Abstract
Aims
Reactive oxygen species (ROS)-mediated intracellular signalling is well described in the vasculature, yet the precise roles of ROS in paracrine signalling are not known. Studies implicate interstitial ROS hydrogen peroxide (H2O2) in vascular disease, and plasma H2O2 levels in the micromolar range are detectable in animal models and humans with hypertension. Recently, H2O2 was shown to cross biological membranes of non-vascular cells via aquaporin (Aqp) water channels. Previous findings suggest that H2O2 activates NADPH oxidase (Nox) enzymes in vascular cells and apoptosis signal-regulating kinase 1 (Ask1) in non-vascular cells. We hypothesized that extracellular H2O2 induces smooth muscle cell (SMC) hypertrophy by a mechanism involving Aqp1, Nox1, and Ask1.
Methods and results
Treatment of rat aortic SMCs (rASMC) with exogenous H2O2 resulted in a concentration-dependent increase in Nox-derived superoxide (O2•–), determined by L-012 chemiluminescence, cytochrome c and electron paramagnetic resonance. Nox1 was verified as the source of O2·– by siRNA. Aqp1 siRNA attenuated H2O2 cellular entry and H2O2-induced O2•– production. H2O2 treatment increased Ask1 activation and induced rASMC hypertrophy in a Nox1-dependent mechanism. Adenoviral-dominant-negative Ask1 attenuated H2O2-induced rASMC hypertrophy and adenoviral overexpression of Ask1 augmented it.
Conclusion
Our results demonstrate for the first time that extracellular H2O2, at pathophysiological concentrations, stimulates rASMC Nox1-derived O2•–, subsequent Ask1 activation and SMC hypertrophy. The data demonstrate a novel pathway by which H2O2 enters vascular cells via aquaporins and activates Nox, leading to hypertrophy, and provide multiple novel targets for combinatorial therapeutics development targeting hypertrophy and vascular disease.
Keywords: NADPH oxidase, Hydrogen peroxide, Aquaporin, Ask1, Hypertrophy
1. Introduction
Reactive oxygen species (ROS) and their cellular sources have been the focus of intense study in recent years. ROS mediate cell and tissue oxidative stress and damage, as well as participate in key signalling pathways, leading to vascular remodelling, cell migration, and differentiation.1,2 However, the role of ROS as paracrine modulators of oxidative stress and signalling has largely gone unstudied. Of the various ROS, hydrogen peroxide (H2O2) is most likely the best candidate for effecting paracrine signalling attributed to its stability and relative diffusibility.2 Indeed, previous data from our laboratory indicate H2O2-mediated feed-forward signalling between the vascular adventitia and medial smooth muscle cells (SMCs) resulting in local ROS production and SMC hypertrophy.3,4
Being a relatively stable ROS, H2O2 is thought to partition into and freely cross membranes and thus pervade cells and tissue in a relatively facile manner. This widely accepted notion was recently challenged in a report showing a role for previously defined water channels, aquaporins, in H2O2 diffusion into HEK 293 and HeLa cells.5 Aquaporins are ubiquitously expressed across species from yeast to humans.6 It is unknown, however, whether these channels function as conduits for H2O2 transport across vascular cells.
The NADPH oxidase (Nox) enzyme family is widely recognized as a robust and critical source of the ROS superoxide anion (O2•–) in the vasculature,1 and as such, is a plausible target of H2O2-mediated feed-forward signalling. However, it is not known whether physiologically relevant concentrations of H2O2 (i.e. as low as 5 µM that are found in animal and human studies of vascular disease) activate Nox isozymes.
Interestingly, the mediators of paracrine H2O2-induced SMC signalling and dysfunction are also still poorly described. It is well accepted that Nox-derived ROS activate p38 MAPK, which is pivotal in SMC hypertrophy.7 In this study, we postulated that H2O2 induces SMC Nox1, which, in turn, activates apoptosis signal-regulating kinase 1 (Ask1), a heretofore described upstream activator of p38 MAPK.8 Ask1 is a viable but never before studied activator of vascular hypertrophy. In summary, our findings are the first to our knowledge to draw a link between extracellular H2O2 entering vascular SMCs via aquaporin 1 (Aqp1) channels and stimulating vascular Nox-derived ROS, which in turn activate Ask1 and hypertrophy. These findings provide multiple novel therapeutic targets aimed at suppressing or abrogating vascular SMC hypertrophy.
2. Methods
Detailed methods are provided in the Supplementary material online.
2.1. Materials
A detailed list of materials used is included in the Supplementary material online.
2.2. Cell culture
Rat aortic smooth muscle cells (rASMC) were purchased from Lonza (Lonza Cologne GmbH, Cologne, Germany). Cells were maintained in DMEM supplemented with 10% FBS and containing 100 U/mLpenicillin and 100 µg/mL streptomycin (Invitrogen). Cells were cultured at 37°C with 5% CO2. Cells between passages 2–9 were used.
2.3. O2•– detection
2.3.1. Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) spin probe CMH (50 μM) was used to examine O2•– production using a Bruker eScan Table-Top EPR spectrometer (Bruker Biospin, USA). 1 × 106 cells/mL were resuspended in Krebs–HEPES buffer (pH 7.4) treated with Chelex resin and containing 25 μM deferoxamine to minimize the deleterious effects of contaminating metals. Spectra were obtained after 1, 10, and 20 min at 37°C.
2.3.2. L-012 chemiluminescence
Cells on white clear-bottom 96-well culture plates were stimulated with varying concentrations of H2O2 for 10–300 min. The media was replaced with PBS (pH 7.4) supplemented with CaCl2 (0.9 mM) and MgSO4 (0.49 mM) and luminol derivative L-012 (400 µM) was added and chemiluminescence measured in a BioTek Synergy 4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA) for at least 30 min post-H2O2 stimulation times. O2•– production was quantified as relative light units (RLU).
2.3.3. Cytochrome c reduction assay
The cytochrome c assay was conducted as described previously.9 Briefly, cells were treated with 50 μM H2O2 for 1 h, lysed and 28 000 × g membrane fraction prepared. Membrane fractions were mixed with cytochrome c. After 5 min baseline measurement, NADPH was added and O2•– production calculated from the initial linear rate of superoxide dismutase (SOD)-inhibitable cytochrome c reduction (at 550 nm; extinction coefficient 21.1 mM−1cm−1).
2.4. Live-cell imaging confocal microscopy of HyPer fluorescence
Cells on coverslip bottomed dishes (MatTek, Ashland, MA, USA) were mounted in a temperature controlled chamber (Tokai Hit, Japan) atop the motorized stage of a Nikon TiE inverted fluorescent microscope with a ×60, 1.4 NA optic (Nikon, Melville, NY, USA). HyPer was excited by 438 and 513 nm (SpectraX, Lumencor, Beaverton OR, USA) and detected using 525/50 nm bandpass filter (Chroma Technology), C11440–22C camera (Hamamatsu), and NIS Elements software. The ratios (513/438) were calculated for one to three cells per stage position, 10 stage positions per plate per experiment.
