Background: LXR inhibits the development of atherosclerosis. It remains unknown whether LXR regulates IL-5 expression, an atheroprotective cytokine, in macrophages.
Results: LXR induces macrophage IL-5 expression in an LXRE-dependent manner. It also induces IL-5 expression in aortic root area of LDLR−/− mice.
Conclusion: Macrophage IL-5 is a target gene for LXR activation.
Significance: The increased IL-5 expression can be related to LXR-induced anti-atherosclerosis.
Keywords: Atherosclerosis, Cholesterol Metabolism, Lipoprotein, Macrophages, Scavenger Receptor, IL-5, LXR, oxLDL
Abstract
IL-5 stimulates production of T15/EO6 IgM antibodies that can block the uptake of oxidized low density lipoprotein by macrophages, whereas a deficiency in macrophage IL-5 expression accelerates development of atherosclerosis. Liver X receptors (LXRs) are ligand-activated transcription factors that can induce macrophage ABCA1 expression and cholesterol efflux, thereby inhibiting the development of atherosclerosis. However, it remains unknown whether additional mechanisms, such as the regulation of macrophage IL-5 expression, are related to the anti-atherogenic properties of LXR. We initially defined IL-5 expression in macrophages where the LXR ligand (T0901317) induced macrophage IL-5 protein expression and secretion. The overexpression of LXR increased, whereas its knockdown inhibited IL-5 expression. Furthermore, we found that LXR activation increased IL-5 transcripts, promoter activity, formation of an LXR·LXR-responsive element complex, and IL-5 protein stability. In vivo, we found that T0901317 increased IL-5 and total IgM levels in plasma and IL-5 expression in multiple tissues in wild type mice. In LDL receptor knock-out (LDLR−/−) mice, T0901317 increased IL-5 expression in the aortic root area. Taken together, our studies demonstrate that macrophage IL-5 is a target gene for LXR activation, and the induction of macrophage IL-5 expression can be related to LXR-inhibited atherosclerosis.
Introduction
The binding and internalization of oxidatively modified low density lipoprotein (oxLDL)4 by scavenger receptors on macrophage surface result in formation of lipid-laden foam cells, which are a prominent part of atherosclerotic lesions (1). Thus, the accumulation of cholesterol/lipids in macrophages is an initial and critical step in the development of atherosclerosis. The degradation of oxidized fatty acids in oxLDL within macrophage/foam cells generates multiple reactive species, such as malondialdehyde (MDA) and 4-hydroxynonenal (2, 3). The autologous molecules, such as the protein moiety of LDL (e.g., apolipoprotein B) and phospholipids (e.g., phosphatidylcholine), can be subjected to modification by these reactive species (4, 5). Many of the modified products can function as excellent immunogens, which result in both the activation of either the innate or adaptive immune response and the production of cytokines, growth factors and other pro-inflammatory mediators (6). Studies with animal models have shown that there are several auto-antibodies to these “oxidation-specific epitopes” (7, 8).
IL-5 is a cytokine that regulates the growth and differentiation of eosinophils (9). In asthma, there is an influx of activated eosinophils into the lung, causing tissue damage (10). IL-5 can promote the differentiation of B-1 cells, a subclass of B cell lymphocytes, into antibody-secreting cells and increases the secretion of T15/EO6 IgM antibodies from the cells. These antibodies are able to bind to the phosphatidylcholine moiety of the oxidized phospholipids in oxLDL, thereby blocking the uptake of oxLDL by macrophages (8). In addition, IL-5 prevents the development of atherosclerosis, whereas IL-5 deficiency will accelerate the development of atherosclerosis (11).
Liver X receptors (LXRs) are ligand-activated transcription factors and consist of LXRα (NR1H3) and LXRβ (NR1H2) (12, 13). LXR activators include natural ligands, such as oxidized cholesterol derivatives (e.g., 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, 24(S),25-epoxycholesterol, and 25-hydroxycholesterol), and synthetic non-sterol LXR ligands (e.g., GW3965 and T0901317). LXRs have been shown to play multiple biological roles (14). LXR regulates the expression of several key molecules in fatty acid synthesis, such as sterol regulatory-binding protein 1c and fatty acid synthase. Both LXRα and LXRβ plays an important role in cholesterol metabolism. For instance, LXRα/β can stimulate the expression of ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1), which are the critical molecules that facilitate excess cellular cholesterol efflux to extracellular cholesterol acceptors, such as apolipoprotein AI/II and HDL. In mouse and rat models, LXR also up-regulates the expression of cholesterol 7α-hydroxylase (14), which is the initial and rate-limiting enzyme for the conversion of cholesterol into bile acids.
Several studies have reported that synthetic LXR ligands inhibit the development of atherosclerosis in animal models (15). The anti-atherogenic properties of the LXR ligands are mainly attributed to the LXR-mediated macrophage ABCA/G1 expression and cholesterol efflux (15, 16). In addition, LXR plays an important role in the regulation of cytokine production and the anti-inflammatory response (17, 18). However, in addition to the regulation of macrophage cholesterol efflux, the function of LXR regarding immune responses in macrophages, such as its influence on the production of anti-atherogenic cytokine, IL-5, has not been elucidated. In this study, we set out to define the role of LXR in the regulation of IL-5 expression in inflammatory cells.
EXPERIMENTAL PROCEDURES
Reagents
Rabbit anti-IL-5 polyclonal antibody was obtained from Boster Biologicals (Wuhan, China). Mouse anti-ubiquitin monoclonal antibody, goat anti-CD68, and MOMA-2 polyclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). TRITC-conjugated goat anti-rabbit IgG and FITC-conjugated rabbit anti-goat IgG were purchased from The Jackson Laboratory (Bar Harbor, ME). FITC-conjugated hamster anti-mouse TCRβ and mouse total IgG and IgM detection ELISA kits were purchased from eBiosciences (San Diego, CA). The ELISA kit for anti-oxLDL antibodies was purchased from Holzel Diagnostika (Koln, Germany). X-tremeGENE HP DAN transfection reagent was purchased from Roche Applied Science. pSilencer 5.1 Retro vector and Amplex® Red cholesterol assay kit were purchased from Invitrogen. T0901317 was purchased from Cayman Chemicals (Rockford, IL). All other reagents were purchased from Sigma-Aldrich except where indicated.
