Abstract
BACKGROUND
A recent study showed that methylphenidate induces emergence from isoflurane anesthesia. Methylphenidate inhibits dopamine and norepinephrine reuptake transporters. The objective of this study was to test the hypothesis that selective dopamine receptor activation induces emergence from isoflurane anesthesia.
METHODS
In adult rats, we tested the effects of chloro-APB (D1 agonist) and quinpirole (D2 agonist) on time to emergence from isoflurane general anesthesia. We then performed a dose–response study to test for chloro-APB-induced restoration of righting during continuous isoflurane anesthesia. SCH-23390 (D1 antagonist) was used to confirm that the effects induced by chloro-APB are specifically mediated by D1 receptors. In a separate group of animals, spectral analysis was performed on surface electroencephalogram recordings to assess neurophysiological changes induced by chloro-APB and quinpirole during isoflurane general anesthesia.
RESULTS
Chloro-APB decreased median time to emergence from 330s to 50s. The median difference in time to emergence between the saline control group (n=6) and the chloro-APB group (n = 6) was 222s (95% CI: 77–534s, Mann-Whitney test). This difference was statistically significant (p = 0.0082). During continuous isoflurane anesthesia, chloro-APB dose-dependently restored righting (n = 6) and decreased electroencephalogram delta power (n = 4). These effects were inhibited by pretreatment with SCH-23390. Quinpirole did not restore righting (n = 6) and had no significant effect on the electroencephalogram (n = 4) during continuous isoflurane anesthesia.
CONCLUSIONS
Activation of D1 receptors by chloro-APB decreases time to emergence from isoflurane anesthesia, and produces behavioral and neurophysiological evidence of arousal during continuous isoflurane anesthesia. These findings suggest that selective activation of a D1 receptor-mediated arousal mechanism is sufficient to induce emergence from isoflurane general anesthesia.
INTRODUCTION
The discovery that anesthetic-induced immobility is mediated primarily in the spinal cord1–3 has led to a growing interest in studying anesthetic mechanisms at the level of neural circuits and systems.4,5 Recent studies suggest that the process of emergence from general anesthesia is distinct from the process of induction,6 and in particular, the roles of ascending arousal pathways in emergence from general anesthesia are becoming recognized.4,5,7 Cholinergic,8–10 noradrenergic,11 histaminergic,12,13 and orexinergic14,15 arousal pathways have been implicated in emergence from general anesthesia, but the role of dopamine remains unclear.
It is widely accepted that dopamine plays an important role in behavioral arousal.16–18 Electrolytic lesions of dopaminergic neurons have been shown to induce a coma-like state,19 and mice with selective loss of dopamine in the brain appear hypoactive and apathetic.20 Dopaminergic neurons in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) send projections to key arousal-promoting brain regions including the dorsal raphe, locus ceruleus, pedunculopontine and laterodorsal tegmental areas, basal forebrain, and the perifornical area of the lateral hypothalamus, and in turn, these arousal-promoting centers also send inputs to the VTA and SNc.16 The existence of these projections alone suggests that dopamine is intimately involved in regulating arousal.
Recent studies show that methylphenidate induces emergence from general anesthesia with isoflurane21 and propofol.22 However, methylphenidate is known to inhibit both dopamine and norepinephrine reuptake transporters with similar affinities (Ki = 250 nM and 150 nM, respectively),23 and both are known to promote arousal. The present study was performed in adult rats to test the hypothesis that selective activation of dopaminergic neurotransmission is sufficient to induce emergence from isoflurane general anesthesia. First, we tested the effects of the specific D1 and D2 dopamine receptor agonists chloro-APB and quinpirole, respectively, on time to emergence from a standardized isoflurane general anesthetic. We then tested the behavioral effects of chloro-APB and quinpirole during continuous isoflurane general anesthesia. In a separate group of rats with preimplanted electrodes we recorded the electroencephalogram during isoflurane general anesthesia, and performed spectral analysis to compare the recordings taken before and after dopamine agonist administration.
MATERIALS AND METHODS
Animal Care and Use
All studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (Boston, Massachusetts), which serves as our Institutional Animal Care and Use Committee. Ten male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used for this study. The age range was approximately 3–6 months, and the weight range was 322–565 grams. The same six rats were used in random order for all behavioral experiments, while a separate group of four rats were used for all electroencephalogram recordings. Each animal was provided with at least 3 days of rest between experiments. Animals were kept on a standard day-night cycle (lights on at 7:00 AM and off at 7:00 PM), and all experiments were performed during the day. Since rats are nocturnal animals, all experiments were conducted during the “night” phase of the rat sleep-wake cycle.
Anesthetizing Protocol
After inducing general anesthesia with isoflurane (2 to 3%) in oxygen, a 24-gauge intravenous catheter was placed in a lateral tail vein, a rectal temperature probe was inserted, and the animal was placed in a cylindrical acrylic anesthetizing chamber as previously described.21 A heating pad was placed under the chamber to maintain rectal temperature between 36.5°C and 37.4°C. Gas was continuously sampled from the distal portion of the chamber, and isoflurane, oxygen, and carbon dioxide concentrations in the chamber were monitored using a calibrated Ohmeda 5250 anesthetic agent analyzer (GE Healthcare, Waukesha, WI).
Preparation and Delivery of Drugs
Isoflurane was purchased from Henry Schein (Melville, NY). The D1 receptor agonist chloro-APB (6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide), the D2 receptor agonist quinpirole (trans-(–)-(4aR)-4,4a,5,6,7,8,8a,9-Octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline monohydrochloride), and the D1 receptor antagonist SCH-23390 (R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride) were purchased from Sigma–Aldrich (St. Louis, MO). All drugs were dissolved in normal saline to a final volume of 0.5 ml, and administered intravenously via the lateral tail vein catheter. Chloro-APB solutions were sonicated in an ultrasound water bath to facilitate dissolution. The IV tubing (approximate volume 0.6 ml) was always flushed with 2 ml of normal saline after drug administration to ensure complete delivery.