2.5. siRNA transfection to suppress Nox1 or Aqp1
Cells were grown to 30–50% confluence on 100 mm, 6-well or 96-well plates and were transfected with scrambled siRNA or three distinct variants of siRNA (5 pmol) against Nox1 or Aqp1 using the transfection reagent Lipofectamine 2000 according to the manufacturer's protocol. Cells were assayed 48 h later.
2.6. Adenoviral transduction for Ask1 gain- and loss-of-function
Adenoviruses for green fluorescent protein (GFP), transgenic (Tg)-, or dominant-negative (DN)-Ask1 were added to 50% confluent cells at a multiplicity of infection (MOI) of 10 and incubated for 24 h at 37°C. For experiments in which siRNA was applied, adenoviruses were added 24 h post-siRNA transfection.
2.7. Quantitative PCR
First cDNA was prepared using 1–2 µg of total RNA (SuperScript™ First-Strand, Invitrogen). Then samples were mixed with primer/probe for Nox1, Nox4, Aqp1, or 18S in 384-well plate (TaqMan® Universal PCR Master Mix, ABI- Applied Biosystems, Inc., Foster City, CA, USA) and qPCR performed in a 7900HT Fast Real-Time PCR System (ABI) for 40 cycles. Relative quantification was obtained using the Ct (threshold cycle) method:
 |
Relative expression was calculated as 2−ΔΔCt.
2.8. Western blot
Cells were lysed with RIPA buffer and SDS–PAGE performed. Membranes were incubated with total Ask1 (Cell Signaling) or phospho-Ask1 (threonine-845, a kind gift from Dr Hidenori Ichijo, University of Tokyo, Japan) antibodies (1:500 dilution). Membranes were probed with goat anti-mouse or goat anti-rabbit secondary antibodies (1:10 000 dilution, Li-Cor Biosciences). Digital imaging was obtained (Odyssey Infra-Red Imaging system, Li-Cor).
2.9. Quantification of cell hypertrophy
Eighty per cent confluent rASMCs were treated with 50 μM H2O2 for 24 h then separated into two equal volumes, one was lysed for protein quantification and the other was lysed for DNA quantification (Hoechst-33258, Invitrogen). Hypertrophy was determined as the protein:DNA ratio normalized to control.
2.10. FACS analysis
Flow cytometry was performed on a BD LSRII cytometer (BD Biosciences). rASMCs were treated as above. Forward scatter (FS) and side scatter (SS) were measured (10 000 events/sample). Quantification was performed using FloJo. Density plots were gated to exclude debris and then quadrant parameters were selected based on the size of 80–85% of cells from control groups. The percentage of events recorded in Quadrant 2 (FS +, SS +) were quantified for each group and taken as a ratio of Quadrant 2 events percentage in control groups.
2.11. Thymidine incorporation
Radiolabelled thymidine incorporation was performed as previously described.10 Briefly, 70–80% confluent rASMCs were incubated with 1 μCi/mL [3H] ± 50 μM H2O2 for 24 h at 37°C, and exposed to 10% trichloroacetic acid for 10 min. Monolayers were then dissolved in 1 N NaOH for radioactivity measurement.
2.12. Cell size measurement
rASMCs were treated as above with 50 μM H2O2 for 24 h at 37°C. Images were captured using a Nikon Eclipse TS100 microscope connected to a Nikon D90 SLR digital camera at an objective lens magnification of ×10. Three to six images were taken per treatment group per experiment. The areas of four individual cells selected at random per image were calculated using Image J (NIH, USA). Areas were then averaged and taken as a ratio of control.
2.13. Statistical analyses
Data are presented as means ± SEM. Data comparisons were performed with Student's t-test, one- or two-way-ANOVA including Bonferroni post hoc analysis. Differences were deemed statistically significant at P < 0.05.
3. Results
3.1. H2O2 activates O2•– production in rASMC
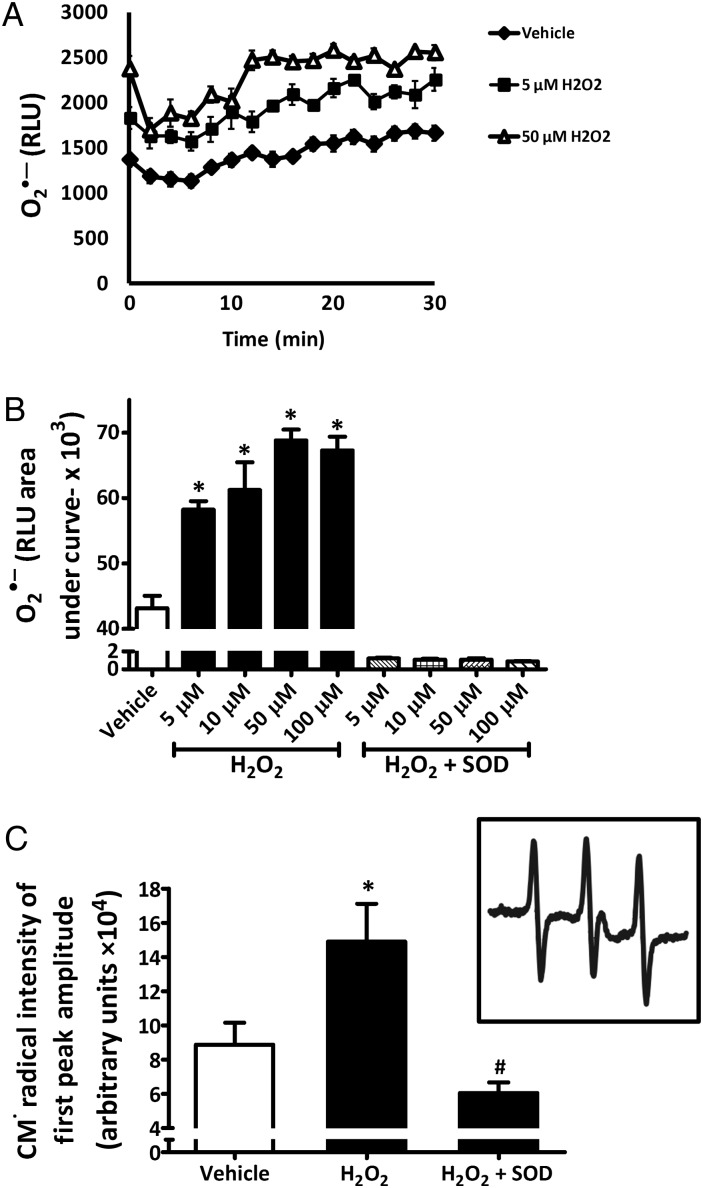
Treatment for 1 h with concentrations as low as 5 μM H2O2 significantly stimulated production of O2•– as demonstrated by an increase in L-012 chemiluminescence. The other concentrations of H2O2 used (10, 50, and 100 µM) also resulted in a significant increase in L-012 chemiluminescence, with the maximal signal observed with 50 µM treatment (Figure 1A and B). Treatment with 50 μM H2O2 for 10–300 min revealed a rise in O2•– levels as early as 10 min post-treatment that was sustained for 3 h (data not shown). SOD treatment (300 U/mL) confirmed this signal as O2•– dependent (Figure 1B). These results were also confirmed by EPR studies using the spin probe CMH, where an increase in CM-radical intensity, indicative of O2•–, was seen in response to 50 μM H2O2 when compared with vehicle-treated cells and this was abolished with the addition of 300 U/mL SOD (Figure 1C). Interestingly, treatment of rASMC with 1 µM H2O2 for 1 h did not stimulate O2•– production (cytochrome c assay; Figure S1, Supplementary material online).
