Abstract
Embryonic stem (ES) cells are derived from the inner cell mass of the blastocyst and can give rise to all cell types in the body. The fate of ES cells depends on the signals they receive from their surrounding environment, which either promote self-renewal or initiate differentiation. Heparan sulfate proteoglycans are macromolecules found on the cell surface and in the extracellular matrix. Acting as low-affinity receptors on the cell surface, heparan sulfate (HS) side chains modulate the functions of numerous growth factors and morphogens, having wide impact on the extracellular information received by cells. ES cells lacking HS fail to differentiate but can be induced to do so by adding heparin. ES cells defective in various components of the HS biosynthesis machinery, thus expressing differently flawed HS, exhibit lineage-specific effects. Here we discuss recent studies on the biological functions of HS in ES cell developmental processes. Since ES cells have significant potential applications in tissue/cell engineering for cell replacement therapies, understanding the functional mechanisms of HS in manipulating ES cell growth in vitro is of utmost importance, if the stem cell regenerative medicine from scientific fiction ever will be made real.
Keywords: proteoglycans, heparan sulfate, embryonic stem cells, development, extracellular signaling
Impact of Heparan Sulfate in Animal Development
Heparan sulfate proteoglycans (HSPGs) at the cell surface and in the extracellular matrix have important functions during embryonic development. They act as essential co-receptors and their interaction with various exogenous factors allows the generation and maintenance of morphogen gradients. The sulfation pattern of the heparan sulfate (HS) side chains, deciding the factors that will bind and the affinity of the interaction, is determined largely during biosynthesis. In addition, after biosynthesis, the HS chains may be modified by the endo–6-O-sulfatases (Sulf 1 and 2, see below) or by heparanase, an endo-glycosidase that generates HS fragments. Biosynthesis occurs in the Golgi compartment and involves the action of several different enzymes, some of which occur in more than one isoform. The enzymes that will be mostly discussed here are Ext1, which, together with Ext2, forms the functional HS polymerase, and the glucosaminyl N-deacetylase/sulfotransferases (Ndsts), which are responsible for the HS N-sulfation that controls the subsequent enzymatic reactions, thereby affecting the overall structure of HS chains. Biosynthesis of HS is covered in two companion mini-reviews (Kreuger & Kjellén 2012; Multhaupt and Couchman 2012).
HS is essential for animal development, as evidenced by mutational studies in model organisms, including Drosophila melanogaster, mouse, Caenorhabditis elegans and zebrafish. In the mouse, complete interruption of HS biosynthesis by obliteration of the polymerases, Ext1 or Ext2 (Lin et al. 2000; Stickens et al. 2005), led to early termination of mouse embryonic development. Selected elimination of the enzymes involved in modification of HS structure resulted in strikingly varied phenotypes in mice. Inhibition of N-sulfation by knockout of Ndst1 caused an overall downregulation of the modification reactions downstream, resulting in HS with greatly reduced charge density (Ringvall et al. 2000). Thus, Ndst1−/− mice showed severe developmental defects in multiple organs, including skeleton, brain, and lungs, and died shortly after birth due to respiratory failure (Fan et al. 2000; Ringvall et al. 2000). In comparison, Ndst2−/− mice did not show any obvious defect, except an abnormal morphology in mast cells (Forsberg E et al. 1999; Humphries et al. 1999). Elimination of the single gene-coded enzymes in HS biosynthesis, glucuronyl C5-epimerase (Glce) and hexuronyl 2-O-sulfotransferase (Hs2st), resulted in similar phenotypes, for example, neonatal lethality with kidney agenesis and multiple skeletal malformations (Bullock et al. 1998; Li et al. 2003). Most glucosaminyl 6-O-sulfotransferase 1 (Hs6st1) knockout embryos died during late gestation, and those that survived were smaller than wild-type littermates, with various developmental abnormalities (Habuchi et al. 2007). Mice deficient in glucosaminyl 3-O-sulfotransferase 1 (Hs3st1) showed intrauterine growth retardation and postnatal lethality, and they displayed a proinflammatory phenotype (Shworak et al. 2002; Shworak et al. 2010).
