Abstract
This study evaluated the incidence, prevalence, and clinical features of seizures in a pedigreed captive colony of baboons. The association of seizures with subspecies, age, sex, and various clinical features was assessed. Records for 1527 captive, pedigreed baboons were reviewed, and 3389 events were identified in 1098 baboons. Of these events, 1537 (45%) represented witnessed seizures, whereas the remaining 1852 presented with craniofacial trauma or episodic changes in behavior that were suggestive, but not diagnostic, of seizure activity. Seizures were generalized myoclonic or tonic–clonic, with two thirds of the events witnessed in the morning. Seizure onset occurred in adolescence (age, 5 y), with an average of 3 seizures in a lifetime. The incidence and prevalence of seizures were 2.5% and 26%, respectively, whereas the prevalence of recurrent seizures (that is, epilepsy) was 15%. Seizures were more prevalent in male baboons, which tended to present with earlier onset and more seizures over a lifetime than did female baboons. Seizures were equally distributed between the subspecies; age of onset and seizure recurrences did not differ significantly between subspecies. Clinical features including age of onset, characteristics, and diurnal presentation of seizures in baboons suggested similarities to juvenile myoclonic epilepsy in humans. Facial trauma may be useful marker for epilepsy in baboons, but its specificity should be characterized.
The Texas Biomedical Research Institute (Texas Biomed; San Antonio, TX) is home to the Southwest National Primate Research Center, which manages the world's largest baboon colony, currently comprising about 2500 baboons. Almost 2000 baboons, stretching across 5 to 7 generations and consisting of primarily olive baboons (Papio hamadryas anubis, 64%), yellow baboons (P. h. cynocephalus, 4%), and their hybrids (29%), belong to a pedigreed colony that is widely used for genetic research.23 Baboons are ideal for the development of genetic models of human disorders due to the many genetic, anatomic, biochemical, and physiologic features shared by humans and baboons.20,21 Researchers at numerous institutions have used baboons as animal models for a broad range of diseases including diabetes, heart disease, osteoporosis, and chronic infectious illnesses.12,21
Baboons are a natural model of idiopathic generalized epilepsy.8 The occurrence of seizures among colony baboons has been noted since the inception of our colony at Texas Biomed more than 50 y ago.9 The seizures occur spontaneously or are triggered by ketamine (used for sedation) or other stressors, such as handling or fighting among baboons. Often the seizures are not witnessed, but the baboons are found lying prone on the ground, presumptively having fallen from an elevated structure. These baboons often undergo craniofacial trauma, including periorbital lacerations or bruising, injury to the muzzle or mouth, and broken teeth. Nonetheless, most of these baboons are otherwise healthy, without evidence of developmental delay or focal neurologic deficits. Some baboons with seizures have been reported to be congenitally blind or demonstrate congenital brain damage,4 whereas others exhibit seizures as a result of head trauma or infectious diseases. For the most part, however, the baboons with seizures have normal brain anatomy.8,11,16,19
The seizures reported in our baboon colony are typically convulsive, either described as brief generalized myoclonic seizures or tonic–clonic seizures,17,18 similar to those described in red baboons (P. h. papio)8 and humans.3 Previous scalp electroencephalographic studies characterizing epilepsy in the baboon colony demonstrated a high prevalence of generalized interictal epileptic discharges.17 As is true for P. h. papio, epileptic baboons in our colony are photosensitive (that is, seizures in the animals can be triggered by visual stimuli such as intermittent light stimulation).9,11,17,18
Electroclinical findings suggest that baboons provide an ideal animal model for idiopathic generalized epilepsies in humans. However, little is known about the natural history of epilepsy in baboons. In the current study, we present clinical data regarding the incidence, prevalence, and characteristics of the seizures; their age of onset and tendency to recur; and the effects of epidemiologic factors, including age, sex, and subspecies, on their expression.
Materials and Methods
We performed a retrospective case-detection survey of veterinary records to evaluate the incidence and prevalence of witnessed and suspected seizures in the pedigreed baboon colony housed at the Southwest National Primate Research Center (Texas Biomed, San Antonio, TX. The review included electronic records generated between 1980 and 2007, with additional chart review for asymptomatic baboons with limited information in the electronic records. The baboons were treated in strict accordance with the US Public Health Service's Guide for the Care and Use of Laboratory Animals6 and the Animal Welfare Act. 1 This study was approved by the IACUC of the University of Texas Health Science Center at San Antonio and Texas Biomed.