Cell Culture
RAW cells (a murine macrophage cell line) and EL-4 (a murine lymphocyte cell line) were purchased from ATCC (Manassas, VA) and cultured in complete RPMI 1640 medium containing 10% FBS, 50 μg/ml penicillin/streptomycin, and 2 mm glutamine, respectively. The cells were switched to serum-free medium and received treatment when the confluence was ∼90% (RAW) or at a density of ∼2 × 105 cells/ml (EL4). Mouse peritoneal macrophages were isolated from wild type C57BL/6 mice as described (19) and received treatment in serum-free medium.
Western Analysis of IL-5 Protein Expression and ELISA of IL-5 Secretion
After treatment, the cells were washed twice with cold PBS and lysed in an ice-cold lysis buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mm PMSF, 50 mm sodium fluoride, 1 mm sodium orthovanadate, 50 μg/ml aprotinin/leupeptin). The lysate was sonicated for 20 cycles followed by centrifugation for 15 min at 16,200 × g at 4 °C with a Microfuge. The supernatant was saved as the whole protein extract. After determination of concentration, the cellular proteins from each sample were loaded on a 15% SDS-PAGE gel. After electrophoresis, the proteins were transferred onto a nylon-enhanced nitrocellulose membrane. The membrane was blocked with a solution of 0.1% Tween 20/PBS (PBS-T) containing 5% dry fat-free milk for 1 h followed by incubation with rabbit polyclonal anti-IL-5 antibody (1:500) overnight at 4 °C. After reblocking with PBS-T containing 5% milk, the blot was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG for 1 h at room temperature. After washing three times for 10 min with PBS-T, the membrane was incubated for 1 min in a mixture of equal volumes of Western blot chemiluminescence reagents 1 and 2. The membrane was then exposed to film before development.
To determine the effects of LXR activation on IL-5 protein secretion, macrophages in 12-well plates were treated with T0901317 at different concentrations (1 ml/well) overnight. The treatment medium was collected and spun for 5 min at 2,600 × g at 4 °C to remove the floating cells. The supernatant was used to determine the secreted IL-5 protein using ELISA kit purchased from R&D Systems (Minneapolis, MN). The cell pellet in the tube and cells in the corresponding well were combined and lysed. The protein content in the cellular lysate was used to normalize IL-5 protein secretion.
In Vivo Study
The protocol for in vivo study with mice was granted by the Ethics Committee of Nankai University and conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
C57BL/6 wild type mice (∼10-week-old females) were randomly divided into two groups (five mice/group) and fed normal chow (control) or normal chow containing T0901317 (T0901317, 5 mg/100 g of food) for 10 days. Based on food consumption, the dose of T0901317 was estimated to be ∼5 mg/kg of body weight. After treatment, the animals were anesthetized and euthanized in a CO2 chamber. Blood was collected and kept for more than 2 h at room temperature. After centrifugation for 20 min at 2,000 × g, the serum was transferred into a new test tube and kept at −20 °C until the assay of IL-5, total IgG/IgM, and anti-oxLDL antibodies levels by ELISA.
To determine the IL-5 protein expression in mouse tissues (liver, spleen, and lymph node), ∼30 mg of tissue was homogenized in the protein lysis buffer. The supernatant of the homogenate was retained as the tissue protein extract. To determine IL-5 protein expression in T cells, mouse spleen and lymph nodes were collected and ground into single cells, respectively. Once cells were filtrated with a 40-μm cell strainer, they were incubated in 3 ml of red cell lysis buffer for 5 min; 7 ml PBS was then added. After centrifugation, the cell pellet was resuspended in 200 μl of PBS, and 1 μl of anti-TCRβ-FITC plus 1 μl of anti-CD3-PerCP Cy5 (eBiosciences) antibodies was added. After incubation for 30 min on ice and washed twice with PBS, the cells were resuspended in 1 ml of serum-free medium and subjected to FACS. About 800,000 TCRβ+CD3+ cells were collected as T cells. The T cells were lysed, and total cellular lysate was used to determine IL-5 protein expression by Western blot.
To determine IL-5 protein expression in the liver, 5-μm paraffin sections from the liver, which was previously fixed in 4% paraformaldehyde followed by embedding in paraffin, were collected. Expression of IL-5 was determined by immunohistochemical staining as described (20). To further define the cell types expressing IL-5, the above sections were blocked with 2% BSA for 2 h at room temperature followed by incubation with rabbit anti-IL-5 polyclonal antibody (1:25 dilution) plus hamster anti-TCRβ-FITC (1:100 dilution) or rabbit anti-IL-5 polyclonal antibody (1:25 dilution) plus goat anti-CD68 polyclonal antibody (1:50 dilution) overnight at 4 °C. After removal of the primary antibodies by washing with PBS for 30 min, the sections stained with anti-IL-5 plus anti-TCRβ antibodies were incubated with TRITC-conjugated goat anti-rabbit IgG alone (1:1000 dilution), whereas the sections stained with anti-IL-5 plus anti-CD68 antibodies were incubated with TRITC-conjugated goat anti-rabbit IgG (1:1000 dilution) plus FITC-conjugated rabbit anti-goat IgG (1:1000 dilution) for 2 h at room temperature, respectively. After washing with PBS for 30 min, all of the sections were stained with DAPI solution for nucleus. Images of all the sections were obtained with a fluorescence microscope.
To determine whether IL-5 is expressed in aortic root, LDL receptor knock-out (LDLR−/−) mice (∼8-week-old females) were randomly divided into two groups and fed a high fat diet (21% fat and 0.5% cholesterol) with or without T0901317 (1 mg/100 g of food) for 20 weeks. After treatment, 5-μm frozen sections of aortic root were collected. IL-5 protein in lesion area was determined by immunohistochemical staining. To further localize IL-5 protein expression in the lesion area, the aortic frozen sections were conducted immunofluorescent staining with anti-IL-5 and MOMA-2 antibodies and DAPI solution.
