Abstract
Nonhuman primates are a valuable model for osteoarthritis. Osteoarthritis has been extensively studied in nonhuman primates in both naturally occurring and induced disease states. However, little published information describes naturally occurring osteoarthritis of the coxofemoral joints of nonhuman primates. We report a case of naturally occurring coxofemoral joint osteoarthritis in a rhesus macaque. This case radiographically resembled hip dysplasia reported in other species and demonstrated a rapid progression in severity of lameness, with accompanying loss of muscle mass in the affected limb. We excised the femoral head and neck to alleviate the pain that accompanied the osteoarthritis. Physical therapy was initiated, and dual-energy X-ray absorptiometry and video recordings were performed to evaluate the macaque's response to surgical intervention. By 3 mo postoperatively, the macaque had regained full use of the affected limb.
Osteoarthritis has been investigated and reported in numerous species of macaques and other Old World nonhuman primates in both retrospective studies of naturally occurring disease and as induced disease models.1-5,7-9,14,15 Nonhuman primates serve as valuable models for osteoarthritis, due to the difficulty of studying osteoarthritis in the human population and the increasing prevalence of osteoarthritis in society.8 Like humans, macaques show an increase in the prevalence and severity of osteoarthritis with increasing age.3-5 The percentage of macaques affected by osteoarthritis has been reported to be as high as 79% in animals older than 15 y.13 Numerous publications referencing osteoarthritis in macaques focus on specific anatomic locations, such as the stifles or spine, as models for osteoarthritis and bone loss or changes in bone mineral density.2-5,8 Little information describes the incidence of osteoarthritis in the coxofemoral joints of macaques, with even less detailing therapies or surgical repair and their response. A retrospective study that evaluated the skeletons of free-ranging macaques described an incidence of coxofemoral osteoarthritis of less than 1%, with no description of lesions present.9 The current report describes a clinical case of osteoarthritis of the coxofemoral joint of a female rhesus macaque and the surgical repair, postoperative treatment, and return to normal function of the affected limb.
Case Report
The subject described here was a 13-y-old female rhesus macaque (Macaca mulatta) that weighed 8 kg. She was born and raised at the Tulane National Primate Research Center (Covington, LA). All procedures were approved by the IACUC of the Tulane National Primate Research Center and were in accordance with the Guide for the Care and Use of Laboratory Animals. At the time of presentation, she was seronegative and virus-negative for simian retrovirus type D and seropositive for simian T lymphotropic virus type 1. All tuberculin skin tests, which were performed semiannually, were negative throughout her history. The case subject was living in the breeding colony of the AAALAC-accredited animal care and use program until 26 mo of age, when she had to be removed for veterinary care for wounding involving the digits and lip that occurred as a result of animal-to-animal aggression. After all wounds had healed, she was returned to a breeding group where she was maintained until approximately 9 y of age, at which time she again had to be removed from her breeding group for veterinary care involving a degloving injury to her tail. After resolution of the degloving injury, she was returned to the breeding colony until she was 11 y old, when she was removed from the breeding colony for assignment to research.
The initial research assignment was to an influenza vaccine research study, in which she was vaccinated by intramuscular, intraconjunctival, and intranasal routes. All intramuscular vaccinations occurred in the vastus lateralis. After completion of the influenza vaccine study, she was enrolled on a protocol investigating the efficacy of microbicides as HIV1 entry inhibitors.
