Abstract
Flavonoids are low-molecular weight, aromatic compounds derived from fruits, vegetables, and other plant components. The consumption of these phytochemicals has been reported to be associated with reduced cardiovascular disease (CVD) risk, attributed to their anti-inflammatory, anti-proliferative, and anti-thrombotic actions. Flavonoids exert these effects by a number of mechanisms which include attenuation of kinase activity mediated at the cell-receptor level and/or within cells, and are characterized as broad-spectrum kinase inhibitors. Therefore, flavonoid therapy for CVD is potentially complex; the use of these compounds as molecular templates for the design of selective and potent small-molecule inhibitors may be a simpler approach to treat this condition. Flavonoids as templates for drug design are, however, poorly exploited despite the development of analogues based on the flavonol, isoflavonone, and isoflavanone subgroups. Further exploitation of this family of compounds is warranted due to a structural diversity that presents great scope for creating novel kinase inhibitors. The use of computational methodologies to define the flavonoid pharmacophore together with biological investigations of their effects on kinase activity, in appropriate cellular systems, is the current approach to characterize key structural features that will inform drug design. This focussed review highlights the potential of flavonoids to guide the design of clinically safer, more selective, and potent small-molecule inhibitors of cell signalling, applicable to anti-platelet therapy.
Keywords: Flavonoids, Anti-platelet drugs, Drug design, Cardiovascular disease, Flavonoid analogues
1. Dietary flavonoids as negative modulators of cardiovascular disease risk
1.1. Epidemiology and clinical trials
The flavonoid group of compounds comprises flavonol and flavononol,1–4 flavan-3-ol, flavan-4-ol, and flavan-3,4-diol subgroups,5–7 flavanone,8,9 flavone,1,2 isoflavone,10,11 as well as anthocyanidin and proanthocyanidin8,12 subgroups.1 The potential value of flavonoids as therapy for cardiovascular diseases (CVD) was recognized with the correlation of the consumption of dietary sources containing high levels of these compounds to lowered risk for these conditions.1,3,13 A number of epidemiological studies described an association between reduction in the incidence of myocardial infarction (MI) and stroke3,13 and dietary intake of flavonoids.
Key studies included a 5-year follow-up study involving 805 men aged 65–84; 38 men from a group of 693 with no history of MI showed an inverse link between flavonoid intake (25 mg/day) and mortality from coronary heart disease.3 Another study demonstrated that lowered incidence of stroke within a cohort of 552 men aged 50–69 correlated with high-dietary intake of flavonoids.13 More recent studies with significantly larger cohorts (1000–90 000 participants), and follow-up periods of up to 11 years, have correlated flavonoid intake with lowered risk of death from CVD,14–18 a protective effect against peripheral arterial occlusive disease19 and prevention of coronary heart disease.20 There are, however, inconsistencies in the evidence base, with some epidemiological studies reporting no association between the dietary intake of flavonols (e.g. quercetin) and ischaemic heart disease21 or plasma antioxidant levels.22
Clinical trials have demonstrated the effects of dietary sources of flavonoids on CVD risk factors,23–25 post-thrombotic syndrome,25 atherosclerosis,24 and vascular health26–29 (Table 1). A meta-analysis of randomized trials describing the effects of chocolate and cocoa consumption on CVD risk showed that flow-mediated dilatation of blood vessels improved after chronic and acute intakes of these flavan-3-ol-rich food sources.23 The flavonoid supplement, Pycnogenol® was significantly more effective than compression stockings for relieving oedema symptoms associated with post-thrombotic syndrome.26 The phenolic content of red wine was suggested to modulate leucocyte adhesion molecules, a marker of atherosclerosis, in patients at high risk from CVD.24 Moreover, chronic cranberry juice (containing high levels of anthocyanins) consumption reduced carotid femoral pulse wave velocity, a clinically relevant measure of arterial stiffness, in subjects with coronary artery disease.27
Table 1.
Flavonoid clinical trials
| Flavonoid | Clinical trial | Outcome of clinical trial | Reference |
|---|---|---|---|
| Flavan-3-ols (chocolate, cocoa, and epicatechin) | Medline, EMBASE, and Cochrane databases for randomized controlled trials of chocolate, cocoa, or flavan-3-ols were reviewed. Forty-two acute or short-term chronic that comprised 1297 participants were assessed. | Insulin resistance was improved by chocolate or cocoa due to significant reductions in serum insulin. Chocolate or cocoa improved flow-mediated dilatation regardless of the dose consumed and reduced diastolic blood pressure, but doses of >50 mg epicatechin/day were needed to induce greater effects on systolic and diastolic blood pressure. | Hooper et al.23 |
| High-flavonoid diet | The Zutphen Elderly Study: A high flavonoid diet [tea (61%), onions (13%), and apples (10%)] was given to 805 men aged 65–84. Flavonoid intake was 25.9 mg daily. The men were then followed-up for 5 years. | Between 1985 and 1990, 43 men died of coronary heart disease. Fatal or non-fatal MI occurred in 38 of 693 men with no history of MI at baseline. Flavonoid intake was significantly inversely associated with mortality from coronary heart disease (P for trend = 0.015). | Hertog et al.3 |
| Pycnogenol® (flavonoid supplement from pine bark) | One hundred and fifty-six patients with a single, major episode of proximal deep vein thrombosis were assigned to one of three groups receiving treatment with either compression stockings (group 1), Pycnogenol® (group 2), or the combination of both (group 3) for 1 year. | Pycnogenol® was significantly more effective from 6 months onwards than compression stockings for relieving oedema symptoms (P < 0.05). Symptoms were more effectively reduced with the combination of Pycnogenol® and compression stockings than with the individual regimen alone (P < 0.05). | Errichi et al.26 |
| Cranberry juice | An acute pilot study with no placebo (n = 15) and a chronic placebo-controlled crossover study (n = 44) that examined the effects of cranberry juice on vascular function in subjects with coronary artery disease, for 4 weeks with a 2-week washout period between juice and placebo. | Improved brachial artery flow-mediated dilation was observed 4 h after consumption of a single 480 mL portion of cranberry juice. Chronic (54% juice, 835 mg total polyphenols, and 94 mg anthocyanins) cranberry juice consumption reduced carotid femoral pulse wave velocity. | Dohadwala et al.27 |
| Grape seeds | In a double-blind, randomized, placebo-controlled intervention study, 28 male smokers were supplemented with 200 mg per day of monomeric and oligomeric flavanols (MOF) from grape seeds. Biomarkers for major pathological processes occurring in the vasculature: disturbances in lipid metabolism and cellular redox balance, and activation of inflammatory cells and platelets were monitored. | In the MOF group, serum total cholesterol and LDL decreased significantly (P ≤ 0.05). MOF supplementation exerted anti-inflammatory effects in blood towards ex vivo added bacterial endotoxin and significantly reduced the expression of inflammatory genes in leucocytes. A global, vascular health index (of all measured effects) showed a significant improvement of overall vascular health by MOF compared with placebo (P ≤ 0.05). | Weseler et al.28 |
| Soya isoflavones | The combined effect of exercise and isoflavones in overweight-to-obese post-menopausal women with clinical risk factors for CVD was assessed over 6 months. The supplementation contained 70 mg/day of isoflavones. | Isoflavones improved leg fat mass % (P = 0.05), but interactions were not observed between isoflavones and exercise. | Choquette et al.29 |
A number of early and recent human clinical trials demonstrate that flavonoid-rich food sources (e.g. chocolate, red wine, and cranberry juice) reduce CVD risk, by improving vascular health and modulating risk factors linked to associated conditions including thrombosis and atherosclerosis.