Time to Emergence After a Standardized Isoflurane General Anesthetic
The inhaled concentration of isoflurane was fixed at 1.5% (approximately 1 median alveolar concentration). After 40 min, rats received normal saline IV, chloro-APB (3 mg/kg IV), or quinpirole (5 mg/kg IV). Isoflurane was continued for 5 additional minutes, after which the rat was taken out of the chamber, and the temperature probe was removed. The animal was placed supine on a warming pad and inspired room air. Time to emergence was defined as the time from termination of isoflurane to return of righting (i.e., all four paws touching the floor).
Emergence During Continuous Isoflurane General Anesthesia
The isoflurane concentration was held at a dose that produced loss of righting with no purposeful movement for 40 consecutive minutes, as described previously.21 Purposeful movements were defined as any movements other than random muscle twitches, such as lifting of the head, opening of the eyes, twisting of the torso, kicking, clawing, chewing, licking, and/or grooming. The intravenous catheter was then flushed with 2 ml of normal saline. Five minutes after this injection, quinpirole (5 mg/kg IV) or chloro-APB was administered. To establish a dose–response relationship, three different doses of chloro-APB (0.03 mg/kg, 0.3 mg/kg, or 3 mg/kg IV) were administered on different days. In a separate experiment, the D1 receptor antagonist SCH-23390 (0.2 mg/kg IV) was administered instead of the normal saline control, followed 5 min later by chloro-APB (3 mg/kg IV). The same six rats were used for each experimental condition, in random order, with at least 3 days of rest between experiments. After intravenous drug administration, each animal continued to inhale the same dose of isoflurane for 30 min, or until restoration of righting occurred.
Electroencephalogram Electrode Placement, Recording, and Spectral Analysis
Extradural electroencephalogram electrodes were implanted surgically at least 7 days before recording, as detailed previously.21,22 Briefly, rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) under isoflurane general anesthesia. A microdrill (Patterson Dental Supply Inc., Wilmington, MA) was used to make four holes at the following stereotactic coordinates: A0L0, A6L3, A6L-3, and A10L2 relative to the lambda.24 An electrode with mounting screw and socket (Plastics One, Roanoke, VA) was screwed into each hole, and the sockets were inserted in a pedestal (Plastics One) before being fixed permanently with dental acrylic cement.
On the day of an experiment, the potential difference between electrodes A0L0 and A6L3 (right somatosensory cortex) or between electrodes A0L0 and A6L-3 (left somatosensory cortex) was recorded based on which signal gave less motion artifact. The signal was referenced to A10L2 and recorded using a QP511 Quad AC Amplifier System (Grass Instruments, West Warwick, RI) and a USB-6009 14-bit data acquisition board (National Instruments, Austin, TX). The sampling rate was 512 Hz, and no line filter was used. Data was filtered between 0.3 Hz and 50 Hz. Baseline recordings were taken for 10 min in the awake state before any drugs were administered. The rats were then anesthetized with isoflurane and placed in the anesthetizing chamber in the prone position with the isoflurane dose fixed at 1.0%. After a minimum isoflurane exposure of 40 min, normal saline or SCH-23390 (0.2 mg/kg IV) was administered and the temperature probe was removed. Five minutes later, either chloro-APB (3 mg/kg IV) or quinpirole (5 mg/kg IV) was administered and isoflurane anesthesia was continued at the same dose for an additional 30 min.
Spectral analysis was performed using Matlab 7.11 (Mathworks, Natick, MA) and the Chronux software (Cold Spring Harbor, NY), as previously described.21,22 Briefly, spectrograms were calculated using sliding windows of 2-s duration stepped through 0.05 s. The resulting spectral estimates have a bandwidth of 1.5 Hz. Mean power spectra were compared before and after quinpirole or chloro-APB administration using the Kolmogorov-Smirnov test. To determine the difference between two spectra, a two-sample Kolmogorov-Smirnov test was performed on the spectral power as a function of frequency computed from the 30 windows in the preagonist and postagonist periods. We used a Bonferroni correction to adjust the significance level for multiple hypothesis testing.
Statistical Analysis of the Effects of Chloro-APB and Quinpirole on Emergence Times, Return of Righting Responses, and Spectrograms
Prism 5.04 (Graphpad Software, San Diego, CA) and Matlab R2010b (Mathworks) were used for statistical analysis, and when possible, results are reported in terms of 95% CI based on Z-tests, t tests, or Mann–Whitney tests. We used a Bayesian Monte Carlo procedure to compute Bayesian 95% CI (credibility) to assess the effect of the different agonists on return of righting during continuous isoflurane general anesthesia as described previously.21,22 The posterior densities for the differences in the proportion of animals that had return of righting were then computed by using standard Matlab simulation procedures. Instead of P values for the Bayesian analyses, we computed the posterior probability that the propensity to right was greater in one group than in the other. A one-way ANOVA was used to assess whether there were significant differences among the final isoflurane doses in each animal group. To provide a conservative check on the assessments made by the 95% CI, the nonparametric tests were also used to assess statistical significance. The Mann-Whitney test was used to test the hypothesis that chloro-APB hastens time to emergence from isoflurane general anesthesia. We used the two-sided Kolmogorov-Smirnov test with a Bonferroni correction to compare spectra in animals before and after agonist administration.
To characterize the righting propensity as a function of dose of chloro-APB and to conduct between group comparisons of differences in righting propensity, we analyzed the data using a Bayesian logistic regression model with an uninformative prior density. In the Bayesian analysis we used a Monte Carlo procedure to compute Bayesian 95% credibility (confidence) intervals for pk – p1. This is the difference in the righting propensities between the group that received dose k, and the group that received normal saline. We define pk as the righting propensity in the group that received dose k of chloro-APB and p1 as the righting propensity in the group that received normal saline. We denote k = 2 as the 0.03 mg dose, k = 3 as the 0.3 mg dose, and k = 4 is the 3 mg dose. Unlike confidence intervals computed using frequentist methods, the Bayesian 95% confidence intervals can be interpreted as having probability 0.95 that the value of pk – p1 lies between the lower and upper limits of the interval based on the data in the current sample.25 We used this type of Monte Carlo algorithm in our previous studies using methylphenidate to induce active emergence from isoflurane and propofol general anesthesia.21,22 The details of this Bayesian analysis are summarized in appendix 1.