Preparation of Plasmid DNA and Determination of IL-5 Promoter Activity
The mouse IL-5 promoter (from −1173 to +45, p1218IL-5) was constructed using PCR with genomic DNA extracted from mouse liver and the following primers: forward, 5′-TAGCCTCGAGGCATGCCTGACACAGTGC-3′; and backward, 5′-TGCCAAGCTTGACTCTGAAGTCTTCAGC-3′. To define the location of LXR responsive element (LXRE or DR4), a shorter IL-5 promoter (from −632 to +45, p677IL-5) was constructed by PCR with the p1218IL-5 DNA and the forward primer of 5′-TAGCCTCGAGAGCAGGGTCTTGTGTGTTCC-3′ and the same backward primer mentioned above. The p677IL-5 promoter with LXRE mutation (p677IL-5M) or deletion (p677IL-5D) was constructed using the Phusion site-directed mutagenesis kit (New England Biolabs, Ipswich, MA) with the p677IL-5 DNA and primers with the corresponding LXRE deletion or mutation. After the sequences were confirmed, the above PCR products were digested using XhoI and HindIII followed by ligation with the pGL4 luciferase reporter vector (Promega, Madison, WI), respectively. To analyze the IL-5 promoter activity, ∼5 × 105/well EL4 cells in 24-well plates were transfected with DNA for IL-5 promoters and Renilla luciferase (for internal normalization) using Gene Tran (Biomiga, San Diego, CA). After 24 h of transfection plus treatment, the cells were lysed, and the cellular lysate was used to determine activities of firefly and Renilla luciferases using the dual-luciferase reporter assay system from Promega.
Extraction of Nuclear Proteins and EMSA of LXR-DNA Binding Activity
After treatment and washing with cold PBS, the cells were suspended in 400 μl of cold buffer A (10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, 0.5 mm PMSF) and incubated for 15 min on ice followed by addition of 50 μl of 10% Nonidet P-40 and vortex for 10 s. After spinning for 30 s at 16,200 × g at 4 °C, the pellet was saved and resuspended in 100 μl of cold buffer C (20 mm HEPES, pH 7.91, 400 mm KCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 1 mm PMSF, 10 μg/ml leupeptin/aprotinin) and kept on ice for 15 min. The mixture was spun again for 5 min, and the supernatant was collected as nuclear proteins and kept at −80 °C until assay.
To determine the binding activity of nuclear proteins to LXRE, the double-stranded oligonucleotides of IL-5 LXRE were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase and purified with a MicroSpin G-25 column. The reaction of nuclear proteins (10 μg/sample) with the 32P-labeled probe in the presence or absence of the indicated competitor was completed. After reaction, the mixture was loaded on a precooled 5% polyacrylamide (38:2) native gel. The complex of nuclear protein·DNA probe was separated from the unbound probe by electrophoresis and detected by radiography after the gel was air-dried.
Reduction of Macrophage LXR Expression by siRNA
The shRNA against LXRα or LXRβ was constructed by subcloning the template oligonucleotides into pSilencer 5.1 Retro vector. The sense and antisense template oligonucleotides for LXRα or LXRβ siRNA were 5′-GATCCGTGCCTGATGTTTCTCCTGATTCAAGAGATCAGGAGAAACATCAGGCATTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAATGCCTGATGTTTCTCCTGATCTCTTGAATCAGGAGAAACATCAGGCACG-3′ or 5′-GATCCGGATTCAGAAGCAGCAACATTCAAGAGATGTTGCTGCTTCTGAATCCTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAAGGATTCAGAAGCAGCAACATCTCTTGAATGTTGCTGCTTCTGAATCCG-3′. To determine the effect of LXR siRNA on IL-5 expression, primary macrophages in 6-well plates were transfected with shRNA against LXRα or LXRβ and scrambled shRNA using Roche X-tremeGENE HP DAN transfection reagent. After 24 h of transfection, the cells were switched to complete medium and continued the culture for 48 h. The cells were then lysed, and the cellular proteins were used to determine the expression of IL-5 and LXRα or LXRβ by Western blot.
Preparation of LXR Expression Vectors and Transfection of RAW Macrophages
The cDNAs encoding mouse LXRα and LXRβ were generated using RT-PCR with total RNA extracted from RAW cells and the following primers: LXRα, forward: 5′-CCGGAATTCATGTCCTTGTGGCTGGAG-3′, and backward: 5′-CGCGGATCCTCACTCGTGGACATCCC-3′; and LXRβ, forward: 5′-TAGCCTCGAGCATGTCTTCCCCCACAAGTTC-3′, and backward: 5′-CGACAAGCTTCTACTCGTGCACATCCCAGATC-3′. After the sequence of LXRα or LXRβ was confirmed, the RT-PCR product was digested with EcoRI and BamHI or XhoI and HindIII followed by subcloning into an expression vector: pEGFP-C2 (pEGFP-LXRα and pEGFP-LXRβ). To determine the effect of overexpression of LXRα or LXRβ on IL-5 protein expression, ∼95% confluent RAW cells in 12-well plates were transfected with DNA for the LXRα or LXRβ expression vector or the pEGFP-C2 vector. After 24 h of transfection, the cells were lysed, and the cellular lysate was used to determine IL-5 and LXRα or LXRβ protein by Western blot.
Isolation of Total Cellular RNA and Real Time RT-PCR
After treatment, RAW or primary macrophages or EL4 cells or mouse tissues were lysed in TRIzol reagent (Invitrogen). The lysate was well mixed with chloroform and spun for 10 min using a Microfuge at 16,200 × g at 4 °C. The top aqueous phase was collected and mixed with an equal volume of isopropanol to precipitate total cellular RNA. The cDNA was then synthesized with 1 μg of total RNA using the RT kit purchased from New England Biolabs. Real time PCR was performed using SYBR green PCR master mix purchased from Bio-Rad with the following primers: IL-5, sense: 5′-AGCACAGTGGTGAAAGAGACCTT-3′, and antisense: 5′-TCCAATGCATAGCTGGTGATTT-3′; and GAPDH, sense: 5′-ACCCAGAAGACTGTGGATGG-3′, and antisense: 5′-ACACATTGGGGGTAGGAACA-3′. Expression of IL-5 mRNA was normalized with GAPDH mRNA.