These data are consistent with flavonoid bioavailability profiles which indicate that these compounds have access to the cells in the circulation (including platelets) at concentrations potentially capable of eliciting modulatory effects. Flavanones (naringenin), flavones (apigenin), flavonols (quercetin), and flavan-3-ols (epicatechin) were detected in the plasma at 5.99,30 0.1,31 10.66,32 and 5.9 μm,33 respectively, after ingestion of supplements and dietary sources. Moreover, elimination of flavonoid metabolites from plasma varies depending on the subclass of compound with half-lives (time for elimination of half of the maximum plasma concentration) ranging from 11–28 h for quercetin,34 1.3–2.9 h for naringenin,30 and 1.9–5.7 h for catechin.35,36
The physiological activities of flavonoids are diminished by factors including lifestyle,37 sex, age, race, other disease states and interactions with drugs,37–41 and genes which may limit their beneficial impact. These compounds would be placed to more effective use as structural guides for the design of anti-platelet therapeutic agents. At present, aspirin (acetylsalicylic acid) is the most widely used antiplatelet drug,42–45 but its use is associated with side effects which include stomach ulcers and bleeding43,46; in addition some patients are resistant to the actions of aspirin. ADP receptor (P2Y12) antagonists include the thienopyridines ticlopidine, clopidogrel, and prasugrel (CS-747; LY-640315).47–49 Prasugrel is increasingly replacing clopidogrel due to more potent and rapid anti-platelet effects with less interpatient variability,48,49 but this drug causes bleeding complications.
Another proposed strategy for the use flavonoids as therapy could involve combining these compounds with existing anti-platelet drugs to investigate potential synergistic inhibitory effects on platelet function. A recent report showed that a mixture of resveratrol, quercetin, and gallic acid, at relative concentrations similar to those found in most red wines, did not inhibit platelet aggregation, but that these compounds potentiated sub-inhibitory concentrations of aspirin.50 Chocolate and cocoa were demonstrated to augment or contribute an additive inhibitory effect to anti-platelet effects of aspirin.51,52 Furthermore, when added to platelets that had been exposed in vivo to aspirin, the flavone apigenin potentiated the inhibitory effect of this drug on platelet aggregation.53
Taken together, these observations between ingestion of flavonoids and CVD risk markers have encouraged research into flavonoid mechanisms of action.
1.2. Flavonoid mechanisms for inhibition of cell function
Flavonoids are well-established as antioxidants,54,55 but these compounds have been demonstrated to attenuate platelet function by working as pro-oxidants to enhance nitric oxide (NO).56 These compounds may also inhibit platelet function by inhibiting reactive oxygen species (ROS)57,58 production, binding to cell-surface receptors,59–61 modifying structural proteins,62,63 and disrupting cell-membrane integrity.64,65 Exploiting flavonoid antioxidant54,55 and pro-oxidant56–58 activities and ability to bind to cell membranes64,65 and structural proteins62,63 would lead to the generation of small-molecule inhibitors which do not discriminate between target cells/tissues. It is therefore, the ability of flavonoids to inhibit kinase activity that is of particular interest, as this property suggests that these compounds may be developed further as selective therapeutic agents.
The anti-inflammatory, anti-thrombotic, and anti-proliferative properties of flavonoids are achieved through modulating the activity of kinases which drive these processes (Figure 1). Red wine polyphenolic compounds containing high levels of the flavonoids, quercetin, and catechin have been previously demonstrated to inhibit the phosphorylation of the serine/threonine kinases, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase1/2 (ERK1/2), c-Jun N-terminal kinase, and protein kinase B (PKB)/Akt in vascular smooth muscle cells66 and endothelial cells.67 Previous reports also show that the polymeric catechin, epigallocatechin gallate (EGCG), inhibits p38 MAPK and ERK1/2 phosphorylation in platelets68 and the tyrosine kinases (Fyn, Lyn, Btk, Syk) in mast cells.69
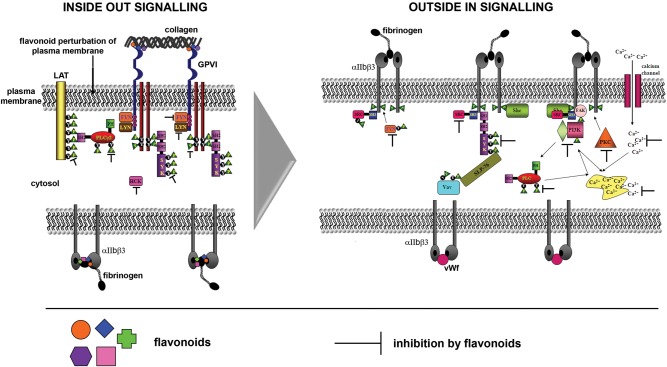
Figure 1.
Inhibition of platelet signalling by flavonoids. Flavonoids inhibit tyrosine kinases (Src, Fyn, Lyn, Hck, Syk), lipid kinases (PI3K), and serine/threonine kinases (PKC) and phospholipases (PLCγ2) involved in inside-out signalling (that initiates platelet activation) and outside-in signalling (that maintains platelet activation). These compounds also inhibit by targeting calcium signalling that is essential for platelet activation, they potentially disrupt fibrinogen and collagen binding to integrin αIIbβ3 and GPVI, respectively, and it is likely that they perturb the platelet plasma membrane.
Flavonoids including quercetin, apigenin, and catechin were demonstrated to inhibit the activities of tyrosine kinases (Fyn, Lyn, Syk),70,71 lipid kinases (phophoinositide-3-kinaseγ: PI3Kγ),71 as well as phosphorylation of the FcRγ chain,71 phospholipase Cγ2,70,71 and the membrane protein, Linker and Activator of T (LAT) cells71 in platelets. The green-tea flavonoid, epigallocatechin-3-gallate and the isoflavone, genistein were shown to inhibit phosphodiesterases and Rac1 proteins which play essential roles in platelet activation, in other cell types.72,73 Furthermore, flavonoid-mediated increases in NO production56 and the blocking of pathways leading to mobilization of calcium from intracellular stores71,74 were shown to result in the inhibition of platelet function.
The disruption of cell signalling by flavonoids may begin at the level of the plasma membrane. These compounds may decrease the fluidity of the lipid bilayer by disrupting protein–protein interactions64,65,75 and binding to cell surface receptors.59–61 Quercetin was previously demonstrated to integrate into the polar head region of dipalmitoylphosphatidylcholine liposomes (used as a phospholipid bilayer model to mimic the plasma membrane) via the A–C ring chromophore complex64 and to bind to thromboxane A2 (TxA2)59 and adenosine diphosphate (ADP)60,61 receptors. This flavonol was reported to enhance the hypotonic integrity of erythrocytes after binding to their membranes.65 Fluorescence resonance energy transfer between the tryptophan residues in erythrocyte membrane proteins and flavonoids showed that individual compounds were in close enough proximity to proteins to potentially disturb their associations.65
Alternatively, flavonoids may be internalized by cells to gain direct access to intracellular signalling enzymes such as kinases.64,70,76–80 Quercetin and the methylated metabolite of this flavonol, tamarixetin, were demonstrated to be internalized by platelets76 and megakaryocytic cells,70 where the intrinsic fluorescent properties of these compounds allowed them to be visualized within the cytosol and associated with the platelet plasma membrane.76
Other possible mechanisms underlying flavonoid inhibitory activities include modifications to cytoskeletal proteins including tubulin62 and actin.63 Quercetin was suggested to inhibit microtubule assembly by masking cysteine residues on tubulin from chemical modification.62 Both tubulin and actin mediate platelet degranulation,81,82 and dense granule secretion from these cells is critically dependent on PKCΔ that mediates translocation of Syk from the cytosol to the membrane in activated platelets.83 Therefore, the blocking of binding sites on the structures of cytoskeletal proteins by flavonoids may disrupt the movement of signalling proteins between intracellular compartments in stimulated platelets.
Flavonoids are therefore non-specific inhibitors, which is not entirely comparable with their potential application as clinically useful drugs. Studies investigating flavonoid inhibition of kinases indicate, however, that the activity and selectivity of these compounds is dependent on their structure, and on this basis, the search for structural elements defining the beneficial effects of these compounds was explored.
