Abstract
Aims
The role of endothelial nitric oxide synthase (eNOS)/NO signalling is well documented in late ischaemic preconditioning (IPC); however, the role of eNOS and its activation in early IPC remains controversial. This study investigates the role of eNOS in early IPC and the signalling pathways and molecular interactions that regulate eNOS activation during early IPC.
Methods and results
Rat hearts were subjected to 30-min global ischaemia and reperfusion (I/R) with or without IPC (three cycles 5-min I and 5-min R) in the presence or absence of the NOS inhibitor l-NAME, phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (LY), and protein kinase A (PKA) inhibitor H89 during IPC induction or prior endothelial permeablization. IPC improved post-ischaemic contractile function and reduced infarction compared with I/R with this being abrogated by l-NAME or endothelial permeablization. eNOSSer1176, AktSer473, and PKAThr197 phosphorylation was increased following IPC. I/R decreased eNOSSer1176 phosphorylation, whereas IPC increased it. Mass spectroscopy confirmed eNOSSer1176 phosphorylation and quantitative Western blots showed ∼24% modification of eNOSSer1176 following IPC. Immunoprecipitation demonstrated eNOS, Akt, and PKA complexation. Immunohistology showed IPC-induced Akt and PKA phosphorylation in cardiomyocytes and endothelium. With eNOS activation, IPC increased NO production as measured by electron paramagnetic resonance spin trapping and fluorescence microscopy. LY or H89 not only decreased AktSer473 or PKAThr197 phosphorylation, respectively, but also abolished IPC-induced preservation of eNOS and eNOSSer1176 phosphorylation as well as cardioprotection.
Conclusion
Thus, Akt- and PKA-mediated eNOS activation, with phosphorylation near the C-terminus, is critical for early IPC-induced cardioprotection, with eNOS-derived NO from the endothelium serving a critical role.
Keywords: Protein phosphorylation, Signalling pathways, Reactive oxygen species, Endothelial nitric oxide synthase structure
1. Introduction
Ischaemic preconditioning (IPC) is a well-documented phenomenon that provides robust intrinsic cardioprotection in all mammalian species as well as humans.1 IPC results in two distinct protective windows that are known as early preconditioning (or classical preconditioning) and late preconditioning (or delayed preconditioning).2 IPC is a multifaceted process with distinct molecular triggers and complex cellular cascades. A number of diverse signalling pathways have been implicated in cardioprotection.1
The endothelial nitric oxide synthase (eNOS)/NO signalling plays a major role in cardioprotection.3–7 Although the role of the eNOS/NO signalling is well-documented in late preconditioning,3,4 the role of eNOS and its regulation in early IPC remains controversial and poorly defined.5–9 Recently, using wild-type (WT) and eNOS-knockout (eNOS−/−) mice, we have shown that early IPC confers robust cardioprotection in WT mice with significantly smaller myocardial infarction, and this protection is lost in eNOS−/− mice.5 This is in agreement with studies where overexpression of eNOS10 or eNOS enhancer11 attenuated myocardial I/R injury. Importantly, a recent report in pigs demonstrated that cardioselective eNOS gene transfer can protect the heart against I/R injury.12 Although these studies strongly suggest an involvement of eNOS in acute or early cardioprotection, the molecular mechanisms regulating the eNOS activation and function remain unclear.5–7
Several studies have demonstrated that Akt,13 protein kinase A (PKA),14 or AMP-activated protein kinase α (AMPKα)15 can phosphorylate eNOS at Serine1177 (note: this corresponds to Serine1176 in rat eNOS), thereby leading to an increase in eNOS activation and cardioprotection; however, questions remain regarding these signalling pathways of eNOS activation, their role in early IPC-induced cardioprotection, and how precisely they modify the structure of eNOS to induce activation. Therefore, the present study was designed to investigate: (i) whether early IPC protects against cardiac I/R injury via a mechanism that is dependent on NO production triggered by eNOS phosphorylation; (ii) what signalling pathways regulate eNOS activation during early IPC; (iii) what precise molecular modifications of eNOS lead to this activation in the heart.
2. Methods
2.1. Isolated rat heart preparation
Animal protocols were performed in accordance with the guidelines of Institutional Laboratory Animal Care and Use Committee at The Ohio State University, and conformed with the Guidelines for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication NO. 85-23, revised 1996). Male Sprague-Dawley rats (300–350 g) were anaesthetized with pentobarbital sodium (60 mg/kg, IP), and the depth of anaesthesia checked by the toe pinch reflex. Under deep anaesthesia, hearts were excised, aorta cannulated, and perfused in the Langendorff mode with constant pressure of ∼80 mmHg using modified Krebs–Henseleit (KH) buffer. Hearts were randomly allocated for different protocols. For detailed procedures, see Supplementary material online.
2.2. Experimental protocols
The experimental protocol for the isolated rat heart studies is illustrated in Figure 1A. The protocol consisted of a control aerobic perfusion period, a treatment period, 30 min of global ischaemia (I), and 120 min of reperfusion (R). The recovery of left ventricular developed pressure (LVDP) was measured at 30 min of R and expressed as a percentage of the initial LVDP before IPC or inhibitor administration. Hearts were assigned randomly to one of the following groups: (i) I/R control (n = 7); (ii) IPC preconditioned with three cycles of 5 min of I and 5 min of R (n = 7); (iii) l-NAME + IPC (n = 7), l-NAME (1 mM) was infused for 5 min prior to the first 5-min IPC ischaemia period and throughout the 3, 5-min IPC R cycles before global ischaemia; (iv) phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (LY) + IPC (n = 5), LY (15 µM) was infused for 5 min prior to the IPC trigger and throughout each preconditioning R cycle; (v) PKA inhibitor H89 + IPC (n = 5), H89 (10 µM) was infused as described for LY. Effects of each inhibitor on functional recovery in the absence of IPC were also investigated. To investigate whether IPC-induced cardioprotection is endothelium dependent, additional experiments were performed for functional recovery and myocardial infarction in endothelium-permeabilized hearts, using brief low level Triton X-100 pretreatment that abolishes eNOS-mediated NO generation with minimal effects on myocardial function.16
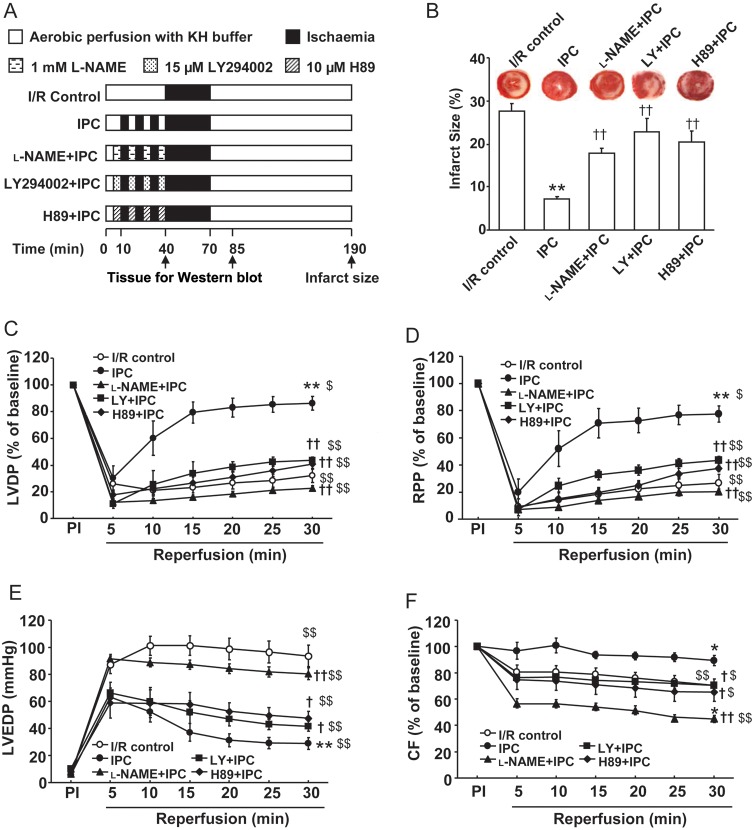
Figure 1.