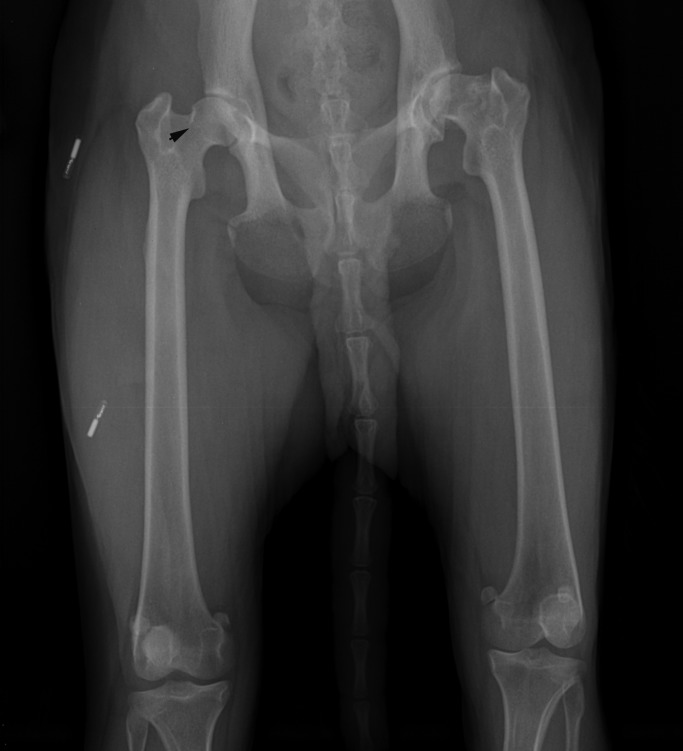
While the macaque was involved in the second research assignment, mild lameness was observed in her left hindlimb, which rapidly progressed to a nonweight-bearing state over the course of approximately 3 wk. During physical examination with the use of ketamine hydrochloride (10 mg/kg IM; Ketaset, Fort Dodge Animal Health, Ft Dodge, IA), loss of muscle mass in the left hindlimb was noted. The remainder of the physical examination was unremarkable. Results of a CBC and chemistry panel were within normal limits. Radiographs were revealed degenerative changes in the left coxofemoral joint (Figure 1). Osteophyte formation was present on the femoral head with femoral head remodeling, consisting of a flattened articular surface and a thickened, irregular femoral neck. The acetabulum was remodeled, demonstrating a change from the normal cup shape appearance to a flattened and widened articular surface. Subchondral bone sclerosis of the femoral head and neck was present also. The degenerative changes demonstrated on radiographs present in our macaque are consistent with those seen in canines diagnosed with hip dysplasia.12
Figure 1.
Preoperative radiographs demonstrating osteoarthritis of the left coxofemoral joint in a mature female rhesus macaque (Macaca mulatta). Remodeling of the femoral head, neck, and acetabulum is present along with subchondral bone sclerosis. Muscle atrophy in the left lower limb is apparent. Solid arrow indicates the Morgan line on the right femoral neck.
The right coxofemoral joint was radiographically normal, with the exception of the presence of the ‘Morgan line,’ which is defined as osteophyte formation on the caudal aspect of the femoral neck adjacent to the trochanteric fossa (Figure 1). The Morgan line is an early and significant indicator of degenerative joint disease.12
Carprofen (4.5 mg/kg SC once daily; Rimadyl, Pfizer, New York, NY) was initiated to treat pain and inflammation. Excision of the femoral head and neck was scheduled after consultation with the principal investigator of the research protocol in which the macaque was assigned. The subject was videorecorded as a baseline measurement to allow evaluation of response to surgery.
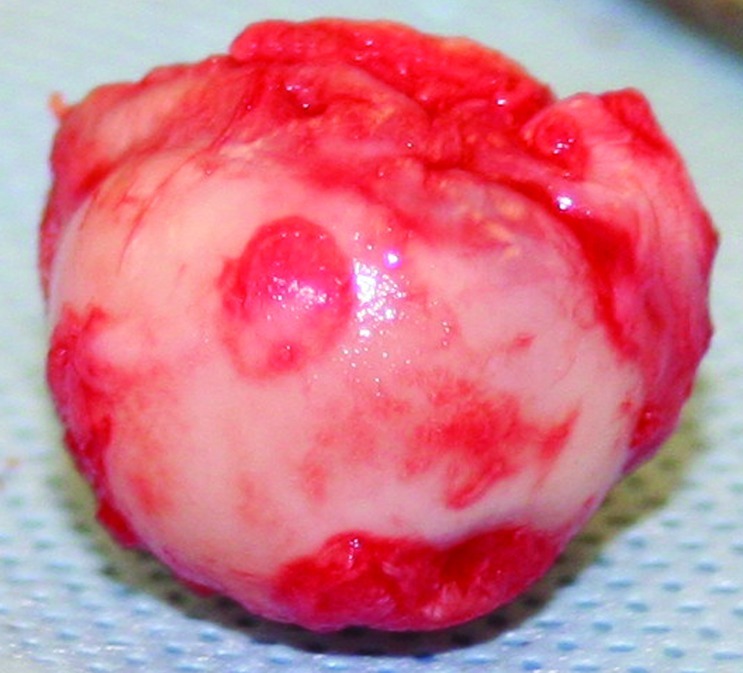
