Abstract
Background
Repeated cycles of chronic intermittent ethanol (CIE) exposure leads to increased voluntary ethanol intake in C57BL/6J mice. This study evaluates the development of tolerance to ethanol’s aversive effects in CIE exposure.
Methods
Adult male C57BL/6J mice were trained to drink 15% ethanol (vs. water) in a limited access procedure and then exposed to CIE (EtOH mice) or air (CTL) for 5 cycles alternating with weekly access to ethanol drinking. Following the 4th CIE cycle, the aversive effects of ethanol were evaluated using a conditioned taste aversion (CTA) paradigm with 1% saccharin as the conditioned stimulus. Several doses of ethanol (0, 1, 2, and 3 g/kg) and LiCl (0.4M, 0.02 ml/g) served as unconditioned stimuli. Finally, mice underwent a 5th CIE cycle to measure blood and brain concentrations following a 2 g/kg ethanol dose.
Results
CIE exposure increased ethanol drinking in EtOH mice while drinking in CTL mice remained stable. The lowest ethanol dose (1 g/kg) did not induce CTA in either group, but the highest dose (3 g/kg) produced CTA in both groups (49% reduction for CTL vs. 25% reduction for EtOH) although the group differences were not statistically significant. However, the 2 g/kg ethanol dose induced a significant aversion in CTL mice (27% reduction) but not in EtOH mice (20% increase), indicating tolerance to ethanol’s aversive effects. LiCl caused a similar aversion in CTL and EtOH mice (50% reduction). Finally, blood and brain ethanol concentrations were not different between CTL and EtOH mice following a 2 g/kg ethanol dose.
Conclusions
The data indicate that CIE exposure produces tolerance to the aversive effects of 2 g/kg ethanol. This effect does not appear to be related to a learning deficit or altered ethanol pharmacokinetics. These data support the notion that tolerance to ethanol’s aversive effects may contribute to excessive ethanol drinking in ethanol-dependent mice.
Keywords: alcohol dependence, alcohol drinking, tolerance, learning, taste aversion
Alcoholism remains a serious, worldwide health problem. Mechanisms that govern drinking behavior involve complex and dynamic processes. At any given time, propensity to imbibe is thought to reflect a balance between the positive (rewarding) and negative (aversive) motivational effects of alcohol (ethanol). Many biological (genetic) and environmental factors impact this balance, and experience with ethanol itself (drinking history) certainly plays a role in influencing the balance between ethanol’s rewarding and aversive effects. The complex interplay among these factors is critical for ultimately shaping decisions about engaging in drinking behavior, including how much, how often, and when to stop.
Numerous factors influence the motivational effects of ethanol, and these may change as the subject gains more experience with the drug (Cunningham et al., 2000). Continued excessive ethanol consumption can lead to the development of dependence, where a shift in the balance between the rewarding and aversive qualities of ethanol may serve to enable and perpetuate excessive levels of drinking. Indeed, ethanol dependence may be characterized as an allostatic state fueled by progressive dysregulation of motivational processes and neural circuitry controlling intake (Becker, 2008; Heilig et al., 2010; Koob, 2003). Such neuroadaptations may play a role in enhancing the rewarding effects of ethanol, thereby fostering the transition from regulated ethanol use to uncontrolled, excessive levels of drinking. Additionally, the ability of ethanol to alleviate withdrawal-related dysphoria may enhance vulnerability to relapse and encourage drinking to greater, more sustained levels (Becker, 2008; Heilig et al., 2010). Another factor that could play an important and permissive role in excessive drinking is tolerance to the aversive effects of ethanol.
Tolerance has long been viewed as playing an important role in the regulation of ethanol self-administration behavior (Cicero, 1980). Ethanol tolerance is a complex and dynamic process that is significantly influenced by genetic, environmental, and experiential factors (Deitrich et al., 1996; Kalant, 1996, 1998; Rigter and Crabbe, 1980; Suwaki et al., 2001). Both pharmacokinetic (metabolic) and pharmacodynamic (functional) processes are operable in producing an overall tolerance effect that may develop within different temporal domains (defined as acute, rapid, and chronic tolerance). Thus, as a consequence of chronic ethanol exposure, the development of tolerance to the aversive effects of ethanol (which ordinarily temper amount consumed) may serve as a permissive factor, enabling higher levels of drinking. This may be particularly relevant in the context of dependence where drinking is typically characterized as excessive.
We have developed a mouse model of ethanol dependence and relapse drinking that demonstrates significant escalation of ethanol consumption following repeated cycles of chronic intermittent ethanol (CIE) exposure via inhalation (Becker, 2008). The increase in voluntary ethanol drinking is sustained well beyond acute withdrawal and produced significant elevation in both blood and brain ethanol concentrations (Becker and Lopez, 2004; Griffin et al., 2009a; Griffin et al., 2009b; Lopez and Becker, 2005). Other studies have also reported elevated ethanol self-administration after chronic ethanol exposure in mice (Chu et al., 2007; Dhaher et al., 2008; Finn et al., 2007) and rats (Funk and Koob, 2007; Gilpin et al., 2008; Gilpin et al., 2009; O’Dell et al., 2004; Richardson et al., 2008; Roberts et al., 1996; Roberts et al., 2000). Whether changes in sensitivity to the rewarding and/or aversive qualities of ethanol underlie excessive drinking associated with these models of dependence is not known at present.