2. Understanding the link between flavonoid structure and activity
2.1. Flavonoid structure–activity relationships
The discovery that key functional groups on the flavonoid structure influence the activity of these compounds on cell function has provoked considerable interest. These compounds share a common structure, based on 2-phenyl-benzogamma-pyrane, i.e. two benzene rings (A and B rings) joined by a third pyranic (C ring) (Figure 2). The well-established free-radical scavenging abilities of flavonoids57,84–94 are dependent on electron-donating hydroxyl group substitutions on the aromatic B ring and heterocyclic C ring.86–88 The C-2–C-3 double-bond conjugated to a C-4 carbonyl group on the C ring is responsible for antioxidant activity through formation of an electron delocalization system.86–88
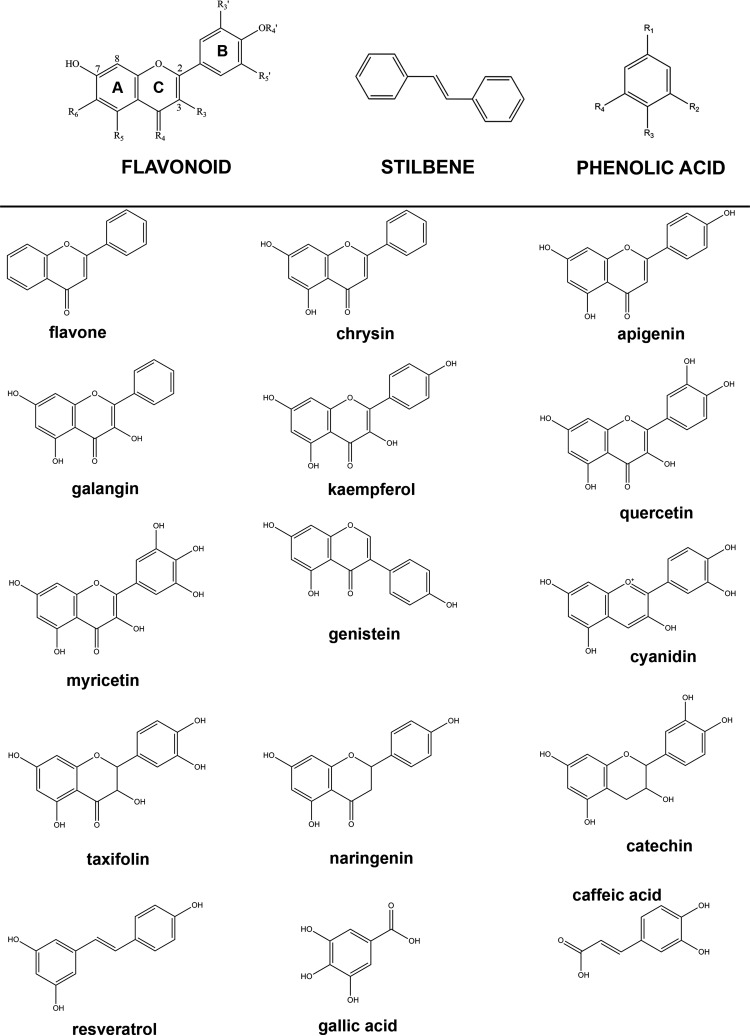
Figure 2.
The structures of polyphenol compounds. Flavonoids form a major subgroup of the polyphenol family of compounds and include flavonols, flavones, flavan-3-ols, phenolic acids, isoflavones, and stilbenes. Flavones (chrysin, apigenin) are characterized by a non-hydroxylated C ring, whereas flavonol (galangin, kaempferol, quercetin, myricetin) C rings contain a C-3 hydroxyl group. Flavononols (taxifolin) are flavonols with a non-planar C ring. Flavan-3-ols (catechin) are defined by a non-planar, C-3 hydroxylated C ring that is not substituted with a C-4 carbonyl group and flavonones (naringenin) are defined by a non-planar, non-hydroxylated C ring containing a carbonyl group. Stilbenes (resveratrol) are characterized by 2 hydroxylated benzene rings in cis or trans conformations and the isoflavones (genistein) are defined by a B ring substituted to the C3 position on the C ring. Cyanidins (cyanidin) are defined by a positively charged C ring, and phenolic acids (caffeic acid and gallic acid) are benzene rings substituted with carboxyl and hydroxyl groups.
Functional groups conjugated to the flavonoid structure through metabolism also affect the antioxidant potential of these compounds. Previous reports demonstrated that the reduction of a stable free radical, 1,1,-diphenyl-2-picrylhydrazyl, was affected by the position of a glucuronide group on the flavan nucleus.95 Quercetin-3-glucuronide prolonged the lag-time of copper-induced low-density lipoprotein oxidation less than quercetin,96 and glucuronides of catechin and epicatechin identified in the plasma of rats following ingestion of the flavan-3-ol monomers, exhibited antioxidant activities which were equivalent to that of their parent compounds.97 Substitution of the C-3 and C-4′ positions on the C and B rings (Figure 2) with a glucuronide group, respectively, was shown to diminish scavenging activity, but only a negligible decrease was observed for a glucuronide group at the C-7 position on the A ring.95 These data are perhaps not unexpected as the hydroxyl groups (C-5 and C-7) on the A ring (Figure 2) are less important for stabilizing the flavonoid radical formed after the reduction–oxidation reaction involved during scavenging.86
Functional groups within and on the periphery of the core flavonoid skeleton (Figure 2), including the chromenone (2H-1-benzopyran) C ring C-2–C-3 double-bond that maintains planarity, variations in the C and B ring hydroxyl group substitutions, and metabolically added sulphate and methyl groups, determine the potency of these compounds for inhibition of platelet signalling and function.70,98,99 The flavonol quercetin was shown to inhibit the aggregation of collagen-stimulated isolated platelets with high potency (IC50: 4.9 ± 1.0 µm) attributed to the planar, C-4 carbonyl substituted C ring and B ring catechol moiety70,98,99 (Figure 2). The flavone apigenin inhibited platelet function with similar high potency (IC50: 5.5 ± 1.0 µm) as quercetin despite the lack of C ring C-3 hydroxyl70 (Figure 2). Low-potency inhibition of platelet function was achieved by the flavan-3-ol, catechin (IC50: 545.2 ± 74.2 µm) comprising a non-planar C ring70,98,99 (Figure 2). Metabolic modification of quercetin with a glucuronide group significantly reduced the potency of the flavonol possibly due to reduced membrane permeability, but the addition of a methyl or a sulphate group to quercetin allowed moderate potency inhibition of platelet function.70
Further studies have improved our understanding of the relative importance of flavonoid functional groups. The C ring C-2–C-3 double-bond responsible for maintaining flavonoid planarity (Figure 2) was reported to be more important for high-potency inhibition of platelet function than the C ring C-4 carbonyl group, as removal of the C-4 carbonyl group on a planar C ring (cyanidin - IC50: 19.2 ± 5.3 µm) resulted in approximately four-fold less potent inhibition than quercetin, whereas with a C-4 carbonyl group on a non-planar C ring (taxifolin - IC50: 312.8 ± 53.6 µm), potency was reduced ∼60-fold compared with quercetin.35 At least two benzene rings are required for potent inhibition of platelet function. The stilbene, resveratrol comprising only two benzene rings in cis or trans conformation, inhibited platelet function with potency (IC50: 5.9 ± 1.7 µm) similar to that of quercetin (unpublished data). Phenolic and benzoic acids, however, inhibited platelet function with IC50 ranging from 6 to 8 µm, indicating that a single-aromatic moiety (Figure 2) is not sufficient for potent inhibition (unpublished data).
Functional groups associated with potent inhibition of platelet (washed platelets) function are also attributed to potent inhibition of signalling stimulated downstream of the activatory collagen receptor, GPVI,58,59,70,71,98,99 ADP,59,98 TxA2,59,98,100,101 arachidonic acid,53,59,98,99 and protease-activated receptors59,102 in these cells. The C-3 C ring hydroxyl (Figure 2) and metabolic sulphation which were not necessary for potent inhibition of platelet function were reported to be important for inhibiting platelet signalling.70 These data are in agreement with earlier studies demonstrating that poly-hydroxylation of flavonoids containing planar C rings were linked to potent inhibition of PI3K,71,103,104 PKC,103,104 and PIM1105 activity. Further support for these findings was reported in structure-activity studies which demonstrated that removal of hydroxyl substituents from the C and B rings was attributed to decreased inhibitory potency of flavonoids for tyrosine kinase activity of the oncogene product, pp130fps.106 Saturation of the C-2–C-3 double bond in addition to exclusion of C and B ring hydroxyl groups (Figure 2) were correlated with the reduced ability of flavonoids to inhibit PI3Kα103 activity, G type (ATP and GTP-dependent) casein kinase107 activity, and the catalytic activity of FOF1 rotary motors in mitochondria.108
Evidence has been previously reported demonstrating that polyphenols display a degree of selectivity. An unmodified flavonol, quercetagetin, was reported to be a more selective inhibitor of the oncogene pim-1 kinase than pim-2 than quercetin, due to an A ring hydroxyl substitution.105 Removal of the C ring C-3 and B ring C-3′ hydroxyls (apigenin) and addition of a C-4′ methyl group to the B ring of quercetin (Figure 2) (tamarixetin) correlated with moderate potency inhibition of Syk and low-potency inhibition of Fyn70 involved in GPVI signalling. Therefore, apigenin and tamarixetin may preferentially inhibit Syk with greater potency than Fyn.