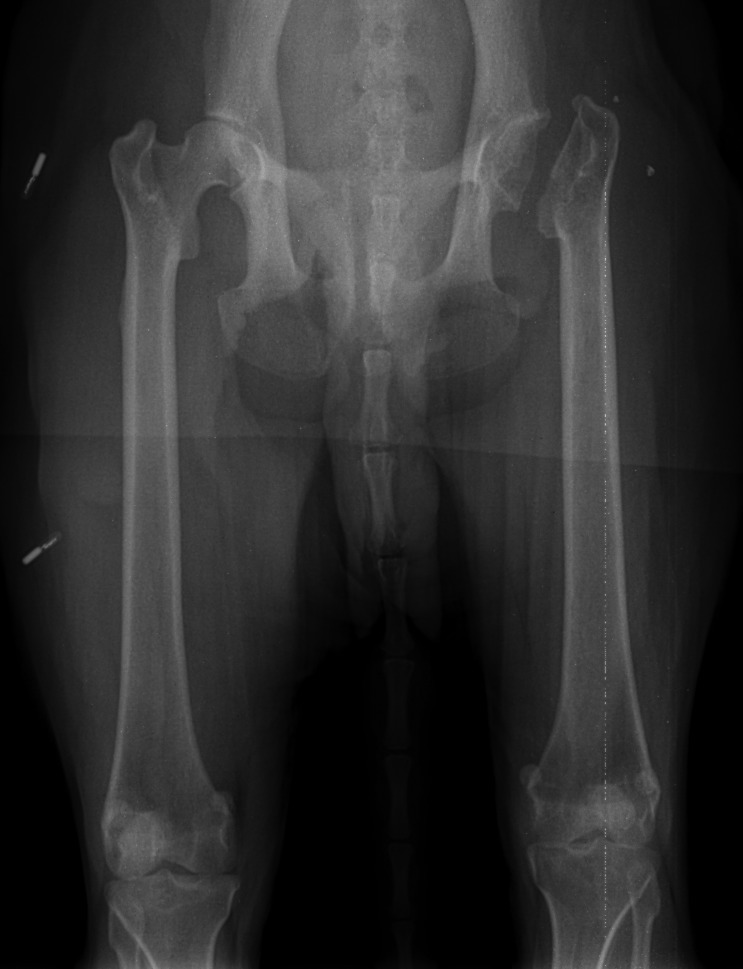
A craniolateral surgical approach was used for excision of the femoral head and neck. The surgical technique was consistent with that published for companion animals.10 A nitrogen-gas–powered saw was used to excise the femoral head and neck (Figure 2). The cut surface of the femur was digitally palpated for confirmation of a smooth surface. Postoperative radiographs were obtained to evaluate the repaired pseudojoint, which demonstrated an excisional surface of the femur extending from the medial surface of the greater trochanter to just proximal to the lesser trochanter with no sharp bony prominences present (Figure 3).
Figure 2.
Excised femoral head demonstrating cartilage erosion and flattening of the femoral head.
Figure 3.
Postoperative radiographs demonstrating left coxofemoral joint after excision of the femoral head and neck.
To provide analgesia postoperatively, carprofen was continued for 5 d at the previously stated dose, and buprenorphine hydrochloride (0.01 mg/kg IM twice daily; Buprenex, Reckitt Benckiser Pharmaceuticals, Richmond, VA) was provided for 3 d postoperatively. To reduce the number of injections administered, meloxicam (0.09 mg/kg PO once daily; Metacam, Boehringer Ingelheim, St Joseph, MO) was initiated at the conclusion of carprofen therapy and continued for 14 d.
Physical therapy was performed under ketamine hydrochloride anesthesia and was initiated beginning 2 d postoperatively to minimize reduced range of motion in the coxofemoral joint. Therapy was continued 3 times weekly for 3 wk. Physical therapy consisted of full extension and flexion of the left coxofemoral and stifle joint, with the animal in right lateral recumbency. Each physical therapy session lasted for approximately 15 min. Nutritional support was administered via feeding tube at each physical therapy time point. After each physical therapy session, the patient was reunited with her social partner in her homecage after recovery from anesthesia. She was videotaped at 1 mo and 3 mo postoperatively to evaluate use of the surgically repaired limb. Dual-energy X-ray absorptiometry was performed at 1 and 3 mo postoperatively to monitor postoperative progress by evaluating muscle mass of the surgically repaired hind limb.