Electronic records were available for 1528 baboons (740 living, 788 dead; 872 female, 586 male; 841 P. h. anubis and 367 hybrid baboons). Electronic records included information about subspecies type, sex, sire, dam, date of birth, health check summaries, and medical interventions. The records were screened for references to witnessed seizures, whether spontaneous (unprovoked) or provoked by ketamine or handling. In addition, they were screened for any treatments for head injuries, particularly brow or muzzle lacerations, which are often (but not exclusively) related to falls during seizures, and observations of peri-ictal behaviors including decreased responsiveness, confusion and lethargy. Electronic or paper records were incomplete for 246 baboons (that is, information regarding sex or subspecies was missing).
The incidence during the period 1991 to 2004 was computed by direct enumeration of the new (living) cases in the at-risk population.13 The prevalence of witnessed seizures was compared between sexes and subspecies by using χ2 tests (www.vassarstats.net). Statistical comparisons were performed for seizure onset and frequency according to sex and subspecies and for seizure onset according to single versus multiple seizures (2-tailed Student t tests).
Results
A total of 3389 events were detected in the records of 1098 baboons. Of these events, 1537 (45%) were witnessed seizures; 1267 (82%) of these witnessed seizures were unprovoked, 212 (14%) were associated with ketamine administration for sedation or anesthesia, and 58 (4%) were associated with handling. The remaining 1852 events consisted of craniofacial trauma, including head, periorbital, and muzzle lacerations or bruising, swollen eyelids or other facial contusions, and broken teeth or, less frequently, reversible behavioral changes, such as lethargy or decreased responsiveness. The durations of spontaneous and provoked seizures were reported for 385 events; the mean duration of a seizure was 75 s (range, 3 to 300 s). The time at which a seizure occurred was provided for 1059 seizures. After exclusion of provoked seizures, 668 (70%) spontaneous seizures occurred in the morning (median time, 1010).
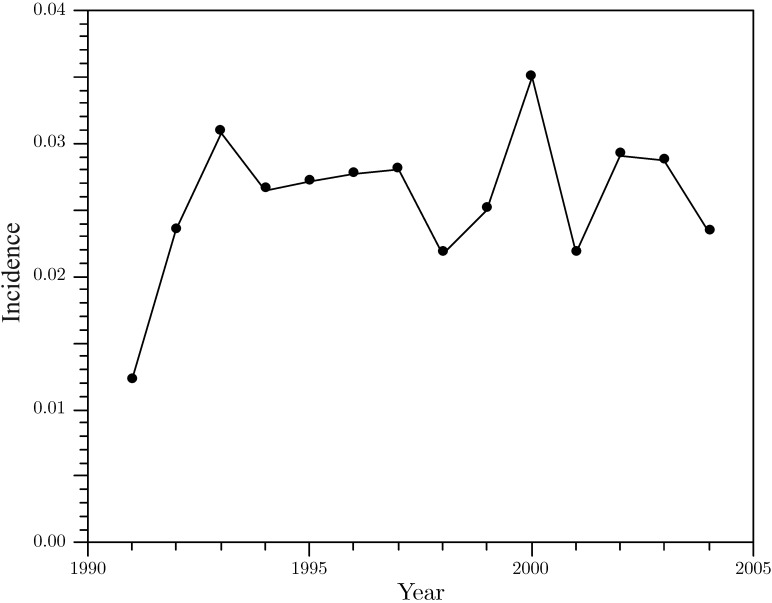
Witnessed seizures were reported for 404 of 1527 baboons, yielding a seizure prevalence of 26%. Most of these seizures were spontaneous and unprovoked. The prevalence of recurrent unprovoked seizures (that is, epilepsy) approached 15%. The annual incidence of seizures (mean ± 1 SD) for the period of 1991 to 2004 (Figure 1) was 0.026 ± 0.005 (or 2.6%). The incidence appeared to be stable over this period. Among the 404 baboons with witnessed seizures, 51% were male (n = 206) and 46% were female (n = 184); 58% were P. h. anubis (n = 236) and 25% were P. h. anubis × P. h. cynocephalus hybrids (n = 102; Table 1). The remaining 3% of the baboons were P. h. hamadryas or other P. h. anubis hybrids. The prevalence of seizures was significantly (χ2 = 34.9, df = 1, P < 0.0001) greater among male (35%, 206 of 586) than female (21%, 184 of 872) baboons but the same between subspecies (28% in both P. h. anubis and hybrid baboons). Single unprovoked seizures were reported in 172 (43%) baboons, whereas 232 baboons had multiple seizures with a mean of 6 (range, 2 to 56) seizures over their lifetimes. Seizures were the only reported events in 173 (43%) baboons, whereas 231 (57%) baboons had craniofacial trauma or episodic behavioral changes in addition to seizures. Craniofacial trauma was almost twice as frequent (mean ± 1 SD, 3 ± 3) in the baboons with seizures than in animals without seizures (2 ± 1). Ketamine-induced seizures were noted in 57 (4%) baboons, and seizures provoked by handling were reported in 35 (2%) baboons, and these were observed predominantly in animals with otherwise spontaneous, unprovoked seizures.
