Abstract
A large number of complex glycosylation mechanisms take place in the Golgi apparatus. In epithelial cells, glycosylated protein molecules are transported to both the apical and the basolateral surface domains. Although the prevailing view is that the Golgi apparatus provides the same lumenal environment for glycosylation of apical and basolateral cargo proteins, there are indications that proteoglycans destined for the two opposite epithelial surfaces are exposed to different conditions in transit through the Golgi apparatus. We will here review data relating proteoglycan and glycoprotein synthesis to characteristics of the apical and basolateral secretory pathways in epithelial cells.
Keywords: proteoglycan, Golgi, polarized transport, MDCK cells
The Golgi apparatus, discovered by histological studies more than a century ago, was established as a central organelle system of the secretory pathway in metazoans in the 1960s and 1970s (Palade 1975). This distinct cellular structure is observed as stacks of membrane-enclosed saccules termed cisternae and is a main site for glycan modification of proteins and lipids. Protein glycosylation in the secretory pathway generates three main classes of glycan structures. By far the most prevalent mechanism is N-linked glycosylation of asparagine residues, initiating already co-translationally in the lumen of the endoplasmic reticulum (ER). Preformed mannose-rich branched glycan structures of 14 monosaccharides are enzymatically transferred en bloc to asn-x-ser/thr sites in translocating polypeptides. The initial glycan structure is subjected to further modifications, as the protein moves forward in the secretory pathway. Another glycosylation mechanism, O-linked glycosylation, starts by addition of an N-acetyl-galactosamine (GalNAc) residue to a serine or threonine. This takes place early in the Golgi apparatus, or just prior to entry into the cis-most Golgi cisternae. Further extension by the action of a number of glycosyltransferases results in branched O-glycans of variable structure. The third major glycosylation mechanism is polymerization of long, linear glycosaminoglycan (GAG) chains onto proteins, resulting in proteoglycans (PGs). The major classes of GAG chains that decorate PGs are chondroitin sulfate (CS)/dermatan sulfate (DS) and heparan sulfate (HS)/heparin. These are all connected to the protein core via a linker tetrasaccharide extending from ser-gly sites in the polypeptide. In addition, some PGs carry keratan sulfate (KS) GAGs, where the connection to the protein core is via N- or O-linked glycosylation. The different classes of GAG chains are defined by their constituent disaccharide units that are enzymatically polymerized in the Golgi apparatus and subsequently further modified by epimerization and sulfation.
The glycan portions of glycoproteins and PGs are important to their functional features at their site of action in intracellular membrane structures or extracellularly. However, glycan structures and the glycosylation process itself are often essential for control of protein folding and anterograde transport of glycoproteins and PGs to their destinations. Alterations in glycan structures, introduced by enzymes encountered in the secretory pathway, are actively used for quality control or sorting (Fiedler and Simons 1995). Forward transport toward the Golgi apparatus of a number of glycoproteins carrying N-glycans has been shown to require ERGIC-53/p58, VIP-36, and related lectins (Hauri et al. 2000). At the terminus of the Golgi apparatus, the trans-Golgi network (TGN), synthesis of glycoproteins and PGs is essentially completed. The TGN is regarded as a major sorting site, organizing further transport of proteins and lipids to intracellular membrane structures (endosomes, lysosomes, or storage granules) or the cell exterior (plasma membrane and secretion).
An additional layer of complexity is introduced to cellular organization and molecular sorting processes in polarized cells, such as neurons, hepatocytes, and endothelial and epithelial cells. In such cell types, the plasma membrane is divided into distinct domains with different protein and lipid composition. Cell surface polarity is also reflected in intracellular transport pathways in the way that stringent sorting mechanisms are required to avoid protein mis-sorting and to maintain cell surface domains of differential molecular compositions. Different classes of glycans have been suggested to be of importance to such sorting mechanisms, as the polarized transport of several proteins is either dependent on or influenced by the glycan portion of the protein (Fiedler and Simons 1995). The cell systems mostly used for studies of polarized protein transport are monolayers of epithelia. In the physiological context, epithelial cells face the lumens of body cavities with their apical membranes, rich in microvilli, whereas the opposite, basolateral domain provides attachment to the underlying tissues and uptake of nutrients from the blood supply. The segregated membrane domains at the opposite sides of an epithelial monolayer, as well as the efficient barrier to domain mixing and intercellular leakage established by tight-junction protein complexes, make epithelial cells the most convenient system for studies of polarized sorting and secretion of macromolecules. We, among others, have used the epithelial Madin-Darby canine kidney (MDCK) cell line to address the role of glycans in polarized transport. More precisely, we have investigated the involvement of GAGs and N-glycan modifications in transport and targeting of secretory proteins. Our results support an impact of GAG modification on PG sorting events, particularly for apical transport of CSPGs. However, we also acknowledge the complexity of polarized transport routes and the glycosylation machineries in polarized cells. The sequential steps of GAG synthesis (for review, see Prydz and Dalen 2000) might occur just before, concomitantly, or just after segregation of PG cargo into transport routes for apical or basolateral delivery (Prydz et al. 2008). The interplay between GAG modification and sorting into the apical and basolateral transport routes is thus not fully understood. Here we will review studies of PGs in polarized epithelial cells and discuss the findings in relation to organization of the secretory pathways and possible GAG synthesizing complexes.