Data Analysis
All of the experiments were repeated at least three times, and the representative results were presented. The data were presented as means ± standard deviation and analyzed using the paired Student's t test (n ≥ 3). The significant difference was considered at p < 0.05.
RESULTS
Activation of LXR Induces Macrophage IL-5 Protein Expression
To determine whether IL-5 is expressed by macrophages, we initially extracted total cellular proteins from mouse tissues, peritoneal macrophages, RAW macrophages, and EL4 cells and then assessed the levels of IL-5 protein by Western blot. As expected, IL-5 protein expression was highest in EL4 cells, a mouse T lymphocyte cell line. In addition to immune tissues, such as lymph node and spleen, IL-5 protein was also observed in mouse liver, primary macrophages, and macrophage cell line (Fig. 1A). We further confirmed the expression pattern by determining IL-5 transcripts in above samples by RT-PCR. The results in Fig. 1B indicate the similar expression profiles of IL-5 mRNA to IL-5 protein.
FIGURE 1.
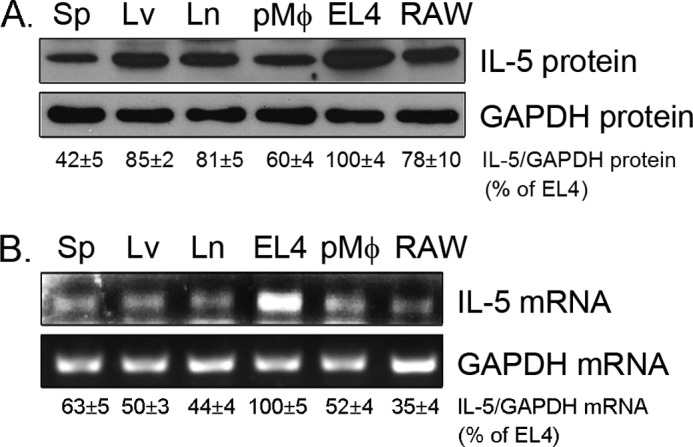
Expression pattern of IL-5 in mouse tissues and macrophages. Total cellular protein or RNA was extracted from mouse tissues or macrophages or EL4 cells. Expression of IL-5 protein (A) and mRNA (B) was determined by Western blot and RT-PCR, respectively. Sp, spleen; Lv, liver; Ln, lymph node; pMφ, peritoneal macrophages. The results of statistical analysis of blots are presented at the bottom of each panel.
To assess the effects of LXR activation on macrophage IL-5 protein expression, we treated RAW cells with an LXR ligand, T0901317, at different concentrations overnight. The results in the top panel of Fig. 2A demonstrate that T0901317 induced IL-5 protein expression in a broad concentration range with a maximum induction at 200 nm. Our results show that the induction occurred quickly (2 h after treatment) and lasted for 24 h. The maximum induction was seen 8 h after treatment (Fig. 2A, bottom panel). Similarly, we investigated the effect of LXR on IL-5 protein expression in primary macrophages isolated from wild type mice. After treatment with T0901317 at different concentrations overnight, IL-5 protein expression in primary macrophages was induced, and the maximum induction was observed with a lower concentration of T0901317 (∼50 nm; Fig. 2B, top panel). The study time course indicated that the peak of induction occurred 12 h after treatment (Fig. 2B, bottom panel).
FIGURE 2.
Regulation of macrophage IL-5 expression by LXR activation. A and B, RAW macrophages (A) or mouse peritoneal macrophages (B) were treated with T0901317 at the indicated concentrations for 8 h or treated with 200 nm T0901317 for the indicated times. C, both RAW and peritoneal macrophages in 12-well plates were treated with T0901317 at the indicated concentrations for 16 h. The treatment medium was used to determine the secreted IL-5 protein by ELISA. *, significantly different from the corresponding controls at p < 0.01 using Student's t test (n = 3). D, RAW cells in 12-well plate were transfected with DNA for the pEGFP-C2 vector, pEGFP-LXRα, or pEGFP-LXRβ expression vector at the indicated concentrations for 24 h. E, primary macrophages in 6-well plates were transfected with shRNA against LXRα or LXRβ or control shRNA at the indicated concentrations for 24 h. Ctrl, control.
To determine the effect of LXR on IL-5 secretion, both RAW and primary macrophages were treated with T0901317 at different concentrations overnight. IL-5 in the treatment medium was determined by ELISA. The results in Fig. 2C show that LXR increased IL-5 secretion. To confirm these observations, we initially transfected RAW macrophages with an LXRα or LXRβ expression vector and determined the effect of overexpressed LXRα or LXRβ on IL-5 protein expression. Compared with the control vector, the overexpressed LXRα or LXRβ increased IL-5 protein expression in macrophages (Fig. 2D). In contrast, the knockdown of LXRα or LXRβ expression by siRNA resulted in decreased IL-5 protein (Fig. 2E).
Induction IL-5 Expression in an LXRE-dependent Manner
To determine whether the induction of macrophage IL-5 protein expression by LXR is completed through transcription, we initially determined the changes in macrophage IL-5 mRNA in response to T0901317 treatment. The results in Fig. 3A indicate a significant increase in IL-5 transcripts by T0910317 treatment, suggesting that the regulation could be at the transcriptional level. We then constructed an IL-5 promoter (p1218IL-5). Consistent with previous reports (9), the results in the left panel of Fig. 3B indicate that IL-5 promoter can be activated by the co-treatment of phorbol 12-myristate 13-acetate plus N6,O2′-dibutyryladenosine 3′:5′-cAMP. Treatment of the transfected cells with T0901317 induced IL-5 promoter activity in a concentration-dependent manner (Fig. 3B, right panel). Taken together, it is clear that LXR induces IL-5 expression at the transcriptional level.
FIGURE 3.