Figure 1.
Incidence of seizures in a captive baboon colony, 1991–2004.
Table 1.
Demographics and clinical features of baboons with seizures
| No. of subjects | Mean age (y [1 SD]) of onset of seizures | Mean no. of seizures | Mean no. of events of facial trauma or behavioral episodes | |
| All | 404 | 5(5) | 3 | 2 |
| Female baboons | 184 | 6(5) | 3 | 2 |
| Male baboons | 206 | 5(4) | 4 | 2 |
| Papio h. anubis | 236 | 6(5) | 3 | 1 |
| Papio h. anubis × | 102 | 5(5) | 4 | 2 |
| Papio h. cynocephalus | ||||
| Single seizures | 172 | 6 (5) | not applicable | 1 |
| Multiple seizures | 232 | 5(4) | 6 | 2 |
The age of onset (mean ± 1 SD) of unprovoked seizures was 5 ± 5 y, with a mean number of 3 witnessed (range, 1 to 62) seizures (Table 1). Compared with female and P. h. anubis baboons, male and hybrid baboons tended to be younger at the onset of seizures and to have more seizures, but these differences were not statistically significant. Baboons with multiple seizures tended to present earlier with their first seizure than did baboons with single seizures, but these differences were not statistically significant.
Discussion
This is the first descriptive study of the incidence and prevalence of seizures in captive baboon colony. Although baboons were established as a model for photosensitivity more than 50 y ago,8 little has been published about the epidemiology of seizures and epilepsy in baboons. The prevalence of seizures in the colony we evaluated was 26%, whereas that of recurrent seizures (that is, epilepsy) was 15%. Seizures were significantly more common in male baboons, but there is little evidence for differences in seizure incidence between P. h. anubis, P. h. cynocephalus, and their hybrids. Affected baboons in this colony presented with their first seizure at about 5 y of age, with only slight differences between sexes (male baboons tended to present earlier than did female baboons) and subspecies (hybrid baboons tended to present earlier than did P. h. anubis baboons). Earlier onset of seizures appeared to be associated with a larger number of spontaneous seizures over a lifetime. Generalized tonic–clonic seizures tended to last longer than 1 min in duration and occurred predominantly during the morning.
The incidence of seizures in the baboon colony we evaluated was 25 per 1000 (2.5%) baboon years, which is much higher than the 135 per 100,000 patient years in human studies.5 Seizures were mainly unprovoked and idiopathic (that is, unrelated to an underlying etiology) in our baboons. As expected, the prevalence of seizures is was much higher in our baboon colony than in human populations: 26% in this baboon colony compared with a cumulative incidence of 10% for lifetime seizures identified in humans.5 Nonetheless, the prevalence of seizures and epilepsy in our baboon colony are more similar to those found in some human pedigrees, particularly in dominantly inherited epilepsies such as the generalized epilepsy with febrile seizures plus syndrome.22 For example, 42 (11%) members of one human pedigree with generalized epilepsy with febrile seizures plus provided a history of seizures, whereas 29 (8%) had a history of epilepsy. In another consanguineous human pedigree with febrile seizures and myoclonic astatic epilepsy, the prevalence of seizures increased to 42%, and almost all of the affected subjects had been diagnosed with epilepsy.14
The genetic basis for epilepsy in baboons has yet to be determined. The prevalence of epilepsy in our colony is not likely to occur in wild baboon populations, because selection pressure is likely strong against young epileptic baboons, which are far more vulnerable to environmental hazards than are captive-reared baboons. The higher prevalence of epilepsy among male baboons differs from the female predominance observed in some types of human idiopathic epilepsies, such as juvenile myoclonic epilepsy and generalized tonic-clonic seizures upon awakening.11 The higher likelihood of recognizing seizures in male baboons may reflect a sex-associated difference in the severity of seizures or epilepsy.18 Male baboons had an earlier onset and greater likelihood for recurrence of seizures than did female baboons, which would make the condition more apparent in male animals. Common seizure types such as eyelid myoclonus are similarly distributed in male and female baboons undergoing scalp EEG studies.17,18
The mean age of seizure onset was 5 y in our baboons, with minor variations across sex and subspecies. Adolescence in baboons typically begins at 4 y, with full maturation into an adult by 7 y.2 Given that every year in a baboon's life corresponds roughly to 3 y of human life, the age of seizure onset in baboons translates to 15 y in humans, with the confidence intervals extending from childhood to the end of the third decade. The age range of affected baboons is roughly that for juvenile myoclonic epilepsy in humans.3 Because craniofacial trauma may present as the initial seizure in some baboons, animals exhibiting such trauma should be followed closely.