Figure 1.
Exogenous H2O2 increases O2•– production in rASMC. (A) Representative trace of L-012 chemiluminescence over time after rASMC treatment with indicated concentrations of H2O2 ± superoxide dismutase (SOD, 300 U/mL). (B) Quantification of all the areas under the curve (AUC) in (A) in relative light units (RLU). (C) Quantification of first-peak amplitudes of CM• radical EPR spectra of rASMC treated with vehicle or 50 μM H2O2 ± SOD (300 U/mL). Inset: representative EPR spectrum of rASMC treated with 50 μM H2O2. Means ± SEM, n = 3, *P < 0.05 vs. vehicle; #P < 0.05 vs. H2O2.
3.2. H2O2-induced O2•– in rASMC is Nox1 derived
To identify the source of O2•– produced in response to H2O2, we used a spectrum of pharmacological inhibitors for cellular sources of O2•–. Prior to 50 μM H2O2 treatment, cells were incubated for 30 min with mitochondrial complex I inhibitor rotenone (50 µM), nitric oxide synthase (NOS) inhibitor l-NAME (100 µM), xanthine oxidase inhibitor febuxostat (100 nM), cyclooxygenase inhibitor indomethacin (10 µM), or flavoprotein inhibitor diphenylene iodonium (DPI) (5 µM). Figure S2, Supplementary material online, shows that only DPI was effective at significantly inhibiting O2•– production in response to H2O2 (77 ± 14% inhibition). Treatment with 300 U/mL SOD or cell-permeant SOD mimetic Tiron (10 mM) both resulted in complete inhibition of the signal (98 ± 0.2 and 99 ± 0.1% inhibition, respectively; Figure S2, Supplementary material online). No significant inhibition was observed with rotenone, l-NAME, febuxostat, or indomethacin.
The Nox isoforms expressed in rodent aortic vascular SMCs are Nox1 and Nox4 with Nox1 appearing to be the isoform most likely involved in O2•– production.1 We acknowledge that some reports have suggested that Nox4 could liberate O2•– under certain conditions.11 However, considering the controversy leaning towards greater H2O2 vs. O2•– production by Nox4, the most likely source of O2•– in these cells is Nox1. Thus, to interrogate the role of Nox1 in H2O2-induced O2•– in rASMC, three distinct siRNA sequences against Nox1 were used to ensure knockdown of Nox1 mRNA. To test for specificity, rASMCs were transfected with each siRNA and assessed for Nox1 and Nox4 mRNA expression by PCR 48 h later. Figure S3, Supplementary material online, shows that both siRNA oligonucleotides (oligo) 1 and 2 were effective at reducing Nox1 mRNA (to 45 ± 6 and 34 ± 10% from scrambled siRNA-transfected cells, respectively) without affecting Nox4 mRNA levels. Oligo 3 did not result in a significant reduction in Nox1 mRNA when compared with scrambled siRNA-transfected cells. Since Nox1 mRNA knockdown was greatest with the use of oligo 2, this sequence was selected for the remainder of studies in this manuscript (Figure S3, Supplementary material online).
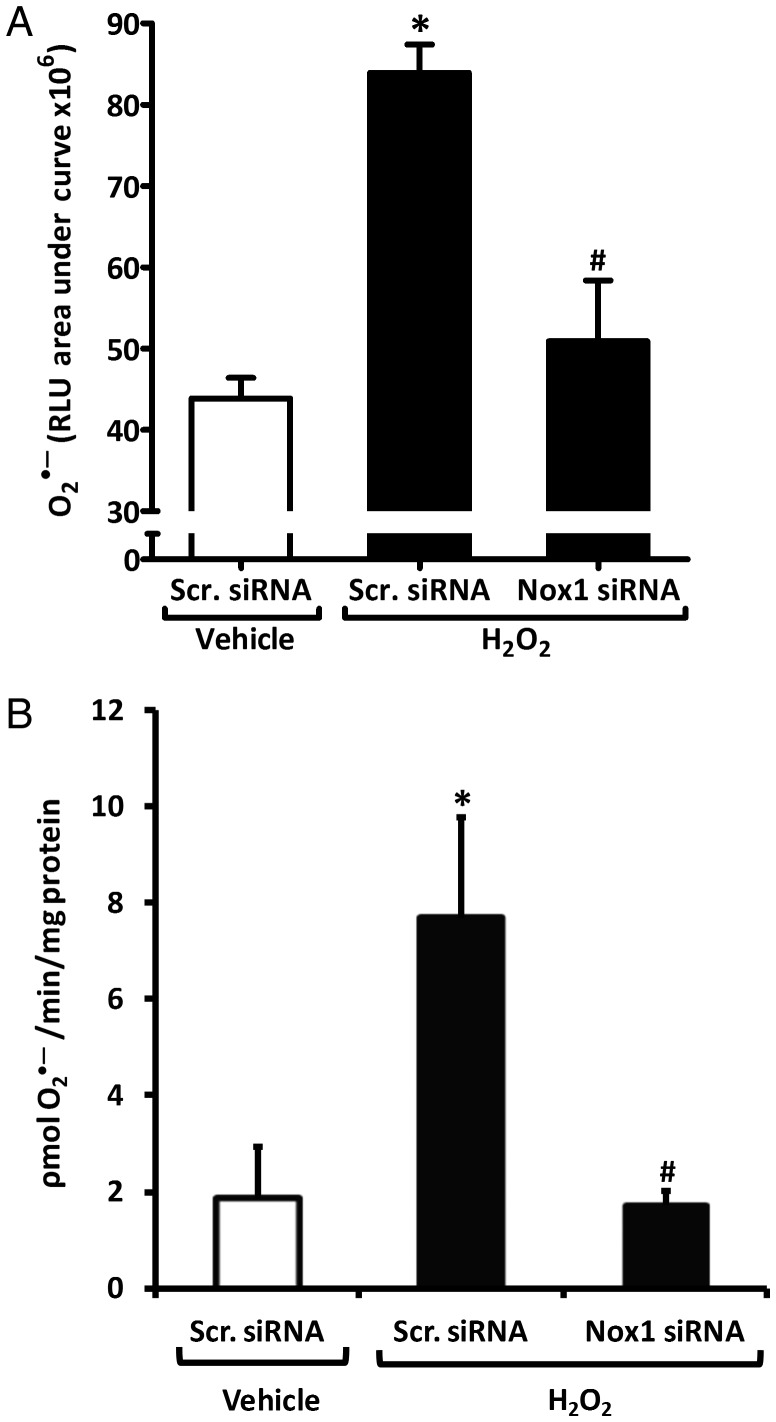
Scrambled siRNA control-transfected rASMCs treated with H2O2 exhibited an approximate doubling of O2•– levels as measured by L-012 chemiluminescence compared with scrambled siRNA-transfected, vehicle-treated cells (84 ± 3 vs. 44 ± 3 × 106 RLU, respectively; Figure 2A). Nox1-siRNA (oligo 2)-transfected rASMC displayed a significant 80% reduction in H2O2-induced O2•– (51 ± 7 × 106 RLU) compared with scrambled siRNA-transfected cells treated with H2O2. No significant difference was detected between Nox1-siRNA-transfected H2O2-treated cells and scrambled siRNA-transfected vehicle-treated cells (Figure 2A). Cytochrome c assay of rASMC membrane fractions confirmed these results (Figure 2B). That is, O2•– levels rose from 2 ± 1 to 8 ± 2 pmol/min/mg protein in H2O2-treated scrambled siRNA-transfected rASMCs compared with vehicle-treated scrambled siRNA-transfected cells; Nox1 siRNA abolished this stimulation (2 ± 0.3 pmol/min/mg protein). H2O2 treatment did not affect Nox1 mRNA levels (Figure S4A and B, Supplementary material online).
