Abstract
B virus (Macacine herpesvirus 1) occurs naturally in macaques and can cause lethal zoonotic infections in humans. Detection of B virus (BV) antibodies in macaques is essential for the development of SPF breeding colonies and for diagnosing infection in macaques that are involved in human exposures. Traditionally, BV infections are monitored for presence of antibodies by ELISA (a screening assay) and western blot analysis (WBA; a confirmatory test). Both tests use lysates of infected cells as antigens. Because WBA often fails to confirm the presence of low-titer serum antibodies detected by ELISA, we examined a recombinant-based ELISA as a potential alternative confirmatory test. We compared a high-throughput ELISA using 384-well plates for simultaneous antibody screening against 4 BV-related, recombinant proteins with the standard ELISA and WBA. The recombinant ELISA results confirmed more ELISA-positive sera than did WBA. The superiority of the recombinant ELISA over WBA was particularly prominent for sera with low (<500 ELISA units) antibody titers. Among low-titer sera, the relative sensitivity of the recombinant ELISA ranged from 36.7% to 45.0% as compared with 3.3% to 10.0% for WBA. In addition, the screening and confirmatory assays can be run simultaneously, providing results more rapidly. We conclude that the recombinant ELISA is an effective replacement for WBA as a confirmatory assay for the evaluation of macaque serum antibodies to BV.
Abbreviations: BV, B virus (Macacine herpesvirus 1); EU, ELISA units; g, glycoprotein; HSV, herpes simplex virus; tELISA, titration ELISA; UN, uninfected; WBA, western blot analysis
B virus (BV; Macacine herpesvirus 1) is a member of the genus Simplexvirus, subfamily Alphaherpesvirinae and family Herpesviridae. The virus occurs naturally in macaques (Macaca spp.) and causes a lethal zoonotic infection in 80% of untreated humans. Because biomedical professionals working with macaques, their cells, or tissues are at risk for becoming infected with BV, it is important to know the status of macaques involved in potential BV exposures. Although cases of BV infection after encounters between tourists and macaques have not been reported, any event that involves direct or fomite-associated contact with macaques has inherent risks. Identification of zoonotic BV infection through the detection of antibodies enables timely antiviral intervention, which is critical to reduce or prevent morbidity and mortality. Similarly rapid detection is important to maintain the biointegrity of SPF captive macaque colonies. The identification of BV in clinical specimens is achieved by using cell culture, PCR, or antibody detection methods. Because BV is shed only rarely from peripheral sites, the identification of BV infection in monkeys and humans currently is based on antibody detection (serology).14,23,28
In our laboratory, current serological diagnosis for B virus infections has been based on 2 principal tests: a titration-based (that is, traditional) ELISA (tELISA) as a screening test and western blot analysis (WBA) as a confirmatory test. Each test uses quality-controlled BV antigens that are prepared from lysates of infected cells.20,22,23 Because BV is the only simplex virus in the Alphaherpesvirinae subfamily that is known to infect macaques,14,28 antibodies interacting with BV antigens are used to indicate BV infection and not an infection due to a crossreacting virus. In practice, tELISA has identified numerous BV antibody-positive sera, the majority of which are low-titer sera from SPF colonies, which fail to be confirmed by WBA, and therefore, are classified as false positives.23 We, therefore, searched for other approaches that could be used for confirmation of tELISA results. One reasonable option was the use of BV recombinant proteins as antigens. Numerous investigators have used recombinant-based assays for routine diagnosis of infections with viruses, including cytomegalovirus,36 Epstein–Barr,6 herpes simplex (HSV1 and HSV2)2,3,17,31,32,34 Crimean–Congo hemorrhagic fever,10 HIV,36 dengue,5,11,27 hepatitis C,24 hepatitis B,8 West Nile,26 influenza,16 Ebola, and Marburg33 viruses.
Screening for the presence of serum IgG molecules against an array of defined and purified recombinant antigens has distinct advantages over assays that use the entire complement of viral antigens that are present in virus-infected cells. This is particularly true for pathogens that require BSL4 laboratories.28,33 The pattern of reactivity obtained against each individual recombinant protein may have diagnostic value, by enabling identification of the stage of infection and the prediction of the prognosis of the disease.3,4,18 However, using a single or only a few recombinant proteins as ELISA antigens can lead to a false-negative result if the antibody repertoire produced after BV infection reacts with other antigenic determinants that are not represented by the particular recombinant antigens used in the test.3,18,28,31,34
Several laboratories have examined the efficacy of using a single BV recombinant antigen (that is, glycoprotein D [gD]) for diagnosing BV infections in macaques25,37 and humans,15 and we previously reported the diagnostic potential of an ELISA that incorporated several recombinant BV antigens.28 We chose 4 recombinant BV glycoproteins as candidate antigens: peptides corresponding to the full-length extracellular domain of gB, gC, and gD and the membrane-associated segment of gG (gGm). Among these antigens, gGm was the most BV-specific, because it failed to crossreact with antibodies induced by HSV1 and HSV2. To validate the use of the recombinant BV antigens for the purpose of BV antibody detection, a panel of antibody-negative (n = 40) and antibody-positive (n = 75) macaque sera that were confirmed to be positive by tELISA and WBA were tested against the panel of the 4 B virus recombinant antigens, all of which showed fairly high sensitivity for detecting antibodies to BV.28
Here, we examine the performance of the recombinant-based ELISA (rELISA) for BV detection by using numerous (>1000) macaque sera, which have a broad range of antibody titers as determined by tELISA. Because manual ELISA to identify antibodies against an array of antigens are too laborious to be cost-effective, we adapted a previously described high-throughput automated single-antigen ELISA performed in 384-well plates to detect antibodies in macaque sera to multiple BV antigens.23 This assay format has been adapted to include antigens from other alphaherpesviruses23 and can be easily modified further for other viruses. We then compared the performance of the rELISA with that of whole-virus tELISA and WBA. The main goal of this study was to determine whether the 384-well rELISA is an effective alternative to WBA as a confirmatory assay for tELISA.
Materials and Methods
Virus and cells.