It is plausible that escalated drinking in our model of dependence involving repeated cycles of CIE exposure could be related, in part, to the development of tolerance to the aversive properties of ethanol. Indeed, there is evidence suggesting an inverse relationship between propensity to drink ethanol and sensitivity to its aversive effects using taste aversion procedures (Green and Grahame, 2008). To evaluate the possibility that tolerance to the aversive effects of ethanol might contribute to escalated drinking in our CIE model, sensitivity to ethanol-induced conditioned taste aversion was compared in ethanol dependent and nondependent mice. It was hypothesized that ethanol dependent (CIE-exposed) mice would demonstrate reduced sensitivity (tolerance) to ethanol’s aversive effects, as defined in the conditioned taste aversion paradigm.
MATERIALS AND METHODS
Subjects
Adult male C57BL/6 mice (25-30 g) purchased from Jackson Laboratories (Bar Harbor, ME) were used as subjects. All mice were individually housed with rodent food (Harland Teklad, Madison, WI) and water available ad libitum. Mice were maintained in an AAALAC-accredited facility, with automated control of temperature, humidity, and light cycles. Body weight was recorded weekly while mice were drinking but daily during CIE exposure cycles. Mice were housed under a 12-hr light/dark cycle (lights on at 0200 hr). Mice were not deprived of food or water at any time during the experiment. All procedures were approved by the Institutional Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals (1996).
General Study Design
The general study design involved use of an ethanol dependence and relapse drinking model developed by our laboratory (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). Briefly, mice were first trained to drink ethanol using a 2-bottle choice limited access procedure (described below). Once stable baseline ethanol intake was established, mice were separated into groups, counterbalanced according to baseline intake so the two groups did not differ in ethanol intake. One group (EtOH group) received repeated weekly cycles of chronic intermittent ethanol (CIE) exposure (16 hr/day × 4 days) to ethanol vapor in inhalation chambers (see below for details). The remaining mice (CTL group) were similarly treated, but maintained in control (air) inhalation chambers. After a 72 hr forced abstinence period following each weekly inhalation exposure cycle, EtOH and CTL mice were given the opportunity to voluntarily drink ethanol under the same limited access conditions as baseline for 5 consecutive days. Thus, each weekly CIE (or air) exposure cycle was followed by a 5-day limited access drinking test cycle, and this pattern of treatment was repeated for three cycles. After a fourth CIE exposure cycle, the groups were further separated to evaluate tolerance to ethanol’s aversive effects using a conditioned taste aversion (CTA) paradigm (described below). A week after CTA testing was completed, mice received an additional (5th) CIE (or air) exposure cycle, and blood and brain tissue was sampled to measure ethanol concentrations.
Limited Access Drinking Procedure
As previously described, a modified sucrose fading procedure was used to train mice to drink ethanol in the home cage under a daily limited access (2 hr/day) schedule (Becker and Lopez, 2004; Griffin et al., 2009a; Griffin et al., 2009b; Lopez and Becker, 2005). Briefly, mice were first presented with 10% (v/v) ethanol in combination with 5% (w/v) sucrose and over several days the sucrose concentration was gradually reduced while the ethanol concentration was increased such that the final solution was 15% ethanol with no sucrose added. The 2 hr daily drinking sessions commenced 30 min before the start of the dark cycle, when mice were presented with a 2-bottle choice to drink ethanol (15% v/v) or tap water as the alternative fluid. The position of the ethanol and water bottles was alternated daily to avoid development of a side preference. The amount of ethanol consumed by each mouse was converted to g/kg based on the milliliters of ethanol consumed (± 0.1 ml) and body weight (± 0.1 g).
Chronic Intermittent Ethanol (CIE) Exposure Procedure
Chronic intermittent ethanol (CIE) exposure was administered in inhalation chambers as detailed in our previous work (Becker and Lopez, 2004; Griffin et al., 2009a; Griffin et al., 2009b; Lopez and Becker, 2005). Ethanol concentration in the inhalation chambers was monitored daily to ensure that the inhalation conditions produced stable blood ethanol concentrations (BEC) above 175 mg/dl (Griffin et al., 2009a). Blood ethanol concentration was assessed once each week by sampling blood from the retro-orbital sinus immediately upon removal from the chamber. Before each 16 hr ethanol exposure, intoxication was initiated in EtOH mice by administration of ethanol (1.6 g/kg) in combination with the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) intraperitoneally (IP) in a volume of 0.02 ml/g body weight. The co-administration of pyrazole is critical to maintain a high and stable level of intoxication during each cycle of ethanol vapor exposure (Griffin et al., 2009a). CTL mice were similarly handled, but administered saline and pyrazole prior to being placed in control (air) inhalation chambers. Thus, all mice received the same number and timing of pyrazole injections prior to final removal from the inhalation chambers.