Figure 2.
Nox1 is the enzymatic source of H2O2-induced O2•– in rASMC. (A) AUC of L-012 chemiluminescence of rASMC transfected with scrambled (Scr.) or Nox1 siRNA and treated with vehicle or 50 μM H2O2. Means ± SEM, n = 12, *P < 0.001 vs. Scr. siRNA vehicle, #P < 0.001 vs. Scr. siRNA H2O2. (B) O2•– production in pmol/min/mg protein quantified by SOD-inhibitable cytochrome c reduction in membrane fractions of rASMC transfected and treated as in (A). Means ± SEM, n = 4, *P < 0.05 vs. Scr. siRNA vehicle, #P < 0.05 vs. Scr. siRNA H2O2.
3.3. Aqp1 is involved in mediating H2O2-induced O2•– production by facilitating H2O2 entry into rASMC
qPCR demonstrated that only Aqp1 is expressed in rASMC. Neither Aqp3 nor Aqp8 was expressed (n = 3 independent experiments, data not shown). To investigate the role of Aqp1, three distinct siRNAs for Aqp1 were tested for the efficacy of mRNA knockdown by qPCR in rASMC (Figure S5, Supplementary material online). Two of the three siRNA sequences, oligo 2 and oligo 3, resulted in a significant reduction in Aqp1 mRNA when compared with scrambled siRNA-transfected cells (down to 38 ± 2 and 37 ± 2% from scrambled siRNA-transfected cells, respectively). Oligo 1 did not result in a significant reduction in Aqp1 mRNA. Aqp1 oligo 3 was chosen for use in the remainder of studies in this manuscript. The Aqp1 siRNAs did not appear to have any effect on the cell shape or size when compared with scrambled siRNA controls.
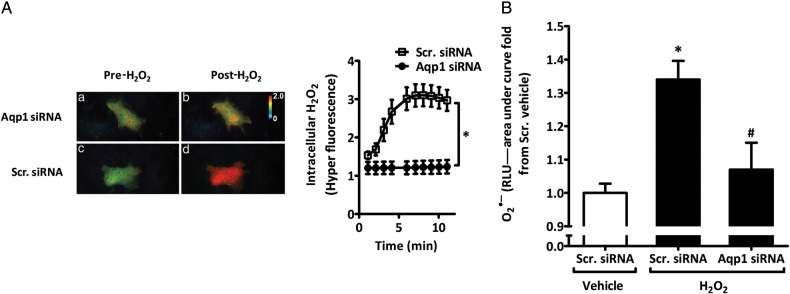
To evaluate whether Aqp1 facilitates H2O2 transport into rASMC, cells were cotransfected with scrambled or Aqp1 siRNA and the highly specific and sensitive genetically encoded intracellular H2O2 fluorescent probe HyPer.5 Live cell imaging was then conducted prior and post-H2O2 addition. As can be seen in Figure 3A, the addition of H2O2 to rASMC resulted in a rapid three-fold increase in HyPer fluorescence, indicating uptake of H2O2. Aqp1 siRNA blocked this increase in HyPer fluorescence.
Figure 3.
Aqp1 is important for H2O2 entry into rASMC and induction of O2•–. (A) Live-cell imaging of changes in HyPer fluorescence of rASMC expressing HyPer plus Scr. or Aqp1 siRNA before (pre) or after (post) treatment with vehicle or 50 μM H2O2. Right panel shows the quantification of HyPer signal vs. time. Data shown as means ± SEM of 10 stage positions per condition, representative of three independent experiments, *P < 0.001 vs. Scr. siRNA. (B) AUC of L-012 chemiluminescence expressed as fold change from Scr. siRNA vehicle of rASMC transfected with Scr. or Aqp1 siRNA and treated with vehicle or 50 μM H2O2. Means ± SEM, n = 40, *P < 0.001 vs. Scr. siRNA vehicle, #P < 0.01 vs. H2O2 Scr. siRNA.
Transfection of rASMC with Aqp1 siRNA effectively and significantly reduced H2O2-induced O2•– relative to scrambled siRNA-transfected rASMC cells treated with H2O2 (1.07 ± 0.08- vs. 1.34 ± 0.06-fold from vehicle-treated scrambled siRNA-transfected cells, respectively; Figure 3B). We observed no significant difference between levels of O2•– in vehicle controls transfected with scrambled siRNA and H2O2-treated rASMCs transfected with Aqp1 siRNA. H2O2 treatment did not affect Aqp1 mRNA levels (Figure S4C, Supplementary material online). Knockdown of Aqp1 mRNA by Aqp1 siRNA had a modest effect (14 ± 0.03% reduction) on Nox1 protein (Figure S6, Supplementary material online).