The accuracy of the estimated number of seizures in baboons is unknown. The reporting of seizures depends on observations by caretakers, who may frequently report prolonged seizures or identify peri-ictal behaviors or injuries but may be less likely to recognize myoclonic seizures, which are extremely brief (especially eyelid myoclonus). Eyelid myoclonus in baboons has been described in the setting of photosensitivity, that is, seizures evoked by intermittent light stimulation, or occurring spontaneously during electroencephalographic studies.17,18 Baboons with frequent seizures were monitored closely by veterinary staff, especially during admissions to the infirmary, but even among these cases, some baboons had such frequent seizures that they were no longer documented. However, because craniofacial trauma and episodic unresponsiveness or confusion can reflect seizure activity, such episodes need to be documented rigorously in medical records to facilitate accurate assessment of seizure frequency. However, the specificity of trauma as a disease marker is unknown, particularly given that baboons frequently contract facial wounds in fights. It is essential to characterize craniofacial wounds according to their location and association with witnessed seizures.
The natural history of epilepsy in our baboon colony is difficult to ascertain. Generalized tonic–clonic seizures appear to remit in some baboons, but those data are confounded by the limitations of observation, particularly of more subtle seizure types, and by colony management practices. Baboons with frequent or severe seizures that no longer thrived or were at high risk for injury were euthanized. In contrast, baboons with infrequent seizures usually were returned to their cages, with some animals eventually dying due to seizure-related injuries or the syndrome of sudden unexpected death in epilepsy.15
Although seizures can occur at any time, our colony appeared to show an increased likelihood of seizure occurrence in the morning.8 Diurnal variation of seizures has also been described in human epilepsy syndromes, and seizures occurring in late stages of sleep or on awakening have been associated with the most common types of idiopathic generalized epilepsies.3 Photosensitivity in both baboons and humans tends to be higher in the morning, during the first hours after awakening.7,8 The diurnal bias toward the morning hours may be in part due to observational bias related to the work schedule of caregivers, allowing them to observe the animals throughout the morning and only during part of the afternoon, with limited observation during the evenings and nights. A similar observational bias may have affected the assessment of seizure duration, in that longer generalized tonic–clonic seizures are more likely to be seen than are brief generalized myoclonic seizures, which last only a few seconds.
Although the natural history of the disease is altered by captivity, the ability to compile observations of seizures in several hundred animals over the course of several decades offers unique insights into the characterization of the baboon model of epilepsy. In addition to the clinical findings in baboons of adolescent-onset of myoclonic and generalized tonic–clonic seizures that occurred predominantly in the morning, we have used scalp electroencephalography to demonstrate generalized ictal and interictal epileptic discharges, both spontaneous and induced by intermittent light stimulation.17,18 These electroclinical findings suggest strong similarities in epilepsy between baboons and humans, particularly resembling juvenile myoclonic epilepsy in humans.3 The availability of accurate pedigree information for the baboon colony we evaluated will enable future qualitative and quantitative genetic analyses to characterize genetic contributions to seizures and related traits in baboons.
Acknowledgments
This study was supported by the National Institute of Neurologic Disorders and Stroke (NIH/NINDS 1 R21 NS065431 to CAS and 1 R01 NS047755 to JTW). This research used primate resources supported by P51 RR013986 and was conducted in facilities constructed with support from Research Facilities Improvement Grants C06 RR013556, C06 RR014578, and C06 RR015456.
References
- 1.Animal Welfare Act as Amended. 2008. 7 USC §2131-2159.
- 2.Coelho AM., Jr1985. Baboon dimorphism: growth in weight, length, and adiposity from birth to 8 years of age, p 125–159. In: Watts, ES, editor. Nonhuman primate models for human growth and development. Monographs in primatology 6. New York (NY): Alan R Liss.