Conditioned Taste Aversion Procedure
For this procedure, saccharin (1% w/v) was used as the conditioned stimulus (CS) and its consumption was paired with vehicle (0.9% NaCl), ethanol (1, 2, or 3 g/kg), or LiCl (0.4M) that served as the unconditioned stimulus (UCS). The volume for IP administration of the UCS was 0.02 ml/g body weight. These ethanol doses were selected to generate a dose-response function, and include doses that have been shown to produce a CTA (Broadbent et al., 2002; Risinger and Cunningham, 1995; Risinger and Cunningham, 1998). The LiCl dose was selected based on prior CTA studies conducted with mice (Ingram, 1982; Kay and Nyby, 1992). The single conditioning trial was conducted 72 hr after the final (4th) CIE exposure cycle. Mice were presented with a single 15 ml graduated bottle containing saccharin for 30 min. Immediately following this saccharin access period, separate groups of EtOH and CTL mice were injected (IP) with the UCS drug/dose as indicated above (N= 8-12/group). The conditioning groups of EtOH and CTL mice were counterbalanced based on ethanol consumption during Test 3 to ensure similar ethanol intake before induction of CTA. At 24 hr following the conditioning trial, mice were again presented with the same saccharin solution in the home cage for a 30 min test session. Saccharin intake was recorded to the nearest 0.1 ml for both pre- and post-conditioning sessions. After these procedures, mice were left undisturbed for the rest of the week in their home cage with full access to food and water.
Blood and Brain Ethanol Measures
A subset of mice from both EtOH (N= 23) and CTL (N= 24) groups were randomly selected from each CTA condition and exposed to an additional (5th) cycle of CIE (or air) exposure. At 72 hr following the inhalation treatment, all mice were injected (IP) with a 2 g/kg ethanol. Mice were sacrificed at 30, 60, or 120 min and blood and brain samples were collected for analysis. Blood samples were centrifuged for phase separation and 5 μl of plasma was injected into an Analox Instrument analyzer (Lunenburg, MA) for measurement of blood ethanol concentration (Lopez and Becker, 2005). After sacrifice, the brain was exposed and a sample of left cortex (10-20 mg) was removed, placed in a conical tube, diluted 1:50 by adding cold de-ionized water, and then mixed using a sonicator for <5 sec. Tubes were then centrifuged and the supernatant was collected for later assay using an enzymatic spectrophotometric assay procedure previously described (Becker and Hale, 1993). Blood ethanol concentrations (BEC) were expressed as mg/dl and brain ethanol concentrations (BrEC) were expressed in mg/g tissue.
Solution Preparation
Ethanol (v/v) solutions were prepared by mixing 95% ethanol with deionized water, and sucrose (w/v) was added when appropriate. Saccharin was presented as a 1% (w/v) solution in deionized water. All solutions were prepared daily and presented at room temperature.
Statistical Analyses
The primary dependent variables were ethanol intake (g/kg), saccharin intake (ml), blood ethanol concentrations (BEC; mg/dl) and brain ethanol concentrations (BrEC; mg/dl). Data were analyzed using factorial ANOVAs, with Group (EtOH vs. CTL) and CTA (saline, 1, 2 or 3 g/kg ethanol, and LiCl) as main factors. Repeated measures were included when appropriate. Post-hoc analyses were performed using Newman-Keuls’ test. For the conditioned taste aversion test, data were expressed as percent change in saccharin intake (post-conditioning - pre-conditioning saccharin intake). Since the percent change values do not follow a normal distribution, these data were analyzed with non-parametric Kruskal-Wallis ANOVA by Ranks followed by Mann-Whitney U Test for post-hoc pair-wise comparisons. For all analyses, significance was set at p<0.05.
RESULTS
Blood Ethanol Concentrations (BEC) During CIE Exposure
Analysis of BEC during the first 4 cycles of exposure indicated a significant main effect of Cycle [F(3,141)= 63.74, p< 0.001]. Mean ± s.e.m. BEC values for Cycles 1-4 were 156.82 ± 5.40, 189.49 ± 6.28, 223.72 ± 6.43, and 264.04 ± 6.94 mg/dl. Post-hoc comparisons indicated that mice reached a significantly higher BEC level after each CIE cycle. Importantly, there were no significant differences in BEC based on the CTA factor [F(4,47)=1.64], nor interaction of CTA and Cycle [F(12,141)=1.41]. The same was true for BEC levels after CTA conditioning and testing (cycle 5). BEC values for this cycle were 229.71±18.28 mg/dl with no effect of the CTA factor [F(4,50)<1] (values are mean±SEM).