3.4. H2O2 induces Nox1-dependent phosphorylation of Ask1 in rASMC
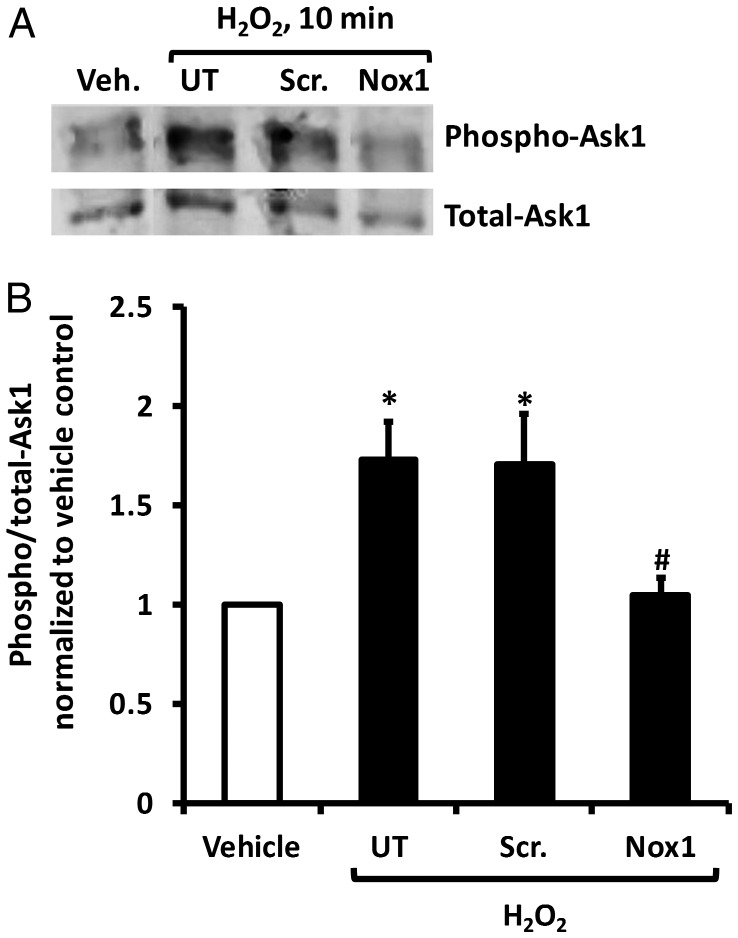
Ask1 is activated by phosphorylation at threonine-845.12,13 To assess Ask1 activation in rASMC, cells were transfected with scrambled or Nox1 siRNA and treated with H2O2. H2O2 treatment resulted in an increase in Ask1 phosphorylation that was reversed by Nox1 siRNA but not by scrambled siRNA (Figure 4A). Cumulative results confirm these findings (Figure 4B). No difference in the ratio of phospho:total Ask1 was observed between untransfected (UT) and scrambled siRNA-transfected cells treated with H2O2. Similarly, no difference was observed in the phospho:total Ask1 ratio between UT vehicle-treated cells and Nox1-siRNA-transfected H2O2-treated cells (Figure 4B). Transfection with scrambled siRNA had no effect on Ask1 phosphorylation in vehicle-treated cells (data not shown). To support these findings, these experiments were repeated in rASMC adenovirally overexpressing Ask1 yielding similar results (Figure S7, Supplementary material online).
Figure 4.
H2O2 induces Ask1 phosphorylation in rASMC in a Nox1-dependent mechanism. (A) Representative Western blot for phospho-Ask1 (threonine-845) and total-Ask1 of lysates of rASMC transfected with Scr. or Nox1 siRNA and treated with 50 μM H2O2 (UT, untransfected cells). (B) The quantification of fluorescence intensity of phospho-Ask1 bands obtained in (A) normalized to total-Ask1. Means ± SEM, n = 6, *P < 0.05 vs. vehicle; #P < 0.05 vs. H2O2 Scr. siRNA.
3.5. H2O2 treatment results in increased hypertrophy, but not proliferation of rASMC, in a mechanism involving Aqp1, Nox1, and Ask1
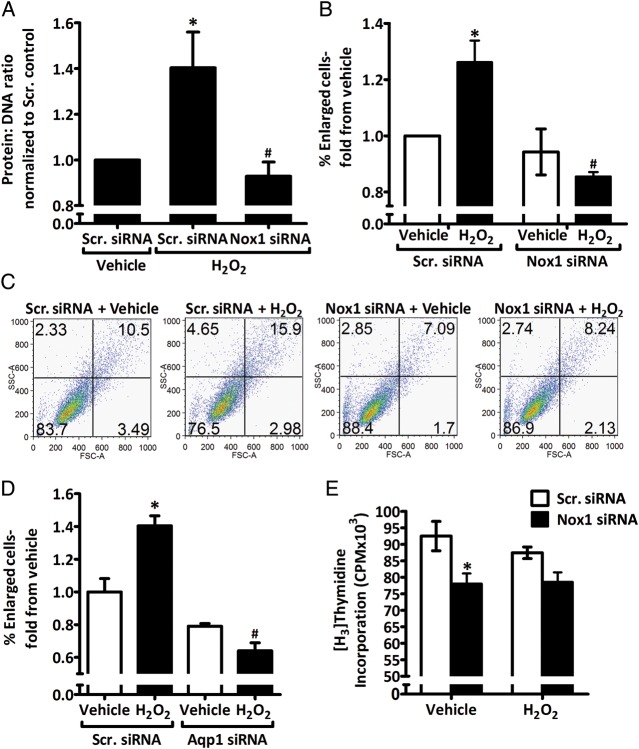
Hypertrophy in rASMC transfected with Nox1 or scrambled siRNA was assessed after H2O2 treatment by two independent methods, quantifying the ratio of the protein:DNA content, and FACS analysis. As seen in Figure 5A, H2O2 treatment resulted in a 1.4 ± 0.16-fold increase in hypertrophy in scrambled siRNA-transfected cells compared with scrambled siRNA-transfected vehicle control. Nox1 siRNA inhibited this increase in hypertrophy in response to H2O2 treatment (0.9 ± 0.06-fold from scrambled siRNA vehicle). These findings were confirmed by FACS analysis: H2O2 treatment increased the percentage of enlarged cells by 1.3 ± 0.08-fold compared with vehicle in scrambled siRNA-transfected cells and this increase in response to H2O2 treatment was abolished in Nox1 siRNA transfected cells (0.9 ± 0.02-fold from scrambled siRNA vehicle; Figure 5B and C). No difference was observed between scrambled siRNA-transfected vehicle-treated cells and Nox1-siRNA-transfected H2O2-treated cells. In addition, the quantification of average cell areas in experiments with similar treatment groups further supported these findings (Figure S8, Supplementary material online). Furthermore, Aqp1 siRNA inhibited H2O2-induced rASMC hypertrophy to a similar extent as Nox1 siRNA, as seen by FACS analysis of the percentage of enlarged cells post-H2O2 treatment (fold from scrambled siRNA vehicle: 1.4 ± 0.06 vs. 0.64 ± 0.05 for scrambled siRNA H2O2 vs. Aqp1 siRNA H2O2 groups, respectively; Figure 5D). There was a trend towards a smaller percentage of enlarged cells in both H2O2- and vehicle-treated Aqp1 siRNA-transfected cells compared with vehicle scrambled siRNA cells, but these were not statistically significant (Figure 5D).