Further functional regulation of HS in embryo development appears linked to the postsynthesis modification by Sulf1 and 2 and the endo-glucuronidase heparanase (Hpse). Although sulf1 and sulf2 single as well as double mutants appear normal at birth, the double mutants die shortly after birth (Ai et al. 2007; Langsdorf et al. 2007; Lamanna et al. 2008). Surprisingly, neither complete elimination of the heparanase gene nor overexpression of the enzyme affected embryo development (Zcharia et al. 2004; Zcharia et al. 2009).
Embryonic Stem Cells and Induced Pluripotent Stem Cells
Pluripotent embryonic stem (ES) cells are permanent cell lines derived from the inner cell mass of the blastocyst (Evans and Kaufman 1981; Martin 1981) and can be maintained and expanded in culture by addition of factors that promote proliferation in the absence of differentiation, also known as self-renewal. They retain the pluripotency of the cells in the early embryo when reintroduced into the blastocyst (Beddington and Robertson 1989) and can give rise to all cell types in the body (Keller 2005). Although some argue that ES cells do not occur in vivo as such, the in vitro differentiation of ES cells can be achieved by reproducing the developmental signaling pathways identified in vivo (Keller 2005). Thus, human ES cells have significant potential applications in tissue and cell engineering and as tools in drug discovery. Applications include the generation of blood cells for blood transfusions, replacement of damaged neurons in Parkinson disease, replenishment of insulin-secreting beta cells in diabetes mellitus, and bone formation in osteoporosis. In addition, the newly discovered means of reprogramming postmitotic cells into induced pluripotent stem (iPS) cells (Takahashi and Yamanaka 2006; Takahashi et al. 2007; Yu et al. 2007) not only offers the possibility to generate disease-specific stem cells for derivation of novel treatment targets but also the production of patient-specific cells that will not cause immune rejection when transplanted back into the patient.
Classically, ES cells are derived and maintained in vitro using combinations of feeder cells, conditioned media, cytokines, growth factors, serum (primarily fetal bovine serum), and serum extracts as multifactorial stimulation of dedicated transcriptional circuitries that lead to the constant transcriptional activation of pluripotency-linked transcription factors such as Oct4, Sox2, and Nanog. Although the cytokine LIF (leukemia inhibitory factor) alone is insufficient to support self-renewal in serum- and feeder cell–free conditions, the activation via the LIF and gp130 receptors of at least four different downstream signal transduction pathways—JAK/STAT (Janus tyrosine kinase/signal transducer and activator of transcription), Ras/ERK1/2 (extracellular signal–related kinases), PI3K (phosphoinositide-3 kinase), and SFK (Src family kinase) pathway—are generally considered most critical for mouse ES (mES) cell maintenance (Anneren 2008; Burdon et al. 2002). Although human ES (hES) cells respond to LIF, the cytokine does not maintain their self-renewal capacity. Instead, hES cells require supplementation with fibroblast growth factor 2 (FGF2) and signaling via members of the FGF receptor (FGFR) tyrosine kinase family (Levenstein et al. 2006). Interestingly, recent breakthroughs have shown that extrinsic stimuli in many respects are dispensable for propagation and self-renewal of mES cells. For example, mES cell self-renewal can be maintained by the use of small-molecule inhibitors to suppress differentiation-inducing signaling from mitogen-activated protein kinase (MAPK) and glycogen synthase kinase 3 (GSK-3) (Ying et al. 2008).
Obviously, significant hurdles remain to be conquered before ES and iPS cells can be used in large-scale applications. Key issues, such as defining and re-creating a suitable culture environment to allow efficient differentiation of the cells, remain to be resolved before the stem cell regenerative medicine from scientific fiction becomes a medical reality.