Induction of IL-5 expression is LXRE-dependent. A, mouse peritoneal macrophages were treated with T0901317 at the indicated concentrations for 6 h. Expression of IL-5 mRNA was determined by real time RT-PCR. *, p < 0.01 versus control (n = 3). B, EL4 cells in 24-well plates were transfected with DNA for p1218IL-5 and Renilla luciferase. After 4 h of transfection, the cells were treated with 32 nm phorbol 12-myristate 13-acetate (PMA) plus 1 mm N6,O2′-dibutyryladenosine 3′:5′-cAMP (Bt2cAMP) or T0901317 at the indicated concentrations overnight followed by determination of activities of firefly and Renilla luciferases. *, p < 0.01 versus control (open bars; n = 3). C, EL4 cells were transfected with DNA for the indicated IL-5 promoters and treated with 200 nm T0901317 overnight. *, p < 0.01 versus controls (n = 3). D, left panel, nuclear proteins were extracted from T0901317-treated RAW cells and used to react with the 32P-labeled IL-5 LXRE probe in the presence or absence of the indicated competitor. Right panel, RAW cells were treated with T0901317 at different concentrations overnight. The binding of nuclear proteins with the 32P-labeled IL-5 LXRE probe was determined using EMSA.
By sequence alignment analysis, we found a putative LXRE or DR4 sequence in the proximal region of IL-5 promoter (from −246 to −231, ATGTGGatcaAAGTTG). The classic sequence of LXRE is AGGTCAXXXXAGGTCA; it is also named DR4 because the six nucleotides separated by any four nucleotides are repeated. To determine the importance of this putative LXRE in IL-5 transcription, we constructed either a shorter IL-5 promoter (p677IL-5) than p1218IL-5 or the shorter promoter with LXRE mutation or deletion (Fig. 3C). We then evaluated the effect of T0901317 on the activity of these promoters. The results in Fig. 3C indicate that T0901317 induced the activity of both p1218IL-5 and p677IL-5 promoters. However, the mutation or deletion of LXRE abolished the inductive effect of LXR on IL-5 promoter activity (Fig. 3C).
We then determined the role of this LXRE in IL-5 transcription by determining the reaction of the LXRE with nuclear proteins. The binding of the labeled IL-5 LXRE to nuclear extract was blocked by the unlabeled wild type IL-5 LXRE but not the mutated IL-5 LXRE nucleotides in a concentration-dependent manner (Fig. 3D, left panel). More importantly, the binding was reduced by ABCA1 LXRE and anti-LXRα or anti-LXRβ antibody (Fig. 3D), indicating the formation of the complex of this putative IL-5 LXRE with LXR. Treatment of cells with T090137 increased the formation of LXR·LXRE complex in a concentration-dependent manner (Fig. 3D, right panel). Taken together, the results in Fig. 3 demonstrate that LXR induces IL-5 expression at the transcriptional level, and the LXRE plays an important role in IL-5 transcription.
Activation of LXR Induces IL-5 Production in Vivo
We also studied the effects of LXR on IL-5 protein stability. The addition of cycloheximide arrests the synthesis of cellular proteins; thus, IL-5 protein was reduced with time. However, in the presence of T0910317, the decline of IL-5 protein expression by cycloheximide was significantly reduced, suggesting that LXR increases IL-5 protein stability (Fig. 4A). Furthermore, we determined the effect of T0901317 on the ubiquitylation of IL-5 protein and observed that the ubiquitinated IL-5 (IL5-Ub) was decreased by the treatment (Fig. 4B).
FIGURE 4.
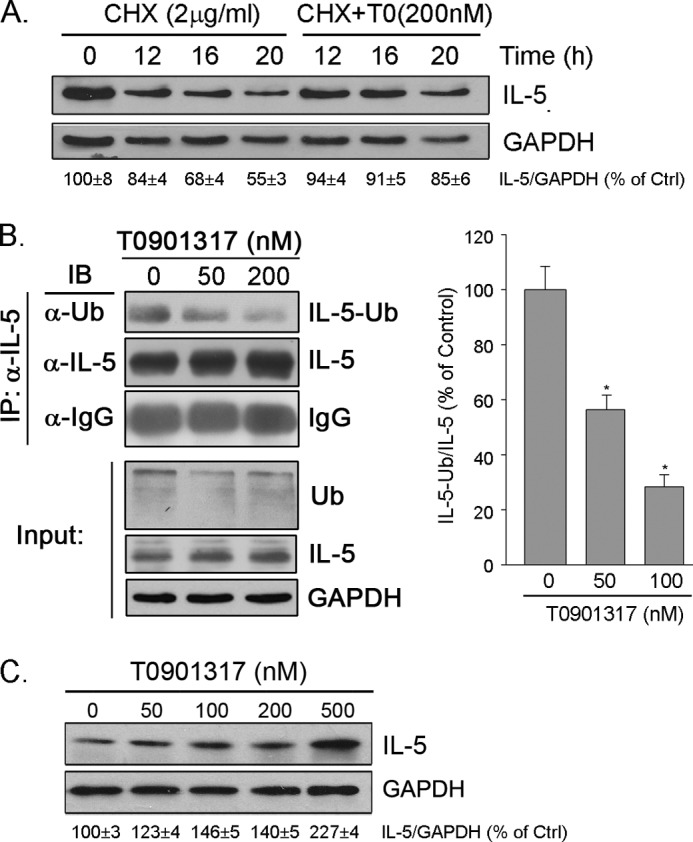
Activation of LXR increases IL-5 protein stability and induces IL-5 expression in EL4 cells. A, RAW macrophages were treated with 2 μg/ml cycloheximide (CHX) or cycloheximide plus 200 nm T0901317 (T0) for the indicated times. B, macrophages were treated with T0901317 at different concentrations overnight and 5 μm MG132 for 5 h before the end of the experiment. After treatment, 200 μg of total cellular protein were used to conduct immunoprecipitation (IP) with anti-IL-5 antibody and protein A-conjugated agarose. The ubiquitinated IL-5 (IL-5-Ub) and total IL-5 protein and the loading in precipitates were determined by Western blot with anti-ubiquitin (Ub) and anti-rabbit IgG antibodies, respectively. The input was determined by Western blot with 20 μg of cellular proteins with anti-IL-5, ubiquitin, and GAPDH antibodies. IB, immunoblot. C, EL4 cells were treated with T0901317 at the indicated concentrations overnight followed by determination of IL-5 protein expression.