BV antigen was prepared by infecting Vero cells (catalog no. CCL-8, American Type Culture Collection, Manassas, VA) with BV (strain E2490, a generous gift from the late Dr RN Hull, Eli Lilly, Indianapolis IN) at a multiplicity of infection of 2, as previously described.7,19-22 Briefly, BV-infected cells were harvested 18 to 24 h after observation of nearly 100% cytopathic effect and resuspended in sterile, deionized water containing a protease inhibitor cocktail. After infected cells were lysed by freeze–thawing to release virus, antigens for ELISA were solubilized by using the nondenaturing detergents Tween-40 (Sigma Chemical, St Louis, Mo) and sodium deoxycholate, each at a final concentration of 1%. Antigens for WBA were prepared by adding 1% SDS to pellets of BV-infected cells immediately after harvesting. Each preparation was subsequently stored at –80 °C. Solubilized infected cells were assayed for protein concentration and assessed against previous antigen and antibody lots. Polyclonal rabbit antibodies prepared against selected BV recombinant proteins25,26 also were used to confirm the presence of all major viral glycoproteins in each antigen preparation. Control antigen was prepared from lysates of passage-matched uninfected cells and validated as described for BV antigen.20,22,23 BV stocks and antigen lysates were prepared within a BSL4 laboratory at Georgia State University in accordance with guidelines from the Centers for Disease Control9 and US Department of Justice's Homeland Security regulations for Select Agents35 by using protocols approved by the University Institutional Biosafety Committee.
Recombinant proteins.
The baculovirus expression system for production of B virus recombinant proteins has been described previously in detail.28 These techniques for the expression and final protein purification were applied to produce gB, gC, gD, and gGm from BV and a recombinant nonviral protein, nectin 1 (negative control). In some experiments, glycoproteins specific to HSV1 (gG1) and HSV2 (gG2) were used as additional negative-control antigens; gG1 and gG2 are used routinely to identify and differentiate antibodies specific for HSV1 or HSV2 infections in humans.2,3,18,31
Sera.
Sera from rhesus (Macaca mulatta) or cynomolgus (M. fascicularis) macaques were obtained from the National B Virus Resource Center (Atlanta, GA) after they were submitted by captive colony managers during routine surveillance or due to a human injury associated with a specific macaque.23
ELISA.
tELISA.
Programs for automated ELISA procedures were written by using SAMI NT (Beckman–Coulter, Fullerton, CA) and Gemini (TECAN, San Jose, CA) software. Test procedures used for the 96-well automated tELISA were adapted to a 384-well format.22,23 The major difference between the 96-well and 384-well formats was the volumes of reagent used: 100 µL per well for substrate and 50 µL per well for all other reagents for the 96-well format compared with 75 µL per well for substrate and 35 µL per well for all other reagents for the 384-well format.22
For the tELISA, in which whole-BV lysate was used as antigen, 1:5 and 1:100 dilutions of each serum were tested in wells coated with the whole-BV lysate and in control wells coated with lysates from uninfected cells (UN); protein concentrations used for coating wells ranged from 20 to 40 µg/mL.
Results were evaluated based on a positive to negative ratio (P:N) that was obtained by dividing the OD value from the virus-coated wells by the OD value from the UN-coated control well. A P:N ratio of 3.5 or greater obtained by using serum diluted 1:5 was classified as positive for the presence of antivirus antibodies. The 1:100 dilution was used to aid in interpretation of high-background sera and to facilitate titer determinations from a standard curve for sera with high OD values.23
The standard curve, expressed in ELISA units (EU) per well, was established from a quality-control experiment that comprised at least 12 repetitions of titrations performed on BV- and UN-coated wells. One EU was defined as the reciprocal of the serum dilution of the standard curve derived from titration of known-positive sera at the intersection of the cutoff value. The cutoff value was determined as follows: 1) the serum dilution that resulted in the OD405 closest to 1.0 on the standard curve derived from BV-coated wells was identified; 2) the OD405 of this same serum dilution tested on UN-coated wells then was determined; 3) the cutoff value was defined arbitrarily as 2 times this value.
For quantitative results, net OD values of diluted (1:5 or 1:100) antibody-positive test sera (P:N ≥ 3.5) were compared with the standard curve for which a predetermined titer had been calculated in the quality-control experiment. The results from the dilution that yielded OD values within the linear range of the standard curve were used for calculating a titer (in EU) for each test serum.22,23 Some of the macaque sera were tested by tELISA in 96-well microtiter plates as previously described21 and some in 384-well microtiter plates.
rELISA
In rELISA, the liquid handler of the automated system distributed each serum sample to an array of antigen-coated wells; each well in the array represented a different antigen.23
We used 2 setups for rELISA, one accommodated 37 serum samples per microtiter plate (setup A) and one that accommodated 32 sera (setup B). A total of 10 microtiter plates could be tested within 6 h by using either of these setups.
In the 384-well rELISA using setup A, 35 µL of each serum (diluted 1:25) was distributed into each of 7 wells, each of which was precoated with a recombinant antigen: 4 BV antigens (gB, gC, gD, and gGm); 2 HSV antigens (gG1 and gG2), and nectin (negative control). Macaques do not naturally possess serum antibodies against HSV1 or HSV2,14 but the sera were screened against gG1 and gG2 for validation purposes.
Antigens for setup A were distributed as follows: a 16-well column of each plate was coated with a recombinant antigen, yielding 7 columns representing the 7 antigens. In each column, row A1 was blank; rows B through G represented 3-fold dilutions (1:20 to 1:4860 for all antigens except gB, for which dilutions were 10-fold higher) of a standard BV antibody-positive serum pool; row H contained a 1:25-dilution of a pooled serum from macaques with high (greater than 5000 EU by tELISA) BV antibody titers; row I contained a 1:25-dilution of a pooled serum from macaques with moderate (50 to less 5000 EU) BV antibody titers; row J contained a 1:25-dilution of a pooled serum from macaques with low (≤50 EU) BV antibody titers; and row K contained a 1:25-dilution of a pooled serum from macaques known to be negative by tELISA for BV antibody. Thirty-seven unknown serum samples (diluted 1:25) were tested in the remaining wells. The first 5 sera (S1–S5) were tested at the bottom of the first third of the plate, samples S6 to S21 in the second third of the plate and samples S22 to S37 in the last section. P:N ratios for antibodies to each glycoprotein were calculated by dividing the OD values of wells coated with the virus glycoprotein of interest by the OD value from the well coated with nectin; a P:N ratio of 3.5 or greater was considered to indicate a positive antibody reaction to a recombinant protein. Some provisional rules were set for the sake of interpreting the diagnostic value of the data. A serum sample that yielded a P:N ratio of greater than or equal to 3.5 reaction to a single BV recombinant antigen was considered to be indeterminate in terms of being indicative of BV infection; sera that yielded P:N ratios of greater than or equal to 3.5 to 2 or more BV recombinant proteins were considered to be positive for BV infection. Reactions to gG1 and gG2 were considered false positives.