Inconsistencies between studies in the observed potency of flavonoids have been reported. Using quercetin as a reference compound that inhibits platelet function with high potency (low µm–nm concentrations), previous studies have shown that quercetin inhibits platelet function with intermediate (20–40 µm) potency59 with apigenin as a more potent inhibitor of thrombus formation in whole blood than quercetin.53 These differences in potency are possibly due to interactions with plasma proteins in platelet preparations.53,59 Certainly, inhibition of platelet function by flavonoids and their metabolites in the circulation may be influenced by several interrelated associations. Flavonoids and their biological metabolites bind to the major plasma carrier protein, human serum albumin, with differential affinities (order of affinity: tamarixetin > quercetin > naringenin > catechin)109 and erythrocytes have been shown to internalize flavonols and flavones to varying degrees in a manner that correlated with structural features.78,80 The main objective of these types of studies is to identify structural features which underlie flavonoid potency, to indicate the manner these compounds may be translated into more selective compounds. Therefore, dietary relevant concentrations or conjugates of flavonoids are not considered. Activity in plasma is, however, an important consideration when measuring flavonoid in vivo effects.
With identification of discrete elements within polyphenol structures that confer potency and selectivity, a focus for beginning to construct analogues is established. The next step will be to explore molecular interactions of flavonoids with kinases to understand the manner that these compounds are positioned within catalytic sites to achieve their effects.
2.2. Structural and computational approaches to understand flavonoid inhibitory mechanisms
Flavonoids have been suggested as mapping agents to guide the development of molecular probes for enzyme/kinase catalytic sites106,107 which determine structural requirements for potent and potentially selective inhibition. A range of functionally diverse kinases (PI3K,71,103,104 PIM1, and PIM2,105 myosin light chain-kinase,106 casein kinase I and II,106 PKC,106 and PKA106) with a central involvement in the growth, proliferation, and functional maintenance of nucleated cells, and key regulatory roles in signal transduction in platelets have been incorporated into initial studies of this nature.
X-ray crystallographic analyses of kinase-flavonoid co-crystals demonstrated that the flavonoid ring systems and their hydroxyl substitutions are involved profoundly in the binding of these compounds to Src-family kinases (Hck),110 lipid kinases (PI3Kγ),111 and serine/threonine kinases (PIM1).105 A quercetin molecule bound to PI3Kγ111 and PIM1105 via an aspartic acid (Asp 964) residue involved in ring stacking with the flavan B ring (Figure 2), and the formation of hydrogen bonds with hydroxyl oxygens on the same ring. The involvement of the C ring C-3 hydroxyl in direct-binding associations was shown as the formation of a hydrogen bond between the functional group and glycine 344 within the crystal structure of Hck.11In contrast, van der Waals interactions occurred between this same functional group on the quercetin molecule and glutamic acid residues within PI3Kγ (Glu 880)111 and PIM1 (Glu 121)105 ATP-binding sites were observed, illustrating redundancy in the interactions with the flavonol C ring hydroxyl (Figure 2) between distinct residues on structurally divergent proteins.
Hydroxyl substituents may also influence flavonoid orientations within binding sites as those varied between flavonoids with unique hydroxylation patterns as well as between different classes of proteins. Quercetin bound to the Hck substrate binding groove show a B ring that orientates towards the solvent,110 whereas the B ring of quercetin bound to PI3Kγ is flipped inside the ATP-binding pocket.111 Interestingly, myricetin (flavonol-B ring hydroxyl groups at C-3′, C-4′, and C-5′) (Figure 2) co-crystallized within the PIM1 ATP-binding site105 was positioned in a similar orientation as quercetin within Hck,110 and was bound to PI3Kγ in a manner that orientated the A ring instead of the B ring towards the solvent.111
The less effective ability of a flavonoid with a non-planar C ring (catechin) to inhibit kinase activity is supported by molecular studies. Within the ATP-binding site of DNA gyrase, EGCG [consisting of a structural homologue of catechin (epicatechin)] was orientated in a manner opposite to that of quercetin,112 and a network of hydrogen bonds was formed between the flavonol and the neighbouring residues,113 but hydrogen bonds only formed between residues and the B ring of the epicatechin moiety.113
Computational tools have also been invaluable for understanding interactions between polyphenols and kinases. Analyses using the GRID algorithm (maps energy values of a functional group throughout and around kinase-binding sites)114 and molecular docking indicated potentially significant variations in binding modes of structurally congeneric flavonoids (quercetin, catechin, and apigenin) in Src-family kinase ATP-binding sites, which was validated with biological data (Wright and Gibbins, 2012, unpublished data). Reports of similar approaches demonstrate that myricetin, which is known to inhibit the cell cycle115 exerts inhibitory effects on cell proliferation by targeting Raf-1.116 Docking studies were performed to understand how myricetin binds to Raf1 without competing with ATP; B-Raf that is highly homologous to Raf-1 was used as the structure of Raf-1 was not available.116 A predicted ternary complex comprising B-Raf, ATP, and myricetin was constructed with myricetin docked to the pocket distinct from, but adjacent to, the ATP-binding site of B-Raf. The hydroxyl groups at positions 3, 5, and 7 of myricetin form hydrogen bonds with the side chains of Lys482, Thr528, and Thr507, respectively, and hydrophobic interactions were formed with Leu504 and Val503.116
Other computational studies have demonstrated flavonoid interactions with housekeeping proteins. By means of molecular-docking operations, stable structures for the binding of the flavone, chrysin to calmodulin were approximated.117 Chrysin was situated in the region that allowed maximum contact with the side chains of the receptor and formation of proper bonds to stabilize the CaM–chrysin complex.117 The phosphodiesterase 4B2 catalytic site was characterized as capable of attracting a flavonoid molecule with electronegative surface charges both sterically and electrostatically.73
Monomeric flavonoids were shown in molecular-docking studies to be accommodated in specific binding sites found on serine proteases involved in blood coagulation and the inflammatory response.118 Larger polyphenols were reported to non-specifically hinder the catalytic pocket serine proteases. The phenyl-ring in position 2 of the phenyl-benzogamma-pyranic core, the ketone carbonyl in position 4, hydroxyl groups in 3, 5, 7, and 3′ (and/or 5′), and 4′ position and C ring 2–3 double-bond were critical for inhibitory activity118 (Figure 2). A pharmacophore model classified the carbonyl group at position 4 as a H-bond acceptor towards Ser 195 acting as the H-bond donor.118
These structural studies prove that the tools for derivation of flavonoid-based drugs are available. At present, coherent methodology consisting of appropriate workflow modules must be developed to correctly translate these compounds into therapeutic agents.
3. The flavonoid structure as a template for drug design
The translation of flavonoids into more potent and selective small-molecules of potential therapeutic value has already begun. Quercetin is the main flavonoid used as a template for drug design. Analogues including LY294002,119,120 quercetin-3-O-amino-esters,121 and penta-O-substituted quercetin analogues119 were previously synthesized using the structure of this flavonol as a foundation (Figure 3).