Ethanol Consumption
The amount of voluntary ethanol consumption (g/kg) during limited access drinking sessions during the last week of baseline and during the three Test Cycles that followed CIE or control-air exposure for dependent (EtOH) mice and non-dependent (CTL) mice are illustrated in Figure 1. Preliminary analyses indicated that ethanol intake did not significantly vary across days within each of the drinking test periods of the study. Therefore, daily intake values were averaged over the last five days of Baseline and over the five days of each Test Cycle drinking period. As demonstrated in our previous work, ethanol consumption escalated over successive cycles of CIE exposure in EtOH mice while intake remained relatively stable in CTL mice over the course of the study. This effect is supported by ANOVA, which indicated significant main effects of Group [F(1,102)= 6.79, p< 0.001] and Test Cycle [F(3,306)= 28.08, p< 0.001], as well as the Group × Test Cycle interaction [F(3,306)= 9.60, p< 0.001]. Post-hoc comparisons based on this interaction term indicated that EtOH mice consumed significantly more ethanol during Test Cycles 1-3 compared to their own baseline level of intake (p< 0.05), and during Test Cycle 3, ethanol intake was significantly greater in EtOH compared to CTL mice (p< 0.05). In contrast, ethanol consumption for CTL mice did not significantly differ from baseline levels of intake throughout the study (Figure 1).
Figure 1. Repeated cycles of CIE exposure increased voluntary ethanol consumption in CIE-exposed (EtOH) mice compared to control (CTL) mice.

Voluntary ethanol intake (g/kg) progressively increased over successive test cycles in EtOH mice (N= 55) while remaining relatively stable in CTL mice (N= 57). * significantly higher ethanol intake in EtOH mice during test cycles compared to their own baseline drinking (p< 0.05); # significantly higher ethanol intake in EtOH mice compared to CTL mice during Test 3 (p< 0.05). Values are mean ± SEM.
These data also were analyzed with the inclusion of CTA group (UCS: 0, 1, 2, 3 g/kg ethanol, LiCl) as an additional between-subjects variable to determine whether the conditioning subgroups differed in ethanol intake. Importantly, 3-way ANOVA indicated no significant effect of CTA group assignment [F(4,102)< 1.0], and this factor did not significantly interact with Group or Test Cycle factors [Fs< 1.0]. This indicates that drinking prior to CTA conditioning was similar across conditioning subgroups (all EtOH groups consumed more ethanol than CTL groups).
Conditioned Taste Aversion
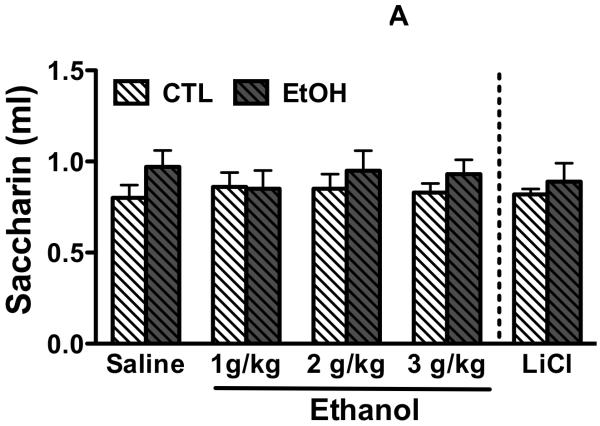
Saccharin intake (ml) during the conditioning phase of the CTA procedure (pre-conditioning) did not significantly differ among EtOH and CTL groups and CTA conditioning groups (Figure 2A). This observation was supported by ANOVA indicating no significant main effects of Group [F(1,102)= 2.75, p> 0.05], CTA Conditioning [F(4,102)< 1.0], or the Group × CTA Conditioning interaction [F(4,102)< 1.0]. These results indicate that CIE exposure did not significantly alter consumption of a novel, sapid solution (saccharin) even though such exposure enhanced ethanol drinking. Importantly, these data also show that CS exposure was similar for all CTA conditioning groups prior to conditioning (USC exposure).
Figure 2. Tolerance to ethanol-induced conditioned taste aversion in CIE-exposed mice.
A. Repeated cycles of CIE exposure did not affect intake of a novel tastant solution (1% saccharin) prior to conditioning. Intake (ml) of the conditioned stimulus (1% saccharin) is presented for EtOH and CTL groups based on conditioning assignment (0, 1, 2, or 3 g/kg ethanol, or 0.4M LiCl used as the unconditioned stimulus). B. Percent change in saccharin intake from pre-conditioning to post-conditioning test day in EtOH and CTL mice. * significant conditioned aversion to saccharin in EtOH and CTL mice that received 3 g/kg ethanol or LiCl, but only in CTL mice that received 2 g/kg ethanol compared to respective vehicle condition (p< 0.05). # significantly different percent change in saccharin intake between EtOH and CTL groups that received 2 g/kg ethanol as the unconditioned stimulus (p< 0.05). Values are mean ± SEM.