The Effect of Altered HS in ES Cells
Over the years, the scientific community has focused more on describing the microenvironments, or so-called niches, surrounding stem cells. Numerous studies have shown that there is a constant signaling (e.g., by auto-, para-, and exocrine secreted factors) through cell-to-cell interactions between stem cells or with the neighboring tissue, as well as through interactions with the extracellular matrix that promote either self-renewal or differentiation initiation. HSPGs are considered the most common and widely acting low-affinity receptors on the cell surface and play a central role in the reception and modulation of a wide range of growth factors, morphogens, and chemokines. Compared with a variety of murine tissues, mouse ES cells express fairly low-sulfated HS (Ledin et al. 2004; Johnson et al. 2007). However, a clear increase in the expression of HS biosynthetic enzymes, which results in increased HS chain sulfation, has been observed during ES cell differentiation (Johnson et al. 2007). Although in vivo studies have shown that disruption of HS biosynthesis, by mutating a good number of the genes involved in this process, clearly affects various stages during animal development, so far studies on ES cells mainly have been done using cell lines deficient in the Ext and Ndst enzymes. In addition, the role of HS in ES cell biology so far has been studied mainly using mouse ES cells, and the studies discussed in this review, if not stated otherwise, have been done in these cells.
Progression from self-renewal to differentiation and the subsequent lineage commitment is the main focus of most recent studies regarding the role and function of HS in ES cells. As stated above, HS sulfation is upregulated upon ES cell differentiation, which in turn alters the ability of the cells to interact with extrinsic factors. Numerous studies have identified various signaling networks, including those initiated by FGF, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), Wnt, and bone morphogenetic protein (BMP) (all of which are modulated by HS interactions), involved in the regulation of cell fates during embryogenesis. Notably, these growth factors are also well established to affect ES cells, either to maintain self-renewal or to induce differentiation. Consequently, an impaired HS function inhibits the transition to differentiation probably by “numbing” the ES cells to their environment and thus keeping the cells in a naive ES cell state.
Studies Using Ext1−/− ES Cells
ES cells lacking Ext1 do not synthesize any HS and are therefore excellent tools for studying the role of HS in differentiation. Several studies have shown that these cells can form phenotypically correct colonies and express high levels of ES cell markers (Johnson et al. 2007; Kraushaar et al. 2010). However, Sasaki and coworkers (2008) show that RNAi silencing of Ext1 leads to a reduced proliferation with subsequent spontaneous differentiation toward extraembryonic endoderm. Maybe the low level of Ext1 expressed by these cells is enough to support HS-mediated signaling.
In contrast, a total lack of Ext1 prevents both spontaneous differentiation as well as directed neural differentiation (Johnson et al. 2007; Kraushaar et al. 2010; Holley et al. 2011). A recent study by Holley and coworkers (2011) showed that Ext1−/− cells do form embryonic bodies (EBs), an in vitro three-dimensional (3D) model for ES cell differentiation, with a subsequent increase in early mesodermal markers. However, these cells failed to terminally differentiate and entirely adopt full lineage restrictions. Notably, the addition of soluble heparin efficiently stimulated these cells to differentiate in a similar manner as their wild-type counterparts by overcoming insufficient signaling by FGF, BMP, and Wnt. In contrast, the addition of heparin to wild-type cells impaired differentiation, most likely by outcompeting cell surface HS for growth factor binding (Holley et al. 2011). (Kraushaar and coworkers (2012) showed that Ext1−/− ES cells were able to differentiate in serum-containing medium supplemented with high levels of FGF2 and exhibited morphological changes associated with differentiation, followed by downregulation of ES pluripotency genes as well as upregulation of endodermal-associated markers. Early markers for neuroectoderm were also detected, albeit at lower levels and at later stages compared with differentiating wild-type ES cells. This is in contrast to the study by Holley and colleagues (2011), who could not detect any mesoderm differentiation. Again, the addition of heparin fully restored differential efficacy, clearly showing the importance of HS in FGF-induced mesoderm differentiation. Similar results were obtained using BMP4, but not Wnt, to overcome the impaired differentiation potential of Ext1−/− ES cells and induce mesoderm. Their results suggest that HS stabilizes BMP4 levels in the medium, thus maintaining and facilitating signaling during the differentiation process.
It was recently shown that Ext1−/− EBs respond to VEGF stimulation and form angiogenic sprouts (Le Jan et al. 2012). Although the subsequent pericyte attachment was slightly defective, this finding suggest that early angiogenic sprouting and tube formation can occur in the absence of HS. Interestingly, the complete loss of HS in the Ext1−/− cells led to increased chondroitin sulfate (CS) biosynthesis, which most likely can compensate for the lack of HS. Although CS was shown to partly substitute for HS deficiency-induced aberrant PDGFB and TGF signaling, both known regulators of pericyte differentiation and attachment (Hirschi et al. 1998; Hellstrom et al. 1999), the signaling input was not sufficient to warrant a complete rescue.