In the second 384-well rELISA setup (setup B), 32 macaque sera were tested simultaneously against an array of BV recombinant antigens (as in setup A) as well as against whole-BV and UN control lysates (as in tELISA). Wells for the recombinant portion of setup B were coated individually with gB, gC, gD, gGm, and nectin (negative control).
For the tELISA section, a titration curve was generated as previously described.21,22 Briefly, a series of 3-fold dilutions of the standard positive macaque pooled serum was tested against the BV-infected and UN whole-cell lysates. Serum dilutions were tested in duplicate starting at 1:100 and ending at 1:24,300 (wells B1 to G4). The high-, moderate-, and low-titer controls were tested in wells H1 to J4, and the negative serum control was in well A2 at 1:5 dilution and in wells A3 and A4 at 1:100 dilution. The high-, moderate-, and low-titer and negative serum controls (1:25 dilution; as in setup A) also were tested against the BV recombinant antigens and nectin control (wells K1 to P4).
The 32 test sera in setup B were placed in wells A5 through P24; each serum was tested at 1:5 and 1:100 dilutions against BV- and UN lysates and at 1:25 dilution against each recombinant antigen, with a P:N ratio of greater than or equal to 3.5 indicating a positive antibody reaction to the antigen.23 The diagnostic value of the results from setup B was interpreted as for setup A. Therefore, a serum sample that reacted positively to 2 or more BV recombinant antigens was considered to be positive for BV infection; sera positive for single antigens only were considered to be indeterminate for diagnosis of BV infection.
WBA.
WBA was performed according to standard laboratory procedures that had been approved for use in BV diagnostic laboratories.23,28,38 The banding patterns that resulted from WBA of test sera were evaluated against those of quality-controlled standards, and each sample's profile was generated through comparison of the immunoreactivity of comigrating bands between each of the control sera and the test serum sample. Control sera used to assess the presence of BV-specific antibodies included pooled sera from tELISA-seronegative macaques, pooled sera from BV-positive macaques, and pooled rabbit antisera against BV recombinant proteins. Blots were prepared from gradient (8% to 14%) SDS–polyacrylamide gels. Results were reported as negative when a test sample yielded no bands that comigrated with those from positive controls, indeterminate when 1 to 3 bands from the test serum comigrated with those from positive controls, and positive when 4 or more bands in the test sample comigrated with those from positive controls.
Statistical analysis.
Here we compared WBA with rELISA as a method for diagnosing BV infection. The sensitivity of WBA and rELISA were defined relative to that of tELISA. Given data from previous studies, we expected results of tELISA to range from negative to greater than 5000 (EU) in 5 stages (stage 1, negative; stage 2, less than or equal to 50 EU; stage 3, greater than 50 to 500 EU; stage 4, greater than 500 to 5000 EU; stage 5, greater than 5000 EU)—that is, an ordinal variable. In addition, the outcome of rELISA can be a binomial variable (negative or positive).
Three statistical tests were used initially: the 3 × 3 contingency test, McNemar test, and κ. The contingency test measures the relationship between 2 methods: if the methods agree at a level greater than random chance, the contingency test yields a statistically significant (P < 0.05) result. The McNemar test measures the symmetry of the disagreement between methods: if both methods have the same accuracy, their results do not differ statistically (P ≥ 0.05). κ measures agreement between observers, with κ < 0.40 indicating poor agreement, 0.40 < κ < 0.75 indicating fair to good agreement, and κ > 0.75 indicating excellent agreement. SPSS version 19.0 (IBM, New York, NY) was used for performing these tests.
A fourth statistical test, noninferiority analysis,30 was used for testing nonnegative samples according to results from rELISA and WBA in comparison with the 5 tELISA stages. The noninferiority (or superiority) test was performed by comparing probability estimates for each assay (percentage of positive sera, classified according to the 5 tELISA titer groups) and their 95% confidence intervals. When the 95% confidence interval of the first probability falls within that of the second probability, the first assay is not inferior to the second. The method for calculating the 95% confidence intervals uses a normal approximation to the binomial probability distribution that requires that there be at least 5 observations in each arm of the distribution. When the sample size was too small, the probability was estimated by adding 2 successes and 2 failures, the adjusted Wald interval.1
Results
Antibody prevalence to BV recombinant proteins in a macaque standard BV-positive serum pool.
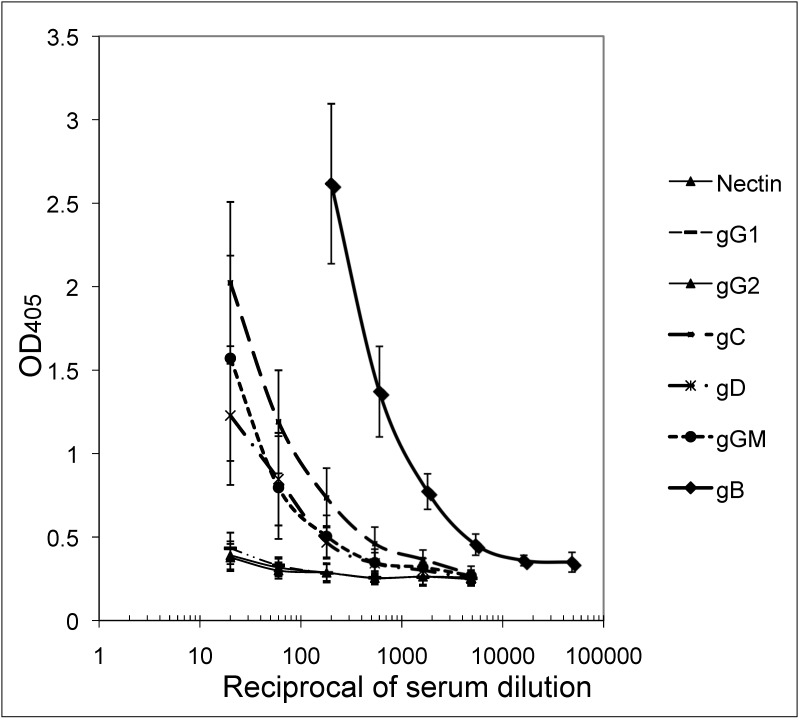
The mean OD values of 24 replicate titrations of a standard BV-positive macaque serum pool against gB, gC, gD, gGm, gG1, gG2, and nectin (Figure 1) were run on different dates (3 or 4 plates per run) over a period of 3 mo. Results demonstrate relatively high levels of reactive antibody to gB in BV-antibody–positive sera, with lower levels of antibodies reactive to the other 3 recombinant BV glycoproteins. No detectable antibodies were found to the HSV1- and HSV2-specific glycoproteins (gG1 and gG2, respectively). The same trend was apparent with the high-, moderate-, and low-titer positive-control serum pools. Only the high-titer positive control serum reacted with all 4 recombinant BV antigens (gB, gC, gD and gGm); the moderate- and low-titer controls reacted with gB only (results not shown).