We considered a result to be statistically significant based on the 95% CI if the interval does not contain zero. In this case, we have p < 0.05. In the case of the Bayesian analyses, the result is statistically significant if the relevant posterior probability was greater than 0.95.
RESULTS
Chloro-APB Reduces Time to Emergence from Isoflurane General Anesthesia
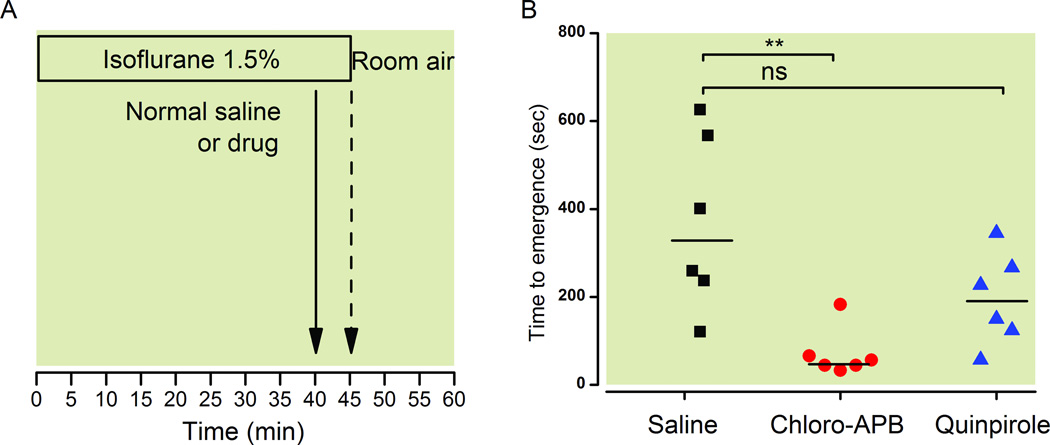
The protocol for this experiment is summarized in figure 1A. As shown in figure 1B, administration of chloro-APB (3 mg/kg IV) caused a significant reduction in time to emergence from isoflurane general anesthesia. The median time to emergence for animals that received normal saline was 330 sec (95% CI: 160 to 577s, n = 6) versus 50 s (95% CI: 12 to 130s, n = 6) for animals that received chloro-APB. The median difference in time to emergence between these two groups was 222 seconds (95% CI: 77 to 534s, Mann-Whitney test). This median difference was statistically significant (p = 0.0082).
Fig. 1.
The D1 receptor agonist chloro-APB decreases time to emergence from isoflurane anesthesia. (A) Rats inhaled isoflurane (1.5%) and received normal saline, chloro-APB (3 mg/kg IV), or the D2 receptor agonist quinpirole (5 mg/kg IV) after 40 min (solid arrow). Five minutes later, the animals were removed from the anesthetizing chamber (dashed arrow). Time to emergence was defined as the time from termination of isoflurane to return of righting (i.e., all four paws touching the floor). (B) Scatter plot of time to emergence for rats that received normal saline, chloro-APB and quinpirole (n = 6 each). The lines represent the medians. ** p < 0.01.
The median time to emergence for rats that received quinpirole (5 mg/kg IV) was 189 s (95% CI: 85 to 305s, n = 6). The median difference in time to emergence between the normal saline control group and the quinpirole group was 155 s (95% CI: −30 to 417s, Mann-Whitney test). This median difference was not statistically significant (p = 0.17). We report that the quinpirole result was not significant based on a sample of six animals per group, assuming a power of 0.8 and a type I error of 0.05, for an anticipated average difference of 199 s with a standard deviation of 172 s, based on our previous results with methylphenidate.21 That is, we designed the current study to detect emergence time effects that were at least as large as those we observed previously with methylphenidate.
Chloro-APB Induces Emergence During Continuous Inhalation of Isoflurane
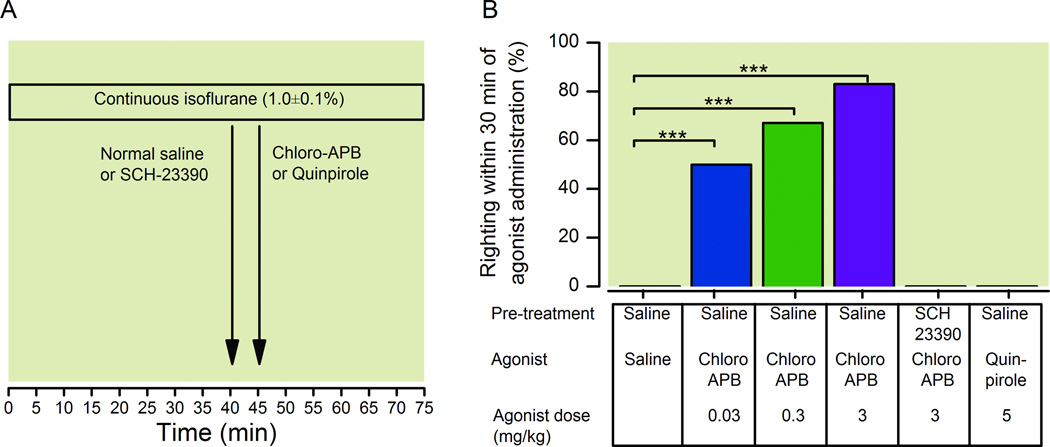
As illustrated in figure 2A, these experiments were conducted during continuous inhalation of isoflurane. The minimum concentration of isoflurane sufficient to maintain loss of righting was established for each rat and this dose was continuously delivered to the chamber throughout the experiment. The average final dose of isoflurane was the same for all six experimental groups (1.0% ± 0.1%, mean ± SD) with no statistically significant difference between groups.
Fig. 2.