Studies Using Ndst1/2−/− ES Cells
ES cells devoid of both Ndst1 and Ndst2 also have been used to study the role of HS in differentiation. These cells produce HS that completely lacks N-sulfation. Apart from a low degree of 6-O-sulfation, the HS was unmodified (Holmborn et al. 2004). Similar to Ext1−/− ES cells, Ndst1/2−/− ES cells also maintained their self-renewal and pluripotency capacity under normal ES cell culture conditions (Holmborn et al. 2004; Lanner et al. 2010). In contrast, angiogenesis was severely delayed in Ndst1/2−/− EB cells, and pericyte formation completely arrested (Le Jan et al. 2012). In comparison to Ext1−/− ES cells, virtually no CS could be detected in Ndst1/2−/− ES cells, which further supports a functional overlap between HS and CS during angiogenesis.
Although it has been suggested that Ndst1/2−/− ES cells generally fail to properly differentiate upon EB formation (Lanner et al. 2010), Forsberg and coworkers show that a stepwise differentiation approach allowing for the formation of intermediate progenitors renders the formation of mesoderm, as well as primitive endo- and ectoderm ((Forsberg M et al. 2012). In their study, the cells formed osteoblasts, although with a lower efficacy compared with wild-type ES cells. However, no formation of adipocytes, which is usually found in concert with osteoblasts under in vitro differentiation, was detected. Under neural-inducing conditions, the Ndst/2−/− ES cells adapted a primitive ectoderm-like state expressing FGF5, but the cells were blocked and could not proceed to neural differentiation. Similar results were obtained when looking at endoderm differentiation where the cells seemed to halt in a Gata4-expressing primitive state. The addition of low levels of heparin induced neural differentiation, presumably by enhancing FGF4 signaling.
Future Studies and Available ES Cell Models
Several key ES cell transcription factors have recently been reported to show diverse but dynamic expression during normal ES cell maintenance in serum (Wray et al. 2010). Functionally, this heterogeneous mixture of cells has distinct differential potential and capacity to form chimeras upon blastocyst injection. When shielding the cultures from exogenous differentiation-inducing serum factors with small-molecule kinase inhibitors, the transcription factor levels are stably high and fluctuate less. We and others have observed that ES cells grown with MAPK and GSK-3 inhibitors form colonies that phenotypically appear more compact than the cells cultured under normal conditions and show very little spontaneous differentiation (C. Tamm, S. P. Galitó, and C. Annerén unpublished observations; Marks et al. 2012). Interestingly, similar phenotypes were found for Ext1−/− ES cells, which lack cell surface HS and thus repress HS-regulated extracellular signaling (Holley et al. 2011). Hence, although not fully investigated, it has been suggested that the pluripotency transcription factor network is inherently stable but is easily altered by the vast amount of extrinsic signaling factors present in serum (Wray et al. 2010). As described above, many of these factors have been shown to rely on HS to function properly, and it is therefore clear that any alterations in the HS chains will have a wide impact on the extracellular information to be received by a cell.
Altogether, the results underscore the need for functional HS for inclusive and committed differentiation. As stated above, the functions of most genes involved in HS biosynthesis have been studied for animal development through targeted mutation of the genes in mice. So far, only a few ES cell lines (i.e., Ext1/2 and Ndst1/2) derived from the mutant mice have been investigated for the functional roles of HS in ES cell biology, and the evidence clearly shows a fundamental role in the mere existence and overall design of the sulfation pattern of HS chains. Further studies by application of ES cells derived from mutant mice will reveal how more subtle and defined changes, brought on by mutations of downstream regulators involved in fine-tuning the definitive HS chains’ architecture, will affect the ES cell self-renewal and differentiation capacity.