Pharmacological characterization of the role of NOS, Akt, and PKA in early IPC. (A) Experimental protocols for I/R and IPC with or without treatment. (B) The infarct size expressed as a percentage of the risk area measured by TTC staining following 30 min of global ischaemia and 120 min of reperfusion for I/R control and IPC protocols in the absence or presence of l-NAME, LY and H89 as depicted in (A). (C–F) Post-ischaemic myocardial recovery following 30-min global ischaemia and 30-min reperfusion for I/R control and IPC protocols with or without l-NAME, LY, and H89 as depicted in (A). Left ventricular developed pressure (LVDP, C), rate-pressure product (RPP, D), and coronary flow (F) are expressed as a percentage of the pre-ischaemic (PI) value (100%); and LV end diastolic pressure (LVEDP, E) is expressed as mmHg. Values are means ± SE. n = 5–7/group. **P < 0.01 vs. I/R control, †P < 0.05, ††P < 0.01 vs. IPC group; $P < 0.05, $$P < 0.01 vs. pre-ischaemic (PI) baseline.
2.3. Infarct size
At the end of 2 h of reperfusion, hearts were collected and the infarct size was measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining. The individual slices were digitally photographed for planimetry using the NIH Image J 1.43 software. For details, see Supplementary material online.
2.4. Immunoblotting analysis
For immunoblotting, hearts were harvested at the end of the treatment period as illustrated in Figure 1A. Tissues were homogenized in ice-cold lysis buffer and subjected to SDS–polyacrylamide gel electrophoresis. Proteins were detected by enhanced chemiluminescence detection reagents for densitometric analysis. For details, see Supplementary material online.
2.5. Fluorescence and immunofluorescence microscopy
Hearts were collected, mounted, and frozen in optimal cutting temperature medium at trigger and reperfusion phases with/without IPC treatments as illustrated in Figure 1A. Tissues were cryosectioned and slices were probed with antibodies for detecting eNOS, peNOS, pAkt, and pPKA by confocal microscopy. To detect NO generation in the process of IPC, tissue sections were incubated with the NO indicator CuFL (1.0 µM) and images were captured and analysed by confocal fluorescence microscopy. For details, see Supplementary material online.
2.6. Detection of NO by electron paramagnetic resonance spin trapping
As described previously,17 Fe-Citrate (63 mg FeSO4.7H2O—200 mg sodium citrate/kg, Fe2+:Citrate = 1:3) was dissolved in phosphate buffered saline (PBS) and injected ip, 24 h and 30 min prior to the onset of perfusion experiments. Then hearts were perfused with KH buffer and after stabilization, infused with 5 mM sodium diethyldithiocarbamate (DETC) for 5 min with or without IPC treatment. Finally, hearts were harvested for detecting NO-Fe2+-DETC signal using low temperature, 77 K, electron paramagnetic resonance (EPR). For details, see Supplementary material online.
2.7. Immunoprecipitation assay
Supernatant of tissue lysates containing equal amounts of proteins were incubated with agarose-conjugated anti-eNOS (Santa Cruz) antibody overnight at 4°C. In a negative control experiment, heart tissue lysates were incubated with irrelevant MnSOD antibody (Millipore) using similar agarose beads. After incubation, immunoprecipitation products were washed once with cold low salt immune complex washing buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS), twice with high-salt washing buffer (50 mM Tris–HCl, pH 7.4, 500 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS), and twice with PBS buffer. Immunoprecipitates were then eluted by SDS–PAGE sample buffer and characterized by immunoblotting with appropriate antibodies.
2.8. Tandem mass spectrometry (MS/MS) to identify eNOS phosphorylation sites
eNOS immunoprecipitation samples from hearts were subjected to 10% SDS–PAGE for separation and the gels were then stained with Coomassie Blue. The eNOS band, which was positioned by both protein ladder and purified human eNOS, was cut and digested with trypsin prior to mass spectrometric (MS) analysis. For detailed MS methods, see Supplementary material online.
2.9. Statistical analysis
All values were expressed as mean ± SE. Comparisons between two groups were statistically evaluated by Student's t-test. To compare data from more than two groups, statistical analysis was performed by one-way or two-way repeated measures ANOVA followed by the Bonferroni test. A difference of P < 0.05 was considered statistically significant.
3. Results
3.1. Involvement of eNOS in early IPC
We first examined the role of the NOS/NO signalling pathway in the trigger phase of IPC. Figure 1 shows the time course of LVDP (C), rate-pressure product (RPP) (D), LV end diastolic pressure (LVEDP) (E), and coronary flow (CF) (F) recovery in hearts subjected to 30-min global ischaemia and 30-min reperfusion without or with IPC, and IPC with or without inhibitors (see Figure 1A). Upon reperfusion, improved recovery of post-ischaemic LV function was seen in IPC hearts with much higher LVDP compared with I/R (86 ± 5 vs. 32 ± 5% of pre-ischaemic (PI) value, P < 0.01, n = 7). A greater recovery of LVDP in the IPC group was concurrent with significantly higher RPP (78 ± 6 vs. 28 ± 4% of PI, P < 0.01, n = 7) and markedly attenuated LVEDP (29 ± 4 vs. 93 ± 8 mmHg, P < 0.01, n = 7). CF recovery with IPC was significantly higher than with I/R (89 ± 4 vs. 70 ± 5% of PI, P < 0.01, n = 7). Importantly, all of these improved post-ischaemic functional parameters with IPC were abolished by the trigger-phase administration of the NOS inhibitor l-NAME, whereas l-NAME pretreatment improved functional recovery despite low CF with I/R alone (Supplementary material online, Table S1). Myocardial infarction was measured after 2 h of reperfusion. IPC significantly reduced myocardial infarction compared with I/R (7 ± 1 vs. 28 ± 2%, P < 0.01, n = 7), and l-NAME abrogated this IPC effect with a larger infarct size (18 ± 1%, P < 0.01, n = 7) (Figure 1B).