Chloro-APB induces emergence during continuous isoflurane general anesthesia. (A) Rats inhaled isoflurane at a dose sufficient to maintain loss of righting for a total of 40 min, and received normal saline or the D1 receptor antagonist SCH-23390. Five minutes later, chloro-APB or quinpirole was administered IV. Isoflurane was continued at the same dose until return of righting occurred or 30 min elapsed. (B) For each drug regimen, the percentage of rats that had restoration of righting within 30 min of dopamine agonist administration is shown (n = 6 each). Normal saline alone elicited no arousal response. Rats that received normal saline followed by chloro-APB exhibited restoration of righting in a dose-dependent manner. Pretreatment with the D1 receptor antagonist SCH-23390 (0.2 mg/kg IV) instead of normal saline inhibited restoration of righting by the highest dose of chloro-APB (3 mg/kg IV). Rats that received normal saline followed by the D2 receptor agonist quinpirole did not exhibit restoration of righting. *** Posterior probability greater than 0.99.
As shown in figure 2B, the rats in the control group that received only normal saline (n = 6) did not exhibit an arousal response, and none had restoration of righting. However, 6/6 rats that received the D1 agonist chloro-APB (0.03 mg/kg IV) exhibited purposeful movements (defined as any movement other than a muscle twitch, such as lifting of the head, opening of the eyes, twisting of the torso, kicking, clawing, chewing, licking, and/or grooming) within 5 min of drug administration, and 3/6 rats had restoration of righting within 30 min, despite continuous inhalation of isoflurane at the same dose.
Chloro-APB restored the righting reflex in a dose-dependent fashion. Restoration of righting occurred in 4/6 and 5/6 rats at chloro-APB doses of 0.3 mg/kg and 3 mg/kg, respectively. Although some rats failed to right themselves within 30 min of drug administration, all doses of chloro-APB produced an arousal response despite continuous isoflurane anesthesia. At the highest dose of 3 mg/kg, chloro-APB induced purposeful movements within 30 s in 6/6 rats. The Bayesian 95% CI for the difference in the propensities to have restoration of righting between rats in the chloro-APB groups (0.03, 0.3 and 3 mg/kg) versus the normal saline group were 0.089 to 0.448, 0.236 to 0.913, and 0.349 to 0.987 respectively. The posterior probability that the difference was greater than 0 exceeded 0.99 for each comparison, indicating that each was statistically significant.
In rats that received the D1 receptor antagonist SCH-23390 (0.2 mg/kg IV) 5 minutes prior to chloro-APB, the highest dose of chloro-APB (3 mg/kg IV) failed to restore the righting reflex. These animals exhibited some sluggish limb movements immediately after the administration of chloro-APB, but showed no other signs of arousal. The Bayesian 95% CI between rats that received normal saline versus SCH-23390 was 0.21 to 0.91, with a posterior probability of 0.998, indicating that the difference was statistically significant. The D2 receptor agonist quinpirole (5 mg/kg IV) failed to elicit an arousal response during continuous isoflurane general anesthesia (n = 6).
SCH-23390 Inhibits Chloro-APB-induced Electroencephalogram Changes during Continuous Inhalation of Isoflurane
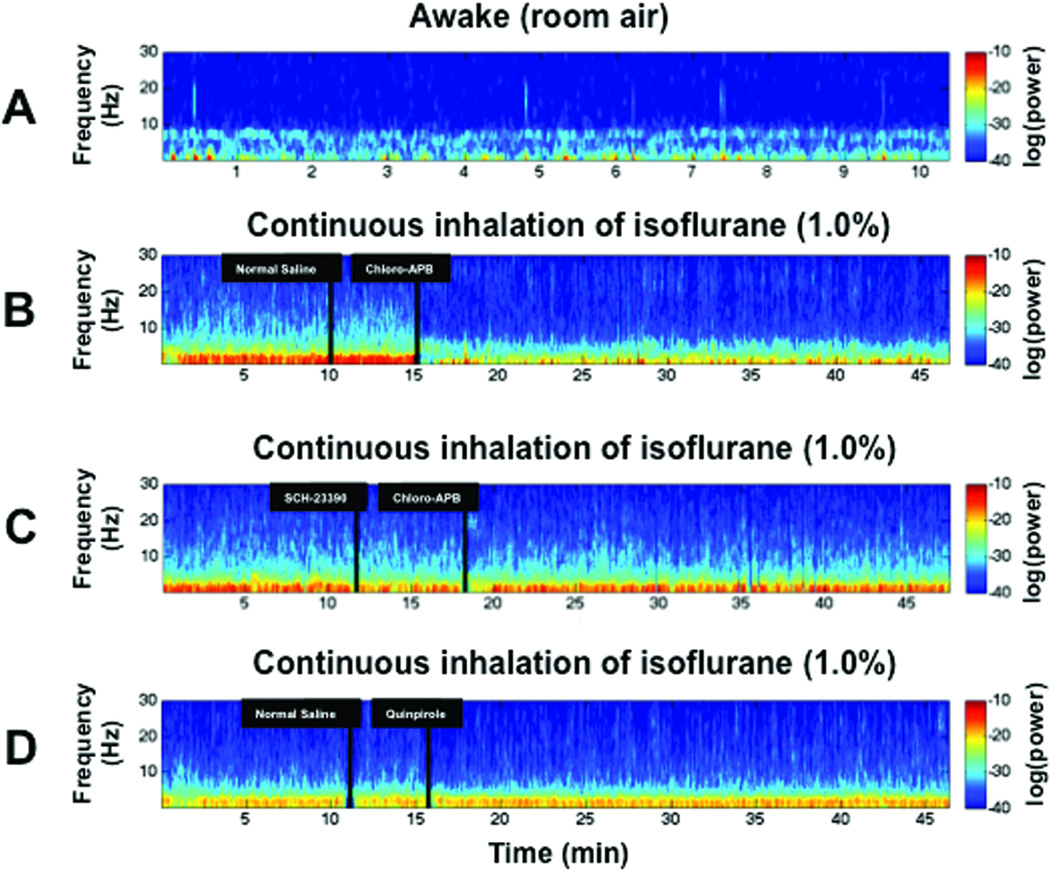
Electroencephalogram data were recorded from rats with preimplanted extradural skull electrodes. These experiments were performed in the prone position to minimize motion artifacts associated with righting attempts. Spectrograms were computed from the continuous electroencephalogram data to analyze changes in electroencephalogram power over time. Typical results from individual rats are shown in figure 3. In awake rats (fig. 3A), electroencephalogram power was mainly in the θ frequency range (4–8 Hz). Continuous inhalation of 1.0% isoflurane (fig. 3B–D) caused a large increase in δ power (0–4 Hz). Injection of normal saline produced no arousal response and no appreciable change in the spectrogram, but administration of the D1 agonist chloro-APB (3 mg/kg IV) induced behavioral signs of arousal (i.e. purposeful movements such as kicking, clawing, etc.) in 4/4 rats, as well as a prompt decrease in δ power on the electroencephalogram (fig. 3B). Pretreatment with the D1 antagonist SCH-23390 (0.2 mg/kg IV) inhibited the chloro-APB-induced arousal response and decrease in electroencephalogram δ power (fig. 3C). The D2 agonist quinpirole (5 mg/kg IV) failed to induce behavioral arousal or electroencephalogram changes during continuous isoflurane general anesthesia (fig. 3D).