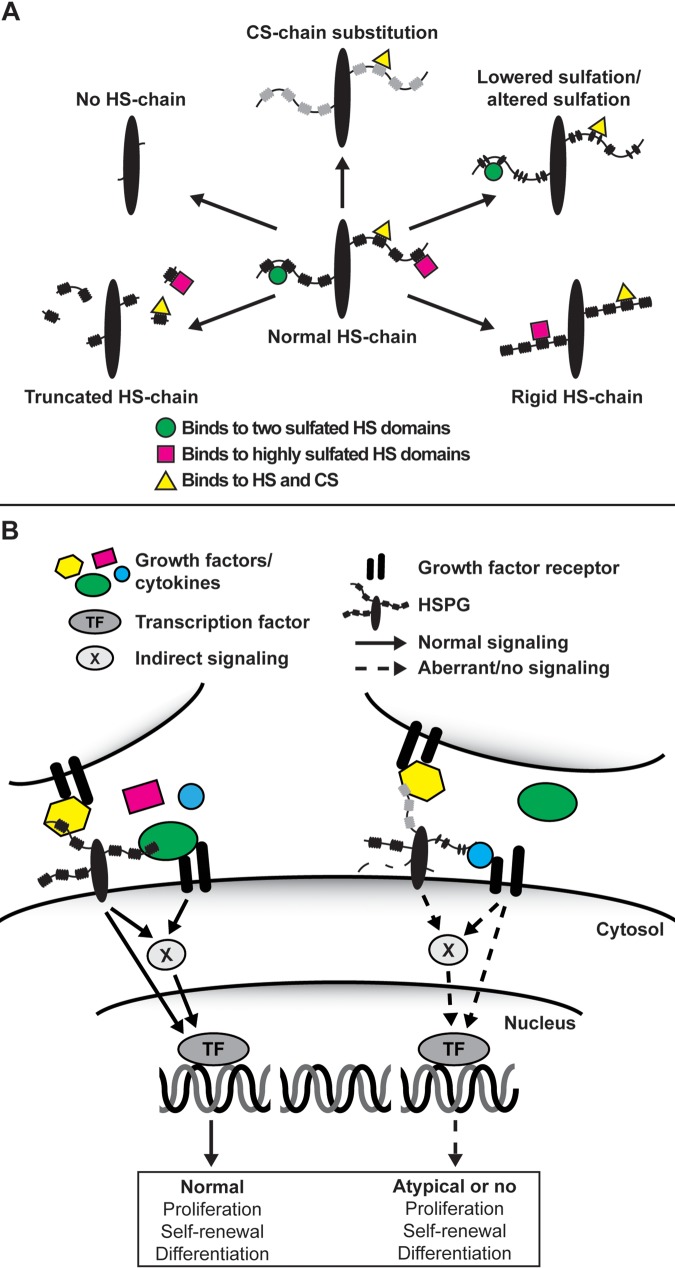
To facilitate the investigation for the roles of HS in ES cell differentiation, apart from the Ext1−/− and Ndst1/2−/− cells discussed above, we have derived Ndst1−/−, Ndst2−/−, Glce−/−, and Hpse −/− ES cells from the corresponding mouse strains generated in our lab. In fact, ES cell lines are being established from most mutant mice strains known to the HS field. Interestingly, our mutant cells, which all express HS with various flaws, demonstrate dissimilar differentiation capacity and lineage commitment. This unique panel of mutant ES cells will allow us to study and compare the functional consequences of completely lacking HS (Ext1−/−) or exhibiting various degrees of structural defects in HS (Figure 1). Elimination/neutralization of extrinsic signaling has revolutionized in vitro maintenance of ES cells; however, the role and function of extracellular signaling in ES cells and early embryogenesis, as well as how it is controlled by HSPGs, still remain of great interest for developmental studies and, maybe more important, for the regulation and harnessing of ES pluripotency and differentiation.
Figure 1.