The effects of ethanol-induced CTA in EtOH and CTL groups are shown in Figure 2B. Data are expressed as percent change in saccharin intake 24 hr following conditioning relative to pre-conditioning levels of saccharin intake. As expected, LiCl induced a strong CTA in both EtOH and CTL groups, as evidenced by significantly reduced saccharin intake following conditioning relative to pre-conditioning intake levels. Additionally, ethanol induced a conditioned aversion to saccharin in a dose-related manner, with the highest dose producing the greatest reduction in saccharin intake following the one-trial conditioning procedure. However, ethanol-induced CTA differed as a function of CIE exposure. These observations were supported by Kruskal-Wallis ANOVA for CTL and EtOH mice. For CTL mice, the analysis indicated a significant effect of the CTA Conditioning factor [H(4,55)= 19.92, p< 0.001]. Post-hoc pair-wise comparisons using Mann-Whitney U Test indicated that CTL mice conditioned with 2 and 3 g/kg (but not 1 g/kg) ethanol or LiCl (0.4M) consumed significantly less saccharin compared to saline treated mice (Z= 2.56, 3.32, and 3.20, respectively; all ps< 0.01). For EtOH mice, the Kruskal-Wallis ANOVA also indicated a significant main effect of CTA Conditioning [H(4,57)= 16.17, p< 0.01]. In this case, pair-wise comparisons indicated that EtOH mice conditioned with 3 g/kg (but not 1 or 2 g/kg) ethanol or LiCl showed a significant aversion to saccharin compared to saline treated mice (Z= 2.44 and 3.78, respectively; ps< 0.05). Follow-up comparisons were also performed between EtOH and CTL mice for each CTA conditioning group. Mann-Whitney U test indicated a significant difference between EtOH and CTL mice for the 2 g/kg ethanol conditioning situation (Z= −2.10, p< 0.05).
Blood and Brain Ethanol Concentrations After 2 g/kg Ethanol
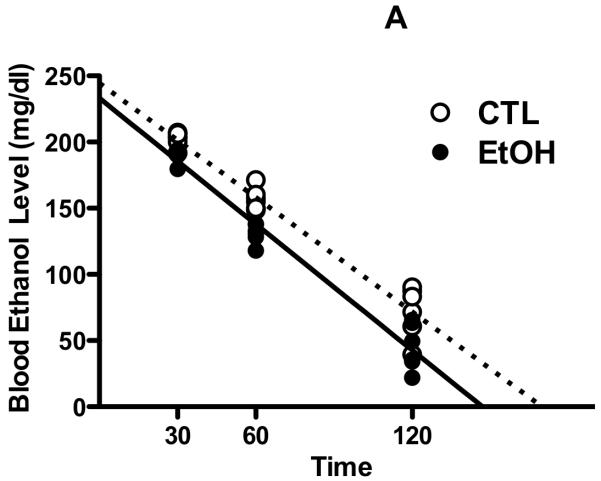
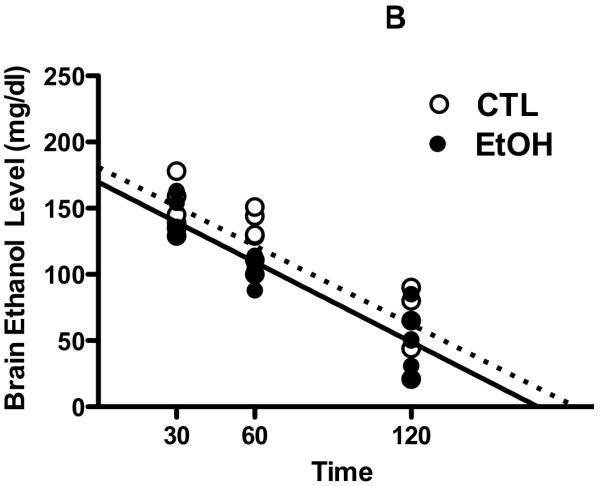
To evaluate whether altered metabolism of ethanol contributes to the apparent tolerance to ethanol’s aversive effects found in the CTA procedure in CIE-exposed mice, ethanol concentrations in blood (Figure 3A) and brain (Figure 3B) samples were analyzed in subset of EtOH and CTL mice at different time points following a 2 g/kg ethanol injection. Analysis of blood ethanol concentrations (BEC) revealed significant main of Group [F(1,30)= 29.29, p< 0.001] and Time [F(2,30)= 402.27, p< 0.001]. Post-hoc comparisons indicated that BEC were significantly higher in CTL vs. EtOH mice and that BEC levels significantly decreased over the time period of analysis. Additionally, regression analysis of BEC data (Figure 3A) revealed no significant difference in the slopes of the regression lines for CTL and EtOH mice [F(1,32)= 1.83, p> 0.05]. Analysis of brain ethanol concentrations (BrEC) indicated only a significant main effect of Time [F(2,27)= 58.80, p< 0.001]. Further, regression analysis of BrEC data (Figure 3B) indicated no significant difference between slopes for EtOH and CTL conditions [F(1,29)< 1.0]. Finally, as expected, there was a positive relationship between BEC and BrEC values for both CTL (r2= 0.78) and EtOH (r2= 0.82) groups, and the slopes of the correlation were significant for CTL and EtOH mice [F(1,15)= 53.40 and F(1,14)= 65.06, respectively; ps< 0.001] (data not shown). In sum, these data indicate that repeated cycles of CIE exposure does not significantly alter ethanol pharmacokinetics, as measured by BEC and BrEC levels following a systemic challenge with 2 g/kg ethanol.