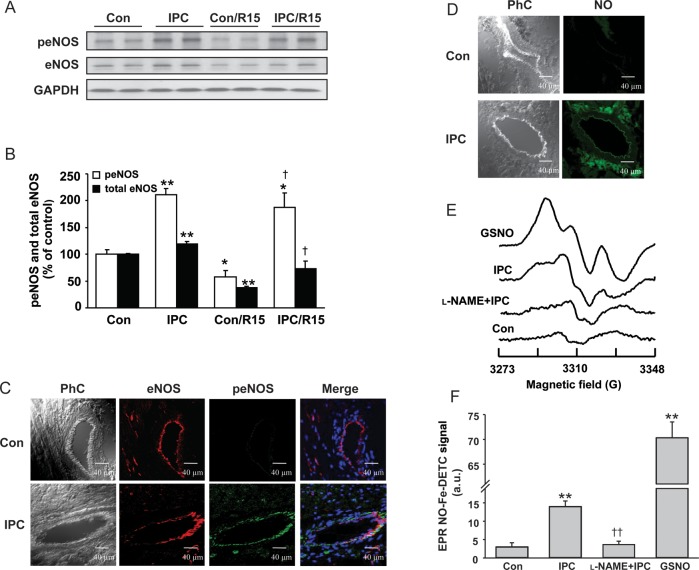
Second, to correlate these findings for eNOS at the molecular level, we evaluated the levels of myocardial total eNOS protein expression and its activation through phosphorylation. Compared with baseline control hearts, IPC stimuli significantly increased both total and phosphorylated eNOS (Figure 2A and B). Importantly, while 30 min of ischaemia followed by 15 min of reperfusion (Con/R15) caused a significant decrease in both total eNOS and phosphorylated eNOS (Figure 2A and B), IPC not only prevented this decrease but also effectively increased the levels of total eNOS and phosphorylated eNOS.
Figure 2.
Activation of eNOS-NO signalling with IPC stimuli. Representative immunoblots (A) and quantification of peNOSSer1176 (peNOS) and total eNOS in hearts (B) subjected to control perfusion (Con), IPC, Con/R15min (30 min ischaemia, 15 min reperfusion) and IPC/R15min (IPC followed by 30 min ischaemia and 15 min reperfusion). Quantitative analyses are expressed as a percentage of control hearts in bar graphs. (C) First column phase contrast images (PhC), second column immunostaining of eNOS (red fluorescence), and third column peNOS (green fluorescence) in hearts subjected to control perfusion and IPC treatment. The fourth column shows the merged peNOS/eNOS image along with 4′,6-diamidino-2-phenylindole (DAPI) staining of the nucleus (blue colour). (D) Fluorescence images of NO (second column, green colour) detected by CuFL (1.0 µM) in heart tissue sections subjected to control perfusion and IPC treatment. (E) EPR spin trapping of NO in hearts using Fe2+-DETC. The signal of the NO-Fe2+-DETC complex was measured from frozen hearts at 77 K. GSNO: In a positive control with GSNO (20 µM) perfusion for 5 min a strong NO-derived triplet signal is seen. IPC: following the IPC trigger phase a smaller but clear NO signal is seen. l-NAME + IPC: in an IPC heart pretreated with l-NAME (1 mM) for 20 min, only trace NO signal is seen. Con: in a normally perfused control heart, little if any detectable NO signal is seen. (F) Mean NO-Fe2+-DETC EPR signal intensities from a series of hearts as described in (E). Values in (A)–(F) are means ± SE. n = 4/group. *P < 0.05, **P < 0.01 vs. control hearts; †P < 0.05 vs. con/R15, ††P < 0.01 vs. IPC group.
Consistent with the Western blot data (Figure 2A and B), confocal immunofluorescence imaging showed an increase in eNOS Ser1176 phosphorylation induced by IPC with this predominantly in the coronary endothelium (Figure 2C). Further studies with the fluorescence NO indicator dye CuFL and the EPR spin trap Fe2+-DETC confirmed that a large increase in NO generation occurred immediately following the trigger phase of IPC, and the NOS inhibitor l-NAME completely blocked this IPC-induced NO generation (Figure 2D–F). Taken together, these findings provide strong evidence that IPC stimuli activate eNOS with NO generation and that this process is essential for early IPC-induced cardioprotection.
3.2. Activation of Akt and PKA following the trigger phase of early IPC
Activation of PI3K/Akt,13 PKA,14 or AMPKα15 has been reported to lead to eNOS activation with Ser1177 phosphorylation and induce cardioprotection; however, it is largely unknown whether these kinases are activated during early IPC. Figure 3A and B shows that IPC caused increased phosphorylation of Akt at Ser473 during the trigger phase compared with control hearts with 2.2-fold increase above control levels. Interestingly, there was an even larger increase in Akt phosphorylation with I/R; and I/R-induced Akt phosphorylation was further enhanced by IPC with values of 220 ± 10% (IPC), 357 ± 34% (Con/R15), 486 ± 37% (IPC/R15) vs. 100 ± 14% (Con; P < 0.01, n = 4). To visualize the cellular localization of IPC-triggered Akt phosphorylation in the heart, immunofluorescence staining of pAkt was performed. The localization of pAkt in the frozen myocardial sections appeared diffuse, with generalized distribution in the cardiac myocytes as well as a distinct focal distribution in the coronary endothelium as shown in Figure 3C.
Figure 3.
Involvement of Akt (A–C) and PKA (D–F) in early IPC. Representative immunoblots of hearts subjected to control perfusion (Con), IPC, Con/R15min and IPC/R15min for pAktSer473 (pAkt) and Akt (A) with quantitative bar graphs of pAkt and Akt in (B). (C) First column phase contrast images (PhC) and second column immunostaining of pAkt (green fluorescence) in hearts subjected to control perfusion and IPC treatment. Representative immunoblots of hearts subjected to Con, IPC, Con/R15min, and IPC/R15min for pPKAThr197 (pPKA) and PKA (D) with quantitative bar graphs of pPKA and PKA in (E). (F) First column phase contrast images (PhC) and second column immunostaining of pPKA (green fluorescence) in hearts subjected to control perfusion and IPC treatment. Values are means ± SE. n = 4/group **P < 0.01 vs. control hearts; †P < 0.05 vs. Con/R15min group.
In addition to the phosphorylation of eNOS (Figure 2) and Akt (Figure 3A–C), IPC also caused marked increase in phosphorylation of PKA at Thr197 during the trigger phase compared with control hearts (343 ± 20 vs. 100 ± 7%, P < 0.01, n = 4; Figure 3D–F). Like Akt, I/R also caused significant PKA phosphorylation [414 ± 20% (Con/R15), vs. 100 ± 7%, P < 0.01, n = 4; Figure 3D and E]; however, IPC had no effect on I/R-induced PKA phosphorylation [408 ± 14% (IPC/R15)] (Figure 3D and E). Immunofluorescence staining of pPKA clearly demonstrated IPC stimuli-induced PKA activation both in the cardiac myocytes and the endothelium (Figure 3F). Interestingly, phosphorylation at Thr172 of AMPKα, another kinase upstream of eNOS, was not increased but rather moderately decreased by IPC stimuli, and no effect was seen of I/R or IPC with I/R on AMPKα phosphorylation (see Supplementary material online, Figure S1).
