the BV tELISA and the heterologous-antigen tELISA. Experiments summarized in Figures 3 and 4 indicate that the tELISA in which the homologous BV antigen was used instead of the heterologous HVP2 and HSV1 antigens resulted in higher sensitivity of detection of BV antibodies in low-titer sera. Differences between the mean OD values obtained by the homologous BV tELISA, as compared with those obtained by the heterologous HVP2 and HSV1 tELISA were significant at higher serum dilutions (1:300, 1:900, and 1:2700) but not at the 1:100 dilution (Figure 4). Sera in the high-titer category (5000 EU or less) were classified similarly regardless whether homologous or heterologous antigens were used (Figure 3). However the percentages of positive sera detected in the low-titer groups (50 EU or less and 500 EU or less) were higher with the BV antigen and lower with the HVP2 and HSV1 antigens. In these groups of sera, the differences were statistically significant at the level of 90% confidence. Note that 18 (26%) of the 70 high-background sera were positive by the BV-based ELISA, 16 (23%) when HVP2 was the antigen, and 14 (20%) by the HSV1-based assay (Table 1). We believe that the higher correlation between the homologous and heterologous ELISAs in this group of high-background sera is due to the relatively high-titer antibodies in the majority of the samples. Of the 18 sera that were positive, 14 (78%) had titers of 5000 EU or greater, 3 (17%) had titers of 500 EU or less, and only 1 (6%) had a titer of 50 EU of less.
In terms of the management of nonhuman primate colonies, the current experiments and our previous studies16 indicated that using the most sensitive screening test available is optimal, particularly given that the prevalence of BV infection has fallen dramatically with the successes of the NIH SPF HIV–AIDS Animal Model program. The data reported here show that the homologous BV tELISA is more sensitive than are heterologous tELISA that are based on HVP2 or HSV1. The ready availability of quality-controlled inactivated BV antigens enables laboratories to use homologous antigens in their inhouse assays for screening colony macaques.
Acknowledgments
We thank Dr Irina Patrusheva for producing the polyclonal rabbit antibodies used in the current study and Dr John A Ward (Department of Clinical Investigation, BAMC, San Antonio, TX) for assisting with the statistical analysis. We also thank the resource medical technologists from the National B Virus Resource Center— Natasha Burnett, Tasha Brooks, and Nina Beato—as well as their predecessors in this lab for their invaluable contributions to this work. This work was supported by the NIH's National Center for Research Resources (P40 RR5162-20), the Elizabeth R Griffin Research Foundation, and the Georgia Research Alliance.
References
- 1.Agresti A, Coull B. 1998. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Stat 52:119–126 [Google Scholar]
- 2.Bernstein DI, Frenkel LM, Bryson YJ, Myers MG. 1990. Antibody response to herpes simplex virus glycoproteins gB and gD. J Med Virol 30:45–49 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention and National Institutes of Health. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Bethesda (MD): Department of Health and Human Services.
- 4.Centers for Disease Control and Prevention.[Internet] 2004. Current CLIA Regulations. [15 October 2011]. Available at: http://wwwn.cdc.gov/clia/regs/toc.aspx.
- 5.Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE. 2002. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine herpesvirus 1). Clin Infect Dis 35:1191–1203 [DOI] [PubMed] [Google Scholar]
- 6.Eberle R, Black DH, Lipper S, Hilliard JK. 1995. Herpesvirus papio 2, an SA8-like α-herpesvirus of baboons. Arch Virol 140:529–545 [DOI] [PubMed] [Google Scholar]
- 7.Eberle R, Hilliard J. 1995. The simian herpesviruses. Infect Agents Dis 4:55–70 [PubMed] [Google Scholar]
- 8.Eberle R, Mou SW, Zaia JA. 1984. Polypeptide specificity of the early antibody response following primary and recurrent genital herpes simplex virus type 2 infections. J Gen Virol 65:1839–1843 [DOI] [PubMed] [Google Scholar]
- 9.Eberle R, Mou SW, Zaia JA. 1985. The immune response to herpes simplex virus: comparison of the specificity and relative titers of serum antibodies directed against viral polypeptides following primary herpes simplex virus type infections. J Med Virol 16:147–162 [DOI] [PubMed] [Google Scholar]
- 10.Elmore D, Eberle R. 2008. Monkey B virus (Cercopithecine herpesvirus 1). Comp Med 58:11–21 [PMC free article] [PubMed] [Google Scholar]
- 11.Henkel RD, McClure HM, Krug P, Katz D, Hilliard JK. 2002. Serological evidence of α-herpesvirus infection in sooty mangabeys. J Med Primatol 31:120–128 [DOI] [PubMed] [Google Scholar]
- 12.Hilliard JK, Ward JA. 1999. B-virus specific-pathogen-free breeding colonies of macaques (Macaca mulatta): retrospective study of 7 years of testing. Lab Anim Sci 49:144–148 [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 14.Kahlon J, Lakeman FD, Ackermann M, Whitley RJ. 1986. Human antibody response to herpes simplex virus-specific polypeptides after primary and recurrent infection. J Clin Microbiol 23:725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz D, Hilliard JK, Eberle R, Lipper SL. 1986a. ELISA for detection of group-common and virus-specific antibodies in human and simian sera induced by herpes simplex and related simian viruses. J Virol Methods 14:99–109 [DOI] [PubMed] [Google Scholar]
- 16.Katz D, Shi W, Krug PW, Henkel R, McClure H, Hilliard JK. 2002. Antibody cross-reactivity of α-herpesviruses as mirrored in naturally infected primates. Arch Virol 147:929–941 [DOI] [PubMed] [Google Scholar]
- 17.Katz D, Shi W, Perelygina L, Wildes MJ, Krug PW, Hilliard JK.2012. An automated ELISA using recombinant antigens for serologic diagnosis of B virus infections in macaques. Comp Med 62:527–534.