Figure 3. Ethanol pharmacokinetics following CIE exposure.
Blood ethanol concentrations (mg/dl) (A) and brain ethanol concentrations (mg/dl) (B) registered in individual EtOH and CTL subjects at 30, 60 and 120 minutes after administration (IP) of a 2 g/kg ethanol dose. Lines represent the slope obtained from linear regression analysis of ethanol concentrations (dotted line = CTL, solid line = EtOH).
DISCUSSION
Results from this study demonstrate that repeated cycles of CIE exposure in mice is associated with escalation of voluntary ethanol drinking while, at the same time, reduced sensitivity to the aversive effects of ethanol, as defined by ethanol-induced CTA. This apparent tolerance to the aversive effects of ethanol exhibited by dependent (EtOH) mice compared to nondependent (CTL) mice does not appear related to a general learning deficit or metabolic tolerance.
The significant progressive increase in voluntary ethanol consumption in CIE-exposed mice compared to controls is consistent with our previous work (Becker and Lopez, 2004; Griffin et al., 2009a; Griffin et al., 2009b; Lopez and Becker, 2005) and work by others using mice (Chu et al., 2007; Dhaher et al., 2008; Finn et al., 2007). Further, results from this study provide additional evidence that the enhanced drinking behavior resulting from repeated cycles of CIE exposure is specific to ethanol. That is, CIE exposure did not alter consumption of a 1% saccharin solution presented prior to CTA conditioning. This is consistent with our previous work indicating that CIE exposure promotes increased voluntary ethanol intake, but such exposure does not influence water intake or consumption of sucrose solutions (Becker and Lopez, 2004). Thus, escalation of drinking in this model of ethanol dependence does not appear to be driven by nonspecific factors such as a general need to hydrate, whether the fluid presented is water or something more attractive (sucrose or saccharin).
A major finding of this study is that repeated cycles of CIE exposure reduced sensitivity to the aversive effects of ethanol, as measured using a CTA procedure. In nondependent (CTL) mice, systemic administration of ethanol (1, 2, 3, g/kg) serving as the UCS reduced consumption of a novel CS (saccharin solution) in a dose-related manner. These results are consistent with other reports indicating that ethanol can effectively support CTA in mice (Broadbent et al., 2002; Green and Grahame, 2008). Reduced sensitivity (tolerance) to this effect exhibited by dependent (EtOH) mice was most apparent at the intermediate dose of ethanol (2 g/kg), since this dose produced no aversion (saccharin intake increased) in EtOH mice while this same dose produced significant reduction in saccharin intake in CLT mice. Additionally, although not statistically significant, there was a trend towards reduced aversion to the highest dose of ethanol (3g/kg), consistent with the development of tolerance to ethanol aversion in EtOH mice. These findings cannot simply be explained by a general learning deficit resulting from repeated cycles of CIE exposure because both EtOH and CTL mice demonstrated an equivalent aversion using a well-characterized LiCl dose (Ingram, 1982; Kay and Nyby, 1992). Further, the noted differences in CTA conditioning between EtOH and CTL groups cannot be attributed to differences in CS exposure since all groups consumed similar amounts of saccharin prior to UCS exposure (ethanol injection).
It could be argued that the greater ethanol-induced CTA displayed by CTL mice compared to EtOH mice was due to that fact that CTA testing was conducted during acute withdrawal from the ethanol dose (2 or 3 g/kg) administered 24 hr earlier. That is, CTL mice were more sensitive than EtOH mice to non-specific withdrawal effects that manifested as greater reduction in saccharin consumption. However, we believe that reduced consumption of the CS (saccharin) reflects a conditioned response to the aversive effects of the UCS (ethanol) rather than an acute withdrawal effect because we have demonstrated ethanol-induced CTA 72 hr after 2 g/kg ethanol injection (unpublished findings). Others have reported robust ethanol-induced CTA when testing occurred 48 hours following ethanol administration (Broadbent et al., 2002; Risinger and Cunningham, 1998). Hence, ethanol-induced CTA is a durable phenomenon that lasts beyond acute withdrawal effects of the drug, especially since with modest doses (e.g., 2 g/kg ethanol) any acute withdrawal effects resolve within 48-72 hours.