3.3. Inhibition of PI3K or PKA abrogates cardioprotection
To further verify the activation of Akt and PKA in the IPC trigger phase, we evaluated post-ischaemic myocardial recovery and salvage in the presence of the PI3K/Akt inhibitor LY or the PKA inhibitor H89. Although LY or H89 pretreatment had no significant effects on I/R-induced functional recovery (Supplementary material online, Table S1), LY or H89 pretreatment abolished the IPC effects on post-ischaemic LVEDP and CF, and significantly inhibited the recovery of LVDP and RPP (Figure 1B–F). Importantly, the infarct limiting effect of IPC was abrogated by LY (23 ± 3 vs. 7 ± 1%, P < 0.01, n = 5–7) or H89 (21 ± 3 vs. 7 ± 1%, P < 0.01, n = 5–7).
3.4. Effects of inhibitors on IPC-induced Akt, PKA, and eNOS phosphorylation
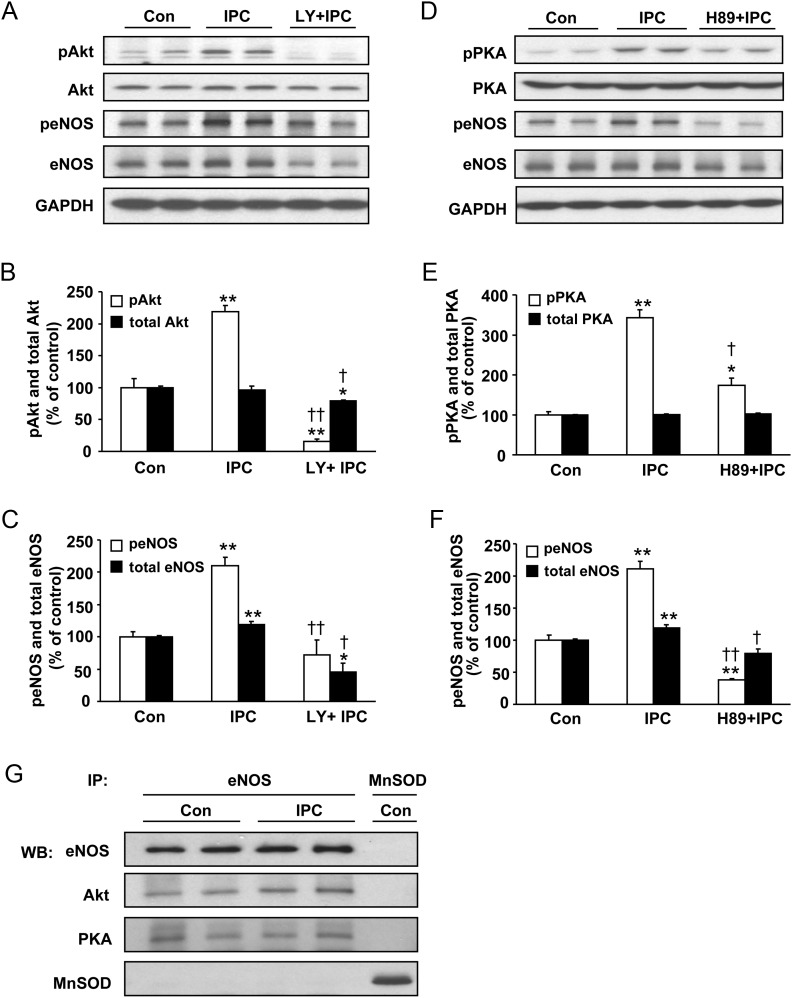
To address whether Akt or PKA activation accounts for eNOS phosphorylation during early IPC, we examined the effects of IPC on eNOS phosphorylation in the absence and presence of pharmacological inhibitors (see Figure 1A). Figure 4A and B shows that pretreatment with LY not only abolished the IPC-induced increase in Akt phosphorylation, but also markedly decreased the level below that of basal Akt phosphorylation seen in control hearts (16 ± 3% with IPC + LY vs. 219 ± 10% with IPC, P < 0.01, n = 4). Importantly, LY abolished the IPC-induced increase in eNOS phosphorylation with only modest non-significant decrease below the basal levels seen in control hearts (73 ± 22% with IPC + LY vs. 211 ± 12% with IPC, P < 0.01, n = 4; Figure 4A and C). LY had a comparable effect on decreasing total eNOS expression (with ∼2.6-fold decrease seen compared with IPC) and this was much greater than its modest effect on Akt expression. Figure 4D and E shows that as expected H89 markedly inhibited IPC-induced PKA phosphorylation (174 ± 18% with IPC + H89 vs. 343 ± 20% with IPC, P < 0.01, n = 4). Importantly, H89 not only largely abolished IPC-induced increase in eNOS phosphorylation (38 ± 3% with IPC + H89 vs. 211 ± 12% with IPC, P < 0.01, n = 4; Figure 4D and F), it also markedly decreased the levels of eNOS phosphorylation to values almost three-fold below the basal levels observed in control hearts. H89 did not have any effect on total PKA levels and for total eNOS it only blocked the IPC-associated increase, lowering eNOS levels to values similar to those in control hearts.
Figure 4.
The effect of inhibition of Akt (A–C) or PKA (D–F) on eNOS phosphorylation following IPC induction; and association of Akt and PKA with eNOS in control and IPC hearts (G). Representative immunoblots of hearts subjected to control perfusion (Con), IPC and IPC + PI3K/Akt inhibitor LY (15 µM) for pAktSer473 (pAkt), Akt, peNOSSer1176 (peNOS) and eNOS (A), with quantitative bar graphs of pAkt and peNOS in (B) and (C). Representative immunoblots of hearts subjected to Con, IPC, and IPC + PKA inhibitor H89 (10 µM) for pPKAThr197 (pPKA), PKA, peNOSSer1176 (peNOS), and eNOS (D), with quantitative bar graphs of pPKA and peNOS in (E) and (F). Values are means ± SE. n = 4/group. *P < 0.05, **P < 0.01 vs. control hearts. †P < 0.05, ††P < 0.01 vs. IPC alone. (G) Immunoprecipitation (IP) of eNOS resulted in co-precipitation of Akt and PKA in heart tissues subjected to control perfusion (Con) or IPC. These data indicate eNOS association with Akt and PKA in control hearts with no change following IPC. A negative control with immunoprecipitation with an MnSOD antibody using similar agarose beads did not pull down any eNOS, Akt, or PKA, whereas MnSOD was pulled down, showing the absence of non-specific association of eNOS, Akt or PKA with the bead. Representative data are shown from three independent experiments.