Figure 1.
Mean OD values (bars, 1 SD) of 24 titrations of the BV-positive macaque serum pool against the 7 recombinant proteins listed.
Evaluation of the rELISA for the detection of BV antibodies in macaque sera.
A total of 1037 macaque sera were tested by using rELISA, 582 sera by using setup A and 455 sera by using setup B. Because analyses revealed no significant differences between the 2 setups for detecting antibodies to BV recombinant proteins, the data were combined for presentation. Nonspecific reactions to the HSV glycoproteins gG1 and gG2 (tested only by using setup A) were low, with 5 of the 582 sera reactive with gG1 (0.9%) and 1 reactive with gG2 (0.2%).
The distribution of sera reactivity (P:N values) against the various recombinant BV antigens is shown in Table 1. According to a P:N cutoff value of 3.5 for antibody reactivity, a total of 409 positive reactions to recombinant antigens were found: 162 reactions to gB (15.6%), 90 reactions to gC (8.7%), 56 reactions to gD (5.4%), and 101 reactions to gGm (9.7%). This distribution analysis demonstrated that antibodies to recombinant gB were more prevalent in macaque sera than were antibodies to gC, gD, and gGm.
Table 1.
Distribution of P:N values calculated for 1037 macaque sera that were tested against recombinant BV gB, gC, gD, and gGm in rELISA
| P:N | gB | gC | gD | gGm |
| <1.5 | 611 | 811 | 826 | 769 |
| 1.5 to <2.5 | 202 | 101 | 110 | 115 |
| 2.5 to <3.5 | 62 | 35 | 45 | 52 |
| 3.5 to <4.5 | 32 | 20 | 12 | 22 |
| 4.5 to <5.5 | 26 | 16 | 7 | 18 |
| 5.5 to <6.5 | 26 | 16 | 5 | 16 |
| 6.5 to <7.5 | 18 | 7 | 3 | 8 |
| 7.5 to <8.5 | 6 | 1 | 1 | 1 |
| 8.5 to <9.5 | 1 | 0 | 2 | 1 |
| 9.5 to <10.5 | 2 | 1 | 0 | 2 |
| ≥10.5 | 51 | 29 | 26 | 33 |
| No. of sera with P:N ≥3.5 | 162 | 90 | 56 | 101 |
| Percentage of sera with P:N ≥3.5 | 15.6 | 8.7 | 5.4 | 9.7 |
Comparison of rELISA, WBA, and tELISA for BV antibody determination in macaque sera.
Of the 1037 sera that were tested by using rELISA, 645 were also tested by tELISA and WBA. The average P:N values obtained with each of the recombinant proteins increased in correlation with increases in tELISA titer values (results not shown). The numbers of sera classified into 5 tELISA titer groups (negative, less than or equal to 50 EU, greater than 50 to less than or equal to 500 EU, greater than 500 to less than or equal to 5000 EU, and greater than 5000 EU) that reacted to either single or multiple recombinant proteins are shown in Table 2. The percentage of sera reacting to gB only was higher in the lower tELISA titer groups (less than or equal to 50 EU, greater than 50 to less than or equal to 500 EU) than in the higher tELISA titer groups (greater than 500 to less than or equal to 5000 EU, greater than 5000 EU). However, the percentage of sera reacting with multiple recombinant proteins was higher in the high tELISA titer groups and lower in the lower tELISA titer groups. This result indicates that the maturation of the immune response after BV infection is reflected not only by the increase in the intensity of the antibody response but also by the increased breadth of the BV antibody repertoire. Furthermore, this pattern of reactivity to the recombinant proteins not only reflects the immune maturation process in BV-infected macaques but also substantiates the correlation between the results obtained by tELISA and rELISA.
Table 2.
rELISA results for macaque sera tested for antibodies to BV
| tELISA-determined titer groups |
|||||||
| rELISA result | Antigen(s) | Negative (n = 455) | ≤50 EU (n = 60) | >50 to ≤500 EU (n = 40) | >500 to ≤5000 EU (n = 40) | >5000 EU (n = 50) | |
| Indeterminate | gB | 5 (1.1) | 13 (26.0) | 11 (27.5) | 6 (15.0) | 8 (16.0) | |
| gC | 2 (0.4) | 1 (1.7) | 0 | 0 | 0 | ||
| gD | 2 (0.4) | 1 (1.7) | 0 | 0 | 0 | ||
| gGm | 13 (2.9) | 1 (1.7) | 1 (2.5) | 0 | 0 | ||
| Positive | gB, gC | 0 | 3 (5.0) | 1 (2.5) | 1 (2.5) | 3 (6.0) | |
| gB, gD | 0 | 1 (1.7) | 0 | 0 | 0 | ||
| gB, gGm | 4 (0.9) | 1 (1.7) | 0 | 1 (2.5) | 3 (6.0) | ||
| gC, gD | 3 (0.7) | 0 | 0 | 0 | 0 | ||
| gD, gGm | 0 | 1 (1.7) | 0 | 0 | 0 | ||
| gB, gC, gD | 0 | 0 | 0 | 4 (10.0) | 2 (4.0) | ||
| gB, gC, gGm | 2 (0.4) | 0 | 3 (7.5) | 6 (15.0) | 17 (34.0) | ||
| gB, gD, gGm | 0 | 0 | 1 (2.5) | 0 | 1 (2.0) | ||
| gB, gC, gD, gGm | 0 | 0 | 1 (2.5) | 19 (47.5) | 12 (24.0) | ||
| No. (%) of indeterminate sera | 22 (4.8) | 16 (26.7) | 12 (30.0) | 6 (15.0) | 8 (16.0) | ||
| No. (%) of positive sera | 9 (1.9) | 6 (10.0) | 6 (15) | 31 (77.5) | 38 (76.0) | ||
| No. (%) of indeterminate + positive sera | 31 (6.8) | 22 (36.7) | 18 (45.0) | 37 (92.5) | 46 (92.0) | ||
| No. (%) of negative sera | 424 (93.2) | 38 (63.3) | 22 (55.0) | 3 (7.5) | 4 (8.0) | ||
A P:N cutoff value of 3.5 was considered as an indication of antibody reactivity. Data are given as the no. (%) of sera that reacted to none (negative), 1 (indeterminate), or 2 or more (positive) of the recombinant BV proteins.