Proteoglycans in Epithelial MDCK Cells
The kidney epithelial MDCK cell line synthesizes mainly HSPGs and CSPGs (Caplan et al. 1987; Svennevig et al. 1995; Erickson and Couchman 2001) and, to a minor extent, KSPGs (Toma et al. 1996). The HS chains are carried by the basement membrane PGs perlecan, agrin, and collagen XVIII (Erickson and Couchman 2001), which are mainly secreted at the basolateral side of the epithelial cell layer (Caplan et al. 1987; Svennevig et al. 1995). The HS GAGs of the GPI-anchored PG glypican have been implicated in basolateral transport in epithelial CaCo-2 and MDCK cells (Mertens et al. 1996). HS GAGs secreted from MDCK cells have been shown to be sulfated in three positions: at N and C-6 of N-acetyl-glucosamine (GlcNAc) and at C-2 of uronic acid (UA) (Safaiyan et al. 2000). 3-O-sulfation, modifying the GlcNAc in position C-3, may alter the biological activity and anticoagulant properties of the GAG chain but has not yet been observed in MDCK cells. However, anticoagulant HS and expression of 3-O-sulfotransferases have been observed in human glomerular epithelial cells (Girardin et al. 2005), in the corresponding basement membrane (Edge and Spiro 1990), and in other tissues (Lawrence et al. 2007). A variable degree of modification results in non-sulfated, di- and trisulfated disaccharide units along the chains. The average sulfate to disaccharide ratio of both apically and basolaterally secreted HS in MDCK cells is close to 1:1 (Dick et al. 2008). However, HS GAGs secreted from MDCK cells are heterogeneously sulfated along the GAG chain, giving rise to stretches of 4 to 12 disaccharide units of more densely sulfated domains interspaced by low or non-sulfated regions (Safaiyan et al. 1999, 2000). MDCK II cells secrete several CSPGs (Erickson and Couchman 2001), which are preferentially transported apically (Svennevig et al. 1995), as are free GAG chains on hexyl-β-D-thioxyloside (Kolset et al. 1999), suggesting that CS GAGs possess sorting information facilitating apical delivery. However, CSPGs are observed in the basement membrane in rat kidney (McCarthy et al. 1990; Schaefer et al. 1998), indicating that CSPGs also are transported to the basolateral side of the epithelial cell layer. A single CS GAG chain does not seem to be sufficient to direct apical transport, since neither amyloid precursor-like protein 2 (Lo et al. 1995) nor the asialoglycoprotein receptor H1 subunit (Kobialka et al. 2009) was sorted apically when carrying one CS chain. CS GAGs secreted from MDCK cells consist of disaccharides that are non-sulfated, monosulfated either by 4-O-sulfation or 6-O-sulfation (C-4 or C-6 in GalNAc), or disulfated, having both C-4 and C-6 modified (Dick et al. 2008; Grøndahl et al. 2009). 2-O-sulfation of epimerized UA, iduronic acid (IdoA), has been demonstrated in IdoA blocks in dermatan sulfate (Pacheco et al. 2009) but has, to our knowledge, not been observed in epithelial cells. Comparing the pools of CS secreted apically and basolaterally, the basolateral fraction is more densely sulfated. The difference in sulfation is partly also a difference in modification. When analyzing the pool of secreted endogenous CSPGs, 4-O-sulfation dominated both in the apical and basolateral fractions, whereas 6-O-sulfation was mainly observed for basolaterally secreted CS GAGs (Dick et al. 2008). A distribution of CS disaccharide units into domains of different sulfate densities, as seen for HS GAGs, has not been demonstrated. If such domain variability exists, this could contribute to the sulfation differences observed for apically and basolaterally secreted CS GAGs.
Model PGs Give Insight into Polarized Synthesis and Transport
To further dissect the mechanisms of polarized synthesis and secretion of PGs, we have employed the use of secreted recombinant model proteins and PGs. This is an attractive approach, as it allows analysis of a single species of PG. Construction of green fluorescent protein (GFP) fusion proteins facilitates confocal microscopy of intracellular location and transport dynamics, as well as easy biochemical detection and purification. Analysis of the GAG chains of the model PG serglycin-GFP (SG-GFP) (Tveit et al. 2005; Vuong et al. 2006) gave strong indications of pathway-specific modifications of PGs. When expressed in MDCK cells, SG-GFP acquired mainly CS chains and was recovered predominantly from the apical medium. However, the GAGs on SG-GFP secreted basolaterally were more intensely sulfated than their apical counterparts. The apical and basolateral sulfation patterns and chain lengths were also different. Disaccharide analysis of the recombinant SG-GFP revealed predominantly CS 4-O-sulfation on the apical CS chains, whereas CS 6-O-sulfation was mainly seen in the basolateral pathway (Tveit et al. 2005; Vuong et al. 2006). Inhibition of 3′-phosphoadenosine-5′-phosphosulfate (PAPS) synthesis by chlorate treatment did not alter the sorting polarity of SG-GFP. However, the two pathways responded differently to the reduced availability of the sulfate donor PAPS. The synthesis machinery gave priority to sulfation of HS GAGs on basolaterally secreted molecules, whereas basolateral CS chains lost sulfate and became longer. The differential response to chlorate treatment supports the view that GAG synthesis and sulfation are regulated differently in the apical and basolateral secretory pathways. Still, pathway-selective CS sulfation might not be a feature of all secreted PGs. When the GAG attachment domain from serglycin was transferred to the non-glycosylated protein rat growth hormone (rGH), a well-established secretory model protein, and expressed in MDCK cells (Hafte et al. 2011), the resulting fusion protein was modified by CS-GAGs and predominantly secreted apically, in line with the apical sorting capability of CS chains. The difference in sulfation seen for SG-GFP in the apical and basolateral directions (Tveit et al. 2005; Vuong et al. 2006), however, was no longer observed, indicating a role of a protein domain in serglycin outside the GAG attachment domain. Differences in CS synthesis have been observed in mouse mammary gland epithelial cells. In a comparative analysis of HS and CS of the transmembrane PGs syndecan-1 and syndecan-4, synthesized simultaneously in the same cell type (Deepa et al. 2004), the HS chains of the two syndecan variants were structurally indistinguishable, whereas their CS chains were structurally and functionally distinct. The differences in CS synthesis might result from segregation of CS sulfotransferases in the Golgi apparatus or pathway-specific variability in their activities. Thus, Golgi organization in epithelial cells has a direct impact on PG synthesis.
Golgi Organization—Affecting GAG Synthesis and Transport
PG synthesis is a result of events in the Golgi apparatus. In the prevailing view of the secretory pathway, secretory proteins move forward in membrane-limited entities. Golgi enzymes reside in these membrane-enclosed cisternae, where their substrates are concentrated, and optimal reaction conditions, such as the proper pH, are provided. Morphological and biochemical analyses of the subcellular location of enzymes, combined with various means of transport inhibition, have confirmed a differential enzyme distribution in the cis-, medial-, and trans-Golgi regions. Especially the use of the brefeldin A (BFA) has been instructive. BFA is an inhibitor of protein trafficking in the endomembrane system of mammalian cells. In many cell types, BFA treatment leads to retrograde transport and fusion of Golgi cisternae components with the ER/intermediate compartment (IC), whereas the TGN remains seemingly intact. Secretory proteins do not reach the TGN, however, since the preceding Golgi cisternae are absent (Lippincott-Schwartz et al. 1991; Elazar et al. 1994; Dinter and Berger 1998). Several studies, using BFA to distinguish events taking place in the TGN from those occurring in cis/medial-Golgi, have demonstrated differential effects on CS- and HS-GAG synthesis (Spiro et al. 1991; Sugumaran et al. 1992; Uhlin-Hansen and Yanagishita 1993). These observations fit with a cis-trans sequential synthesis of HS- and CS-GAGs, where HS chain synthesis on protein cores seems to be mediated by enzymes that have returned from cis- and medial-Golgi to the ER in BFA-treated cells, whereas enzymes responsible for CS chain synthesis are localized more distally in the secretory pathway, presumably in the TGN, and are therefore inaccessible to secretory cargo proteins in the presence of BFA.