Fig. 3.
Spectral analysis of electroencephalogram data reveals a decrease in δ power induced by chloro-APB that is inhibited by SCH-23390. Warm colors (e.g., red) represent higher power at a given frequency, while cool colors (e.g., blue) represent lower power. (A) A representative spectrogram computed from a rat in the awake state shows predominance of θ power (4–8 Hz). (B) A representative spectrogram computed from a rat inhaling isoflurane (1.0%) shows predominance of δ power (<4 Hz) before and after administration of normal saline. However, administration of chloro-APB (3 mg/kg IV) promptly induced a decrease in δ power. (C) A representative spectrogram computed from a rat that received the D1 receptor antagonist SCH-23390 (0.2 mg/kg IV) instead of normal saline shows that similar to the rat in (B), δ power is dominant during inhalation of isoflurane (1.0%), before and after administration of SCH-23390. However, after the administration of SCH-23390, chloro-APB (3 mg/kg IV) failed to induce a decrease in δ power. (D) A representative spectrogram computed from a rat that received normal saline followed by the D2 receptor agonist quinpirole (5 mg/kg IV) shows that quinpirole did not induce a decrease in δ power.
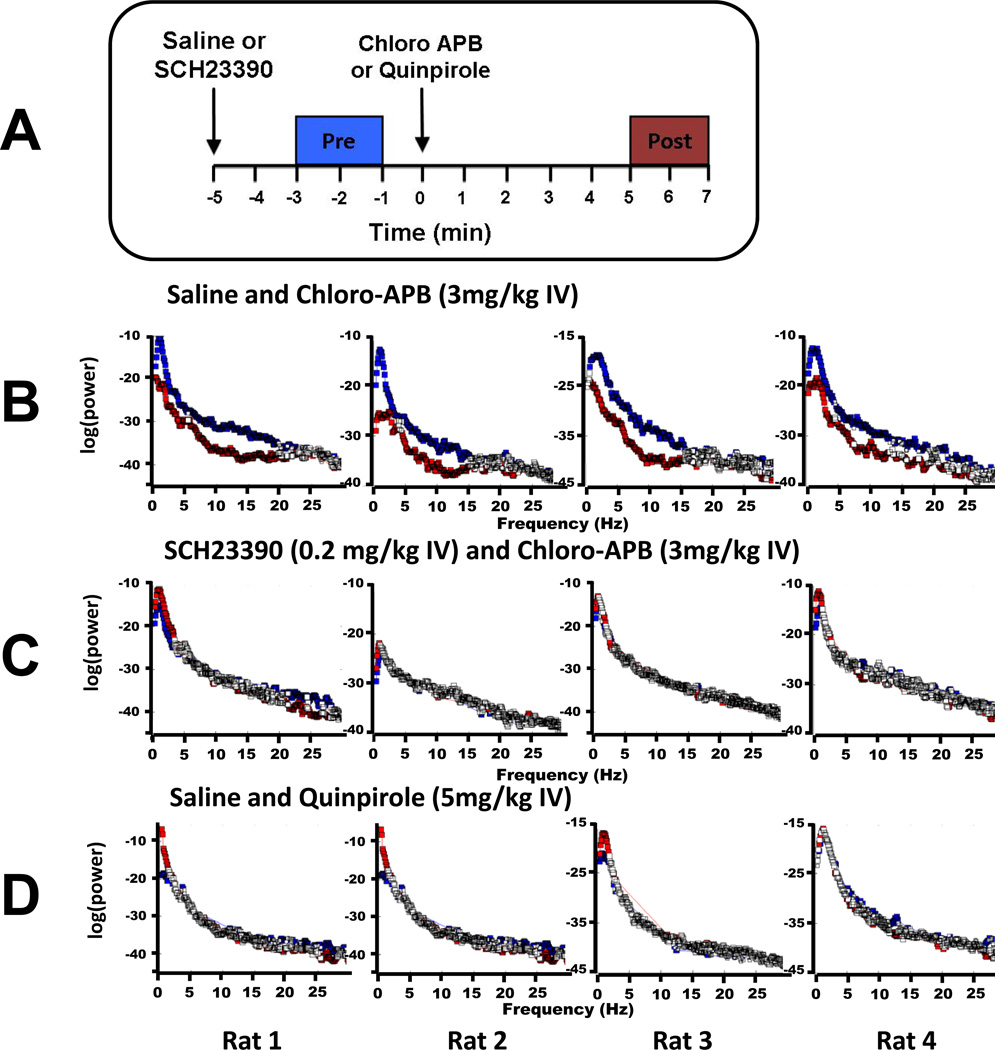
Figure 4 shows power spectra computed from four different rats during continuous isoflurane general anesthesia. Figure 4A illustrates the 2-min “pre-drug” (blue) and “postdrug” periods used for analysis. Because the time to onset of the arousal response was somewhat variable, the “post-drug” period began 5 min after drug administration. At any given frequency, statistically significant differences between power spectra are depicted in color, while the white boxes show differences that do not reach statistical significance. As shown in figure 4B, treatment with chloro-APB (3 mg/kg IV) induced a statistically significant decrease in power at most frequencies under 15 Hz for 4/4 rats (p < 0.05). Decrease in power was more pronounced at δ and α (8–12 Hz) frequencies. As shown in figure 4C, rats that received SCH-23390 (0.2 mg/kg IV) before chloro-APB had only small changes in δ power, with no statistically significant changes at most frequencies between 0–30 Hz. Similarly, administration of quinpirole (5 mg/kg IV) induced only minor, insignificant changes in electroencephalogram power at frequencies under 30 Hz (fig. 4D).