Of the 645 sera tested, 64 (13.9%) reacted only with a single recombinant antigen. In the current study, these sera were regarded as being indeterminate for diagnosis of BV infection. The 90 sera (13.5%) that reacted with multiple recombinant BV antigens were regarded as being positive for BV infection. The total number of BV-indeterminate and -positive sera (that is, nonnegative sera) was 154 (23.9%). By comparison, of the 645 sera tested by WBA, 10 (1.6%) were indeterminate and 75 sera (11.6%) were positive, yielding a total number of 81 (12.6%) nonnegative sera (Table 3).
Table 3.
Comparison of results obtained for 645 macaque sera tested for antibodies to BV by using rELISA and WBA
| WBA |
||||
| rELISA | Indeterminate | Negative | Positive | Total |
| Indeterminate | 2 | 51 | 10 | 63 |
| Negative | 3 | 485 | 3 | 491 |
| Positive | 5 | 24 | 62 | 91 |
| Total | 10 | 560 | 75 | 645 |
rELISA and WBA results showed 85.1% agreement and 1.96% disagreement. Among the disagreements, WBA classified 12.4% of samples lower (that is, more negative) than did rELISA and 2.5% higher (that is, more positive) than did rELISA. The contingency test yielded a significant (P < 0.001) difference, indicating that the methods agree at a level greater than random chance. The McNemar test was significant (P < 0.001), indicating that the methods are asymmetric and do not have the same accuracy. κ was 0.537, indicating that there was fair to good agreement between methods (P < 0.001).
To estimate the agreement between the tELISA and the other 2 methods (rELISA and WBA), we arranged the data in 2 × 2 tables in which negative results by tELISA were compared with negative results by rELISA or WBA and positive sera by tELISA were compared with nonnegative (indeterminate and positive combined) sera by rELISA or WBA. This arrangement enabled statistical evaluation by using the contingency test, McNemar test, and κ statistic. Of the 455 sera negative by tELISA, 424 (93.2%) were negative by rELISA. In addition, 123 (64.7%; 95% confidence interval, 57.9% to 71.5%) of the 190 sera that were positive by tELISA were nonnegative by rELISA. Therefore the agreement between tELISA and rELISA was 84.8%. The result of the contingency test was significant (P < 0.001), indicating that the methods agree at a level greater than random chance. The McNemar test (P < 0.001) indicated that tELISA and rELISA are asymmetric and do not have the same accuracy. The κ statistic was 0.480, denoting fair to good agreement between methods (P < 0.001).
Of the 455 sera BV-negative by tELISA, 449 (98.7%) were negative by WBA; 79 (41.6%; 95% confidence interval, 34.6% to 48.6%) of the 109 sera that were BV-positive by tELISA were nonnegative by WBA, yielding an agreement of 81.9% between tELISA and WBA. The contingency test (P < 0.001) showed that the methods agree at a level greater than random chance. The McNemar test indicated that the methods are asymmetric and do not have the same accuracy (P < 0.001), and κ was 0.480, indicating fair to good agreement between methods (P < 0.001).
rELISA confirmed more samples that were BV-positive by tELISA than did WBA (64.7% compared with 41.6%) and therefore may be more sensitive than WBA. Because the 95% confidence intervals do not overlap, this finding is statistically significant according to the noninferiority test.
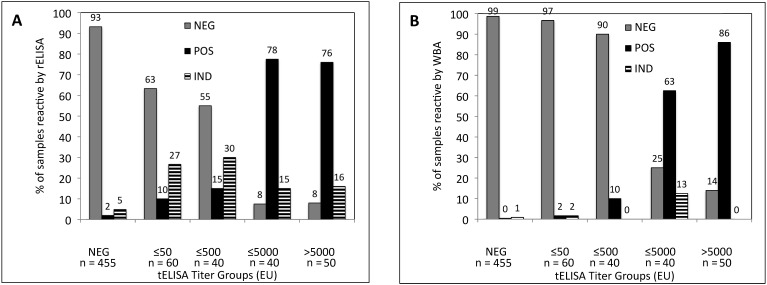
To better understand the relationship between the antibody titers obtained by using tELISA and the results from rELISA and WBA, we arranged test sera into 5 groups according to their tELISA-determined titer: negative, less than or equal to 50 EU, greater than 50 to less than or equal to 500 EU, greater than 500 to less than or equal to 5000 EU, and greater than 5000 EU. We then determined the percentages of sera in each tELISA group that were negative, indeterminate, or positive by rELISA (Figure 2 A) and WBA (Figure 2 B). The figure reveals that rELISA confirmed more tELISA-determined BV-positive samples than did WBA in the tELISA low-titer groups.
Figure 2.
Percentages of sera in each tELISA titer group that were negative, indeterminate, or positive for antibodies to BV by (A) rELISA and (B) WBA. NEG, negative; ≤50, 1 to 50 EU; ≤500, greater than 50 to 500 EU; ≤5000, greater than 500 to 5000 EU; >5000, more than 5000 EU.