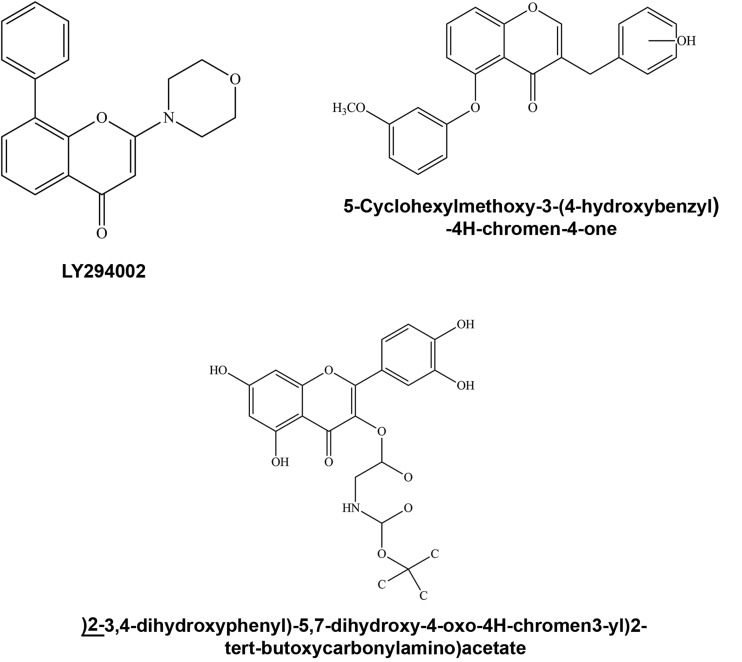
Figure 3.
Flavonoid analogues. Analogues based on quercetin (LY294002 and quercetin-3-O-amino-esters) and the flavonoid chromone moeity (5-cyclohexylmethoxy-3-(4-hydroxybenzyl)-4H-chromen-4-one).
Substitution of the C-8 position of the A ring with a benzene ring and omission of hydroxyl groups (LY294002) enhanced both potency and specificity for inhibition of PI3K. The small-molecule inhibitor was greater than two-fold (IC50 –1.4 μm) more potent than quercetin (IC50–3.8 μm) for blocking the activity of PI3K.120 LY294002 was reported as selective for PI3K, due to complete inhibition by the compound, but PI4K, PKC, PKA, MAPK, S6 kinase, epidermal growth factor receptor tyrosine kinase activity, DAG kinase, or ATPase were not inhibited.120 Addition of a methyl group to the C-7 position on the A ring (LY805921) did not lower inhibitory potency to as great an extent as a poly-hydroxylated and methylated heterocycle at the C ring C-3 position (LY802132) (Figure 2) or removal of the B ring catechol moiety (LY002079).119
Isoflavonone and isoflavanone compounds have also been used as templates for the design of more potent analogues for inhibiting interleukin activity122,123 (Figure 3). Planarity of the chromen-4-one ring (Figure 2), a phenolic hydroxyl at the C-4 position on the B ring and a benzyloxy at the C-5 position were identified as functional groups necessary for high potency (IC50 of 15.3 µM) inhibition of interleukin-5 (IL-5).123 Chromone analogues synthesized based on these data included 5-cyclohexylmethoxy-3-(4-hydroxybenzyl)-4H-chromen-4-one and 5-cyclohyxylmethoxy-3-(hydroxymethyl)-4H-chromen4-one. The analogues inhibited IL-5 activity with higher potencies (IC50 of 3 and 7.6 µm, respectively) than parent compounds.122
Although these reported studies provide useful information, they did not explore the diversity of the flavonoid family of compounds. Strategies for comprehensive exploitation of polyphenolic compounds could involve screens of a range of compounds with clear structural differences, against key kinases involved in cell signalling.124–126 Previous reports employing this approach have focused on high-throughput screens of quercetin inhibition of Src and MAPK activity.124 These approaches may involve the development of flavonoid-centred combinatorial libraries.
4. Conclusions
The scene is set for drug discovery through exploitation of dietary polyphenols. Although, long-term dietary consumption of flavonoids as a means to lower CVD risk is still an option, barriers to increasing consumption of flavonoid rich foods, particularly fruits and vegetables, and possible lack of specificity could have a significant impact on the efficacy of this strategy for risk reduction.
To explore the potential of these compounds as templates applied to the construction of potent, selective small molecule inhibitors, thorough screenings of their structural interactions with molecular targets are necessary. This approach can be accelerated through the use of computational tools together with structural assays, before follow-up investigations involving biological validation of actual/approximated interactions between kinases and polyphenols. This platform of methodologies may include modelled kinases as well as those with solved structures, and therefore allow the inclusion of as broad a range of kinases as possible. Furthermore, this rational screening strategy performed to understand features on the flavonoid structure which confer selectivity may involve recombinant kinase libraries and model cell systems (e.g. platelets).
The development of a new generation of flavonoid-based inhibitory agents may overcome the common problem encountered with the small therapeutic window of or lack of efficacy of existing anti-platelet drugs.
Conflict of interest: none declared.
Funding
Research in the authors’ laboratory is supported by grants from the Medical Research Council, British Heart Foundation, Wellcome Trust, and the Biotechnology and Biological Sciences Research Council.
References
- 1.Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379–2383. doi:10.1021/jf00024a011. [Google Scholar]
- 2.Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J Chromatogr A. 1998;799:101–110. doi: 10.1016/s0021-9673(97)01061-3. doi:10.1016/S0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 3.Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. The Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. doi:10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 4.Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–595. doi:10.1021/jf960339y. [Google Scholar]
- 5.Macheix JJ, Fleuriet A, Billot J. Fruit Phenolics. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- 6.Hollman PCH, Arts ICW. Flavonols, flavones and flavanols—nature, occurrence and dietary burden. J Food Sci Agric. 2000;80:1081–1093. [Google Scholar]
- 7.Arts IC, van De Putte B, Hollman PC. Catechin contents of foods commonly consumed in the Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J Agric Food Chem. 2000;48:1752–1757. doi: 10.1021/jf000026+. doi:10.1021/jf000026+ [DOI] [PubMed] [Google Scholar]
- 8.Mouly PP, Arzouyan CR, Gaydou EM, Estienne JM. Differentiation of citrus juices by factorial discriminant analysis using liquid chromatography of flavanone glycosides. J Agric Food Chem. 1994;42:70–79. doi:10.1021/jf00037a011. [Google Scholar]
- 9.Tomas-Barberan FA, Clifford MN. Dietary hydroxybenzoic acid derivatives and their possible role in health protection. J Sci Food Agric. 2000;80:1024–1032. doi:10.1002/(SICI)1097-0010(20000515)80:7<1024::AID-JSFA567>3.0.CO;2-S. [Google Scholar]
- 10.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124:825–832. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Wang HJ, Murphy PA, Hendrich S. Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. J Nutr. 2000;130:798–801. doi: 10.1093/jn/130.4.798. [DOI] [PubMed] [Google Scholar]
- 12.Mazza G, Maniati E. Anthocyanins in Fruits, Vegetables, and Grains. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 13.Keli SO, Hertog MGL, Feskens EJM, Kromhout D. Dietary flavonoids, antioxidant vitamins, and the incidence of stroke. Arch Int Med. 1996;154:637–642. [PubMed] [Google Scholar]
- 14.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. doi:10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and risk of ischaemic stroke and CVD mortality in middle aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2008;100:890–895. doi: 10.1017/S0007114508945694. [DOI] [PubMed] [Google Scholar]
- 16.Sagara M, Kanda T, NJelekera M, Teramoto T, Armitage L, Birt N, et al. Effects of dietary intake of soy protein and isoflavones on cardiovascular disease risk factors in high risk, middle-aged men in Scotland. J Am Coll Nutr. 2004;23:85–91. doi: 10.1080/07315724.2004.10719347. [DOI] [PubMed] [Google Scholar]
- 17.de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favourable metabolic cardiovascular risk profile in postmenopausal US women: the Framingham study. J Nutr. 2002;132:276–282. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 18.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. doi:10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagiou P, Samoli E, Lagiou A, Skalkidis Y, Katsouyanni K, Petridou E, et al. Flavonoid classes and risk of peripheral arterial occlusive disease: a case-control study in Greece. Eur J Clin Nutr. 2006;60:214–219. doi: 10.1038/sj.ejcn.1602291. doi:10.1038/sj.ejcn.1602291. [DOI] [PubMed] [Google Scholar]
- 20.Mennen LI, Sapinho D, de Bree A, Arnault N, Bertrais S, Galan P, et al. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr. 2004;134:923–926. doi: 10.1093/jn/134.4.923. [DOI] [PubMed] [Google Scholar]