- 3.Dreifuss FE. 1989. Juvenile myoclonic epilepsy: characteristics of a primary generalized epilepsy. Epilepsia 30 Suppl 4:S1–S7 [DOI] [PubMed] [Google Scholar]
- 4.Fox B, Owston MA, Kumar S, Dick EJ. 2011. Congenital anomalies in the baboon (Papio spp.). J Med Primatol 40:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser WA, Annegers JF, Rocca WA. 1996. Descriptive epidemiology of epilepsy: contribution of population-based studies from Rochester, Minnesota. Mayo Clin Proc 71:576–586 [DOI] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press.
- 7.Kasteleijn-Nolst Trenité DGA. 1989. Photosensitivity in epilepsy: electrophysiological and clinical correlates. Acta Neurol Scand Suppl 125:3–149 [PubMed] [Google Scholar]
- 8.Killam EK. 1979. Photomyoclonic seizures in the baboon, Papio papio. Fed Proc 38:2429–2433 [PubMed] [Google Scholar]
- 9.Killam EK, Starck LG, Killam KF. 1967. Photic stimulation in 3 species of baboons. Life Sci 6:1569–1574 [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Juarez IE, Alonso ME, Medina MT, Durón RM, Bailey JN, López-Ruiz M, Ramos-Ramirez R, León L, Pineda G, Castroviejo IP, Silva R, Mija L, Perez-Gosiengfiao K, Machado-Salas J, Delgado-Escueta AV. 2006. Juvenile myoclonic epilepsy subsyndromes: family and long-term follow-up. Brain 129:1269–1280 [DOI] [PubMed] [Google Scholar]
- 11.Naquet R, Catier J, Menini C.1975. Neurophysiology of photically induced epilepsy in Papio papio, p 107–118. In: Meldrum BS, Marsden CD. Advances in neurology, vol 10. New York (NY): Raven Press. [PubMed]
- 12.Rogers J, Hixson JE. 1997. Insights from model systems: baboons as an animal model for genetic studies of common human disease. Am J Hum Genet 61:489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothman KJ, Greenland S.1998. Modern epidemiology, 2nd ed. Philadelphia (PA): Lippincott Williams and Wilkins.
- 14.Scheffer IE, Berkovic SF. 1997. Generalized epilepsy with febrile seizures plus: a genetic disorder with heterogeneous clinical phenotypes. Brain 120:479–490 [DOI] [PubMed] [Google Scholar]
- 15.Szabó CA, Knape KD, Leland MM, Feldman J, McCoy KJM, Hubbard GB, Williams JT. 2009. Mortality in captive baboons with seizures: a new model for SUDEP? Epilepsia 50:1995–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabó CA, Kochunov P, Knape KD, McCoy KJM, Leland MM, Lancaster JL, Fox PT, Williams JT, Rogers J. 2011. Cortical sulcal areas in baboons (Papio hamadryas spp.) with generalized interictal epileptic discharges on scalp EEG. Epilepsy Res 93:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabó CA, Leland MM, Knape KD, Elliott JJ, Haines VL, Williams JT. 2005. Clinical and EEG phenotypes of epilepsy in the baboon (Papio hamadryas spp.). Epilepsy Res 65:71–80 [DOI] [PubMed] [Google Scholar]
- 18.Szabó CA, Leland MM, Sztonák L, Restrepo S, Haines R, Mahaney MC, Williams JT. 2004. Scalp EEG for the diagnosis of epilepsy and photosensitivity in the baboon. Am J Primatol 62:95–106 [DOI] [PubMed] [Google Scholar]
- 19.Ticku MK, Lee JC, Murk S, Mhatre MC, Story JL, Kagan-Hallet K, Luther JS, MacCluer JW, Leland MM, Eidelberg E. 1992. Inhibitory and excitatory amino acid receptors, c-fos expression, and calcium-binding proteins in the brain of baboons (Papio hamadryas) that exhibit ‘spontaneous’ grand mal epilepsy. Epilepsy Res Suppl 9:141–149 [PubMed] [Google Scholar]
- 20.VandeBerg JL, Williams-Blangero S. 1996. Strategies for using nonhuman primates in genetic research on multifactorial disease. Lab Anim Sci 46:146–151 [PubMed] [Google Scholar]
- 21.VandeBerg JL, Williams-Blangero S.2003. Nonhuman primates in genetic research on common diseases, p 114–121. In: International perspectives: the future of nonhuman primate resources. Washington (DC): National Academies Press.
- 22.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. 1998. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nat Genet 19:366–370 [DOI] [PubMed] [Google Scholar]
- 23.Williams-Blangero S, VandeBerg JL, Blangero J, Konigsberg L, Dyke B. 1990. Genetic differentiation between baboon subspecies: relevance for biomedical research. Am J Primatol 20:67–81 [DOI] [PubMed] [Google Scholar]