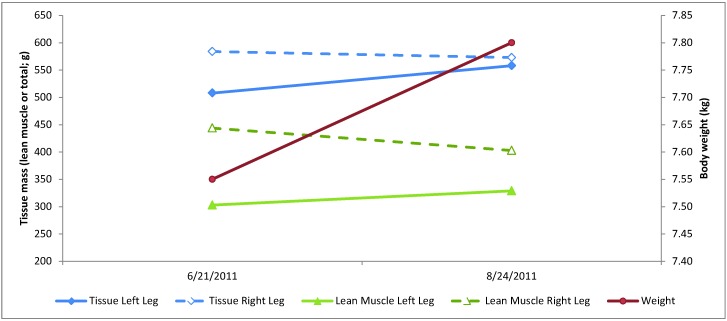
Video recordings at 1 mo postoperative demonstrated full weight-bearing and, when compared with preoperative recordings, showed increased use of the left hindlimb, with ability to rotate the coxofemoral pseudojoint during normal postural adjustments. Recordings at 3 mo postoperatively revealed virtually no detectable difference in ambulation and movement between the 2 hindlimbs. Gait appeared normal, and the assessment at that point was that full function of the operated joint had returned. The 1- and 3-mo postoperative scans demonstrated an increase in total tissue mass and lean muscle mass of the left hindlimb. In addition, the total tissue mass and lean muscle mass of the right hindlimb (compensating limb) decreased during this time period, illustrating increased use of the affected limb (Figure 4). The videorecordings and results from dual-energy X-ray absorptiometry demonstrate that our subject has not displayed some of the postsurgical complications that have been documented in companion animals, such as muscle atrophy, shortening of the surgically repaired limb, and reduction of hip extension.10
Figure 4.
Postoperative increases in lean muscle mass and total tissue mass in the left hindlimb and decreases in these parameters in the right hindlimb, demonstrating increased use of the surgically repaired left coxofemoral joint and less dependence on the contralateral (right) hindlimb.
Discussion
Hip dysplasia in animals is normally an inherited, developmental disorder that is age-related and that can be adversely affected by environmental factors such as rapid growth and excessive exercise.6,12 It is seen most often in large-breed dogs, but similar disease has been reported to occur in humans, gorillas, bears, horses, cattle, and cats.10 A genetic basis has been identified for this disease, but the pattern of inheritance is multifactoral.10 Hip dysplasia typically presents bilaterally, but unilateral disease has been reported in 11% of the canine cases.12 Overnutrition plays an important role in disease progression, and it is the main nongenetic factor related to disease.12 Similarly, in the human population, factors that affect the risk of development of osteoarthritis include sex, age, genetic predisposition, mechanical stress, trauma, and obesity.3,8
We investigated several of the risk factors for hip dysplasia in humans in regard to the current case. Historical weight records for this female macaque were within 1 SD of or below the average weights of female rhesus monkeys (maximum age, 13 y) in the breeding colony. Our female rhesus macaque maintained a weight between 7 and 8 kg for most of her adult life, with a maximum weight of 9 kg. She was fed the same commercial nonhuman primate diet as that for all other animals in the breeding colony. These findings indicate that overnutrition and obesity are unlikely predisposing causes of the coxofemoral joint disease seen in the current case.
To identify a potential genetic basis for the degenerative joint disease in our macaque, we reviewed the medical histories of related animals. The medical records of 7 offspring contained no reports of arthritic degenerative changes in the coxofemoral or any other joint. In addition, no degenerative musculoskeletal changes were noted in any sibling. In addition, the macaque's lineage had no history of joint abnormalities through 2 generations of dams. The related dams survived to the ages of 13 and 20 y. Although the lack of disease of the coxofemoral joints of relatives does not absolutely rule out a genetic basis for disease in this case, it reduces the likelihood of this factor.
Trauma does not appear to be a contributing factor for the development of osteoarthritis in the coxofemoral joint of our subject. Although our macaque experienced 2 reported incidents of trauma due to animal-to-animal aggression, neither event involved the coxofemoral joint or related areas. In addition, any injections associated with research were limited to the musculature of the lower limb and were not associated with the coxofemoral joint.