- 21.Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 22.Van der Gaag MS, Van der Berg R, Van der Berg H, Schaafsma G, Hendriks HFJ. Moderate consumption of beer, red wine and spirits has counteracting effects on plasma antioxidants in middle-aged men. Eur J Clin Nutr. 2000;54:586–591. doi: 10.1038/sj.ejcn.1601061. doi:10.1038/sj.ejcn.1601061. [DOI] [PubMed] [Google Scholar]
- 23.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. doi:10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 24.Chiva-Blanch G, Urpi-Sarda M, Lorach R, Rotches-Ribalta M, Guillen M, Casas R, et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: a randomized clinical trial. Am J Clin Nutr. 2012;95:326–334. doi: 10.3945/ajcn.111.022889. doi:10.3945/ajcn.111.022889. [DOI] [PubMed] [Google Scholar]
- 25.Desch S, Kobler D, Schmidt J, Sonnabend M, Adams V, Sareban M, et al. Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients . Am J Hypertens. 2010;23:694–700. doi: 10.1038/ajh.2010.29. [DOI] [PubMed] [Google Scholar]
- 26.Errichi BM, Belcaro G, Hosoi M, Cesarone MR, Dugall M, Feragalli B, et al. Prevention of post thrombotic syndrome with Pycnogenol® in a twelve month study. Panminerva Med. 2011;53(Suppl . 1):21–27. [PubMed] [Google Scholar]
- 27.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–940. doi: 10.3945/ajcn.110.004242. doi:10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weseler AR, Ruijters EJ, Drittij-Reijnders MJ, Reesink KD, Haenen GR, Bast A. Pleiotropic benefit of monomeric and oligomeric flavonols on vascular health – a randomized controlled clinical pilot study. PLoSOne. 2011;6:e28460. doi: 10.1371/journal.pone.0028460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choquette S, Riesco E, Cormier E, Dion T, Aubertin-Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr. 2011;105:1199–1209. doi: 10.1017/S0007114510004897. doi:10.1017/S0007114510004897. [DOI] [PubMed] [Google Scholar]
- 30.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131:235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 31.Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metab. 2006;50:167–172. doi: 10.1159/000090736. doi:10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–2145. doi: 10.1111/j.1538-7836.2004.01067.x. doi:10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- 33.Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, et al. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavonol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 34.Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, et al. Pharmacokinetics and Bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. doi:10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- 35.Donovan JL, Bell JR, Kasim-Karakas S, German JB, Walzem RL, Hansen RJ, et al. Catechin is present as metabolites in human plasma after consumption of red wine. J Nutr. 1999;129:1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- 36.Bell JRC, Donovan JL, Wong R, Waterhouse AL, German JB, Walzem RL, et al. (+)-catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am J Clin Nutr. 2000;71:103–108. doi: 10.1093/ajcn/71.1.103. [DOI] [PubMed] [Google Scholar]
- 37.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Int Med. 1996;125:384–389. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. doi:10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra S, Bailey DG, Paine MF, Watkins PB. Seville orange juice-felodipine interaction: comparison with dilute grapefruit juice and involvement of furocoumarins. Clin Pharmacol Ther. 2001;69:14–23. doi: 10.1067/mcp.2001.113185. doi:10.1067/mcp.2001.113185. [DOI] [PubMed] [Google Scholar]
- 40.Lilja JJ, Juntti-Patinen L, Neuvonen PJ. Orange juice substantially reduces the bioavailability of the β-adrenergic–blocking agent celiprolol. Clin Pharmacol Ther. 2004;75:184–190. doi: 10.1016/j.clpt.2003.11.002. doi:10.1016/j.clpt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Bailey DG, Dresser GK, Kreeft JH, Munoz C, Freeman DJ, Bend JR. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68:468–477. doi: 10.1067/mcp.2000.110774. doi:10.1067/mcp.2000.110774. [DOI] [PubMed] [Google Scholar]
- 42.Patrignani P. Aspirin insensitive eicosanoid biosynthesis in cardiovascular disease. Thromb Res. 2003;110:281–286. doi: 10.1016/s0049-3848(03)00382-7. doi:10.1016/S0049-3848(03)00382-7. [DOI] [PubMed] [Google Scholar]
- 43.Barrett NE, Holbrook L, Jones S, Kaiser WJ, Moraes LA, Rana R, et al. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154:918–939. doi: 10.1038/bjp.2008.151. doi:10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 45.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. doi:10.1016/S0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 46.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. doi:10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michelson AD. P2Y12 antagonism: promises and challenges. Arterioscler Thromb Vasc Biol. 2008;28:S33–S38. doi: 10.1161/ATVBAHA.107.160689. doi:10.1161/ATVBAHA.107.160689. [DOI] [PubMed] [Google Scholar]
- 48.Angiolillo DJ, Bates ER, Bass TA, Arbor A. Clinical profile of prasugrel, a novel thienopyridine. Am Heart J. 2008;156:S16–S22. doi: 10.1016/j.ahj.2008.06.005. doi:10.1016/j.ahj.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine anti-platelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25:357–374. doi: 10.1111/j.1527-3466.2007.00027.x. doi:10.1111/j.1527-3466.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 50.Crescente M, Jessen G, Momi S, Holtje HD, Gresele P, Cerletti C, et al. Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level. Functional and modelling studies. Thromb Haemost. 2009;102:336–346. doi: 10.1160/TH09-01-0057. [DOI] [PubMed] [Google Scholar]
- 51.Pearson DA, Paglieroni TG, Rein D, Wun T, Schramm DD, Wang JF, et al. The effects of flavonol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106:191–197. doi: 10.1016/s0049-3848(02)00128-7. doi:10.1016/S0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- 52.Zubair MH, Zubair MH, Zubair MN, Aftab T, Asad F. Augmentation of anti-platelet effects of aspirin. J Pak Med Assoc. 2011;61:304–307. [PubMed] [Google Scholar]
- 53.Navarro-Nunez L, Lozano ML, Palomo M, Martinez C, Vicente V, Castillo J, et al. Apigenin inhibits platelet adhesion and thrombus formation and synergizes with aspirin in the suppression of the arachidonic acid pathway. J Agric Food Chem. 2008;56:2970–2976. doi: 10.1021/jf0723209. doi:10.1021/jf0723209. [DOI] [PubMed] [Google Scholar]
- 54.Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. doi:10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Perron NR, Garcia CR, Pinzon JJR, Chaur MN, Brumaghim JL. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J Inorg Biochem. 2011;105:745–753. doi: 10.1016/j.jinorgbio.2011.02.009. doi:10.1016/j.jinorgbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Freedman JE, Parker C, Li L, Perlman JA, Frei B, Ivanov V, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. doi:10.1161/01.CIR.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 57.Pignatelli P, Ghiselli A, Buchetti B, Carnevale R, Natella F, Germano G, et al. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atheroscler. 2006;188:77–83. doi: 10.1016/j.atherosclerosis.2005.10.025. doi:10.1016/j.atherosclerosis.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 58.Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, et al. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- 59.Guerrero JA, Lozano ML, Castillo J, Benavente-Garcia O, Vicente V, Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. doi:10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson KA, Moro S, Manthey JA, West PL, Ji XD. Interactions of flavones and other phytochemicals with adenosine receptors. Adv Exp Med Biol. 2002;505:163–171. doi: 10.1007/978-1-4757-5235-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji XD, Melman N, Jacobson KA. Interactions of flavonoids and other phytochemicals with adenosine receptors. J Med Chem. 1996;39:781–788. doi: 10.1021/jm950661k. doi:10.1021/jm950661k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta K, Panda D. Perturbation of microtubule polymerisation by quercetin through tubulin binding: a novel mechanism of its antiproliferative activity. Biochem. 2002;41:13029–13038. doi: 10.1021/bi025952r. doi:10.1021/bi025952r. [DOI] [PubMed] [Google Scholar]