Fig. 4.
Electroencephalogram power spectra computed for each of 4 animals during continuous isoflurane general anesthesia. (A) The 2-min windows used to compute power spectra before dopamine agonist administration (blue, “PRE”), and after administration (red, “POST”). (B) Power spectra computed from animals that received normal saline prior to chloro-APB, showing results of the Kolmogorov-Smirnov test for the 2-min periods before and after chloro-APB administration. At a 0.05 significance level (with Bonferonni correction) the Kolmogorov-Smirnov test rejects the null hypothesis at all frequencies except those marked with white squares. Statistically significant decreases in power occurred at most frequencies under 15 Hz. (C) Power spectra computed from animals that received the D1 receptor antagonist SCH-23390 (0.2 mg/kg IV) prior to chloro-APB (3 mg/kg IV). After SCH-23390, chloro-APB failed to induce statistically significant changes in the power spectrum. (D) Power spectra computed from animals that received normal saline followed by quinpirole (5 mg/kg IV). Quinpirole induced no statistically significant changes in the power spectrum.
DISCUSSION
The present study was conducted to test the hypothesis that specific dopamine receptor agonists induce emergence from isoflurane anesthesia. The results show that the D1 agonist chloro-APB decreases time to emergence after isoflurane general anesthesia, and induces emergence during continuous inhalation of isoflurane. In addition, chloro-APB induces changes in electroencephalogram power consistent with arousal. The chloro-APB-induced arousal response is inhibited by the D1 antagonist SCH-23390, strongly suggesting that the arousal effect is specifically mediated by D1 receptors. In contrast, the D2 agonist quinpirole failed to induce emergence from isoflurane general anesthesia.
In a previous study it was shown that methylphenidate, an inhibitor of dopamine and norepinephrine reuptake transporters, induces emergence from isoflurane general anesthesia.21 It was also shown that droperidol inhibits the arousal-promoting actions of methylphenidate, but because droperidol inhibits both dopaminergic and adrenergic receptors, the specific neurotransmitter systems responsible for the effects of methylphenidate were not clear. The present results show that activation of D1 receptors alone is sufficient to induce emergence from general anesthesia with isoflurane. It has been reported that chloro-APB and other D1 agonists are respiratory stimulants that reverse opioid-induced respiratory depression,26 but not analgesia.27 Therefore it is likely that chloro-APB produced a large reduction in time to emergence from isoflurane general anesthesia (fig. 1) by a combination of increased minute ventilation and increased arousal, similar to methylphenidate.21
Qualitatively, the behavioral arousal response induced by chloro-APB was not as pronounced as the response observed previously with methylphenidate. That is, chloro-APB generally induced less vigorous movements compared to methylphenidate. The electroencephalogram changes were also less pronounced: while methylphenidate and chloro-APB both induced a decrease in δ power, methylphenidate increased θ power21 whereas chloro-APB did not. Rather, chloro-APB induced a decrease in power at most frequencies under 30 Hz. Taken together, these results suggest that chloro-APB induces an attenuated arousal response compared to methylphenidate. This is likely because methylphenidate activates both dopaminergic and adrenergic neurotransmission, whereas chloro-APB acts selectively at D1 receptors.
We selected chloro-APB and quinpirole because they are widely used in animal studies as specific dopamine receptor agonists, and they are readily available. Although there is evidence that quinpirole may also interact with receptors other than D2 receptors,28 it was ineffective at inducing emergence from isoflurane anesthesia. Therefore we conclude that D2 receptors (and any other putative molecular targets of quinpirole) are unlikely to play an important role in emergence from general anesthesia. However, because we only tested a single, high dose of quinpirole, it is unlikely but still possible that lower doses may have produced an arousal response.
There are five types of G protein–coupled dopamine receptors, broadly divided into two classes: D1-like and D2-like.29 The D1 subfamily includes the D1 and D5 receptors, which are postsynaptic and mediate excitation by activating G-proteins that stimulate cyclic adenosine monophosphate synthesis. Stimulation of the D1 receptor depolarizes neurons ascending to the thalamus, the lateral hypothalmus and the basal forebrain, and neurons descending to the dorsal raphe nucleus and the locus ceruleus.16 Some of these neuronal groups project to the nonspecific thalamocortical system while others project through the ventral extrathalamic pathway, both of which stimulate cortical activation.30 A variety of specific D1 agonists have been shown to increase wakefulness and spontaneous grooming, while reducing both rapid eye movement and non-rapid eye movement sleep.31,32 It was previously reported that administration of a D1 receptor agonist, SKF-38393, decreases time to emergence from pentobarbital anesthesia in rabbits33 and rats.34 Taken together with our finding that chloro-APB induces restoration of righting and electroencephalogram changes consistent with arousal during continuous isoflurane general anesthesia, it is likely that a D1 receptor-mediated arousal mechanism plays an important role in emergence from general anesthesia.