MDCK cells are less sensitive to BFA treatment, compared with most other cell types. Their Golgi apparatus is structurally intact, even in the presence of high doses of BFA. Still, secretory transport pathways are affected (Tveit et al. 2009). Both CS- and HS-GAG chain synthesis and sulfation in polarized MDCK cells are reduced by BFA. The apically secreted GAGs are affected at lower doses than basolaterally secreted GAGs (Fjeldstad et al. 2002; Tveit et al. 2009). Moreover, when analyzing the effect of BFA on GAG synthesis in more detail, secreted HS GAGs were clearly affected. The amount of secreted HS GAGs was reduced and displayed reduced N- and 6-O-sulfation (Kolset et al. 2002). Chlorate inhibits sulfate activation, carried out by PAPS synthase, converting sulfate and adenosine triphosphate (ATP) to PAPS, and reduces therefore the availability of PAPS. In MDCK cells, the addition of chlorate affects GAG synthesis differently than what is observed in the presence of BFA. CS sulfation is clearly more sensitive to low chlorate concentrations than HS sulfation is, whereas BFA reduces both (Fjeldstad et al. 2002). Also, HS GAGs secreted from MDCK cells treated with BFA (Kolset et al. 2002) and chlorate (Safaiyan et al. 1999), respectively, differ in structure: the latter leaves N-sulfation intact, whereas O-sulfation is reduced; the former clearly affects N-sulfation in addition to 6-O-sulfation. These observations argue that both substrate availability and Golgi transport dynamics are involved in regulating GAG synthesis.
GAG Synthesizing Enzymes—Complex Formation and Localization
Synthesis of both HS- and CS-GAGs is initiated early in the secretory pathway by O-glycosylation, adding xylose to a serine residue of the protein core. Further linker tetrasaccharide formation and modification must be completed before the involvement of polymerizing enzymes, epimerases, and sulfotransferases. The initial activities of xylosyltransferases I and II (XT I and II) (Götting et al. 2000; Schön et al. 2006), galactosyltransferases β4Gal-T7 (Esko et al. 1987; Almeida et al. 1999), β3Gal-T6 (Bai et al. 2001), and glucuronyltransferase I (Kitagawa et al. 1998) all reside in the early secretory pathway. When disrupting secretory transport by BFA, XT I and II redistributed to the ER (Schön et al. 2006). When expressing a modified decorin carrying a KDEL ER-retrieval signal in COS-7 cells, the GAG attachment site carried a pentasaccharide consisting of the four linker sugars and the first GalNAc of a CS disaccharide. Also, the N-glycans were endo-H sensitive, indicating retrieval of decorin-KDEL before reaching the medial-Golgi (Jönsson et al. 2003). The transferases involved in linker region synthesis are affected by sulfate and phosphate modifications on individual saccharides (Gulberti et al. 2005; Tone et al. 2008). In the case of CS GAG chain initiation, sulfation of linker galactose 1 and 2 increases the activity of the subsequent CS GalNAc-transferase I, committing the polymer to CS synthesis (Gulberti et al. 2012). This suggests that early events influence whether a CS/DS chain is formed, whereas the GAG polymerization itself is shown to take place later, in the TGN.
The GAG polymerizing and modifying enzymes are thought to act in a coordinated fashion, facilitating concomitant synthesis and sulfation of GAGs. In vitro incubation of Golgi-enriched subcellular fractions after chlorate treatment of MDCK cells revealed no increase in sulfate incorporation (K. Prydz, unpublished observation). This suggests that post-synthesis sulfation of undersulfated GAG chains does not occur. However, the degree of co-localization and cooperation between the enzymes involved is uncertain. Oligomers of CS synthases 1 to 3 facilitate efficient synthesis of CS GAGs (Kitagawa et al. 2003; Izumikawa et al. 2008). Pull-down experiments, as well as morphological data, demonstrate enzyme complex formation in the Golgi apparatus, with functional consequences for CS synthesis (Izumikawa et al. 2008; Ogawa et al. 2010). A differential combination of CS synthesizing enzymes is a potential regulatory mechanism of GAG synthesis, but the distribution of these complexes within the Golgi apparatus and their relation to Golgi organization and transport have not been resolved. For instance, CS 4-O-sulfation of decorin reappear more rapidly than 6-O-sulfation after reversal of BFA treatment in rat fibroblasts (Moses et al. 1997). Enzymes involved in HS GAG synthesis and modification, EXT1 and 2 (McCormick et al. 2000), EXTL 3 (Busse et al. 2007), and NDSTs (Ledin et al. 2006; Presto et al. 2008), oligomerize and form multienzyme complexes in the Golgi apparatus. This “gagosome” model suggests that competition between various enzymes for participation in functional complexes could regulate chain length and sulfation intensity of HS GAGs.