- 63.Bohl M, Czupalla C, Tokalov SV, Hoflack B, Gutzeit HO. Identification of actin as quercetin-binding protein: an approach to identify target molecules for specific ligands. Anal Biochem. 2005;346:295–299. doi: 10.1016/j.ab.2005.08.037. doi:10.1016/j.ab.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 64.Pawlikowska-Pawlega B, Gruszecki WI, Misiak L, Paduch R, Piersiak T, Zarzyka B, et al. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim Biophys Acta. 2007;1768:2195–2204. doi: 10.1016/j.bbamem.2007.05.027. doi:10.1016/j.bbamem.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects International. Int J Biol Macromol. 2007;41:42–48. doi: 10.1016/j.ijbiomac.2006.12.003. doi:10.1016/j.ijbiomac.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Oak MH, Chataigneau M, Keravis T, Chataigneau T, Beretz A, Andriantsitohaina R, et al. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1001–1007. doi: 10.1161/01.ATV.0000070101.70534.38. doi:10.1161/01.ATV.0000070101.70534.38. [DOI] [PubMed] [Google Scholar]
- 67.Kobuchi H, Roy S, Sen CK, Nguyen HG, Packer L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol. 1999;277:C403–C411. doi: 10.1152/ajpcell.1999.277.3.C403. [DOI] [PubMed] [Google Scholar]
- 68.Lill G, Voit S, Schror K, Weber AA. Complex effects of different green tea catechins on human platelets. FEBS Lett. 2003;546:265–270. doi: 10.1016/s0014-5793(03)00599-4. doi:10.1016/S0014-5793(03)00599-4. [DOI] [PubMed] [Google Scholar]
- 69.Maeda-Yamamoto M, Inagaki N, Kitaura J, Chikumoto T, Kawahara H, Kawakami Y, et al. O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells. J Immunol. 2004;172:4486–4492. doi: 10.4049/jimmunol.172.7.4486. [DOI] [PubMed] [Google Scholar]
- 70.Wright B, Moraes LA, Kemp CF, Mullen W, Crozier A, Lovegrove JA, et al. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. Br J Pharmacol. 2010;159:1312–1325. doi: 10.1111/j.1476-5381.2009.00632.x. doi:10.1111/j.1476-5381.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hubbard GP, Stevens JM, Cicmil M, Sage T, Jordan PA, Williams CM, et al. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signalling pathway. J Thromb Haemost. 2003;1:1079–1088. doi: 10.1046/j.1538-7836.2003.00212.x. doi:10.1046/j.1538-7836.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Han G, Fan B, Zhou Y, Zhou X, Wei L, et al. Green tea (-)-epigallocatechin-3-gallate down-regulates VASP expression and inhibits breast cancer cell migration and invasion by attenuating Rac1 activity. Eur J Pharmacol. 2009;606:172–179. doi: 10.1016/j.ejphar.2008.12.033. doi:10.1016/j.ejphar.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 73.Peluso MR. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. Exp Biol Med. 2006;231:1287–1299. doi: 10.1177/153537020623100802. [DOI] [PubMed] [Google Scholar]
- 74.Deana R, Turetta L, Donella-Deana A, Donà M, Brunati AM, De Michiel L, et al. Green tea epigallocatechin-3-gallate inhibits platelet signalling pathways triggered by both proteolytic and non-proteolytic agonists. Thromb Haemost. 2003;89:866–874. [PubMed] [Google Scholar]
- 75.Rawel HM, Meidtner K, Kroll J. Binding of selected phenolic compounds to proteins. J Agric Food Chem. 2005;53:4228–4235. doi: 10.1021/jf0480290. doi:10.1021/jf0480290. [DOI] [PubMed] [Google Scholar]
- 76.Wright B, Gibson T, Spencer J, Lovegrove JA, Gibbins JM. Platelet-mediated metabolism of the common dietary flavonoid, quercetin. PLoS One. 2010;5:e9673. doi: 10.1371/journal.pone.0009673. doi:10.1371/journal.pone.0009673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gryglewski RJ, Korbut R, Robak J, Swies J. On the mechanism of antithrombotic action of flavonoids. Biochem Pharmacol. 1987;36:317–322. doi: 10.1016/0006-2952(87)90288-7. doi:10.1016/0006-2952(87)90288-7. [DOI] [PubMed] [Google Scholar]
- 78.Fiorani M, Accorsi A, Cantoni O. Human red blood cells as a natural flavonoid reservoir. Free Rad Res. 2003;37:1331–1338. doi: 10.1080/10715760310001615998. doi:10.1080/10715760310001615998. [DOI] [PubMed] [Google Scholar]
- 79.Fiorani M, De Sanctis R, De Bellis R, Dacha M. Intracellular flavonoids as electron donors for extracellular ferricyanide reduction in human erythrocytes. Free Rad Biol Med. 2002;32:64–72. doi: 10.1016/s0891-5849(01)00762-6. doi:10.1016/S0891-5849(01)00762-6. [DOI] [PubMed] [Google Scholar]
- 80.Fiorani M, Accorsi A. Dietary flavonoids as intracellular substrates for an erythrocyte trans-plasma membrane oxidoreductase activity. Br J Nutr. 2005;94:338–345. doi: 10.1079/bjn20051504. doi:10.1079/BJN20051504. [DOI] [PubMed] [Google Scholar]
- 81.Cerecedo D, Stock R, González S, Reyes E, Mondragón R. Modification of actin, myosin and tubulin distribution during cytoplasmic granule movements associated with platelet adhesion. Haematologica. 2002;87:1165–1176. [PubMed] [Google Scholar]
- 82.Berry S, Dawicki DD, Agarwal KC, Steiner M. The role of microtubules in platelet secretory release. Biochim Biophys Acta. 1989;1012:46–56. doi: 10.1016/0167-4889(89)90009-8. doi:10.1016/0167-4889(89)90009-8. [DOI] [PubMed] [Google Scholar]
- 83.Pula G, Schuh K, Nakayama K, Nakayama KI, Walter U, Poole AW. PKCdelta regulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035–4044. doi: 10.1182/blood-2006-05-023739. doi:10.1182/blood-2006-05-023739. [DOI] [PubMed] [Google Scholar]
- 84.Brown JE, Khodr H, Hider RC, Rice-Evans CA. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem J. 1998;330:1173–1178. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Justino GC, Santos MR, Canario S, Borges C, Florencio MH, Mira L. Plasma quercetin metabolites: structure-antioxidant activity relationships. Arch Biochem Biophys. 2004;432:109–121. doi: 10.1016/j.abb.2004.09.007. doi:10.1016/j.abb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. doi:10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 87.Van Acker SABE, Van Den Berg DJ, Tromp MNJL, Griffoen DH, Van Bennekom WP, Van Der Vijgh WJF, et al. Structural aspects of antioxidant activity of flavonoids. Free Rad Biol Med. 1996;20:275–283. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 88.Bors W, Heller W, Michel C, Saran M. Radical chemistry of flavonoid antioxidants. Adv Exp Med Biol. 1990;264:165–170. doi: 10.1007/978-1-4684-5730-8_25. doi:10.1007/978-1-4684-5730-8_25. [DOI] [PubMed] [Google Scholar]
- 89.Velioglu YS, Mazza G, Goa L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46:4113–4117. doi:10.1021/jf9801973. [Google Scholar]
- 90.Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Rad Res. 1999;30:153–162. doi: 10.1080/10715769900300161. doi:10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- 91.Rice-Evans C, Miller NJ. Antioxidants—the case for fruits and vegetables in the diet. Br Food J. 1995;97:35–40. doi:10.1108/00070709510100163. [Google Scholar]
- 92.Kanner J, Franke E, Granit R, German B, Kinsella JE. Natural antioxidants in grapes and wines. J Agric Food Chem. 1994;42:64–69. doi:10.1021/jf00037a010. [Google Scholar]
- 93.De Whalley CV, Rankin SM, Hoult RS, Jessup W, Leake DS. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem Pharmacol. 1990;39:1743–1750. doi: 10.1016/0006-2952(90)90120-a. doi:10.1016/0006-2952(90)90120-A. [DOI] [PubMed] [Google Scholar]