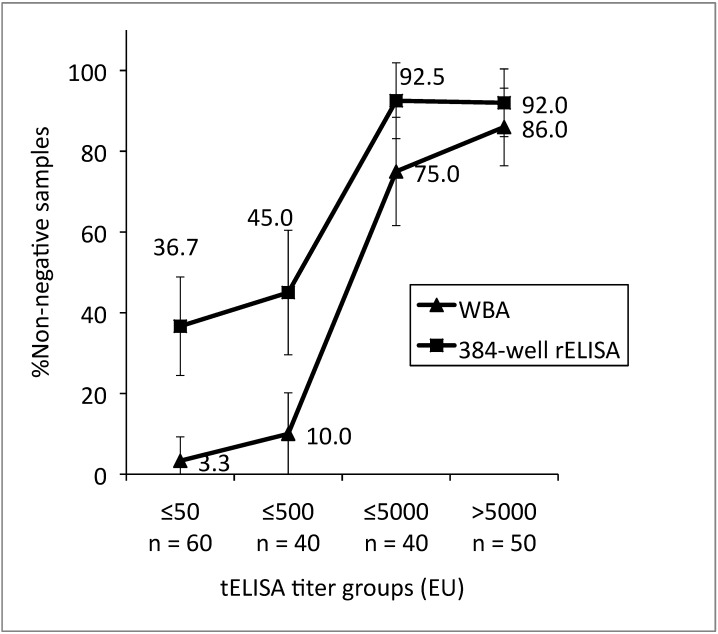
Nonnegative results as determined by WBA and rELISA were analyzed further by using the noninferiority test (Figure 3). For the 2 lowest-titer groups (less than or equal to 50 and greater than 50 to less than or equal to 500 EU), the 95% confidence interval of the relative sensitivity estimate for WBA fell below that of rELISA, indicating that the relative sensitivity of WBA is inferior to that of rELISA for sera of these titers. At higher titers, the relative sensitivity of WBA was not inferior to that of rELISA for sera with tELISA.
Figure 3.
Comparison of relative sensitivity estimates (percentage of nonnegative samples) for WBA and rELISA grouped according to tELISA-determined titer (NEG, negative; ≤50, 1 to 50 EU; ≤500, greater than 50 to 500 EU; ≤5000, greater than 500 to 5000 EU; >5000, more than 5000 EU). Bar, 95% confidence interval.
Discussion
Viruses of the Simplexvirus genus to which BV belongs exhibit a complex host–parasite relationship. Acute infection, whether primary or reactivated, can be asymptomatic or clinically mild. Once an animal is infected, it is infected for life. Generally, the virus establishes latency in neurons of sensory ganglia either in the dorsal root or cranial sensory root. Reactivations may occur at intervals throughout the lifetime of the animal and can be diagnosed by PCR or virus isolation, albeit infrequently, or by determination of BV serum antibodies. Given that clinical symptoms and shedding of virus are rare in macaques, the typically used markers of infection are serum antiviral antibodies, and the screening assay of choice is ELISA. Because BV infection is usually latent, virus isolation, the diagnostic ‘gold standard,’ is rarely successful, making it difficult to determine the accuracy of ELISA.14,23,26 A similar situation has been noted for human herpes simplexvirus infections.18,34 Previous data from our lab demonstrate that many sera designated as low-titer (less than 500 EU) by ELISA and even some of those labeled as high-titer (greater than 5000 EU) are negative by WBA. Some of these serum samples are true positives that WBA failed to confirm and were regarded as ELISA false-positives when WBA was used as a confirmatory test.23
Although we did not perform serial testing in the study, it is reasonable to consider that the low-titer sera may represent primary or early infections and that high-titer sera may represent reactivated infections. Various HSV studies have suggested that many of the early immunologic responses are against denaturation-sensitive epitopes and therefore may not be detected by WBA.12,13 Because antigenic denaturation is minimal during ELISA, antibodies to both linear and conformational epitopes can be detected.4,12,13,19,25,28
We previously demonstrated the diagnostic potential of an ELISA using several recombinant BV antigens.28 We here extended our automated high-throughput ELISA for detecting antibodies to BV22,23 to a 384-well format for the detection of antibodies against recombinant BV antigens. We compared data from our new rELISA with results from WBA to determine whether rELISA was an effective alternative confirmatory assay for the screening tELISA. The specificities of WBA and rELISA (relative to tELISA) were 98.7% and 93.2%, respectively. Of 190 sera that were positive by tELISA, 123 were nonnegative (indeterminate or positive) by rELISA and 79 were nonnegative by WBA, resulting in overall relative sensitivity estimates of 64.7% and 41.6%, respectively. The agreement between all tests (tELISA, rELISA, and WBA) ranged from 81.0% to 86.4%, and the methods agree at a level greater than random chance (P < 0.001), with fair to good agreement (κ, 0.480 to 0.613). We found that the sensitivities of WBA and rELISA varied, depending on the tELISA-established titer of the serum tested. Specifically, WBA and rELISA confirmed fewer low-titer than high-titer sera as nonnegative. However, rELISA detected more nonnegative samples among the low-titer sera than did WBA. The relative sensitivity estimates for WBA in the low-titer groups ranged between 3.3% and 10.0%, compared with 36.7% and 45.0% for rELISA. Among high-titer sera, the relative sensitivity of WBA was 75.0% to 86.0%, compared with 92.0% to 92.5% for rELISA.