In addition to the synthesizing enzymes, availability of precursors influences GAG synthesis. UDP-sugars and PAPS are synthesized in the cytoplasm and translocated into the Golgi lumen by nucleotide sugar transporters (NSTs) and PAPS transporters (PAPSTs). Translocation into the restricted Golgi luminal space increases the concentration 50- to 100-fold (Hirschberg and Snider 1987). The NSTs harboring translocation activities required for PG synthesis have all been cloned (Ishida and Kawakita 2004) and display variable substrate specificity (Ashikov et al. 2005). Homodimer formation has been reported for the GDP-mannose transporter in yeast (Gao and Dean 2000). Also, interaction between a UDP-gal transporter and a galactosyltransferase has been described (Sprong et al. 2003). However, to what extent partitioning and distribution of NSTs and PAPSTs within the Golgi complex contribute to variability in PG structure is not known. We have investigated the role of PAPST1 in the synthesis of GAGs in polarized MDCK cells (Dick et al. 2008). By stable expression of recombinant PAPST1-GFP in MDCK cells, CS sulfation in the apical pathway was increased. When isolating Golgi vesicles from these cells by subcellular fractionation, we could observe increased PAPS uptake and GAG synthesis in vitro (Dick et al. 2008). The effect on CS sulfation could be explained by elevated PAPS concentration in the Golgi lumen. However, the differential effect on CS sulfation in the apical and basolateral pathways suggests pathway-specific changes. Figure 1 shows the location of PAPST1-GFP at one side of the Golgi, presumably in the TGN. Further investigations into possible domain formations, involving the NSTs and PAPSTs, together with the synthesizing enzymes (already suggested to operate in complexes), are needed to clarify the effects of possible co-localization and/or complex formation.
Figure 1.
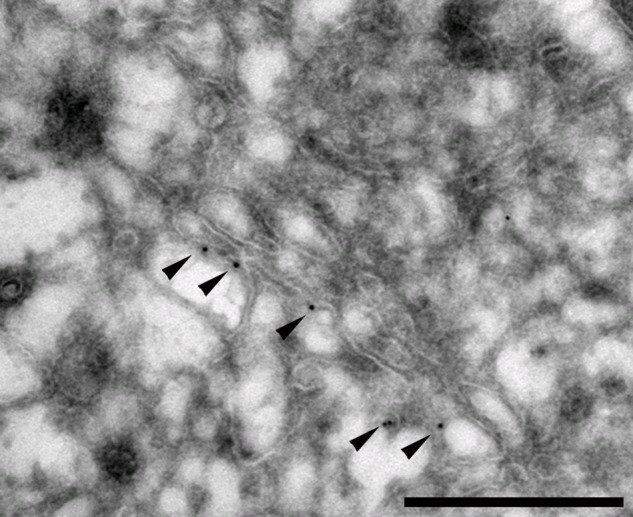
3′-Phosphoadenosine-5′-phosphosulfate transporter 1 (PAPST1) distribution in the Golgi apparatus of polarized cells. Madin-Darby canine kidney (MDCK II) cells expressing PAPST1–green fluorescent protein (GFP) were grown on polycarbonate filters for 4 days, fixed, and sectioned into 70-nm-thin sections in the cryomicrotome. Prior to examination by transmission electron microscope, the section was immunogold labeled using anti-GFP (Ab6556; Abcam, Cambridge, UK) and protein-A-Gold, followed by staining with uranyl acetate. Gold particles labeling PAPST1-GFP are observed at one side of the Golgi structure (black dots indicated by arrowheads). Scale bar = 500 nm.
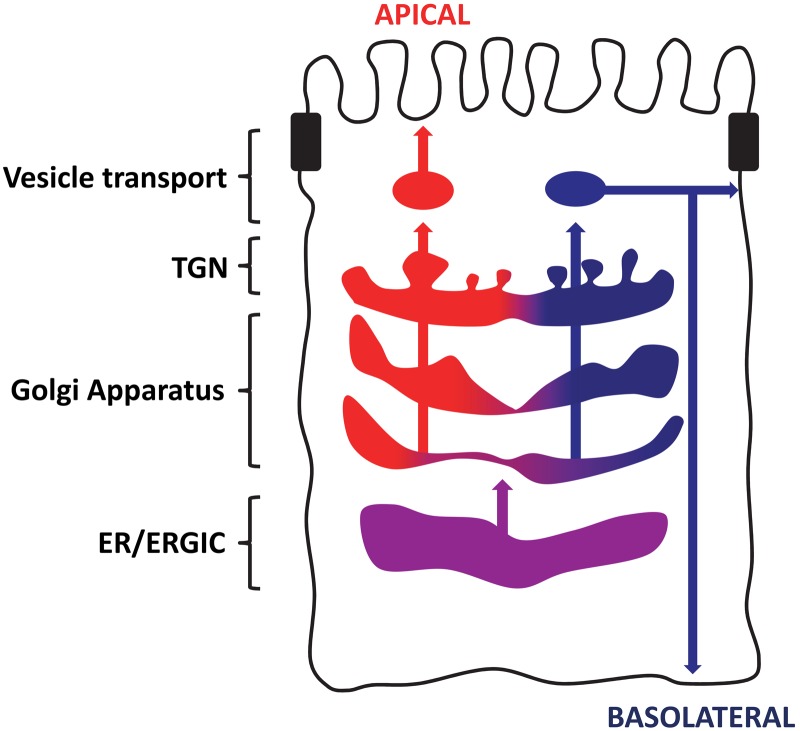
Enzymes involved in modification of the GAG chains, the epimerases and sulfotransferases (reviewed in Kusche-Gullberg and Kjellen 2003), have been observed in oligomeric complexes. The HS-modifying C-5-epimerase, which converts glucuronic acid to iduronic acid, and the 2-O-sulfotransferase do interact (Pinhal et al. 2001). The 6-O-sulfotransferase involved in HS sulfation forms oligomers required for Golgi localization and enzyme activity (Nagai et al. 2004). In MDCK cells, we have observed a tendency for there to be more 6-O-sulfated CS in the basolateral pathway (Tveit et al. 2005; Dick et al. 2008). This could be due to partitioning of enzymes in the Golgi apparatus into different synthesizing domains or pathways, as depicted in Fig. 2 and discussed in Prydz et al. (2008).
Figure 2.
The Golgi apparatus in polarized epithelial cells segregates cargo from common origin in the endoplasmic reticulum (ER)/ERGIC (purple) into the apical (red) and basolateral (blue) pathways. The pathway-specific glycosylation of secreted cargo indicates a partitioning early in the Golgi, before completion of glycosaminoglycan (GAG) synthesis and modification. This implies a segregation of the Golgi itself into domains facilitating apical or basolateral glycosylation and modification. TGN, trans-Golgi network.