The true prevalence of hip dysplasia in macaques is difficult to determine because there are few reports in the literature. In one study, the skeletal remains of wild Japanese macaques, (Macaca fuscata fuscata) were surveyed to investigate bone and joint disorders in this species.9 The study included 107 Japanese macaque skeletons, which were evaluated for fractures, osteoarthritis, vertebral osteophytosis, and other pathologies. Only one case of osteoarthritis of the hips was revealed, which occurred in an adult female. No details were given to describe the severity or type of lesion that was present.9
When compared with the published data on the incidence of spontaneous osteoarthritis of the coxofemoral joint in macaques, even fewer publications are found that describe the surgical repair or treatment for such conditions. Our literature search yielded only a single report of femoral head ostectomy, which occurred in a juvenile rhesus macaque to treat Legg–Calve–Perthes disease.11 As in our case, the animal responded well to surgical therapy, regaining a good range of motion and improved gait, and although recovery was not complete at the time of publication, the prognosis for the animal was determined to be good.11
Although we could rule out the most common etiologies noted for coxofemoral joint disease in other species and humans, including trauma, overnutrition, obesity, and genetics, the etiology underlying this case of unilateral hip dysplasia remains unclear. The Morgan line was identified on radiographs of the right coxofemoral joint and may be an indicator that more severe degenerative changes will occur in the future. At the time of writing, our macaque has regained full use of her lower left limb, as demonstrated through increased activity with normal, full range of motion seen in video recordings and under direct observation and through the changes in lean muscle mass of the left and right hindlimbs appreciated through dual-energy X-ray absorptiometry.
Acknowledgments
This study was supported by NIH grants P51 RR00164-50/P51 OD011104-51 and U19 AI76982. We are grateful to Larissa Devlin for her assistance with this case.
References
- 1.Bessis N, Lemeiter D, Laroche L, Fournier C, Huizinga T, Brok H, Hart B, Boissier MC. 2007. Engraftment of cutaneous fibroblasts within synovial membrane in a nonhuman primate: short-term results. Joint Bone Spine 74:48–51 [DOI] [PubMed] [Google Scholar]
- 2.Black A, Tilmont EM, Handy AM, Scott WW, Shapses SA, Ingram DK, Roth GS, Lane MA. 2001. A nonhuman primate model of age-related bone loss: a longitudinal study in male and premenopausal female rhesus monkeys. Bone 28:295–302 [DOI] [PubMed] [Google Scholar]
- 3.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. 1996. Ostoearthritis in cynomologous macaques III: effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res 11:1209–1217 [DOI] [PubMed] [Google Scholar]
- 4.Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkley N. 1999. Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab 84:4144–4148 [DOI] [PubMed] [Google Scholar]
- 5.Colman RJ, Lane MA, Binkley N, Wegner H, Kemnitz JW. 1999. Skeletal effects of aging in male rhesus monkeys. Bone 24:17–23 [DOI] [PubMed] [Google Scholar]
- 6.Ettinger SJ, Feldman EC.1995. Textbook of veterinary internal medicine. Philadelphia (PA): W B Saunders Publishing.
- 7.Kramer P, Simkin P, Morris LN, Wener M. 2007. Markers of cartilage and bone matrix metabolism differ between the sera of macaque and squirrel monkeys. J Med Primatol 36:143–147 [DOI] [PubMed] [Google Scholar]
- 8.Miller LM, Novatt JT, Hamerman D, Carlson CS. 2004. Alterations in mineral composition observed in osteoarthritic joints of cynomologous monkeys. Bone 35:498–506 [DOI] [PubMed] [Google Scholar]
- 9.Nakai M. 2003. Bone and joint disorders in wild Japanese macaques from Nagano Prefecture, Japan. Int J Prim 24:179–195 [Google Scholar]
- 10.Slatter D.1993. Textbook of small animal surgery. Philadelphia (PA): W B Saunders Publishing.
- 11.Smedley JV, Lomax LG, Williams JF, Barras PW, Hasselschwert DL. 2004. Legg–Calve–Perthes disease in a rhesus macaque (Macaca mulatta). Comp Med 54:585–588 [PubMed] [Google Scholar]
- 12.Thrall DE.editor. 1998. Textbook of veterinary diagnostic radiology. Philadelphia (PA): W B Saunders Publishing
- 13.Videan EN, Lammey ML, Lee DR. 2011. Diagnosis and treatment of degenerative joint disease in a captive male chimpanzee (Pan troglodytes). J Am Assoc Lab Anim Sci 50:263–266 [PMC free article] [PubMed] [Google Scholar]
- 14.Vierboom MPM, Jonker M, Bontrop RE, Hart B. 2005. Modeling human arthritic diseases in nonhuman primates. Arthritis Res Ther 7:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vierboom MPM, Jonker M, Tak PP, Hart BA. 2007. Preclinical models of arthritic disease in nonhuman primates. Drug Discov Today 12:327–335 [DOI] [PubMed] [Google Scholar]