To study the interactions of these kinases in the induction of early IPC, we measured the total Akt and pAkt in hearts treated with PKA inhibitor H89, and also total PKA and pPKA in hearts treated with PI3K/Akt inhibitor LY. LY slightly reduced IPC-induced pPKA activation without altering total PKA, thus suggesting a possible modest effect of Akt on PKA activation, while H89 had no significant effect on IPC-induced total Akt or pAkt (see Supplementary material online, Figure S2).
3.5. eNOS, Akt, PKA complexation
Since it has been reported that eNOS kinase complexation can occur,13 we performed experiments to determine in control- and IPC-treated hearts if eNOS is complexed with either Akt or PKA. Immunoprecipitation of eNOS from hearts resulted in clear co-precipitation of Akt and PKA (Figure 4G), which suggests that Akt and PKA are bound to eNOS and together form complexes that may play important roles in the process of eNOS activation and cardioprotection induced by IPC. Of note, in control experiments with MnSOD immunoprecipitation from heart homogenate using similar agarose beads, no eNOS, Akt, or PKA bands were seen indicating an absence of non-specific binding.
3.6. Coronary endothelial eNOS function and IPC-induced cardioprotection
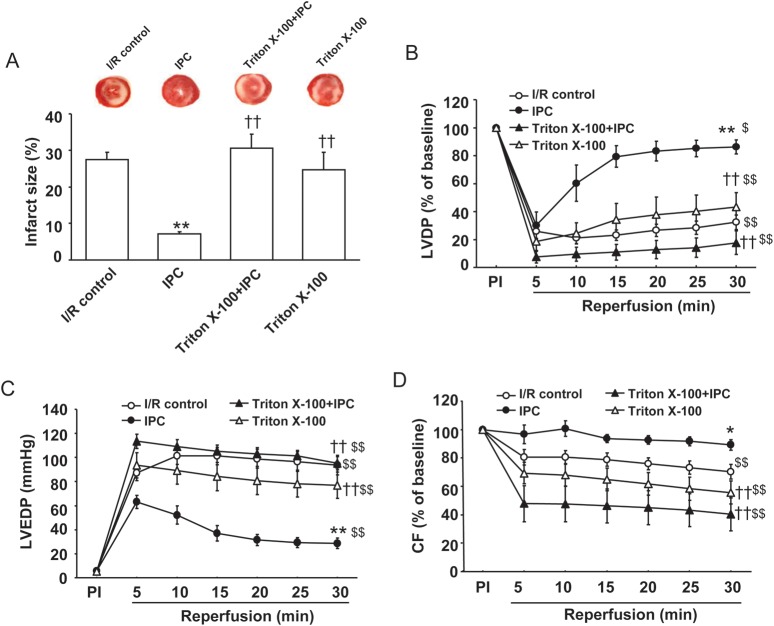
To determine the importance of coronary endothelial eNOS function additional experiments were performed in endothelium-permeabilized hearts, as described previously using brief low level Triton X-100 pretreatment that abolishes eNOS-mediated NO generation with minimal effects on myocardial function.16 Triton X-100 pretreatment abolished the IPC effects on post-ischaemic LVEDP and CF, and significantly inhibited the recovery of LVDP (Figure 5A–D). Importantly, the infarct limiting effect of IPC was abrogated by Triton X-100 (31 ± 4 vs. 7 ± 1%, P < 0.01, n = 6–7). In Triton X-100 pretreated hearts subjected to I/R, functional recovery was similar to that in I/R control hearts (see Supplementary material online, Table S1).
Figure 5.
The effect of coronary endothelium permeabilization on I/R-induced injury and IPC-induced cardioprotection. (A) The infarct size (as described above in Figure 1) for I/R control and IPC protocols in the absence or presence of Triton X-100 (0.25%). (B–D) Post-ischaemic myocardial recovery following 30-min global ischaemia and 30-min reperfusion for I/R control and IPC protocols. LVDP (B) and coronary flow (D) are expressed as a percentage of the pre-ischaemic (PI) value (100%); and LVEDP (C) is expressed as mmHg. Values are means ± SE. n = 5–7/group. *P < 0.05, **P < 0.01 vs. I/R control; ††P < 0.01 vs. IPC group; $P < 0.05, $$P < 0.01 vs. pre-ischaemic (PI) baseline.
3.7. Mapping the molecular sites and prevalence of eNOS phosphorylation
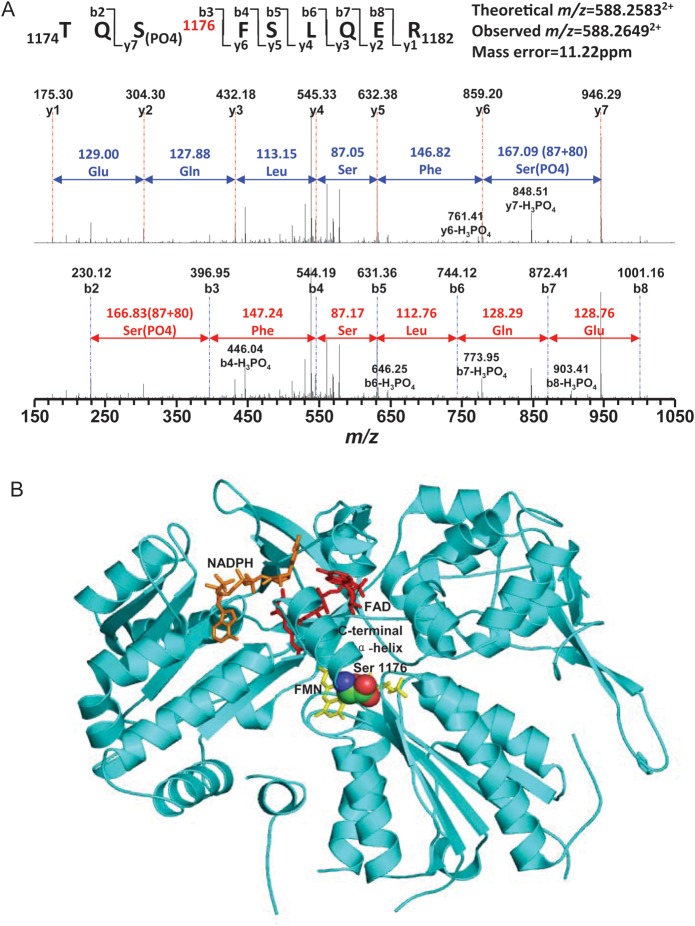
MS/MS can provide mapping of phosphorylation sites in proteins. MS/MS was performed to definitively demonstrate that IPC results in the predicted eNOS modifications. IPC-induced eNOS phosphorylation was detected at Ser1176 that in rat corresponds to Ser1177 of human eNOS (Figure 6A). From the structure of the eNOS reductase as predicted from the homologous crystal structure of the nNOS reductase,18 this site is close to the C-terminus of the protein in close proximity to the flavin mononucleotide (FMN), which is required to transfer electrons to the haeme of the oxygenase domain for NO synthesis (Figure 6B). It can be visualized that these phosphorylations would displace the C-terminal α-helix away from the FMN enabling the enhanced rate of the electron flow to the haeme that has been reported with human eNOS with Ser1177 phosphorylation.19 This is the first direct demonstration that this eNOS modification occurs in the heart and is linked to cardioprotection.