Figure 5.
H2O2 induces increase in rASMC hypertrophy, but not proliferation, in an Aqp1- and Nox1-dependent mechanism. (A) Quantification of the protein:DNA ratio of lysates from rASMC transfected with Scr. or Nox1 siRNA and treated with vehicle or 50 μM H2O2 for 24 h expressed as fold from Scr. vehicle. (B) FACS analysis of % enlarged cells of total rASMC treated as in (A) expressed as fold from Scr. vehicle. (C) Representative FACS analysis image of B. (D) FACS analysis quantified as in (B) of rASMC transfected with Scr. or Aqp1 siRNA and treated with vehicle or 50 μM H2O2 for 24 h. (E) [3H]thymidine incorporation measurement in counts per minute (CPM) of rASMC treated as in (A). Means ± SEM, n = 3–4. *P < 0.05 vs. Scr. siRNA vehicle. #P < 0.05 vs. H2O2 Scr. siRNA.
Treatment of cells with H2O2 elicited no effect on rASMC proliferation compared with vehicle treatment in scrambled siRNA-transfected cells as measured by radio-labelled thymidine incorporation (Figure 5E). Similarly, H2O2 treatment had no effect on proliferation compared with vehicle treatment in Nox1-siRNA-transfected cells. There was a significant inhibition of proliferation by Nox1siRNA when compared with scrambled siRNA in vehicle-treated cells (78 ± 3 vs. 92 ± 4 × 103 CPM, respectively).
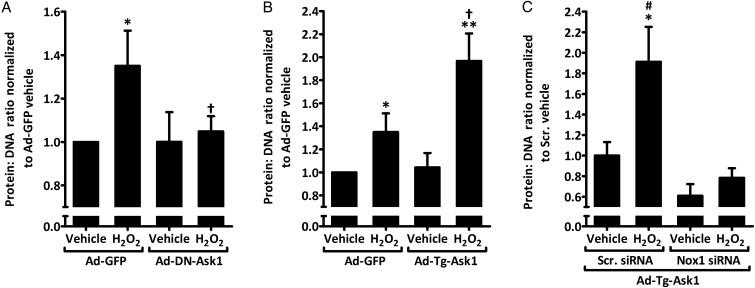
To test the role of Ask1 on hypertrophy, rASMCs were transfected with adenovirus expressing dominant negative Ask1 (Ad-DN-Ask1) or GFP (Ad-GFP). As shown in Figure 6A, H2O2 caused a 1.35 ± 0.16-fold rise in the protein:DNA ratio compared with vehicle treatment in cells transduced with GFP control adenoviruses. In the presence of Ad-DN-Ask1, this increase was abolished. No difference was detected between vehicle and H2O2 treatments in cells transduced with Ad-DN-Ask1. Ask1 overexpression (transgenic Ask1, Ad-Tg-Ask1) in rASMC resulted in an enhanced increase in hypertrophy in response to H2O2 compared with Ad-GFP cells treated with H2O2 (1.97 ± 0.24- vs. 1.35 ± 0.16-fold from Ad-GFP vehicle control, respectively; Figure 6B). No difference was detected under vehicle treatment between Ad-GFP and Ad-Tg-Ask1-transduced cells.
Figure 6.
H2O2-induced Nox1-dependent increase in rASMC hypertrophy is augmented by Ask1 overexpression and attenuated by dominant-negative Ask1 expression. (A and B) Quantification of the protein:DNA ratio of lysates from rASMC transduced with adenoviruses expressing GFP control (Ad-GFP), dominant-negative Ask1 (Ad-DN-Ask1), or transgenic Ask1 (Ad-Tg-Ask1) and treated with vehicle or 50 μM H2O2 for 24 h expressed as fold from Ad-GFP vehicle. Means ± SEM, n = 5, *P < 0.05 vs. Ad-GFP vehicle. **P < 0.001 vs. Ad-Tg-Ask1 vehicle. †P < 0.05 vs. Ad-GFP H2O2. (C) Quantification of the protein:DNA ratio of lysates from rASMC transduced with transgenic Ask1 (Ad-Tg-Ask1), transfected with Scr. or Nox1 siRNA and treated with vehicle or 50 μM H2O2 for 24 h expressed as fold from Scr. siRNA vehicle Ad-Tg-Ask1. Means ± SEM, n = 5, *P < 0.01 vs. Scr. siRNA vehicle Ad-Tg-Ask1, #P < 0.001 vs. H2O2 Nox1 siRNA Ad-Tg-Ask1.
Co-transfection of Nox1 siRNA into Ad-Tg-Ask1-transduced rASMC abolished H2O2-induced hypertrophy when compared with H2O2-treated scrambled siRNA-transfected Ad-Tg-Ask1-tansduced cells (0.78 ± 0.09- vs. 1.91 ± 0.34-fold from scrambled siRNA Ad-Tg-Ask1 vehicle control, respectively; Figure 6C). No significant difference was detected between H2O2-treated Nox1 siRNA-transfected Ad-Tg-Ask1 cells and vehicle-treated scrambled siRNA-transfected Ad-Tg-Ask1 cells. Similarly, there was no significant difference under vehicle treatment of Ad-Tg-Ask1 cells between scrambled and Nox1 siRNA transfections.
4. Discussion
Previously our laboratory and others demonstrated that ROS are important autocrine mediators of medial SMC signalling and hypertrophy. Previous findings suggested a paracrine feed-forward ROS signalling mechanism in the vascular wall; however, the signalling pathways involved in this response are not known. The findings of this study elucidate a novel pathway by which ROS effect medial hypertrophy and demonstrate for the first time that: (i) low micromolar concentrations of H2O2 induce O2•– production in rASMCs; (ii) Nox1 oxidase is the source of H2O2-induced O2•– production; (iii) the water channel Aqp1 is involved in H2O2-mediated O2•– production in rASMC through facilitating H2O2 transport into the cells; (iv) Ask1 is activated in vascular cells in response to pathophysiologically relevant concentrations of H2O2; and (v) Ask1 activation is involved in the propagation of rASMCs hypertrophy. Thus, these data support a mechanism by which vascular ROS can enter SMC via Aqp1, stimulate Nox1 and O2•– production, leading to Ask1-mediated vascular SMC hypertrophy.