Effects of pH
As proteins move along the secretory pathway, they encounter gradually more acidic stations, from a neutral ER via a slightly acidic IC (Palokangas et al. 1998; Appenzeller-Herzog et al. 2004) to a pH around 6.0 in the TGN (Seksek et al. 1995). The slightly acidic pH in the secretory pathway is required for a number of posttranslational modifications, such as glycosylation, sulfation, proteolytic processing, and binding of ionic copper (Weisz 2003; Paroutis et al. 2004; Caldwell and Howell 2008).
To acidify the organelles, V-ATPase is considered responsible. This proton pump consists of two distinct domains: V0, which is transmembrane, and V1, which is cytoplasmic and anchored to the membrane via interaction with the V0 domain. In kidney tubuli epithelial cells, such as MDCK cells, the apical plasma membrane domain is particularly active in proton pumping. Consequently, the V-ATPase is more abundant in the apical secretory pathway than in the corresponding basolateral pathway in these cells (Gong et al. 2010). To overcome the membrane potential generated by pumping protons into organelles, anion channels letting anions, mainly chloride, into the organelles contribute to the generation of an acidic environment (Maeda et al. 2008). The pH is also regulated by a proton leakage, as well as possibly by anion leakage, into the cytoplasm (Caldwell and Howell 2008; Carraro-Lacroix et al. 2010).
Several compounds have been used to disrupt pH gradients within the secretory pathway. Bafilomycin A1 (Baf) and concanamycin (conc) are specific inhibitors of the V-ATPase, whereas chloroquine and ammonium chloride are weak bases, and nigericin is an ionophor. Neutralization by such drugs has been shown to affect glycosylation of proteins and lipids, as well as transport and sorting in the secretory pathway. For example, terminal sialylation of N- and O-glycans in the TGN is inhibited when the pH gradient is dissipated (Thorens and Vassalli 1986; Gawlitzek et al. 2000; Axelsson et al. 2001; Campbell et al. 2001). Alterations in glycosylation induced by pH elevation could be due to changes in enzyme expression, localization or activity, altered interactions with partners, or volume increase of Golgi compartments. Although mRNA and enzyme levels for β1,4-galactosyltransferase (β1,4-GT) and β-galactoside α-2,3-sialyltransferase (ST3Gal-I) remained unchanged in the presence of ammonium chloride, the enzymatic activities decreased as much as 50% to 60% (Gawlitzek et al. 2000). In a later study, ST3Gal-I failed to terminally sialylate an N-glycosylated reporter protein (CEA), when the pH was elevated by as little as 0.2 units by chloroquine (Rivinoja et al. 2009). This implies that fine tuning of pH is required to locally regulate steps in protein glycosylation.
Enzyme interactions and localization may also be affected when pH is elevated (Chen et al. 2000; Hassinen et al. 2011). Enzymes in the N-glycosylation pathway, galactosyltransferase-I (GalT-I) and β-galactosamide α-2,6-sialyltranferase-I (ST6Gal-I), are capable of forming homo- and heterodimers in live cells (Hassinen et al. 2010). Fluorescence resonance energy transfer (FRET) analysis revealed that glycosyltransferases form glycosylation pathway-specific heteromeric complexes, such as GalT-I·ST6Gal-I in N-glycosylation and polypeptide N-acetylgalactosaminyltransferase-6 (ppGalNAcT-6)·Glycoprotein-N-acetylgalactosamine β-3-galactosyl transferase (C-1GalT-I) in mucin-type O-glycosylation (Hassinen et al. 2011). In contrast, complex formation between enzymes from each of the two glycosylation pathways was not observed. Non-reducing SDS-PAGE and size exclusion chromatography revealed a mass of 180 to 190 kD for the GalT1·ST6Gal-I complex, indicative of homo- and heteromeric tetramers. Heteromeric complexes displayed higher enzymatic activities and were more pH-sensitive than homomers (Hassinen et al. 2011). Homodimerization is also sensitive to pH. When ST6Gal-I was extracted at pH 6.8, it was observed as a homodimer, but when extracted at pH 8.0, the enzyme was more soluble and monomeric (Chen et al. 2000). Several glycosyltransferases have been shown to change cellular localization in response to dissipation of the pH gradient. GalNAc transferase 2, β1,2 GlcNAc transferase I, and β1,4 GT 1, normally localized in the Golgi apparatus and TGN, were relocalized to the cell surface after the pH gradient was disrupted by Baf (Axelsson et al. 2001; Rivinoja et al. 2009).
Synthesis of GAGs is affected by elevated pH in the Golgi apparatus. In several tissues from cystic fibrosis transmembrane conductance regulator (CFTR) knockout mice, CS/DS was more intensely sulfated than in control mice (Hill et al. 1997). A role of CFTR in Golgi pH regulation is, however, not generally established (Machen et al. 2001). We have investigated GAG synthesis in MDCK cells after elevating Golgi pH. Adding 0.5 µM Baf to MDCK cells enhanced sulfation of apically secreted CS/DS GAGs, whereas HS GAG chains became longer (Grøndahl et al. 2009). The effect of Baf treatment differed in the apical and basolateral pathways. This indicated that PG synthesis in the apical secretory route through the Golgi apparatus is more sensitive to inhibition of the V-ATPase. One would expect that most of the apically targeted V-ATPases travel through the apical secretory pathway and are active already in transit through the Golgi apparatus. As a result, the apical pathway will have a lower internal pH compared with the baslolateral route. Indeed, changes to the apically secreted GAGs induced by Baf neutralization led to major changes to GAG synthesis in the apical route, whereas basolaterally secreted GAGs were less affected (Grøndahl et al. 2009).
Conclusion
Accumulating evidence demonstrates that glycosylation mechanisms in the Golgi apparatus are regulated by several important mechanisms in parallel, and that manipulation of these mechanisms leads to significant changes in the output of PGs and glycoproteins. Several studies indicate that the apical and basolateral secretory routes in epithelial MDCK cells offer different conditions for glycosaminoglycan synthesis, implying an early segregation of the two pathways (Fig. 2). Further studies concentrating on both Golgi organization and proteoglycan and glycoprotein synthesis are required to address this possibility.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a FUGE grant from the Norwegian Research Council of Norway to GLYCONOR. L. K. Akslen-Hoel is an EMBIO (MLS) Fellow funded by University of Oslo.