IL-5 has been observed in multiple tissues/cell types, such as Th2 cells, eosinophils, mast cells, and macrophages (9). To determine whether LXR influences IL-5 expression in cell types other than macrophages, we treated EL4 cells with T0901317 and determined the changes in IL-5 protein levels. Similar to macrophages, LXR can induce IL-5 protein expression in EL4 cell, indicating the common inductive effect on IL-5 expression in different cell types (Fig. 4C).
To define the physiological relevance of LXR activation on IL-5 expression, we conducted in vivo studies in which we initially fed wild type mice normal chow or chow containing T0901317 (5 mg/100 g of food) for 10 days. Analysis of serum IL-5 using ELISA indicated that T0901317 significantly increased the IL-5 protein levels in circulation (18.8 versus 38.1 pg/ml, 203%, p < 0.01; Fig. 5A). In tissues, T0901317 induced IL-5 protein expression in the liver, lymph nodes, and spleen (Fig. 5B). Because T cells are a major source of IL-5 production, we also determined the effect of administration of T0901317 on IL-5 expression in T cells isolated either from spleen or lymph nodes. The results in Fig. 5C indicate that T0901317 also increased T cell IL-5 expression in vivo. In addition, we determined the effect of LXR ligand administration on serum antibody levels; the results in Fig. 5D indicate that T0901317 significantly increased levels of total IgM, anti-oxLDL antibodies but not total IgG. However, LXR activation had little effect on B-1 cell population in spleen (data not shown), which might be because we defined splenic B-1 cells by CD19+CD23− rather than CD19+B220low, and the induction of IgM antibodies to oxLDL by IL-5 occurs in peritoneal B-1 cells (11). We also determined the expression levels of IL-5 protein by immunohistochemical assay, and the results confirmed the inductive effect of T0901317 treatment on IL-5 protein expression in the liver (Fig. 5E, left panel). To disclose the cell types expressing IL-5 in the liver after LXR activation, we did a double immunofluorescent staining assay. The results in the right panel of Fig. 5E demonstrate that LXR moderately induced IL-5 expression in T cells and significantly induced IL-5 expression in Kupffer cells in the liver.
FIGURE 5.
Activation of LXR induces IL-5 production in vivo. C57BL/6 wild type mice (10-week-old females; n = 5) were fed normal chow (control) or chow containing T0901317 (5 mg/100 g of food) for 10 days. After treatment, the following assays were completed. A, serum IL-5 concentrations were determined using an ELISA kit. *, p < 0.001 versus control (n = 10; each serum sample was analyzed in duplicate). B, IL-5 protein expression in mouse tissues was determined by Western blot. C, T cells isolated from spleen or lymph nodes were sorted with anti-TCRβ plus anti-CD3 antibodies. After lysis, IL-5 protein expression in the T cells was determined by Western blot. D, serum total IgG, IgM, and anti-oxLDL antibodies levels were determined using ELISA kits. *, p < 0.01 versus control (n = 5). E, left panel, immunohistochemical analysis of IL-5 protein in the liver; right panel, IL-5 protein in T cells or Kupffer cells in the liver was determined by immunofluorescent staining. Bars, 100 μm.
LXR ligands have been reported to inhibit the development of atherosclerosis in LDLR−/− mice (21, 22). To determine whether the inhibitory effect of LXR ligand on atherosclerosis is related to the induction of IL-5 expression in macrophages within aortic lesions, LDLR−/− mice were fed a high fat diet or the diet containing T0901317 (1 mg/100 g food) for 20 weeks. After treatment, the mouse aortic root was used to determine IL-5 protein expression and the presence of macrophages. Compared with control mice, the smaller size of lesion was observed in the animals receiving LXR ligand treatment (Fig. 6A). In addition, the results of immunohistochemical staining indicate that T0901317 induced IL-5 protein expression in aortic lesions (Fig. 6A). More importantly, the immunofluorescent staining results suggest that the increased IL-5 protein was mainly contributed by macrophages (Fig. 6B).
FIGURE 6.
Activation of LXR induces IL-5 expression in LDLR−/− mouse aortic lesions. LDLR−/− mice were fed a high fat diet or the diet containing T0901317 (1 mg/100 g of food) for 20 weeks. Mouse aortic root was collected and used to determine the presence of IL-5 protein and macrophages by immunohistochemical staining (A) or immunofluorescent staining (B), respectively. MOMA-2, monocyte/macrophage marker. a or b is the part in arterial wall of control or T0-treated mouse with greater magnification to demonstrates the location of IL-5 protein within macrophages. Bars, 50 μm.
DISCUSSION
IL-5 has been reported to link adaptive and natural immunity in inhibiting atherosclerosis (11). In this study, we report for the first time that LXR activation induces macrophage IL-5 expression. LXR also increases IL-5 protein and IgM production in the circulation and IL-5 expression in multiple tissues in vivo. More importantly, LXR induces IL-5 expression in macrophages within aortic lesions. These findings suggest the following: 1) IL-5 is widely expressed in various tissues/cell types, and the expression of IL-5 can be regulated by different mechanisms, and 2) in addition to promoting ABCA1 expression and cholesterol efflux, the anti-atherogenic function of LXR can be related its inductive effect on IL-5 expression in macrophages.