- 18.Katz D, Shi W, Wildes M, Hilliard JK. 2002. Automation of serological diagnosis for herpes B virus infections using robot-assisted integrated workstations. J Lab Autom 7:108–113 [Google Scholar]
- 19.Kuller L, Wantabe R, Anderson D, Grant R. 2005. Development of whole-virus multiplex flow cytometric assay for antibody screening of a specific-pathogen-free primate colony. Diagn Microbiol Infect Dis 53:185–193 [DOI] [PubMed] [Google Scholar]
- 20.Lachmann RH, Sadarangani M, Atkinson HR, Efstathiou S. 1999. An analysis of herpes simplex virus gene expression during latency establishment and reactivation. J Gen Virol 80:1271–1282 [DOI] [PubMed] [Google Scholar]
- 21.Ludwig H, Pauli G, Gelderblom HR, Darai G, Koch HG, Flugel RM, Norrild B, Daniel MD.1983. B Virus (Herpesvirus simiae), p 385–428. In: Roizman B, editor. The herpesviruses. New York (NY): Academic Press.
- 22.Manz RA, Hauser AE, Hiepe F, Radbruch A. 2005. Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386 [DOI] [PubMed] [Google Scholar]
- 23.McGavern DB, Kang SS. 2011. Illuminating viral infections in the nervous system. Nat Rev Immunol 11:318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motulsky H.1995. Intuitive biostatistics, p 11–21. New York (NY): Oxford University Press.
- 25.O'Fegan P.2000. Validation, p 211–238. In: Gosling JP, editor. Immunoassays, practical approach. New York (NY): Oxford University Press.
- 26.Ohsawa K, Lehenbauer TW, Eberle R. 1999. Herpesvirus papio 2: alternative antigen for use in monkey B virus diagnostic assays. Lab Anim Sci 49:605–616 [PubMed] [Google Scholar]
- 27.Perelygina L, Patrusheva I, Hombaiah S, Zurkuhlen H, Wildes MJ, Patrushev N, Hilliard JK. 2005. Production of herpes B virus recombinant glycoproteins and evaluation of their diagnostic potential. J Clin Microbiol 43:620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perelygina L, Patrusheva I, Zurkuhlen H, Hilliard JK. 2002. Characterization of B virus glycoprotein antibodies induced by DNA immunization. Arch Virol 147:2057–2073 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka S, Mannen K, Sato H. 2004. Use of Herpesvirus papio 2 as an alternative antigen in immunoblotting assay for B virus diagnosis. J Vet Med Sci 66:529–532 [DOI] [PubMed] [Google Scholar]
- 30.Turgeon ML.2009. Acquired immunodeficiency syndrome, p 319–320. In: Immunology and serology in laboratory medicine. St Louis (MO): Elsevier Health Sciences.
- 31.Ward JA, Hilliard JK. 2002. Herpes B virus specific-pathogen-free breeding colonies of macaques: serologic test results and the B virus status of the macaque. Contemp Top Lab Anim Sci 41:36–41 [PubMed] [Google Scholar]
- 32.Weigler BJ. 1992. Biology of B virus in macaque and human hosts: a review. Clin Infect Dis 14:555–567 [DOI] [PubMed] [Google Scholar]
- 33.Whitley R, Hilliard JK.2007. Cercopithecine herpesvirus (B virus), p 2888–2903. In: Fields BN, Knipe DM, Howley PM. Fields virology, 5th ed, vol 2. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams and Wilkins.
- 34.Yamamoto H, Oshava K, Walz SE, Mitchen JL, Wantyabe Y, Eberle R, Origasa H, Sato H. 2005. Validation of an enzyme-linked immunosorbent assay kit using Herpesvirus papio 2 (HVP2) antigen for detection of Herpesvirus simiae (B virus) infection in rhesus monkeys. Comp Med 55:244–248 [PubMed] [Google Scholar]