The D2-like class of dopamine receptors (which includes D2, D3 and D4 receptors) mediate inhibition via activation of G-proteins that inhibit cyclic adenosine monophosphate synthesis, suppress calcium currents, and activate potassium currents.16 Unlike D1 receptors, D2 receptors are found both pre- and postsynaptically, and have a more complicated effect on arousal. Biphasic, dose dependent effects on sleep have been observed,35 with decreased wakefulness occurring at low doses of quinpirole (0.015 mg/kg) and increased wakefulness with concomitant reductions in rapid eye movement and non-rapid eye movement sleep occurring at higher doses (1 mg/kg).36 It has been hypothesized that the effects at low doses of D2 agonists are mediated through activation of the presynaptic auto-receptors, while the increase in wakefulness after larger doses depends on activation of the postsynaptic D2 receptors.16 In the present study, large doses of quinipirole (5 mg/kg IV) produced no appreciable arousal effect in rats anesthetized with isoflurane. In previous studies using rabbits and rats anesthetized with pentobarbital, the same dose of quinpirole failed to reduce time to emergence,33 which is consistent with our results. While D2 agonists have been shown to affect the sleep-wake cycle, the arousal-promoting effects may be too weak to induce arousal during general anesthesia.
The VTA and SNc are the two major sources of dopamine in the brain.16 The nigrostrial pathway which projects from SNc to the striatum is a component of the basal ganglia that is crucial for movement control,37 and loss of SNc neurons leads to Parkinson’s Disease. There are two main pathways that arise from the VTA: the mesolimbic pathway and the mesocortical pathway. The mesolimbic pathway that projects to the nucleus accumbens, amygdala, and hippocampus plays a key role in processing reward, motivation, emotion, and reinforcement.38 The mesocortical pathway, which has a major projection to the prefrontal cortex, complements the function of the mesolimbic pathway and aids in cognition.39
Dopaminergic neurons in the VTA and the SNc innervate brain areas involved in sleep regulation, including the serotonergic cells of the dorsal raphe nucleus, the noradrenergic cells of the locus ceruleus, the cholinergic cells of the pedunculopontine and laterodorsal tegmental nuclei and the basal forebrain and neurons that modulate behavioral states in the thalamus.16 In turn these areas, as well as orexin-containing neurons located in the lateral hypothalamus, have reciprocal inputs to the VTA and SNc. Electrolytic lesions of the VTA and SNc in cats have been reported to induce a “total lack of behavioral arousal,”19 and selective loss of dopamine in mice causes marked hypoactivity.20 All of these data support the idea that dopaminergic neurons in the VTA and/or SNc are important for behavioral arousal.
However, the specific role of dopamine in arousal remains controversial, because dopaminergic neurons in the VTA and SNc do not significantly change their mean firing rates during the sleep–wake cycle,40,41 and neurotoxic lesions of the ventral midbrain (which includes the VTA and SNc) have been reported to cause contradictory insomnia and hyperactivity.42 Recently, a group of wake-active dopaminergic neurons was identified in the ventral periaqueductal gray area, and it has been suggested that these cells may be important for regulating arousal.43 However, further characterization of these neurons has not been reported. More studies are needed to determine which population of dopaminergic neurons is responsible for the arousal effects that lead to active emergence from general anesthesia.
In summary, the results of the present study demonstrate that activation of D1 dopamine receptors is sufficient to induce emergence from isoflurane general anesthesia, while activation of D2 receptors does not produce an arousal response under identical experimental conditions. These findings suggest that methylphenidate-induced arousal during general anesthesia is mediated, at least in part, by activation of D1 receptors. D1 receptors may be a rational target for the development of novel therapeutic agents that induce emergence from general anesthesia.
Final Box Summary.
What we already know about this topic:
Methylphenidate, which inhibits dopamine and norepinephrine reuptake transporters, induced emergence from isoflurane anesthesia in prior studies, but whether selective dopamine receptor activation could also have a similar effect is not known.
What this article tells us that is new:
Activation of D1 receptors by chloro-APB decreased time to emergence from isoflurane anesthesia, and produced behavioral and neurophysiological evidence of arousal during continuous isoflurane anesthesia, consistent with a D1-mediated arousal response that promotes emergence from isoflurane anesthesia."
Acknowledgments
DISCLOSURE OF FUNDING
Supported by grants DP1-OD003646 and K08-GM094394 from the National Institutes of Health, Bethesda, Maryland, and the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Appendix
Bayesian Logistic Regression
To define our Bayesian logistic regression model, we note that our experiment used the same six rats at each dose level. However, the dose levels were studied in random order and the experiments within a given rat were separated by 3 days. Therefore, a reasonable assumption is that the responses are independent. Let xk be the kth dose level of chloro-APB. These are x1 = 0,x2 =0.03,x3=0.3 and x4 = 3 mg, where the zero dose is normal saline. Let zk = log(xk) where for x1 = 0 we take x1= 0.003 to avoid problems with an undefined number. Let nk be the number of righting response observed from the six animals at the kth dose level. Assume the logistic regression model44
| (1) |
It follows that the joint density of the data n = (n1,n2,n3,n4) or equivalently the likelihood of θ = (θ1, θ2) is
| (2) |
where z=(z1,z2,z3,z4). If we assume f(θ), a prior density for θ, then by Bayes’ rule the posterior density of θ is
| (3) |
where is the normalizing constant. We approximate the posterior density f(θ|n,z) as the Gaussian density whose mean is θ̂ the maximum likelihood estimate of θ and whose covariance matrix is I(θ̂)−1, the inverse of the observed Fisher information matrix.25
This approximation of the posterior density is equivalent to computing the Gaussian approximation to the posterior under the assumption that the prior density of θ is uninformative.25 We estimated θ̂ using the Matlab logistic regression procedure.
Bayesian Monte Carlo Analysis
Given the approximate posterior density f(θ|n,z), we compute the posterior densities f(pk – p1|n,z) for k = 2,3,4. by using the following Monte Carlo algorithm:
Draw θ from f(θ|n,z)
Compute pk =[1+exp(θ1 + θ2 zk)]−1 exp(θ1 + θ2 zk) and p1 = [1 + exp(θ1 + θ2 z1)]−1 exp(θ1 + θ2 z1).
Compute pk – p1
Do 1 to 3 10,000 times.