We demonstrate that low micromolar concentrations of H2O2 (in the physiological range in plasma) are capable of increasing rASMC O2•– production. For ease of use in initial comprehensive measurements of concentration response and time course experiments, the highly sensitive L-012 chemiluminescence method was used. The data demonstrated a stimulation of vascular SMC O2•– production by concentrations of H2O2 as low as 5 µM in a concentration-dependent manner. We confirmed these data using two widely accepted methods for O2•– detection, EPR and cytochrome c assays. O2•– detection was confirmed by two O2•– scavengers, cell-impermeant SOD and cell-permeant Tiron. The inhibition by SOD demonstrated a trend towards lower O2•– production even when compared with vehicle (Figure 1C). This effect is in line with low-level basal ROS production in these cells, which have been deemed operant in normal cellular signalling. Interestingly, our data suggest that O2•– production in vascular SMC is predominantly extracellular since non-cell-permeant SOD was capable of completely ablating O2•– detection (Figure 1B and C, and Figure S2, Supplementary material online). This difference in localization of O2•– production from that which was previously reported14,15 could, however, be specific to the means by which H2O2 elicits Nox1 activation in comparison with receptor-mediated agonists. One hypothesis as to how this Nox1-derived extracellular O2•–, which cannot easily cross biological membranes, carries out its action on Ask1 inside the cell is that it initiates a propagated response via H2O2. Extracellular O2•– dismuted to H2O2 is expected to enter the cell and activate Ask1. It should be noted also that undetectable intracellular O2•– production in response to exogenous H2O2 cannot be entirely excluded. Studies are ongoing to delineate the precise mechanism by which H2O2 activates Nox1 in these cells.
Interestingly, plasma levels of 3–5 μM H2O2 have been reported in patients with hypertension,16 with some studies showing plasma levels up to 50 µM.17,18 Moreover, studies have suggested that vascular cells produce H2O2, accumulating micromolar levels of the oxidant under stress stimuli, which are likely exacerbated by infiltrating leukocytes in inflammation.19,20 Previous studies using exogenous H2O2 typically employed concentrations >100 μM,19,21,22 and, to our knowledge, none used concentrations lower that 50 µM in vascular SMC. Thus our studies are the first to demonstrate that H2O2 concentrations, increasing from physiological to pathophysiological levels (Figure 1 and Figure S1, Supplementary material online) in the cardiovascular system, are capable of propagating ROS production and hypertrophy.
Our findings indicate that Nox1 is the source of H2O2-stimulated O2•– in rASMC. Using a broad spectrum of pharmacological inhibitors of cellular oxidases we were able to preliminarily conclude that Nox-like activity was responsible for the SMC O2•– production since only iodonium compound DPI abolished O2•– levels. Although previous studies showed that H2O2 stimulates O2•– production from xanthine oxidase, mitochondria, and uncoupled endothelial NOS in endothelial cells,21,23 our data do not support H2O2-induced O2•– production from these enzyme classes in SMCs. With this in mind, we interrogated the ability of Nox1 siRNA to inhibit H2O2-stimulated O2•–. Nox1 is the identifying anchoring subunit of Nox1 oxidase, which co-associates with p22phox in SMC caveolae to comprise the catalytic cytochrome portion of the enzyme system. Incidentally, cytosolic subunits p47phox and NOXA1 are also implicated as essential for the active Nox1 oxidase complex in rASMC.1 In preliminary studies, we tested the ability of three distinct oligonucleotides targeting Nox1 for their ability to specifically suppress Nox1 mRNA. Two of the three siRNA were effective, with the greatest inhibition (∼70%) rendered by oligonucleotide 2 (oligo 2, Figure S3, Supplementary material online). Nox1 siRNA oligo 2 ablated the four-fold increase in O2•– in response to H2O2 (in the presence of control siRNA), suggesting for the first time Nox1 as the major effector of H2O2 in SMCs (Figure 2B). Our data are supported by previous data showing that antisense oligonucleotides to p22phox attenuated H2O2-stimulated ROS production in rASMC.19 In that paper, however, the minimum effective concentration of H2O2 was 100 μM and knockdown of p22phox per se was not sufficient to delineate the role of specific Nox isoforms, since p22phox is critical for the activity of isoforms 1-4.
Our data show that suppressing Aqp1 using siRNA attenuated H2O2 entry into the cell as well as H2O2-induced O2•– production in rASMC. These data reveal for the first time a role for Aqp1 in paracrine vascular ROS signalling and challenge the widely accepted notion that H2O2 gains entry into vascular cells solely by virtue of its permeability across membranes. To date, only three studies addressed the role of aquaporins in mediating H2O2 transport across cellular membranes, and none was in cardiovascular cells.5,6,24 Moreover, those previous studies demonstrate that Aqp3 and Aqp8 contribute to H2O2 transport yet none provide evidence for a role of Aqp1 in H2O2 transport. Those findings distinct from our current findings are likely explained by tissue and species differences in Aqp1 expression and regulation. Indeed, qPCR experiments (data not shown) demonstrated that only Aqp1, and not Aqp3 nor Aqp8, is expressed in rAMSC. This is consistent with previous findings by Herrera et al.25 These differences notwithstanding, the data could foster a paradigm shift in our understanding of the means by which H2O2 enters cells and mediates its paracrine effects. At a more basic level, the data appear to provide the first evidence for a role of aquaporins in H2O2 transport across parenchymal cells.
Western blot analysis in the present study showed the activation of Ask1 in response to H2O2 treatment, and siRNA transfection revealed a role for Nox1 in this process. Although Ask1 is activated by a number of stress stimuli including ROS in multiple cell types, its role as a downstream target of Nox enzymes was until now unknown. Ask1 was shown to be activated by high micromolar concentrations of H2O2 in HeLa, HEK293 cells and mouse embryonic fibroblasts.8,13,26 Despite this evidence, only one study in neurons suggests a link between Ask1 activation and Nox enzymes.27 Indeed, the current data support a direct link between Nox1 and Ask1 and demonstrate that activation of Ask1 is downstream of Nox1-derived O2•– production in H2O2-treated rASMC. Nonetheless, direct activation of Ask1 by exogenous H2O2 cannot be ruled out. What is clear, however, is that knockdown of Nox1 was sufficient to abolish both endogenous and overexpressed Ask1 activation in response to H2O2 (Figure 4 and Supplementary material online, Figure S7). Interestingly, knockdown of Aqp1 in cells overexpressing Ask1 was not enough to block Ask1 phosphorylation in response to H2O2 (Figure S9, Supplementary material online). We attribute this negative result to be due to a technical limitation of an overexpressed Ask1 on the one hand and a less than optimal antibody for endogenous phospho-Ask1 detection on the other hand.