References
- Almeida R, Levery SB, Mandel U, Kresse H, Schwientek T, Bennett EP, Clausen H. 1999. Cloning and expression of a proteoglycan UDP-galactose:beta-xylose beta1,4-galactosyltransferase I: A seventh member of the human beta4-galactosyltransferase gene family. J Biol Chem. 274:26165–26171 [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Roche AC, Nufer O, Hauri HP. 2004. pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J Biol Chem. 279:12943–12950 [DOI] [PubMed] [Google Scholar]
- Ashikov A, Routier F, Fuhlrott J, Helmus Y, Wild M, Gerardy-Schahn R, Bakker H. 2005. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J Biol Chem. 280:27230–27235 [DOI] [PubMed] [Google Scholar]
- Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. 2001. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 11:633–644 [DOI] [PubMed] [Google Scholar]
- Bai X, Zhou D, Brown JR, Crawford BE, Hennet T, Esko JD. 2001. Biosynthesis of the linkage region of glycosaminoglycans: cloning and activity of galactosyltransferase II, the sixth member of the beta 1,3-galactosyltransferase family (beta 3GalT6). J Biol Chem. 276:48189–48195 [DOI] [PubMed] [Google Scholar]
- Busse M, Feta A, Presto J, Wilen M, Gronning M, Kjellen L, Kusche-Gullberg M. 2007. Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem. 282:32802–32810 [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Howell KE. 2008. Pores galore for the Golgi. Nat Cell Biol. 10:1125–1126 [DOI] [PubMed] [Google Scholar]
- Campbell BJ, Yu LG, Rhodes JM. 2001. Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj J. 18:851–858 [DOI] [PubMed] [Google Scholar]
- Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD. 1987. Dependence on pH of polarized sorting of secreted proteins. Nature. 329:632–635 [DOI] [PubMed] [Google Scholar]
- Carraro-Lacroix LR, Lessa LM, Bezerra CN, Pessoa TD, Souza-Menezes J, Morales MM, Girardi AC, Malnic G. 2010. Role of CFTR and ClC-5 in modulating vacuolar H+-ATPase activity in kidney proximal tubule. Cell Physiol Biochem. 26:563–576 [DOI] [PubMed] [Google Scholar]
- Chen C, Ma J, Lazic A, Backovic M, Colley KJ. 2000. Formation of insoluble oligomers correlates with ST6Gal I stable localization in the Golgi. J Biol Chem. 275:13819–13826 [DOI] [PubMed] [Google Scholar]
- Deepa SS, Yamada S, Zako M, Goldberger O, Sugahara K. 2004. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors: a novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J Biol Chem. 279:37368–37376 [DOI] [PubMed] [Google Scholar]
- Dick G, Grøndahl F, Prydz K. 2008. Over-expression of the 3′-phosphoadenosine 5′-phosphosulfate (PAPS) transporter 1 increases sulfation of chondroitin sulfate in the apical pathway of MDCK II cells. Glycobiology. 18:53–65 [DOI] [PubMed] [Google Scholar]
- Dinter A, Berger EG. 1998. Golgi-disturbing agents. Histochem Cell Biol. 109:571–590 [DOI] [PubMed] [Google Scholar]
- Edge AS, Spiro RG. 1990. Characterization of novel sequences containing 3-O-sulfated glucosamine in glomerular basement membrane heparan sulfate and localization of sulfated disaccharides to a peripheral domain. J Biol Chem. 265:15874–15881 [PubMed] [Google Scholar]
- Elazar Z, Orci L, Ostermann J, Amherdt M, Tanigawa G, Rothman JE. 1994. ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J Cell Biol. 124:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson AC, Couchman JR. 2001. Basement membrane and interstitial proteoglycans produced by MDCK cells correspond to those expressed in the kidney cortex. Matrix Biol. 19:769–778 [DOI] [PubMed] [Google Scholar]
- Esko JD, Weinke JL, Taylor WH, Ekborg G, Roden L, Anantharamaiah G, Gawish A. 1987. Inhibition of chondroitin and heparan sulfate biosynthesis in Chinese hamster ovary cell mutants defective in galactosyltransferase I. J Biol Chem. 262:12189–12195 [PubMed] [Google Scholar]
- Fiedler K, Simons K. 1995. The role of N-glycans in the secretory pathway. Cell. 81:309–312 [DOI] [PubMed] [Google Scholar]
- Fjeldstad K, Pedersen ME, Vuong TT, Kolset SO, Nordstrand LM, Prydz K. 2002. Sulfation in the Golgi lumen of Madin-Darby canine kidney cells is inhibited by brefeldin A and depends on a factor present in the cytoplasm and on Golgi membranes. J Biol Chem. 277:36272–36279 [DOI] [PubMed] [Google Scholar]
- Gao XD, Dean N. 2000. Distinct protein domains of the yeast Golgi GDP-mannose transporter mediate oligomer assembly and export from the endoplasmic reticulum. J Biol Chem. 275:17718–17727 [DOI] [PubMed] [Google Scholar]
- Gawlitzek M, Ryll T, Lofgren J, Sliwkowski MB. 2000. Ammonium alters N-glycan structures of recombinant TNFR-IgG: degradative versus biosynthetic mechanisms. Biotechnol Bioeng. 68:637–646 [DOI] [PubMed] [Google Scholar]
- Girardin EP, Hajmohammadi S, Birmele B, Helisch A, Shworak NW, de Agostini AI. 2005. Synthesis of anticoagulantly active heparan sulfate proteoglycans by glomerular epithelial cells involves multiple 3-O-sulfotransferase isoforms and a limiting precursor pool. J Biol Chem. 280:38059–38070 [DOI] [PubMed] [Google Scholar]
- Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. 2010. Vacuolar H+-atpase apical accumulation in kidney intercalated cells is regulated by pKa and amp-activated protein kinase. Am J Physiol Renal Physiol. 298:1162–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. 2000. Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol. 304:517–528 [DOI] [PubMed] [Google Scholar]
- Grøndahl F, Tveit H, Prydz K. 2009. Neutralization of endomembrane compartments in epithelial MDCK cells affects proteoglycan synthesis in the apical secretory pathway. Biochem J. 418:517–528 [DOI] [PubMed] [Google Scholar]
- Gulberti S, Jacquinet JC, Chabel M, Ramalanjaona N, Magdalou J, Netter P, Coughtrie MW, Ouzzine M, Fournel-Gigleux S. 2012. Chondroitin sulfate N-acetylgalactosaminyltransferase-1 (CSGalNAcT-1) involved in chondroitin sulfate initiation: impact of sulfation on activity and specificity. Glycobiology. 22:561–571 [DOI] [PubMed] [Google Scholar]