The histogram of the 10,000 pk – p1 values is a Monte Carlo approximation to the posterior density f(pk – p1|n,z). The lower and upper limits of the 95% Bayesian credibility (confidence) interval are 250th smallest value 9,750th smallest value in the Monte Carlo sample respectively. We compute the probability that pk > p1 as Pr(pk > p1|n,z) ≐ ℓ/10,000, where ℓ is the number of times that pk > p1 in the Monte Carlo sample.21,22
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PRIOR PRESENTATION OF WORK
This work has been presented, in part, at annual meetings of the Society for Anesthesia and Sleep Medicine (Chicago, Illinois, October 14, 2011), the American Society of Anesthesiologists (Chicago, Illinois, October 15, 2011), and the Society for Neuroscience (Washington, DC, November 13, 2011).
REFERENCES
- 1.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Rampil IJ. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology. 1994;80:606–610. doi: 10.1097/00000542-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 5.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: Evidence for neural inertia. PLoS One. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudetz AG, Wood JD, Kampine JP. Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology. 2003;99:1125–1131. doi: 10.1097/00000542-200311000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 10.Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–717. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115:733–742. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009;111:725–733. doi: 10.1097/ALN.0b013e3181b061a0. [DOI] [PubMed] [Google Scholar]
- 13.Luo T, Leung LS. Involvement of tuberomamillary histaminergic neurons in isoflurane anesthesia. Anesthesiology. 2011;115:36–43. doi: 10.1097/ALN.0b013e3182207655. [DOI] [PubMed] [Google Scholar]
- 14.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Maze M, Franks NP. The involvement of hypothalamic sleep pathways in general anesthesia: Testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Van Dort CJ, Baghdoyan HA, Lydic R. Neurochemical modulators of sleep and anesthetic states. Int Anesthesiol Clin. 2008;46:75–104. doi: 10.1097/AIA.0b013e318181a8ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 19.Jones BE, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58:157–177. doi: 10.1016/0006-8993(73)90830-5. [DOI] [PubMed] [Google Scholar]
- 20.Palmiter RD. Dopamine signaling as a neural correlate of consciousness. Neuroscience. 2011;198:213–220. doi: 10.1016/j.neuroscience.2011.06.089. [DOI] [PubMed] [Google Scholar]
- 21.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: Insights on efficacy and safety. Neuropharmacology. 2009;57:608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Vijn PC, Sneyd JR. I.V anaesthesia and EEG burst suppression in rats: Bolus injections and closed-loop infusions. Br J Anaesth. 1998;81:415–421. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 25.DeGroot MH, Schervish MJ. Probability and statistics. 3rd edition. Boston: Addison-Wesley; 2002. [Google Scholar]
- 26.Lalley PM. Dopamine1 receptor agonists reverse opioid respiratory network depression, increase CO2 reactivity. Respir Physiol Neurobiol. 2004;139:247–262. doi: 10.1016/j.resp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Lalley PM. D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regul Integr Comp Physiol. 2005;289:R45–R51. doi: 10.1152/ajpregu.00868.2004. [DOI] [PubMed] [Google Scholar]
- 28.Gilliland SL, Alper RH, Levant B. Pharmacology of quinpirole-stimulated [35S]GTPgammaS binding: Discrepancy with receptor binding profile. Eur J Pharmacol. 2000;392:125–128. doi: 10.1016/s0014-2999(00)00124-2. [DOI] [PubMed] [Google Scholar]
- 29.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 30.Jones BE. From waking to sleeping: Neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Monti JM, Fernandez M, Jantos H. Sleep during acute dopamine D1 agonist SKF 38393 or D1 antagonist SCH 23390 administration in rats. Neuropsychopharmacology. 1990;3:153–162. [PubMed] [Google Scholar]
- 32.Trampus M, Ferri N, Adami M, Ongini E. The dopamine D1 receptor agonists, A68930 and SKF 38393, induce arousal and suppress REM sleep in the rat. Eur J Pharmacol. 1993;235:83–87. doi: 10.1016/0014-2999(93)90823-z. [DOI] [PubMed] [Google Scholar]
- 33.Horita A, Carino MA. D-1 agonist, SKF 38393, but not a D-2 agonist, produces a cholinergically mediated analeptic effect in rabbits. Pharmacol Biochem Behav. 1991;39:449–452. doi: 10.1016/0091-3057(91)90206-h. [DOI] [PubMed] [Google Scholar]
- 34.Horita A, Carino MA, Nishimura Y. D1 agonist SKF 38393 antagonizes pentobarbital-induced narcosis and depression of hippocampal and cortical cholinergic activity in rats. Life Sci. 1991;49:595–601. doi: 10.1016/0024-3205(91)90258-d. [DOI] [PubMed] [Google Scholar]
- 35.Monti JM, Hawkins M, Jantos H, D'Angelo L, Fernandez M. Biphasic effects of dopamine D-2 receptor agonists on sleep and wakefulness in the rat. Psychopharmacology (Berl) 1988;95:395–400. doi: 10.1007/BF00181955. [DOI] [PubMed] [Google Scholar]
- 36.Monti JM, Jantos H, Fernandez M. Effects of the selective dopamine D-2 receptor agonist, quinpirole on sleep and wakefulness in the rat. Eur J Pharmacol. 1989;169:61–66. doi: 10.1016/0014-2999(89)90817-0. [DOI] [PubMed] [Google Scholar]
- 37.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 38.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 39.Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, Rodriguez M, Olanow CW. The basal ganglia in Parkinson's disease: Current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–S46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- 40.Trulson ME, Preussler DW, Howell GA. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci Lett. 1981;26:183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- 41.Trulson ME, Preussler DW. Dopamine-containing ventral tegmental area neurons in freely moving cats: Activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83:367–377. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- 42.Lai YY, Shalita T, Hajnik T, Wu JP, Kuo JS, Chia LG, Siegel JM. Neurotoxic N-methyl-D-aspartate lesion of the ventral midbrain and mesopontine junction alters sleep-wake organization. Neuroscience. 1999;90:469–483. doi: 10.1016/s0306-4522(98)00429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawaitan Y. In all likelihood: Statistical modelling and inference using likelihood. Oxford: Oxford University Press; 2001. [Google Scholar]