Formation of lipid-laden macrophage/foam cells is a critical step in atherogenesis where oxLDL is the major component within these cells (2). oxLDL demonstrates other multiple atherogenic properties, such as the promotion of macrophage recruitment and retention, and the activation of the transcription of inflammatory genes (23). The interaction of oxLDL with the immune system has been demonstrated (24–27). T cell clones responsive to oxLDL have been isolated from human lesions (27, 28). Autoantibodies against oxidation-specific epitopes from oxLDL have been found in humans and in animal models (26, 29, 30). In general, the elevated IgG titers to oxLDL are positively associated with progression of lesion development, whereas IgM titers to oxLDL are negatively associated (7, 31, 32). A study with natural monoclonal antibodies cloned from apolipoprotein E-deficient mouse spleen identified an IgM anti-oxLDL antibody, EO6. This antibody demonstrates a high affinity for MDA modified LDL, copper-oxidized LDL, and other forms of modified LDL (29). Further studies show that EO6 inhibits scavenger receptor function and reduces macrophage lipid accumulation, indicating an atheroprotective role for this antibody (33, 34). Interestingly, EO6 shares a high genetic and structural identity with the classic anti-phosphorylcholine B-cell clone, T15, which is able to protect against common infectious pathogens, such as pneumococci (33–35). A pneumococcal vaccination in LDLR−/− mice results in high circulating levels of oxLDL-specific IgM and a persistent expansion of oxLDL-specific T15 IgM-secreting B cells primarily in the spleen. Consequently, lesion development in vaccinated mice is inhibited (36, 37). Consistently, immunization of mice with MDA-LDL leads to expansion of MDA-LDL-specific Th2 cells with prominent secretion of IL-5, and, in turn, the secreted IL-5 stimulates innate B-1 cells to produce and secrete T15/EO6 IgM antibodies. Concomitantly, fewer lesions are observed in MDA-LDL-immunized LDLR-deficient mice (11). In our study, we observed that associated with the induction of IL-5 expression, the serum total IgM and anti-oxLDL antibodies levels were modestly increased by LXR activation (Fig. 5D). It has been reported that IL-5 induces IgM antibodies to oxLDL in peritoneal B-1 cells but not in other antibody-secreting cells, such as B-2 cells, splenic marginal zone B cells, and follicular B cells (11). Similarly, we were not able to observe that the B-1 cell population in spleen was significantly increased by LXR activation. In IL-5 knock-out (IL-5−/−) mice, induction of IgG1 antibody to oxLDL by MDA-LDL immunization is impaired (11). Thus, the possible source for LXR-induced plasma IgM and anti-oxLDL antibodies levels and the role of IL-5 involved may need to be further investigated.
IL-5 controls the production, activation, and localization of eosinophils (9). Therefore, IL-5 plays an important role in asthma and in allergies (38). Induction of T15/EO6 antibodies suggests an additional physiological relevance of IL-5. It has been observed that selective deficiency of IL-5 expression in macrophages accelerates lesion development (11). Clinical observations demonstrate the positive association between plasma IL-5 levels and antibody titers to copper oxLDL and IgM to MDA-LDL and an inverse association between plasma IL-5 levels and bifurcational intima-media thickness (39). In addition, patients with asthma have elevated IL-5 levels but decreased atherosclerosis (40).
It is believed that IL-5 expression can be regulated at the transcriptional level because the highly conserved element, namely consensus lymphokine element 0 (CLE0), exists in the IL-5 promoter (41, 42). The CLE0 element binds factors of the AP-1 family together with NF-AT regulatory proteins in response to T cell activation signals that are generated by phorbol 12-myristate 13-acetate in combination with cAMP or anti-CD28 (9). In our study, we observed that LXR increases IL-5 transcripts and promoter activity (Fig. 3, A and B). Furthermore, we found a putative LXRE in the proximal region of IL-5 promoter, and we demonstrated that either the mutation or deletion of this LXRE abolishes the induction of IL-5 promoter activity (Fig. 3C). By EMSA, we confirmed the specific binding of this LXRE with LXR protein and observed that LXR ligand enhances the specific binding (Fig. 3D). In addition, LXR activation reduces ubiquitination of IL-5; thus, it increases IL-5 protein stability (Fig. 4, A and B). Although the role of LXR activation in protein ubiquitination has not been fully elucidated, LXR ligands increase LXRα protein levels by suppressing ubiquitination and degradation of LXRα protein (43). The presence of ligands inhibits the interaction of LXRα protein with an ubiquitin E3·ligase protein complex (43). Despite the fact that the precise mechanisms by which LXR activation influences IL-5 protein ubiquitination require more investigation, our study indicates that IL-5 expression can be regulated at both transcriptional and post-transcriptional levels. Our study also defines an important signaling pathway that can affect IL-5 expression. The finding that LXR activation enhances IL-5 production in macrophages of the aorta (Fig. 6) may suggest the involvement of increased IL-5 in LXR-inhibited atherosclerosis.
This work was supported by the Ministry of Science and Technology of China Grant 2010CB945003 to JH; the National Science Foundation of China (NSFC) Grants 30971271 and 81272460 to JH and 81000128 to YD; Tianjin Municipal Science and Technology Commission of China Grants 08ZCKFSH04400 and 11JCYBJC10600 to JH, and funds from the J & S Gross Foundation awarded to DH.
- oxLDL
- oxidatively modified low density lipoprotein
- LXR
- liver X receptor
- LXRE
- LXR-responsive element
- LDLR
- low density lipoprotein receptor
- MDA
- malondialdehyde
- TRITC
- tetramethylrhodamine isothiocyanate
- E3
- ubiquitin-protein isopeptide ligase.