On a functional level, the current study establishes a role for Aqp1, Nox1 and Ask1 in H2O2-induced hypertrophy of rASMC. Using protein to DNA ratio measurements, FACS analysis, average cell area quantification, and a three-pronged approach utilizing Aqp1 or Nox1 siRNA transfection and adenoviral transduction of DN or Tg Ask1, we conclude that H2O2 enters the cells via Aqp1 and induces Nox1 activation, which in turn activates Ask1 leading to SMC hypertrophy. Importantly, by the use of DN Ask1, we have demonstrated the essential role of Ask1 in this pathway. H2O2 has been implicated in the development of SMC hypertrophy and pro-hypertrophic signalling pathways;2,28 however, the precise mechanisms have been elusive. Based on the current findings implicating Ask1 in rASMC hypertrophy, and recent findings from our laboratory implicating medial SMC p38 MAPK activation in response to adventitia-derived H2O2,4 it is reasonable to speculate a signalling cascade involving the p38 MAPK pathway. Indeed, the activation of p38 MAPK downstream of Nox-derived ROS has been demonstrated.7 However, based on the literature, links between Ask1 and p38 MAPK are expected to be complex and currently outside the scope of this study.
Induction of rASMC proliferation by H2O2 was ruled out by assessing radiolabelled thymidine incorporation; that is, H2O2 did not stimulate thymidine incorporation of rASMCs (Figure 5E). Of note is the observation of a significant inhibition of thymidine incorporation by Nox1 siRNA in vehicle-treated cells, which is consistent with previous findings.29 Furthermore, there was no effect on cell count or appearance of cell death in rASMC treated with H2O2 in our studies, consistent with no effect on apoptosis of the concentrations used in this study. This is in line with the literature showing that apoptosis is typically observed at millimolar concentrations of H2O2.19,26,30
In summary, our findings challenge existing dogma on the biological action of H2O2 in the vasculature in a number of ways. Our results demonstrate that circulating or interstitial pathophysiological concentrations of H2O2 may induce paracrine O2•– production in rASMC. The findings suggest a novel mechanism by which H2O2 enters SMCs via Aqp1 and induces Nox1-derived O2•– production and SMC hypertrophy. This, combined with the fact that Nox1 siRNA completely blocked the O2•– signal, Ask1 phosphorylation and SMC hypertrophy and the fact that DN Ask1 blocked SMC hypertrophy, is consistent with a role for Ask1 activation in this pathway. Given the growing evidence that H2O2 acts on vascular cells as both a paracrine and autocrine signalling mediator, our findings have important implications for hypertension- and inflammatory disease-related SMC growth. From a therapeutic standpoint, the data provide key insights into the development of drugs to prevent medial thickening via new multiple targets.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the following: P.J.P. receives support from National Institutes of Health (R01HL079207, P01HL103455–01) and is an Established Investigator of the American Heart Association. All Vascular Medicine Institute investigators receive support from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania, PA.
Supplementary Material
Acknowledgments
We would like to thank Dr Hidenori Ichijo for supplying us with the phospho-Ask1 antibody, and Dr. Jae J Song (Yonsei University College of Medicine, Seoul, Republic of Korea) for GFP control and Ask1 and dominant negative Ask1 adenoviruses. We would also like to thank Drs Iulia D. Popescu and John F. McDyer (University of Pittsburgh) for their assistance with FACS experiments.
Conflict of interest: none declared.
References
- 1.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, et al. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. doi:10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csanyi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. doi:10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Ormsby A, Oja-Tebbe N, Pagano PJ. Gene transfer of NAD(P)H oxidase inhibitor to the vascular adventitia attenuates medial smooth muscle hypertrophy. CircRes. 2004;95:587–594. doi: 10.1161/01.RES.0000142317.88591.e6. [DOI] [PubMed] [Google Scholar]
- 4.Cascino T, Csanyi G, Al Ghouleh I, Montezano AC, Touyz RM, Haurani MJ, et al. Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxid Redox Signal. 2011;15:1507–1515. doi: 10.1089/ars.2010.3631. doi:10.1089/ars.2010.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. doi:10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. doi:10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 7.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 8.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. doi:10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, et al. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. doi:10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song GJ, Barrick S, Leslie KL, Sicari B, Fiaschi-Taesch NM, Bisello A. EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells. J Mol Cell Cardiol. 2010;49:1012–1021. doi: 10.1016/j.yjmcc.2010.08.025. doi:10.1016/j.yjmcc.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. doi:10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. doi:10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 13.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. doi:10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. doi:10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 15.Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, et al. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. doi:10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacy F, O'Connor DT, Schmid-Sch'nbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. JHypertens. 1998;16:291–303. doi: 10.1097/00004872-199816030-00006. doi:10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 17.Varma SD, Devamanoharan PS. Hydrogen peroxide in human blood. Free Radic Res Commun. 1991;14:125–131. doi: 10.3109/10715769109094124. doi:10.3109/10715769109094124. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486:10–13. doi: 10.1016/s0014-5793(00)02197-9. doi:10.1016/S0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 19.Li WG, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H2O2-induced O2 production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Zweier JL. A real-time electrochemical technique for measurement of cellular hydrogen peroxide generation and consumption: evaluation in human polymorphonuclear leukocytes. Free Radic Biol Med. 2001;31:894–901. doi: 10.1016/s0891-5849(01)00665-7. doi:10.1016/S0891-5849(01)00665-7. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. doi:10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 22.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett. 2005;579:2533–2540. doi: 10.1016/j.febslet.2005.03.057. doi:10.1016/j.febslet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 23.Witting PK, Rayner BS, Wu BJ, Ellis NA, Stocker R. Hydrogen peroxide promotes endothelial dysfunction by stimulating multiple sources of superoxide anion radical production and decreasing nitric oxide bioavailability. Cell Physiol Biochem. 2007;20:255–268. doi: 10.1159/000107512. [DOI] [PubMed] [Google Scholar]
- 24.Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J. 2008;414:53–61. doi: 10.1042/BJ20080287. doi:10.1042/BJ20080287. [DOI] [PubMed] [Google Scholar]
- 25.Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol. 2007;292:F1443–F1451. doi: 10.1152/ajprenal.00353.2006. doi:10.1152/ajprenal.00353.2006. [DOI] [PubMed] [Google Scholar]
- 26.Nadeau PJ, Charette SJ, Landry J. REDOX reaction at ASK1-Cys250 is essential for activation of JNK and induction of apoptosis. Mol Biol Cell. 2009;20:3628–3637. doi: 10.1091/mbc.E09-03-0211. doi:10.1091/mbc.E09-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, et al. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke. 2008;39:3049–3056. doi: 10.1161/STROKEAHA.108.517284. doi:10.1161/STROKEAHA.108.517284. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. doi:10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 29.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. doi:10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Niu XL, Madamanchi NR. Leukocyte antigen-related protein tyrosine phosphatase negatively regulates hydrogen peroxide-induced vascular smooth muscle cell apoptosis. J Biol Chem. 2008;283:34260–34272. doi: 10.1074/jbc.M806087200. doi:10.1074/jbc.M806087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.