For the current study, we arbitrarily defined an indeterminate result on rELISA as a reaction to 1of the 4 BV glycoproteins and a positive result as a reaction to at least 2 recombinant proteins. However, an indeterminate result on rELISA (as defined here) might not be equivalent to an indeterminate result on WBA. We found that the most prevalent antibody reaction was to gB: 75.4% of the indeterminate results were due to reactions to gB, and 97.8% of the sera that were defined as positive according to rELISA reacted with gB in addition to at least one other recombinant antigen. Therefore, an antibody reaction to gB may represent a true positive early antibody response that will be followed by development of antibodies to other antigens later during infection. A similar prevalence of gB antibodies in BV-infected macaques was observed previously,28 and gB was a major target for antibodies during HSV infections in humans.3,4,13,23,32,34 By comparison, many sera with WBA-indeterminate results may truly be BV-negative because of failure to detect conformational epitopes and because of the well-known difficulties in interpreting weak or unclear band patterns.12,13,17,23,29,28
Several laboratories have reported the use of gD for diagnosing BV infections in macaques25,37 and humans.15 In view of our present and previous findings showing that antibodies to gB are the most prevalent in BV-infected macaques, gD is not the best choice for use as a single antigen to diagnose BV infections in macaques. In addition, neither gB nor gD can be used to differentiate antibodies induced by BV from those that are induced by HSV types 1 or 2 and therefore are inappropriate for use in human diagnostics. However, gB can be used for identification of BV antibodies in macaques, and in studies where a single purified antigen was used, the type-common antigen HSV1 gB was the antigen of choice for diagnosing HSV infections.4,19,32 Previous reports of the applicability of gG to differentiate antibodies induced by HSV1 from those induced by HSV2 have been questioned since investigators have shown that using type-specific gG1 and gG2 as single antigens may lead to false-negative diagnosis of HSV infection, given that type-common antigens may appear earlier.2,3,18,34
Other issues that we have confronted in our studies is that rELISA did not confirm all tELISA-positive sera and that low-titer sera can be interpreted as false positives by tELISA or as false negatives by rELISA. According to our present data, BV-lysate–based ELISA remains the most sensitive method for the capture of BV-induced antibodies. Lysates of virus-infected cells present more viral antigens than those represented by the array of selected recombinant antigens.2,3,12,13,18,34
In addition, a few samples that were BV-negative by tELISA were positive by rELISA, suggesting that these results were false positives. However, the possibility that these responses represent true positives cannot be ruled out at this time. Perhaps the recombinant glycoproteins in the test array are poorly represented in the virus-lysate antigen, thereby resulting in a false-negative result by tELISA.31
The use of automation and a ‘singleplex’ (one antigen source per well) format enabled us to test more than 1000 macaque sera for antibodies reactive with recombinant BV proteins. There are many advantages for using purified recombinant antigens instead of virus lysates in serology.3,4,18,28,33 Providing that they yield similarly sensitive assays, recombinant virus antigens are safe, because they eliminate the need for propagating BV. In contrast, preparing lysates from cells infected with this potentially deadly virus prompts serious concerns regarding laboratory safety. The design of the automated rELISA enabled objective reading of results against an array of proteins that undergo minimal denaturation and meets the criteria necessary for a confirmatory assay that offers an alternative form of antigen presentation. Additional advantages of laboratory automation have been discussed previously.22,23 In particular, labor-intensive manual procedures are replaced by high-throughput testing of biohazardous materials that is more cost-effective and safe. Moreover, the singleplex design enabled us to create an ELISA in which sera were screened simultaneously for antibodies to lysates from BV-infected cells and to 4 recombinant BV antigens. Confirmatory testing by WBA of only tELISA-positive sera usually was performed on the day after tELISA testing.
Obtaining results simultaneously from the screening assay (tELISA) and confirmatory test (rELISA) allows the reporting of findings 1 d sooner than after conventional confirmatory assays using WBA; in addition, individual serum samples are handled only once for testing. By comparison, the manual test procedures involved in performing WBA are tedious and time-consuming, and results cannot be quantified but instead must be evaluated subjectively.17,18
Overall, our results indicate that rELISA for the confirmation of BV infection is less subjective, more reliable, and more sensitive than is WBA for macaque sera, particularly low-titer sera. We therefore conclude that our rELISA can replace WBA as a confirmatory assay for serologic diagnosis of BV infection in macaques. However, in the absence of a gold standard for BV infections, we must expect discrepancies between screening and confirmatory serologic tests. These discrepancies can only be resolved through follow-up investigations and the determination of seroconversion or a change in clinical status.
Acknowledgments
We thank the resource medical technologists from the National B Virus Resource Center—Martin Wildes, Natasha Burnett, Tasha Brooks, and Nina Beato—for their invaluable contributions to this work.
This work was supported by NIH (P40 RR0050-62-20), the Elizabeth Griffin Research Foundation, and the Georgia Research Alliance.
References
- 1.Agresti A, Coull B. 1998. Approximate is better than exact for interval estimation of binomial proportions. Am Stat 52:119–126 [Google Scholar]
- 2.Ashley RL, Miltoni J, Lee F, Nahmias A, Corey L. 1988. Comparison of western blot (immunoblot) and glycoprotein G specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 26:662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley RL, Wald A. 1999. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin Microbiol Rev 12:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein DI, Frenkel LM, Bryson YJ, Myers MG. 1990. Antibody response to herpes simplex virus glycoproteins gB and gD. J Med Virol \30:45–49 [DOI] [PubMed] [Google Scholar]
- 5.Buchy P, Yoksan S, Peeling RW, Hunsperger E. [Internet]. 2007. Laboratory tests for the diagnosis of dengue virus infection. [Cited date]. Available at: http://www.who.int/tdr/publications/publications/swg_dengue_2.htm
- 6.Buisson M, Fleurent B, Mak M, Morand M, Chain L, Ng A, Guan M, Chin D, Seignurin JM. 1999. Novel immunoblot assay using 4 recombinant antigens for diagnosis of Epstein–Barr virus primary infection and reactivation. J Clin Microbiol 37:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbelo PD, Hoshino Y, Leahy H, Krogmann T, Hornung RL, Iadarola MJ, Cohen JI. 2009. Serological diagnosis of human herpes simplex virus type 1 and 2 infections by luciferase immunoprecipitation system assay. Clin Vaccine Immunol 16:366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cambron P, Jacquet J-M, Hoet B, Lievens M. 2009. Development and technical and clinical validation of a quantitative enzyme-linked immunosorbent assay for the detection of human antibodies to hepatitis B surface antigen in recipients of recombinant hepatitis B virus vaccine. Clin Vaccine Immunol 16:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention and National Institutes of Health. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Bethesda (MD): Department of Health and Human Services.