(A) Hypothetical heparan sulfate (HS) chain structural consequences and effects on ligand interaction upon various mutations in the HS biosynthesis machinery. Depending on the enzyme being modified, various degrees of structural defects in the HS chain will occur ranging from a complete lack of HS chains (Ext1−/−) and exhibiting different degrees of sulfation (various HS sulfotransferase knockouts) to chain length (Hpse−/−) and HS chain rigidity (Glce−/−). The loss of HS in the Ext1−/− cells can also lead to a compensatory increase in chondroitin sulfate (CS) biosynthesis. (B) In wild-type embryonic stem (ES) cells (left), heparan sulfate proteoglycans (HSPGs) located on the same or an adjacent cell function as co-receptors for growth factors and their receptors or, upon ligand binding, induce downstream signal transduction on their own. Although not shown in the figure, HSPGs also facilitate cell adhesion to the extracellular matrix and, when secreted, are involved in the formation of the extracellular matrices that, for example, sequester growth factors and morphogens. However, in the various mutant ES cells (right), in which the HS synthesis and modification machinery have been targeted, an altered HS chain will change the extracellular information input received by the cell, subsequently affecting processes such as self-renewal capacity and pluripotency.
Finally, although the role of HS in ES cell biology mainly has been studied in mES cells, a recent study by Levenstein et al. (2008) showed that mouse embryonic fibroblast–secreted HSPGs stabilize basic fibroblast growth factor (FGF2) and directly mediate binding of FGF2 to hES cells, and HS removal impaired proliferation. No doubt, HS plays an essential role also in hES cell biology, fine-tuning the extracellular input received by the cells and thus directing the interaction with adjacent cells and matrices in its surrounding niche.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: C.T. is supported by Linnéstiftelsen and Polysackaridforskning AB. L.K. is supported by the Swedish Research Council, the Swedish Cancer Society and Polysackaridforskning AB. J-P.L. is supported by the Swedish Research Council, the Swedish Cancer Society, and the Swedish Heart-Lung Foundation.
References
- Ai X, Kitazawa T, Do AT, Kusche-Gullberg M, Labosky PA, Emerson CP., Jr 2007. SULF1 and SULF2 regulate heparan sulfate–mediated GDNF signaling for esophageal innervation. Development. 134:3327–3338 [DOI] [PubMed] [Google Scholar]
- Anneren C. 2008. Tyrosine kinase signalling in embryonic stem cells. Clin Sci (Lond). 115:43–55 [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. 1989. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 105:733–737 [DOI] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA. 1998. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 12:1894–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon T, Smith A, Savatier P. 2002. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 12:432–438 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature. 292:154–156 [DOI] [PubMed] [Google Scholar]
- Fan G, Xiao L, Cheng L, Wang X, Sun B, Hu G. 2000. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 467:7–11 [DOI] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L. 1999. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 400:773–776 [DOI] [PubMed] [Google Scholar]
- Forsberg M, Holmborn K, Kundu S, Dagalv A, Kjellen L, Forsberg-Nilsson K. 2012. Undersulfation of heparan sulfate restricts differentiation potential of mouse embryonic stem cells. J Biol Chem. 287:10853–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K. 2007. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem. 282:15578–15588 [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. 1999. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 126:3047–3055 [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D’Amore PA. 1998. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell–induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 141:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley RJ, Pickford CE, Rushton G, Lacaud G, Gallagher JT, Kouskoff V, Merry CL. 2011. Influencing hematopoietic differentiation of mouse embryonic stem cells using soluble heparin and heparan sulfate saccharides. J Biol Chem. 286:6241–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmborn K, Ledin J, Smeds E, Eriksson I, Kusche-Gullberg M, Kjellen L. 2004. Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J Biol Chem. 279:42355–42358 [DOI] [PubMed] [Google Scholar]
- Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, Sharpe AH, Stevens RL. 1999. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 400:769–772 [DOI] [PubMed] [Google Scholar]
- Johnson CE, Crawford BE, Stavridis M, Ten Dam G, Wat AL, Rushton G, Ward CM, Wilson V, van Kuppevelt TH, Esko JD, et al. 2007. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein–expressing neural progenitor cells. Stem Cells. 25:1913–1923 [DOI] [PubMed] [Google Scholar]