- 94.Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr. 1998;128:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto N, Moon JH, Tsushida T, Nagao A, Terao J. Inhibitory effect of quercetin metabolites and their related derivatives on copper ion-induced lipid peroxidation in human low-density lipoprotein. Arch Biochem Biophys. 1999;372:347–354. doi: 10.1006/abbi.1999.1516. doi:10.1006/abbi.1999.1516. [DOI] [PubMed] [Google Scholar]
- 96.Janisch MK, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Rad Res. 2004;38:877–884. doi: 10.1080/10715760410001728415. doi:10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- 97.Harada M, Kan Y, Naoki H, Fukui Y, Kageyama N, Nakai M, et al. Identification of the major antioxidant metabolites in biological fluids of the rat with ingested (+)-catechin and (−)-epicatechin. Biosci Biotech Biochem. 1999;63:973–977. doi: 10.1271/bbb.63.973. doi:10.1271/bbb.63.973. [DOI] [PubMed] [Google Scholar]
- 98.Beretz A, Cazenave JP, Anton R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: Structure-activity relationships. Agents Actions. 1982;12:382–387. doi: 10.1007/BF01965408. doi:10.1007/BF01965408. [DOI] [PubMed] [Google Scholar]
- 99.Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids: Structure-activity relations. Biochem Pharmacol. 1984;33:1525–1530. doi: 10.1016/0006-2952(84)90423-4. doi:10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- 100.Navarro-Nunez L, Castillo J, Lozano ML, Martinez C, Benavente-Garcia O, Vicente V, et al. Thromboxane A2 receptor antagonism by flavonoids: structure-activity relationships. J Agric Food Chem. 2009;57:1589–1594. doi: 10.1021/jf803041k. doi:10.1021/jf803041k. [DOI] [PubMed] [Google Scholar]
- 101.Guerrero JA, Navarro-Nunez L, Lozano ML, Martinez C, Vicente V, Gibbins JM, et al. Flavonoids inhibit the platelet TxA(2) signalling pathway and antagonise TxA(2) receptors (TP) in platelets and smooth muscle cells. Br J Clin Pharmacol. 2007;64:133–144. doi: 10.1111/j.1365-2125.2007.02881.x. doi:10.1111/j.1365-2125.2007.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Navarro-Nunez L, Rivera J, Guerrero JA, Martinez C, Vicente V, Lozano ML. Differential effects of quercetin apigenin and genistein on signalling pathways of protease-activated receptors PAR(1) and PAR(4) in platelets. Br J Pharmacol. 2009;158:1548–1556. doi: 10.1111/j.1476-5381.2009.00440.x. doi:10.1111/j.1476-5381.2009.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, et al. Relationship between flavonoids structure and inhibition of phosphatidylinositol 3-kinase: A comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. doi:10.1016/S0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 104.Gamet-Payrastre L, Manenti S, Gratacap MP, Tulliez J, Chap H, Payrastre B. Flavonoids and the inhibition of PKC and PI 3-kinase. Gen Pharmacol. 1999;32:279–286. doi: 10.1016/s0306-3623(98)00220-1. doi:10.1016/S0306-3623(98)00220-1. [DOI] [PubMed] [Google Scholar]
- 105.Holder S, Zemskova M, Zhang C, Tabrizizad M, Bremer R, Neidigh JW, et al. Characterisation of a potent and selective small-molecule inhibitor of the PIM1 kinase. Mol Cancer Ther. 2007;6:163–172. doi: 10.1158/1535-7163.MCT-06-0397. doi:10.1158/1535-7163.MCT-06-0397. [DOI] [PubMed] [Google Scholar]
- 106.Hagiwara M, Inoue S, Tanaka T, Nunoki K, Ito M, Hidaka H. Differential effects of flavonoids as inhibitors of tyrosine protein kinases and serine/threonine protein kinases. Biochem Pharmacol. 1988;37:2987–2992. doi: 10.1016/0006-2952(88)90286-9. doi:10.1016/0006-2952(88)90286-9. [DOI] [PubMed] [Google Scholar]
- 107.Cochet C, Feige JJ, Pirollet F, Keramidas M, Chambaz EM. Selective inhibition of a cyclic nucleotide independent protein kinase (G-type casein kinase) by quercetin and related polyphenols. Biochem Pharmacol. 1982;31:157–1361. doi: 10.1016/0006-2952(82)90028-4. doi:10.1016/0006-2952(82)90205-2. [DOI] [PubMed] [Google Scholar]
- 108.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. doi:10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dufour C, Dangles O. Flavonoid–serum albumin complexation: determination of binding constants and binding sites by fluorescence spectroscopy. Biochim Biophys Acta. 2005;1721:164–173. doi: 10.1016/j.bbagen.2004.10.013. doi:10.1016/j.bbagen.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 110.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. doi:10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 111.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. doi:10.1016/S1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 112.Plaper A, Golob M, Hafner I, Oblak M, Solmajer T, Jeralab R. Characterization of quercetin binding site on DNA gyrase. Biochem Biophys Res Commun. 2003;306:530–536. doi: 10.1016/s0006-291x(03)01006-4. doi:10.1016/S0006-291X(03)01006-4. [DOI] [PubMed] [Google Scholar]
- 113.Gradisar H, Pristovsek P, Plaper A, Jerala R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J Med Chem. 2007;50:264–271. doi: 10.1021/jm060817o. doi:10.1021/jm060817o. [DOI] [PubMed] [Google Scholar]
- 114.Goodford PJ. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem. 1985;28:849–857. doi: 10.1021/jm00145a002. doi:10.1021/jm00145a002. [DOI] [PubMed] [Google Scholar]
- 115.Zhang XH, Zou ZQ, Xu CW, Shen YZ, Li D. Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting the activity of the cyclin B/Cdc2 complex. Mol Med Report. 2011;4:273–272. doi: 10.3892/mmr.2011.417. [DOI] [PubMed] [Google Scholar]
- 116.Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010;79:1455–1461. doi: 10.1016/j.bcp.2010.01.004. doi:10.1016/j.bcp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li L, Wei DQ, Wang JF, Chou KC. Computational studies of the binding mechanism of calmodulin with chrysin. Biochem Biophys Res Commun. 2007;358:1102–1107. doi: 10.1016/j.bbrc.2007.05.053. doi:10.1016/j.bbrc.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 118.Cuccioloni M, Mozzicafreddo M, Bonfili L, Cecarini V, Eleuteri AM, Angeletti M. Natural occurring polyphenols as template for drug design. Focus on serine proteases. Chem Biol Drug Des. 2009;74:1–15. doi: 10.1111/j.1747-0285.2009.00836.x. doi:10.1111/j.1747-0285.2009.00836.x. [DOI] [PubMed] [Google Scholar]
- 119.Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Comm. 1992;186:624–631. doi: 10.1016/0006-291x(92)90792-j. doi:10.1016/0006-291X(92)90792-J. [DOI] [PubMed] [Google Scholar]
- 120.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 121.Huang H, Jia Q, Ma J, Qin G, Chen Y, Xi Y, et al. Discovering novel quercetin-3-O-amino acid-esters as a new class of Src tyrosine kinase inhibitors. Eur J Med Chem. 2009;44:1982–1989. doi: 10.1016/j.ejmech.2008.09.051. doi:10.1016/j.ejmech.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thanigaimalai P, Le Hoang TA, Lee KC, Sharma VK, Bang SC, Yun JH, et al. Synthesis and evaluation of novel chromone analogs for their inhibitory activity against interleukin-5. Eur J Med Chem. 2010;45:2531–2536. doi: 10.1016/j.ejmech.2010.02.041. doi:10.1016/j.ejmech.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 123.Jung SH, Cho SH, Dang TH, Lee JH, Ju JH, Kim MK, et al. Structural requirement of isoflavonones for the inhibitory activity of interleukin-5. Eur J Med Chem. 2003;38:537–545. doi: 10.1016/s0223-5234(03)00064-3. doi:10.1016/S0223-5234(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 124.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. doi:10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bain J, McLauchlan H, Elliott M, Cohen P. The selectivity of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. doi:10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. doi:10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]