Figure 6.
Mass spectrometric identification of eNOS phosphorylation at Ser1176 in rat hearts subjected to IPC. (A) MS/MS spectrum of doubly protonated molecular ion, m/z = 588.26492+ of amino acids 1174–1182 of the eNOS C-terminal region. Rat eNOS was purified from IPC hearts by immunoprecipitation with eNOS antibody. The phosphorylation site of eNOS at Ser1176 in rat heart was determined from tryptic fragment 1174TQS(PO4)1176FSLQER1182. MW difference of 80 Da is seen between fragment ions y6 and y7 compared with the native fragment ions, which confirmed phosphorylation of Ser1176. The sequence-specific ions were labelled as y and b ions. (B) Molecular model of the eNOS reductase site with phosphorylation at Ser1176 showing its critical position in the C-terminal α-helix close to the critical FAD cofactor.
Further experiments with quantitative immunoblotting of the levels of Ser1176 eNOS compared with that of fully phosphorylated eNOS, prepared by pretreatment with 1000 ng PKA for 30 min at 30 °C, indicated that following IPC induction in the heart ∼24% modification of Ser1176 was present (see Supplementary material online, Figure S3A and B). Thus, prominent modification of eNOS with phosphorylation near the C-terminus is present following induction of IPC.
4. Discussion
The major findings in the present study are that: (i) the IPC trigger stimulus induces distinct eNOS phosphorylation at Ser1176 leading to increased cardiac NO generation that mediates robust early cardioprotection; (ii) this increased eNOS phosphorylation requires both PI3K/Akt and PKA signalling pathways with PI3K/Akt of critical importance in maintaining and increasing eNOS levels; (iii) pharmacological inhibition of PI3K/Akt or PKA pathways not only block their respective phosphorylation sites, but also abolishes myocardial eNOS phosphorylation and abrogates the cardioprotective effects of early IPC; (iv) both Akt and PKA bind to eNOS and are required for its activation in IPC; and (v) induction of cardioprotection is eNOS dependent and intact coronary endothelial eNOS function is required. Thus, these findings demonstrate that eNOS activation secondary to phosphorylation by both Akt and PKA is of critical importance for the induction of early IPC-induced cardioprotection.
4.1. Early IPC and eNOS
The role of eNOS in cardioprotection is well recognized in delayed IPC;3,4 however, there are conflicting reports regarding its role in early IPC.6–9 The role of eNOS in early IPC was first reported in isolated hearts of eNOS-knockout mice,20 with this lowering the preconditioning threshold.7 With consistent reports of attenuated myocardial I/R injury with eNOS overexpressing mice10 or eNOS enhancer,11 there is now increasing evidence for the crucial role of eNOS in early IPC.5–7 Our recent in vivo study has shown that early IPC induces robust cardioprotection in WT mice, but this early IPC-induced protection is absent in eNOS-knockout mice.5 Consistent with these prior observations, our functional, pharmacological, molecular, and confocal imaging data in this study provide comprehensive evidence that eNOS is necessary for triggering early IPC. Recently, in a rat model of in vivo myocardial I/R and early IPC, we observed that IPC preserves the expression of total eNOS and its phosphorylated state in ischaemic myocardium compared with that in I/R.21 Taken together, it is evident that eNOS is activated by IPC stimuli and is required for IPC-induced early cardioprotection.
The main focus of this study was on the role of eNOS and NO in acute IPC. eNOS is the main effector of endothelial cells and eNOS located in the coronary endothelium accounts for most of the NO production in the heart.22 Consistent with our prior study, endothelial permeabilization does not exacerbate I/R injury,16 but it abolished the acute cardioprotective effects of IPC. This is consistent with the effects of the NOS inhibitor l-NAME on I/R, where decreased not increased I/R injury is seen,23–26 but acute IPC is also abolished. Thus, eNOS in the coronary endothelium is activated by IPC stimuli and is required for IPC-induced early cardioprotection.
4.2. eNOS activation and myocardial protection
Studies with delayed IPC3,4 have demonstrated a critical role of eNOS in myocardial salvage against I/R injury; however, the molecular mechanisms of eNOS activation remain unclear. Akt has been implicated as a crucial up-stream effector for eNOS in the regulation of vessel relaxation27 and cardioprotection against I/R injury28. Activation of Akt can be both, beneficial and detrimental depending on the intensity and duration of effects.29 Diverse effects have been reported between normal adaptive/protective responses with two to six-fold increase in Akt activation vs. pathological/maladaptive responses with high level (15–80-fold) Akt activation.29 Interestingly, we observe that Akt activation occurs when there is an event of I/R, and a two to five-fold increase in Akt phosphorylation is seen in whole cardiac homogenate (Figure 3A and B). LY pretreatment abolished the early IPC-induced Akt (Figure 4A and B) and eNOS (Figure 4A and C) phosphorylation concurrent with the loss of IPC-induced post-ischaemic recovery and myocardial salvage (Figure 1), thus suggesting an important role of PI3K/Akt pathway in early IPC-induced cardioprotection.
Despite an important role of Akt in eNOS regulation, other up-stream effectors of eNOS signalling can also be involved in early IPC-induced cardioprotection. Transient accumulation of cyclic AMP in the myocardium during IPC has been reported to activate PKA and limit infarct size.30 PKA-mediated cardioprotection has been attributed to inhibition of calpain, activation of p38MAPK and inactivation of RhoA;30,31 however, the preischaemic administration of Tongxinluo, used clinically to treat acute coronary syndrome, is reported to reduce I/R injury by up-regulating eNOS phosphorylation via PKA.32 Thus, there may be a signalling link between PKA and eNOS in early IPC.
We found that PKA was activated in the trigger phase of early IPC (Figure 3D–F). Importantly, the PKA inhibitor H89 not only prevented PKA phosphorylation (Figure 4D and E), it also abolished the IPC-induced eNOS phosphorylation (Figure 4D and F) and the protective effect of early IPC on post-ischaemic myocardial function and infarction (Figure 1). These findings are consistent with a previous report that PKA was involved in the trigger phase of β1-adrenergic preconditioning.33 Thus, PKA-dependent eNOS activation is involved in the protective effect of early IPC; however, like Akt phosphorylation, the cardioprotective effect of PKA phosphorylation in relation to eNOS activation was seen only with preceding IPC.