- Gulberti S, Lattard V, Fondeur M, Jacquinet JC, Mulliert G, Netter P, Magdalou J, Ouzzine M, Fournel-Gigleux S. 2005. Phosphorylation and sulfation of oligosaccharide substrates critically influence the activity of human beta1,4-galactosyltransferase 7 (GalT-I) and beta1,3-glucuronosyltransferase I (GlcAT-I) involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 280:1417–1425 [DOI] [PubMed] [Google Scholar]
- Hafte TT, Fagereng GL, Prydz K, Grøndahl F, Tveit H. 2011. Protein core-dependent glycosaminoglycan modification and glycosaminoglycan-dependent polarized sorting in epithelial Madin-Darby canine kidney cells. Glycobiology. 21:457–466 [DOI] [PubMed] [Google Scholar]
- Hassinen A, Pujol FM, Kokkonen N, Pieters C, Kihlstrom M, Korhonen K, Kellokumpu S. 2011. Functional organization of Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 286:38329–38340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen A, Rivinoja A, Kauppila A, Kellokumpu S. 2010. Golgi N-glycosyltransferases form both homo- and heterodimeric enzyme complexes in live cells. J Biol Chem. 285:17771–17777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H, Appenzeller C, Kuhn F, Nufer O. 2000. Lectins and traffic in the secretory pathway. FEBS Lett. 476:32–37 [DOI] [PubMed] [Google Scholar]
- Hill WG, Harper GS, Rozaklis T, Boucher RC, Hopwood JJ. 1997. Organ-specific over-sulfation of glycosaminoglycans and altered extracellular matrix in a mouse model of cystic fibrosis. Biochem Mol Med. 62:113–122 [DOI] [PubMed] [Google Scholar]
- Hirschberg CB, Snider MD. 1987. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 56:63–87 [DOI] [PubMed] [Google Scholar]
- Ishida N, Kawakita M. 2004. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch. 447:768–775 [DOI] [PubMed] [Google Scholar]
- Izumikawa T, Koike T, Shiozawa S, Sugahara K, Tamura J, Kitagawa H. 2008. Identification of chondroitin sulfate glucuronyltransferase as chondroitin synthase-3 involved in chondroitin polymerization: chondroitin polymerization is achieved by multiple enzyme complexes consisting of chondroitin synthase family members. J Biol Chem. 283:11396–11406 [DOI] [PubMed] [Google Scholar]
- Jönsson M, Eklund E, Fransson LA, Oldberg A. 2003. Initiation of the decorin glycosaminoglycan chain in the endoplasmic reticulum-Golgi intermediate compartment. J Biol Chem. 278:21415–21420 [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Izumikawa T, Uyama T, Sugahara K. 2003. Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J Biol Chem. 278:23666–23671 [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Tone Y, Tamura J, Neumann KW, Ogawa T, Oka S, Kawasaki T, Sugahara K. 1998. Molecular cloning and expression of glucuronyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 273:6615–6618 [DOI] [PubMed] [Google Scholar]
- Kobialka S, Beuret N, Ben-Tekaya H, Spiess M. 2009. Glycosaminoglycan chains affect exocytic and endocytic protein traffic. Traffic. 10:1845–1855 [DOI] [PubMed] [Google Scholar]
- Kolset SO, Prydz K, Fjeldstad K, Safaiyan F, Vuong TT, Gottfridsson E, Salmivirta M. 2002. Effect of brefeldin A on heparan sulphate biosynthesis in Madin-Darby canine kidney cells. Biochem J. 362:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset SO, Vuong TT, Prydz K. 1999. Apical secretion of chondroitin sulphate in polarized Madin-Darby canine kidney (MDCK) cells. J Cell Sci. 112:1797–1801 [DOI] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Kjellen L. 2003. Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol. 13:605–611 [DOI] [PubMed] [Google Scholar]
- Lawrence R, Yabe T, Hajmohammadi S, Rhodes J, McNeely M, Liu J, Lamperti ED, Toselli PA, Lech M, Spear PG, et al. 2007. The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues. Matrix Biol. 26:442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledin J, Ringvall M, Thuveson M, Eriksson I, Wilen M, Kusche-Gullberg M, Forsberg E, Kjellen L. 2006. Enzymatically active N-deacetylase/N-sulfotransferase-2 is present in liver but does not contribute to heparan sulfate N-sulfation. J Biol Chem. 281:35727–35734 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 56:801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. 1991. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 67:601–616 [DOI] [PubMed] [Google Scholar]
- Lo AC, Thinakaran G, Slunt HH, Sisodia SS. 1995. Metabolism of the amyloid precursor-like protein 2 in MDCK cells: polarized trafficking occurs independent of the chondroitin sulfate glycosaminoglycan chain. J Biol Chem. 270:12641–12645 [DOI] [PubMed] [Google Scholar]
- Machen TE, Chandy G, Wu M, Grabe M, Moore HP. 2001. Cystic fibrosis transmembrane conductance regulator and H+ permeability in regulation of Golgi pH. JOP. 2:229–236 [PubMed] [Google Scholar]
- Maeda Y, Ide T, Koike M, Uchiyama Y, Kinoshita T. 2008. GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat Cell Biol. 10:1135–1145 [DOI] [PubMed] [Google Scholar]
- McCarthy KJ, Couchman JR. 1990. Basement membrane chondroitin sulfate proteoglycans: localization in adult rat tissues. J Histochem Cytochem. 38:1479–1486 [DOI] [PubMed] [Google Scholar]
- McCormick C, Duncan G, Goutsos KT, Tufaro F. 2000. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci U S A. 97:668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens G, Van Der Schueren B, Van Den Berghe H, David G. 1996. Heparan sulfate expression in polarized epithelial cells: the apical sorting of glypican (GPI-anchored proteoglycan) is inversely related to its heparan sulfate content. J Cell Biol. 132:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses J, Oldberg A, Eklund E, Fransson LA. 1997. Biosynthesis of the proteoglycan decorin—identification of intermediates in galactosaminoglycan assembly. Eur J Biochem. 248:767–774 [DOI] [PubMed] [Google Scholar]