- 10.Chinikar S, Ghiasi SM, Ghalyanchi-Langeroudi A, Goya MM, Shirzadi MR, Zeinali M, Haeri A. 2009. An overview of Crimean–Congo hemorrhagic fever in Iran. Iranian J Microbiol 1:7–12 [Google Scholar]
- 11.Cuzzubbo AJ, Endy TP, Nisalak A, Kalayanarooj S. 2001. Use of recombinant envelope proteins for serological diagnosis of dengue virus infection in an immunochromatographic assay. Clin Diagn Lab Immunol 8:1150–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberle R, Mou SW, Zaia JA. 1984. Polypeptide specificity of the early antibody response following primary and recurrent genital herpes simplex virus type 2 infections. J Gen Virol 65:1839–1843 [DOI] [PubMed] [Google Scholar]
- 13.Eberle R, Mou SW, Zaia JA. 1985. The immune response to herpes simplex virus: comparison of the specificity and relative titers of serum antibodies directed against viral polypeptides following primary herpes simplex virus type infections. J Med Virol 16:147–162 [DOI] [PubMed] [Google Scholar]
- 14.Elmore D, Eberle R. 2008. Monkey B virus (Cercopithecine herpesvirus 1). Comp Med 58:11–21 [PMC free article] [PubMed] [Google Scholar]
- 15.Fujima A, Ochiai Y, Saito A, Omori Y, Noda A, Kazuyama Y, Shoji H, Tanabayashi K, Ueda F, Yoshikawa Y, Hondo R. 2008. Discrimination of antibody to herpes B virus from antibody to herpes simplex virus types 1 and 2 in human and macaque sera. J Clin Microbiol 46:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hydayatullah T. 2009. Recombinant hemagglutinin of influenza A virus as a tool to improve serological diagnosis of influenza. Int J Integrat Biol 7:85–92 [Google Scholar]
- 17.Hogrefe W, Su X, Song J, Ashley R, Kong L. 2002. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2, western blotting, and gG2 inhibition. J Clin Microbiol 40:3635–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikoma M, Liljeqvist J-A, Groen J, Glazenburg KL, The TH, Welling-Wester S. 2002. Use of a fragment of glycoprotein G2 produced in the baculovirus expression system for detecting herpes simplex virus type 2-specific antibodies. J Clin Microbiol 40:2526–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahlon J, Lakeman FD, Ackermann M, Whitley RJ. 1986. Human antibody response to herpes simplex virus-specific polypeptides after primary and recurrent infection. J Clin Microbiol 23:725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz D, Hilliard JK, Eberle R, Lipper SL. 1986. ELISA for detection of group-common and virus-specific antibodies in human and simian sera induced by herpes simplex and related simian viruses. J Virol Methods 14:99–109 [DOI] [PubMed] [Google Scholar]
- 21.Katz D, Shi W, Krug PW, Henkel R, McClure H, Hilliard JK. 2002. Antibody crossreactivity of alphaherpesviruses as mirrored in naturally infected primates. Arch Virol 147:929–941 [DOI] [PubMed] [Google Scholar]
- 22.Katz D, Shi W, Wildes M, Hilliard JK. 2002. Automation of serological diagnosis for herpes B virus infections using robot-assisted integrated workstations. J Lab Autom 7:108–113 [Google Scholar]
- 23.Katz D, Shi W, Wildes MJ, Krug PW, Hilliard JK. 2012. Reassessing the detection of B virus-specific serum antibodies. Comp Med 62: 516–526 [PMC free article] [PubMed] [Google Scholar]
- 24.Kesli R. 2011. Evaluation of assay methods and false positive results in the laboratory diagnosis of hepatitis C virus infection. Archives of Clinical Microbiology 4:1–4 [Google Scholar]
- 25.Liao Q, Guo H, Tang M, Touzjian N, Lerche NW, Lu Y, Yee JL. 2011. Simultaneous detection of antibodies to 5 simian viruses in nonhuman primates using recombinant viral protein based multiplex microbead immunoassays. J Virol Methods 178:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnarelli LA, Busmich SL, Anderson JF, Ledizet M, Koski RA. 2008. Serum antibodies to West Nile virus in naturally exposed and vaccinated horses. J Med Microbiol 57:1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, Enria DA, Farrar J, Gubler DJ, Guzman MG, Halstead SB, Hunsperger E, Kliks S, Margolis HS, Nathanson CM, Nguyen VC, Rizzo N, Vázquez S, Yoksan S. 2010. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol 8:S30–S38 [DOI] [PubMed] [Google Scholar]
- 28.Perelygina L, Patrusheva I, Hombaiah S, Zurkuhlen H, Wildes MJ, Patrushev N, Hilliard JK. 2005. Production of herpes B virus recombinant glycoproteins and evaluation of their diagnostic potential. J Clin Microbiol 43:620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perelygina L, Patrusheva I, Zurkuhlen H, Hilliard JK. 2002. Characterization of B virus glycoprotein antibodies induced by DNA immunization. Arch Virol 147:2057–2073 [DOI] [PubMed] [Google Scholar]
- 30.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW. 2006. Reporting of noninferiority and equivalence randomized trials. An Extension of the CONSORT Statement. JAMA 295:1152–1160 [DOI] [PubMed] [Google Scholar]
- 31.Prince HE, Ernst CE, Hogrefe WR. 2000. Evaluation of an enzyme immunoassay system for measuring herpes simplex virus (HSV) type 1-specific and HSV type 2-specific IgG antibodies. J Clin Lab Anal 14:13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Revello MG, Gualandri R, Manservigi R, Gerna G. 1991. Development and evaluation of an ELISA using secreted recombinant glycoprotein B for determination of IgG antibody to herpes simplex virus. J Virol Methods 34:57–70 [DOI] [PubMed] [Google Scholar]
- 33.Saijo M, Nikura M, Ikegami T, Kurane I, Kurata T, Morikawa S, Morikawa S. 2006. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol 13:444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid DS, Brown DR, Nisenbaum R, Burke RL, Alexander D, Ashley R, Pellett PE, Reeves WC. 1999. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J Clin Microbiol 37:376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Select Biological Agents and Toxins. 2003. 42 CFR Part 73. Possession, Use, and Transfer of Biological Toxins.
- 36.Talha SM, Salminen T, Chugh DA, Swaminathan S, Soukka T, Pettersson K, Khanna N. 2010. Inexpensive designer antigen for antiHIV antibody detection with high sensitivity and specificity. Clin Vaccine Immunol 17:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabayashi K, Mukai R, Yamada A. 2001. Detection of B virus antibody in monkey sera using glycoprotein D expressed in mammalian cells. J Clin Microbiol 39:3025–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward JA, Hilliard JK. 2002. Herpes B virus-specific pathogen-free breeding colonies of macaques: serologic test results and the B-virus status of the macaque. Contemp Top Lab Anim Sci 41:36–41 [PubMed] [Google Scholar]
- 39.Weber B, Berger A, Rabenau H. 2001. Human cytomegalovirus infection: diagnostic potential of recombinant antigens for cytomegalovirus antibody detection. J Virol Methods 96:157–170 [DOI] [PubMed] [Google Scholar]