4.3. Akt, PKA, and eNOS activation during ischaemia and reperfusion
Our observations raise an interesting question; since increased Akt and increased PKA activation with down stream eNOS phosphorylation and NO production are cardioprotective when triggered during the IPC stimulus, why is increased Akt and PKA activation also seen during control I/R (Figure 3) that is associated with myocardial injury (Figure 1). Similarly, why are the levels of peNOSSer1176 decreased with I/R (Figure 2A and B) despite Akt and PKA activation (Figure 3). The latter observation may be due in part to the more than two-fold loss of eNOS that was observed with I/R. In addition, the loss of cardioprotection may be secondary to superoxide generation during I/R that scavenges NO with the formation of the cytotoxic oxidant peroxynitrite34 and also alters the function of eNOS decreasing NO generation.35
Indeed oxidant stress following I/R is an important mechanism well-documented to cause myocardial injury. I/R greatly stimulates the production of superoxide in the endothelium and cardiac myocytes by pathways including xanthine oxidoreductase and the mitochondrial respiratory chain.36 Furthermore, this primary oxidant stress has been shown to oxidize the essential NOS cofactor tetrahydrobiopterin (BH4), that is required for NO production inducing an ‘uncoupling’ of eNOS, switching eNOS from cardioprotective NO generation to the production of cytotoxic superoxide and secondary reactive oxygen species.37 Following 30 min of cardiac ischaemia over 80% depletion of BH4 has been demonstrated with a profound loss of NOS-dependent endothelial function with switch of the enzyme from NO to superoxide generation.35 Thus, since in the post-ischaemic heart the production of NO from eNOS is decreased with uncoupling to produce superoxide, it is not surprising that the protection conferred with IPC through down stream eNOS activation is lost. Interestingly, we have previously observed that eNOS phosphorylation at Ser1177 not only activates NO production from eNOS with a shift to activation at low calcium levels, but also similarly enhances superoxide production from the uncoupled enzyme when BH4 is depleted as is the case with I/R.38 Therefore, since I/R switches eNOS from NO generation to superoxide production, activation of the enzyme would no longer be expected to exert cardioprotection.
4.4. Role of Akt and PKA in eNOS phosphorylation and cadioprotective signalling
eNOS phosphorylation, is modulated by the balanced actions of protein kinases (Akt, PKA, PKC, AMPK), protein phosphatases (PP1, PP2A), and protein–protein interactions.39,40 Regulation of eNOS by phosphorylation is relatively complex because eNOS activity is increased by phosphorylation at Ser1177 but decreased by Thr495 phosphorylation.39,41 Importantly, regulation of eNOS phosphorylation by specific kinases and phosphatases can vary depending on the activating stimulus and/or the tissue bed.39,42 For instance, PKA signalling has been shown to phosphorylate Ser1177 and dephosphorylate Thr495 in endothelial cells where PP1 and PP2A act selectively at different magnitude to dephosphorylate these sites. In contrast, PKC signalling causes eNOS phosphorylation of Thr495 and dephosphorylation of Ser1177.41 Interestingly, physiological stimuli such as shear stress stimulate eNOS phosphorylation at Ser1177 by Akt-dependent and Akt-independent mechanisms.42,43 Thus, this complexity reflects the importance of precise and optimal control of eNOS phosphorylation/dephosphorylation.
eNOS phosphorylation is a pivotal molecular switch in vasodilation and cardioprotection,44 and a coordinated interaction between Akt and PKA has been shown to regulate eNOS/NO activity in response to physiological stimuli.42 In the present study, inhibition of either PI3K/Akt or PKA abolished IPC-induced post-ischaemic myocardial recovery and salvage (Figure 1), indicating a requirement for both kinase pathways to mediate protection. Importantly, PI3K/Akt inhibition abolished the IPC-induced increase in eNOS expression and phosphorylation with 2.9-fold and 2.5-fold decreases in peNOSSer1176 and total eNOS, respectively (Figure 4A and C). In contrast, PKA inhibition markedly reduced IPC-induced eNOS phosphorylation with 5.6-fold decrease but caused only a modest 28% decrease on total eNOS levels (Figure 4D and F). Thus, it is evident that the activation of PI3K/Akt signalling pathways enhances and maintains the levels of eNOS through inhibition of degradation and/or enhanced synthesis. In contrast, the role of PKA seems to be largely based on the activation of the process of eNOS Ser1176 phosphorylation itself.
Since both Akt and PKA kinase pathways can similarly activate eNOS and are both required to confer cardioprotection there may be cross-talk between them. To this end, we observed that PKA inhibitor H89 did not interfere with IPC-induced Akt phosphorylation (see Supplementary material online, Figure S2B); however, PI3K/Akt inhibitor LY significantly decreased IPC-induced PKA phosphorylation (see Supplementary material online, Figure S2A). Interestingly, our immunoprecipitation studies indicated the presence of complexation of both Akt and PKA with eNOS. This complexation (Figure 4G) was observed in the basal state; and following the IPC trigger this was maintained with increased phosphorylation of both kinases and eNOS. Since, the eNOS/NO signalling pathway has emerged as a central hub for many cardioprotective strategies;1 further studies are needed to address the fundamental basis of the cross-talk between different signalling mechanisms.
4.5. Conclusion
In summary, our findings provide strong evidence that eNOS/NO signalling is crucial for cardioprotection. The specific novel aspects of this study include MS identification and the quantitation of the phosphorylation sites of activated eNOS, localization of Akt and PKA signalling proteins and their sites of activation, and the critical importance of the functional endothelium and NO in acute IPC. Importantly, both Akt and PKA are associated and form a complex with eNOS and mediate eNOS phosphorylation at Ser1176 near the C-terminus of the enzyme leading to its activation with NO production that plays a pivotal role in triggering early IPC-induced cardioprotection. This understanding of the importance and molecular process of eNOS activation in the induction of IPC can now guide efforts to design potent cardioprotective therapies suitable for clinical translation.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institutes of Health grants HL38324, HL63744, and HL65608 to J.L.Z.
Supplementary Material
Acknowledgements
The authors thank Dr Chun-An Chen for and scientific advice and Craig Hemann for technical support.
Conflict of interest: none declared.