- Keller G. 2005. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19:1129–1155 [DOI] [PubMed] [Google Scholar]
- Kraushaar DC, Rai S, Condac E, Nairn A, Zhang S, Yamaguchi Y, Moremen K, Dalton S, Wang L. 2012. Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J Biol Chem. May 3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraushaar DC, Yamaguchi Y, Wang L. 2010. Heparan sulfate is required for embryonic stem cells to exit from self-renewal. J Biol Chem. 285:5907–5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Kjellén L. 2012. Regulation of heparan sulfate biosynthesis. J Histochem Cytochem. 60:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna WC, Frese MA, Balleininger M, Dierks T. 2008. Sulf loss influences N-, 2-O-, and 6-O-sulfation of multiple heparan sulfate proteoglycans and modulates fibroblast growth factor signaling. J Biol Chem. 283:27724–27735 [DOI] [PubMed] [Google Scholar]
- Langsdorf A, Do AT, Kusche-Gullberg M, Emerson CP, Jr, Ai X. 2007. Sulfs are regulators of growth factor signaling for satellite cell differentiation and muscle regeneration. Dev Biol. 311:464–477 [DOI] [PubMed] [Google Scholar]
- Lanner F, Lee KL, Sohl M, Holmborn K, Yang H, Wilbertz J, Poellinger L, Rossant J, Farnebo F. 2010. Heparan sulfation–dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. Stem Cells. 28:191–200 [DOI] [PubMed] [Google Scholar]
- Le Jan S, Hayashi M, Kasza Z, Eriksson I, Bishop JR, Weibrecht I, Heldin J, Holmborn K, Jakobsson L, Soderberg O, et al. 2012. Functional overlap between chondroitin and heparan sulfate proteoglycans during VEGF-induced sprouting angiogenesis. Arterioscler Thromb Vasc Biol. 32:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledin J, Staatz W, Li JP, Gotte M, Selleck S, Kjellen L, Spillmann D. 2004. Heparan sulfate structure in mice with genetically modified heparan sulfate production. J Biol Chem. 279:42732–42741 [DOI] [PubMed] [Google Scholar]
- Levenstein ME, Berggren WT, Lee JE, Conard KR, Llanas RA, Wagner RJ, Smith LM, Thomson JA. 2008. Secreted proteoglycans directly mediate human embryonic stem cell–basic fibroblast growth factor 2 interactions critical for proliferation. Stem Cells. 26:3099–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. 2006. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 24:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U. 2003. Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. J Biol Chem. 278:28363–28366 [DOI] [PubMed] [Google Scholar]
- Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, Matzuk MM. 2000. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 224:299–311 [DOI] [PubMed] [Google Scholar]
- Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Francis Stewart A, Smith A, et al. 2012. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 149:590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 78:7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaupt HAB, Couchman JR. 2012. Heparan sulfate biosynthesis: methods for investigation of the heparanosome. J Histochem Cytochem. 60:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringvall M, Ledin J, Holmborn K, van Kuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellen L, Forsberg E. 2000. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 275:25926–25930 [DOI] [PubMed] [Google Scholar]
- Sasaki N, Okishio K, Ui-Tei K, Saigo K, Kinoshita-Toyoda A, Toyoda H, Nishimura T, Suda Y, Hayasaka M, Hanaoka K, et al. 2008. Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem. 283:3594–3606 [DOI] [PubMed] [Google Scholar]
- Shworak NW, HajMohammadi S, de Agostini AI, Rosenberg RD. 2002. Mice deficient in heparan sulfate 3-O-sulfotransferase-1: normal hemostasis with unexpected perinatal phenotypes. Glycoconj J. 19:355–361 [DOI] [PubMed] [Google Scholar]
- Shworak NW, Kobayashi T, de Agostini A, Smits NC. 2010. Anticoagulant heparan sulfate to not clot—or not? Prog Mol Biol Transl Sci. 93:153–178 [DOI] [PubMed] [Google Scholar]
- Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. 2005. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 132:5055–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676 [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Smith AG. 2010. The ground state of pluripotency. Biochem Soc Trans. 38:1027–1032 [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. 2008. The ground state of embryonic stem cell self-renewal. Nature. 453:519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science. 318:1917–1920 [DOI] [PubMed] [Google Scholar]
- Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP. 2009. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One. 4:e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, Vlodavsky I. 2004. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 18:252–263 [DOI] [PubMed] [Google Scholar]