Reduced sensitivity to ethanol-induced CTA in mice with a history of CIE exposure is consistent with previous reports showing that prior exposure to ethanol subsequently diminishes ethanol’s capability for producing CTA (Berman and Cannon, 1974; Cannon and Carrell, 1987; Eckardt, 1976; Hunt and Rabin, 1988; Lessov et al., 2001; Rabin et al., 1988; Risinger and Cunningham, 1995). In these studies ethanol was repeatedly injected prior to its pairing with the CS. In a recent study, exposure to ethanol vapor in inhalation chambers reduced subsequent ethanol-induced CTA in adolescent mice (Diaz-Granados and Graham, 2007). In another study, female high-preferring (P) rats given the opportunity to drink ethanol continuously for 33 days showed an attenuated ethanol-induced CTA compared to non-drinking controls (Stewart et al., 1991). To our knowledge, results from the present study are the first to demonstrate reduced sensitivity to the aversive effects of ethanol (as defined by CTA) in the same subjects exhibiting escalation of drinking as a function of CIE exposure.
Prior studies have indicated an inverse relationship between propensity to drink ethanol and sensitivity to its aversive effects, as defined by taste aversion procedures (Green and Grahame, 2008). For example, a significant negative correlation was demonstrated between voluntary ethanol intake and the magnitude of CTA induced by ethanol in a panel of inbred mouse strains (Broadbent et al., 2002). Similarly, rats selectively bred for high ethanol preference/drinking (P, sP lines) have been shown to be generally less sensitive to ethanol-induced CTA than their low ethanol preference/drinking counterparts (NP, sNP lines) (Brunetti et al., 2002; Froehlich et al., 1988). A similar finding has been reported for mice selectively bred for high (HAP) vs. low (LAP) alcohol preference (Chester et al., 2003). Results from this study generally support this relationship, with reduced sensitivity to the aversive effects of ethanol demonstrated in concert with increased voluntary ethanol drinking, secondary to the development of ethanol dependence.
The possibility that development of metabolic tolerance to ethanol might contribute to the apparent tolerance to ethanol-induced CTA was also examined in this study. While there were small differences in BEC (but not BrEC) between groups following a challenge dose of 2 g/kg ethanol, the rate of elimination from blood and brain did not differ between EtOH and CTL mice, suggesting that the mice metabolized ethanol at the same rate regardless of their history of chronic ethanol exposure. Therefore, it appears that the relative tolerance to ethanol’s aversive effects in dependent mice is unrelated to gross differences in ethanol metabolism. Further, it should be noted that abundant evidence indicates that learned aversions, such as that induced by ethanol in the CTA paradigm, are centrally mediated by discrete brain regions (Jimenez and Tapia, 2004; McGaugh, 2004; Miranda et al., 2002). Thus, the absence of differences in BrEC between EtOH and CTL groups supports the idea that pharmacokinetic differences did not contribute to the development of tolerance to ethanol-induced CTA in the present study.
Results obtained in this study provide support for the idea that tolerance to ethanol’s aversive effects may play a role in the escalation of voluntary ethanol drinking observed over successive cycles of CIE exposure. Indeed, such tolerance may serve as a permissive factor, favoring greater ethanol consumption. In this vein, we have previously demonstrated that repeated cycles of CIE exposure produced tolerance to the discriminative stimulus effects of ethanol using a standard operant discrimination conditioning paradigm (Becker and Baros, 2006). Thus, reduced sensitivity to feedback about the intoxicating effects of ethanol along with reduced sensitivity to the aversive effects of the drug may contribute to enhanced intake in dependent subjects.
At the same time, there is evidence to suggest that CIE exposure increases the rewarding effects of ethanol. Studies employing operant self-administration procedures have demonstrated augmented motivation to self-administer ethanol (increased responding and consumption) in ethanol-dependent mice (Chu et al., 2007; Lopez et al., 2006; 2008) and rats (Gilpin et al., 2008; Gilpin et al., 2009; O’Dell et al., 2004; Roberts et al., 1996; 2000). Further, progressive ratio testing has revealed enhanced reinforcing efficacy of ethanol following repeated CIE exposure (Brown et al., 1998; Lopez et al., 2008). Additionally, the potential for ethanol to alleviate negative affect and other symptoms of withdrawal serves as a powerful motivational force that promotes and sustains high levels of drinking (Becker, 2000; Heilig et al., 2010). Collectively, these data support the notion that with prolonged excessive ethanol consumption, the relative balance between rewarding/reinforcing and aversive properties of ethanol is shifted away from aversion in favor of reinforcement. Thus, the combination of enhanced rewarding effects (through both positive and negative reinforcement) along with reduced sensitivity (tolerance) to the aversive qualities of ethanol intoxication may, in large part, drive excessive drinking associated with dependence. Elucidating neurobiological mechanisms underlying changes in sensitivity to both the rewarding and the aversive effects of ethanol is key to understanding motivational processes that are critical for regulating and controlling alcohol consumption, as well as adaptations in such processes that mediate transition to uncontrolled, harmful levels of drinking characteristic of dependence.
Acknowledgements
Supported by grants RO1 AA018036, P50 AA10716 and VA Medical Research (HCB). The authors thank Jessica Mixson Ramirez, Kay Fernandes and Melissa Overstreet for excellent technical assistance in conducting this work.