References
- 1.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. doi:10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 2.Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann N Y Acad Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. doi:10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- 3.Qiu Y, Rizvi A, Tang XL, Manchikalapudi S, Takano H, Jadoon AK, et al. Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, et al. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. doi:10.1161/01.RES.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 5.Talukder MA, Yang F, Shimokawa H, Zweier JL. eNOS is required for acute in vivo ischemic preconditioning of the heart: effects of ischemic duration and sex. Am J Physiol Heart Circ Physiol. 2010;299:H437–H445. doi: 10.1152/ajpheart.00384.2010. doi:10.1152/ajpheart.00384.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muscari C, Bonafe F, Gamberini C, Giordano E, Tantini B, Fattori M, et al. Early preconditioning prevents the loss of endothelial nitric oxide synthase and enhances its activity in the ischemic/reperfused rat heart. Life Sci. 2004;74:1127–1137. doi: 10.1016/j.lfs.2003.10.001. doi:10.1016/j.lfs.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Bell RM, Yellon DM. The contribution of endothelial nitric oxide synthase to early ischaemic preconditioning: the lowering of the preconditioning threshold. An investigation in eNOS knockout mice. Cardiovasc Res. 2001;52:274–280. doi: 10.1016/s0008-6363(01)00394-7. doi:10.1016/S0008-6363(01)00394-7. [DOI] [PubMed] [Google Scholar]
- 8.Weselcouch EO, Baird AJ, Sleph P, Grover GJ. Inhibition of nitric oxide synthesis does not affect ischemic preconditioning in isolated perfused rat hearts. Am J Physiol. 1995;268:H242–H249. doi: 10.1152/ajpheart.1995.268.1.H242. [DOI] [PubMed] [Google Scholar]
- 9.Nakano A, Liu GS, Heusch G, Downey JM, Cohen MV. Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischemic preconditioning. J Mol Cell Cardiol. 2000;32:1159–1167. doi: 10.1006/jmcc.2000.1152. doi:10.1006/jmcc.2000.1152. [DOI] [PubMed] [Google Scholar]
- 10.Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, et al. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:1517–1523. doi: 10.1161/01.ATV.0000224324.52466.e6. doi:10.1161/01.ATV.0000224324.52466.e6. [DOI] [PubMed] [Google Scholar]
- 11.Frantz S, Adamek A, Fraccarollo D, Tillmanns J, Widder JD, Dienesch C, et al. The eNOS enhancer AVE 9488: a novel cardioprotectant against ischemia reperfusion injury. Basic Res Cardiol. 2009;104:773–779. doi: 10.1007/s00395-009-0041-3. doi:10.1007/s00395-009-0041-3. [DOI] [PubMed] [Google Scholar]
- 12.Szelid Z, Pokreisz P, Liu X, Vermeersch P, Marsboom G, Gillijns H, et al. Cardioselective nitric oxide synthase 3 gene transfer protects against myocardial reperfusion injury. Basic Res Cardiol. 2010;105:169–179. doi: 10.1007/s00395-009-0077-4. doi:10.1007/s00395-009-0077-4. [DOI] [PubMed] [Google Scholar]
- 13.Bellis A, Castaldo D, Trimarco V, Monti MG, Chivasso P, Sadoshima J, et al. Cross-talk between PKA and Akt protects endothelial cells from apoptosis in the late ischemic preconditioning. Arterioscler Thromb Vasc Biol. 2009;29:1207–1212. doi: 10.1161/ATVBAHA.109.184135. doi:10.1161/ATVBAHA.109.184135. [DOI] [PubMed] [Google Scholar]
- 14.Inserte J, Garcia-Dorado D, Ruiz-Meana M, Agullo L, Pina P, Soler-Soler J. Ischemic preconditioning attenuates calpain-mediated degradation of structural proteins through a protein kinase A-dependent mechanism. Cardiovasc Res. 2004;64:105–114. doi: 10.1016/j.cardiores.2004.06.001. doi:10.1016/j.cardiores.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjoquist PO, et al. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. doi:10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 16.Giraldez RR, Panda A, Zweier JL. Endothelial dysfunction does not require loss of endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2000;278:H2020–H2027. doi: 10.1152/ajpheart.2000.278.6.H2020. [DOI] [PubMed] [Google Scholar]
- 17.Wei G, Dawson VL, Zweier JL. Role of neuronal and endothelial nitric oxide synthase in nitric oxide generation in the brain following cerebral ischemia. Biochim Biophys Acta. 1999;1455:23–34. doi: 10.1016/s0925-4439(99)00051-4. doi:10.1016/S0925-4439(99)00051-4. [DOI] [PubMed] [Google Scholar]
- 18.Knudsen GM, Nishida CR, Mooney SD, Ortiz de Montellano PR. Nitric-oxide synthase (NOS) reductase domain models suggest a new control element in endothelial NOS that attenuates calmodulin-dependent activity. J Biol Chem. 2003;278:31814–31824. doi: 10.1074/jbc.M303267200. doi:10.1074/jbc.M303267200. [DOI] [PubMed] [Google Scholar]
- 19.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. doi:10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 20.Wei G, Zweier JL. Endothelial nitric oxide synthase is essential for actute ischemic preconditioning. FASEB J. 2001;15:A465. [Google Scholar]
- 21.Zweier JL, Yang F, Nishijima Y, Chen CA, Yang C, Varadharaj S, et al. Novel rapid-multiple-short-cycle preconditioning stimuli induces robust cardioprotective signaling mechanisms and protects the heart against in vivo ischemia reperfusion injury: an effective approach with clinical justification. FASEB J. 2012;26:1136. [Google Scholar]
- 22.Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. doi:10.1161/01.RES.79.3.363. [DOI] [PubMed] [Google Scholar]
- 23.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. doi:10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 24.Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. doi:10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- 25.Csonka C, Szilvassy Z, Fulop F, Pali T, Blasig IE, Tosaki A, et al. Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation. 1999;100:2260–2266. doi: 10.1161/01.cir.100.22.2260. doi:10.1161/01.CIR.100.22.2260. [DOI] [PubMed] [Google Scholar]
- 26.Genade S, Genis A, Ytrehus K, Huisamen B, Lochner A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J Pineal Res. 2008;45:449–458. doi: 10.1111/j.1600-079X.2008.00615.x. doi:10.1111/j.1600-079X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 27.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, et al. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. doi:10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. doi:10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. doi:10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanada S, Asanuma H, Tsukamoto O, Minamino T, Node K, Takashima S, et al. Protein kinase A as another mediator of ischemic preconditioning independent of protein kinase C. Circulation. 2004;110:51–57. doi: 10.1161/01.CIR.0000133390.12306.C7. doi:10.1161/01.CIR.0000133390.12306.C7. [DOI] [PubMed] [Google Scholar]
- 31.Sadat U. Signaling pathways of cardioprotective ischemic preconditioning. Int J Surg. 2009;7:490–498. doi: 10.1016/j.ijsu.2009.06.004. doi:10.1016/j.ijsu.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Li XD, Yang YJ, Geng YJ, Jin C, Hu FH, Zhao JL, et al. Tongxinluo reduces myocardial no-reflow and ischemia-reperfusion injury by stimulating the phosphorylation of eNOS via the PKA pathway. Am J Physiol Heart Circ Physiol. 2010;299:H1255–H1261. doi: 10.1152/ajpheart.00459.2010. doi:10.1152/ajpheart.00459.2010. [DOI] [PubMed] [Google Scholar]
- 33.Robinet A, Hoizey G, Millart H. PI 3-kinase, protein kinase C, and protein kinase A are involved in the trigger phase of beta1-adrenergic preconditioning. Cardiovasc Res. 2005;66:530–542. doi: 10.1016/j.cardiores.2005.02.010. doi:10.1016/j.cardiores.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. doi:10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 35.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, et al. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. doi:10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci USA. 1988;85:4046–4050. doi: 10.1073/pnas.85.11.4046. doi:10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. doi:10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 38.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. doi:10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. doi:10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry. 2002;41:15845–15853. doi: 10.1021/bi026732g. doi:10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 41.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. doi:10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 42.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. doi:10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 43.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. doi:10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 44.Kukreja RC, Xi L. eNOS phosphorylation: a pivotal molecular switch in vasodilation and cardioprotection? J Mol Cell Cardiol. 2007;42:280–282. doi: 10.1016/j.yjmcc.2006.10.011. doi:10.1016/j.yjmcc.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.