- Nagai N, Habuchi H, Esko JD, Kimata K. 2004. Stem domains of heparan sulfate 6-O-sulfotransferase are required for Golgi localization, oligomer formation and enzyme activity. J Cell Sci. 117:3331–3341 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Shionyu M, Sugiura N, Hatano S, Nagai N, Kubota Y, Nishiwaki K, Sato T, Gotoh M, Narimatsu H, et al. 2010. Chondroitin sulfate synthase-2/chondroitin polymerizing factor has two variants with distinct function. J Biol Chem. 285:34155–34167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco B, Maccarana M, Malmström A. 2009. Dermatan 4-O-sulfotransferase 1 is pivotal in the formation of iduronic acid blocks in dermatan sulfate. Glycobiology. 19:1197–1203 [DOI] [PubMed] [Google Scholar]
- Palade G. 1975. Intracellular aspects of the process of protein synthesis. Science. 189:867. [DOI] [PubMed] [Google Scholar]
- Palokangas H, Ying M, Vaananen K, Saraste J. 1998. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol Biol Cell. 9:3561–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P, Touret N, Grinstein S. 2004. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda). 19:207–215 [DOI] [PubMed] [Google Scholar]
- Pinhal MA, Smith B, Olson S, Aikawa J, Kimata K, Esko JD. 2001. Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci U S A. 98:12984–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presto J, Thuveson M, Carlsson P, Busse M, Wilen M, Eriksson I, Kusche-Gullberg M, Kjellen L. 2008. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc Natl Acad Sci U S A. 105:4751–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz K, Dalen KT. 2000. Synthesis and sorting of proteoglycans. J Cell Sci. 113:193–205 [DOI] [PubMed] [Google Scholar]
- Prydz K, Dick G, Tveit H. 2008. How many ways through the Golgi maze? Traffic. 9:299–304 [DOI] [PubMed] [Google Scholar]
- Rivinoja A, Hassinen A, Kokkonen N, Kauppila A, Kellokumpu S. 2009. Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J Cell Physiol. 220:144–154 [DOI] [PubMed] [Google Scholar]
- Safaiyan F, Kolset SO, Prydz K, Gottfridsson E, Lindahl U, Salmivirta M. 1999. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J Biol Chem. 274:36267–36273 [DOI] [PubMed] [Google Scholar]
- Safaiyan F, Lindahl U, Salmivirta M. 2000. Structural diversity of N-sulfated heparan sulfate domains: distinct modes of glucuronyl C5 epimerization, iduronic acid 2-O-sulfation, and glucosamine 6-O-sulfation. Biochemistry. 39:10823–10830 [DOI] [PubMed] [Google Scholar]
- Schaefer L, Hausser H, Altenburger M, Ugorcakova J, August C, Fisher LW, Schaefer RM, Kresse H. 1998. Decorin, biglycan and their endocytosis receptor in rat renal cortex. Kidney Int. 54:1529–1541 [DOI] [PubMed] [Google Scholar]
- Schön S, Prante C, Bahr C, Kuhn J, Kleesiek K, Götting C. 2006. Cloning and recombinant expression of active full-length xylosyltransferase I (XT-I) and characterization of subcellular localization of XT-I and XT-II. J Biol Chem. 281:14224–14231 [DOI] [PubMed] [Google Scholar]
- Seksek O, Biwersi J, Verkman AS. 1995. Direct measurement of trans-Golgi pH in living cells and regulation by second messengers. J Biol Chem. 270:4967–4970 [DOI] [PubMed] [Google Scholar]
- Spiro RC, Freeze HH, Sampath D, Garcia JA. 1991. Uncoupling of chondroitin sulfate glycosaminoglycan synthesis by brefeldin A. J Cell Biol. 115:1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong H, Degroote S, Nilsson T, Kawakita M, Ishida N, Van Der Sluijs P, Van Meer G. 2003. Association of the Golgi UDP-galactose transporter with UDP-galactose:ceramide galactosyltransferase allows UDP-galactose import in the endoplasmic reticulum. Mol Biol Cell. 14:3482–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran G, Katsman M, Silbert JE. 1992. Effects of brefeldin A on the localization of chondroitin sulfate-synthesizing enzymes: activities in subfractions of the Golgi from chick embryo epiphyseal cartilage. J Biol Chem. 267:8802–8806 [PubMed] [Google Scholar]
- Svennevig K, Prydz K, Kolset SO. 1995. Proteoglycans in polarized epithelial Madin-Darby canine kidney cells. Biochem J. 311:881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Vassalli P. 1986. Chloroquine and ammonium chloride prevent terminal glycosylation of immunoglobulins in plasma cells without affecting secretion. Nature. 321:618–620 [DOI] [PubMed] [Google Scholar]
- Toma L, Pinhal MA, Dietrich CP, Nader HB, Hirschberg CB. 1996. Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J Biol Chem. 271:3897–3901 [DOI] [PubMed] [Google Scholar]
- Tone Y, Pedersen LC, Yamamoto T, Izumikawa T, Kitagawa H, Nishihara J, Tamura J, Negishi M, Sugahara K. 2008. 2-O-phosphorylation of xylose and 6-O-sulfation of galactose in the protein linkage region of glycosaminoglycans influence the glucuronyltransferase-I activity involved in the linkage region synthesis. J Biol Chem. 283:16801–16807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit H, Akslen LK, Fagereng GL, Tranulis MA, Prydz K. 2009. A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells. Traffic. 10:1685–1695 [DOI] [PubMed] [Google Scholar]
- Tveit H, Dick G, Skibeli V, Prydz K. 2005. A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin-Darby canine kidney cells. J Biol Chem. 280:29596–29603 [DOI] [PubMed] [Google Scholar]
- Uhlin-Hansen L, Yanagishita M. 1993. Differential effect of brefeldin A on the biosynthesis of heparan sulfate and chondroitin/dermatan sulfate proteoglycans in rat ovarian granulosa cells in culture. J Biol Chem. 268:17370–17376 [PubMed] [Google Scholar]
- Vuong TT, Prydz K, Tveit H. 2006. Differences in the apical and basolateral pathways for glycosaminoglycan biosynthesis in Madin-Darby canine kidney cells. Glycobiology. 16:326–332 [DOI] [PubMed] [Google Scholar]
- Weisz OA. 2003. Organelle acidification and disease. Traffic. 4:57–64 [DOI] [PubMed] [Google Scholar]