REFERENCES
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC. Alcohol withdrawal: Neuroadaptation and sensitization. CNS Spectrums. 1999;4:38–65. [Google Scholar]
- Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Alcohol dependence, withdrawal and relapse. Alcohol Res Health. 2008;31:348–361. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Baros AM. Effect of duration and pattern of chronic ethanol exposure on tolerance to the discriminative stimulus effects of ethanol in C57BL/6J mice. J Pharmacol Exp Ther. 2006;319:871–878. doi: 10.1124/jpet.106.108795. [DOI] [PubMed] [Google Scholar]
- Berman RF, Cannon DS. The effect of prior ethanol experience on ethanol-induced saccharin aversions. Physiol Behav. 1974;12:1041–1044. doi: 10.1016/0031-9384(74)90152-8. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Brown G, Jackson A, Stephens DN. Effects of repeated withdrawal from chronic ethanol on oral self-administration of ethanol on a progressive ratio schedule. Behav Pharmacol. 1998;9:149–161. [PubMed] [Google Scholar]
- Brunetti G, Carai MA, Lobina C, Melis S, Serra S, Vacca G, Gessa GL, Colombo G. Differences in ethanol-induced conditioned taste aversion in Sardinian alcohol-preferring and Sardinian alcohol-nonpreferring rats. Alcohol. 2002;26:167–172. doi: 10.1016/s0741-8329(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Cannon DS, Carrell LE. Effect of taste aversion learning on ethanol self-administration. Pharmacol Biochem Behav. 1987;28:53–56. doi: 10.1016/0091-3057(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, Grahame NJ. High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res. 2003;27:12–18. doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ. Alcohol self-administration, tolerance and withdrawal in humans and animals: theoretical and methodological issues. In: Rigter H, Crabbe JC, editors. Alcohol Tolerance and Dependence. Elsevier/North-Holland; New York, NY: 1980. pp. 1–51. [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Radcliffe R, Erwin VG. Pharmacological effects in the development of physiological tolerance and physical dependence. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 431–476. [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res. 2008;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ. Alcohol-induced conditioned taste aversion in rats. Effect of concentration and prior exposure to alcohol. J Stud Alcohol. 1976;37:334–346. doi: 10.15288/jsa.1976.37.334. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: Is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009a;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009b;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA, Rabin BM. Attenuation of a radiation-induced conditioned taste aversion after the development of ethanol tolerance. Life Sci. 1988;43:59–66. doi: 10.1016/0024-3205(88)90237-8. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Lithium chloride-induced taste aversion in C57BL/6J and DBA/2J mice. J Gen Psychol. 1982;106:233–249. [PubMed] [Google Scholar]
- Jimenez B, Tapia R. Biochemical modulation of NMDA receptors: role in conditioned taste aversion. Neurochem Res. 2004;29:161–168. doi: 10.1023/b:nere.0000010445.27905.aa. [DOI] [PubMed] [Google Scholar]
- Kalant H. Current state of knowledge about the mechanisms of alcohol tolerance. Addict Biol. 1996;1:133–141. doi: 10.1080/1355621961000124756. [DOI] [PubMed] [Google Scholar]
- Kalant H. Research on tolerance: What can we learn from history? Alcohol Clin Exp Res. 1998;22:67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- Kay E, Nyby J. LiCl aversive conditioning has transitory effects on pheromonal responsiveness in male house mice (Mus domesticus) Physiol Behav. 1992;52:105–113. doi: 10.1016/0031-9384(92)90439-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Risinger FO, Phillips TJ. Attenuation of ethanol-induced conditioned taste aversion in mice sensitized to the locomotor stimulant effects of ethanol. Behav Neurosci. 2001;115:146–153. doi: 10.1037/0735-7044.115.1.146. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Overstreet MP, Becker HC. Repeated chronic ethanol exposure and withdrawal increases ethanol self-administration in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:138A. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increase both self-administration and the reinforcing value of ethanol in C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:163A. [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11417–11422. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Lee J. Attenuation and cross-attenuation in taste aversion learning in the rat: Studies with ionizing radiation, lithium chloride and ethanol. Pharmacol Biochem Behav. 1988;31:909–918. doi: 10.1016/0091-3057(88)90404-2. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigter H, Crabbe JC. Alcohol Tolerance and Dependence. Elsevier/North-Holland; New York, NY: 1980. [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res. 1998;22:1234–1244. [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: Animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Stewart RB, McBride WJ, Lumeng L, Li TK, Murphy JM. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology (Berl) 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Suwaki H, Kalant H, Higuchi S, Crabbe JC, Ohkuma S, Katsura M, Yoshimura M, Stewart RC, Li TK, Weiss F. Recent research on alcohol tolerance and dependence. Alcohol Clin Exp Res. 2001;25:189S–196S. doi: 10.1097/00000374-200105051-00031. [DOI] [PubMed] [Google Scholar]