REFERENCES
- 1. Ley K., Miller Y. I., Hedrick C. C. (2011) Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 31, 1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitra S., Deshmukh A., Sachdeva R., Lu J., Mehta J. L. (2011) Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am. J. Med. Sci. 342, 135–142 [DOI] [PubMed] [Google Scholar]
- 3. Esterbauer H., Schaur R. J., Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 [DOI] [PubMed] [Google Scholar]
- 4. Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. (1989) Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. U.S.A. 86, 1372–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. (1989) Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84, 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hulthe J. (2004) Antibodies to oxidized LDL in atherosclerosis development. Clinical and animal studies. Clin. Chim. Acta 348, 1–8 [DOI] [PubMed] [Google Scholar]
- 7. Palinski W., Ord V. A., Plump A. S., Breslow J. L., Steinberg D., Witztum J. L. (1994) ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 14, 605–616 [DOI] [PubMed] [Google Scholar]
- 8. Palinski W., Tangirala R. K., Miller E., Young S. G., Witztum J. L. (1995) Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15, 1569–1576 [DOI] [PubMed] [Google Scholar]
- 9. Karlen S., De Boer M. L., Lipscombe R. J., Lutz W., Mordvinov V. A., Sanderson C. J. (1998) Biological and molecular characteristics of interleukin-5 and its receptor. Int. Rev. Immunol. 16, 227–247 [DOI] [PubMed] [Google Scholar]
- 10. Hogan S. P., Koskinen A., Foster P. S. (1997) Interleukin-5 and eosinophils induce airway damage and bronchial hyperreactivity during allergic airway inflammation in BALB/c mice. Immunol. Cell Biol. 75, 284–288 [DOI] [PubMed] [Google Scholar]
- 11. Binder C. J., Hartvigsen K., Chang M. K., Miller M., Broide D., Palinski W., Curtiss L. K., Corr M., Witztum J. L. (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Apfel R., Benbrook D., Lernhardt E., Ortiz M. A., Salbert G., Pfahl M. (1994) A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 14, 7025–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. (1995) LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9, 1033–1045 [DOI] [PubMed] [Google Scholar]
- 14. Im S. S., Osborne T. F. (2011) Liver X receptors in atherosclerosis and inflammation. Circ. Res. 108, 996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parikh N., Frishman W. H. (2010) Liver X receptors. A potential therapeutic target for modulating the atherosclerotic process. Cardiol. Rev. 18, 269–274 [DOI] [PubMed] [Google Scholar]
- 16. Tangirala R. K., Bischoff E. D., Joseph S. B., Wagner B. L., Walczak R., Laffitte B. A., Daige C. L., Thomas D., Heyman R. A., Mangelsdorf D. J., Wang X., Lusis A. J., Tontonoz P., Schulman I. G. (2002) Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 99, 11896–11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph S. B., Bradley M. N., Castrillo A., Bruhn K. W., Mak P. A., Pei L., Hogenesch J., O'Connell R, M., Cheng G., Saez E., Miller J. F., Tontonoz P. (2004) LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119, 299–309 [DOI] [PubMed] [Google Scholar]
- 18. Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. (2003) Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 [DOI] [PubMed] [Google Scholar]
- 19. Zhou X., Yin Z., Guo X., Hajjar D. P., Han J. (2010) Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J. Biol. Chem. 285, 6316–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duan Y., Chen Y., Hu W., Li X., Yang X., Zhou X., Yin Z., Kong D., Yao Z., Hajjar D. P., Liu L., Liu Q., Han J. (2012) Peroxisome proliferator-activated receptor γ activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J. Biol. Chem. 287, 23667–23677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terasaka N., Hiroshima A., Koieyama T., Ubukata N., Morikawa Y., Nakai D., Inaba T. (2003) T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 536, 6–11 [DOI] [PubMed] [Google Scholar]
- 22. Joseph S. B., McKilligin E., Pei L., Watson M. A., Collins A. R., Laffitte B. A., Chen M., Noh G., Goodman J., Hagger G. N., Tran J., Tippin T. K., Wang X., Lusis A. J., Hsueh W. A., Law R. E., Collins J. L., Willson T. M., Tontonoz P. (2002) Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. U.S.A. 99, 7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. New Engl. J. Med. 320, 915–924 [DOI] [PubMed] [Google Scholar]
- 24. Linton M. F., Fazio S. (2001) Class A scavenger receptors, macrophages, and atherosclerosis. Curr. Opin. Lipidol. 12, 489–495 [DOI] [PubMed] [Google Scholar]
- 25. Frostegård J. (2002) Autoimmunity, oxidized LDL and cardiovascular disease. Autoimmun. Rev. 1, 233–237 [DOI] [PubMed] [Google Scholar]
- 26. Miller Y. I., Chang M. K., Binder C. J., Shaw P. X., Witztum J. L. (2003) Oxidized low density lipoprotein and innate immune receptors. Curr. Opin. Lipidol. 14, 437–445 [DOI] [PubMed] [Google Scholar]
- 27. Binder C. J., Chou M. Y., Fogelstrand L., Hartvigsen K., Shaw P. X., Boullier A., Witztum J. L. (2008) Natural antibodies in murine atherosclerosis. Curr. Drug Targets 9, 190–195 [DOI] [PubMed] [Google Scholar]
- 28. Stemme S., Faber B., Holm J., Wiklund O., Witztum J. L., Hansson G. K. (1995) T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U.S.A. 92, 3893–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palinski W., Hörkkö S., Miller E., Steinbrecher U. P., Powell H. C., Curtiss L. K., Witztum J. L. (1996) Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 98, 800–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chou M. Y., Hartvigsen K., Hansen L. F., Fogelstrand L., Shaw P. X., Boullier A., Binder C. J., Witztum J. L. (2008) Oxidation-specific epitopes are important targets of innate immunity. J. Int. Med. 263, 479–488 [DOI] [PubMed] [Google Scholar]
- 31. Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. (1994) Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler. Thromb. 14, 32–40 [DOI] [PubMed] [Google Scholar]
- 32. Tsimikas S., Palinski W., Witztum J. L. (2001) Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21, 95–100 [DOI] [PubMed] [Google Scholar]
- 33. Hörkkö S., Bird D. A., Miller E., Itabe H., Leitinger N., Subbanagounder G., Berliner J. A., Friedman P., Dennis E. A., Curtiss L. K., Palinski W., Witztum J. L. (1999) Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 103, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karvonen J., Päivänsalo M., Kesäniemi Y. A., Hörkkö S. (2003) Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 108, 2107–2112 [DOI] [PubMed] [Google Scholar]
- 35. Caligiuri G., Nicoletti A., Poirier B., Hansson G. K. (2002) Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 109, 745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Binder C. J., Hörkkö S., Dewan A., Chang M. K., Kieu E. P., Goodyear C. S., Shaw P. X., Palinski W., Witztum J. L., Silverman G. J. (2003) Pneumococcal vaccination decreases atherosclerotic lesion formation. Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9, 736–743 [DOI] [PubMed] [Google Scholar]
- 37. Shaw P. X., Hörkkö S., Chang M. K., Curtiss L. K., Palinski W., Silverman G. J., Witztum J. L. (2000) Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kouro T., Takatsu K. (2009) IL-5- and eosinophil-mediated inflammation. From discovery to therapy. Int. Immunol. 21, 1303–1309 [DOI] [PubMed] [Google Scholar]
- 39. Sämpi M., Ukkola O., Päivänsalo M., Kesäniemi Y. A., Binder C. J., Hörkkö S. (2008) Plasma interleukin-5 levels are related to antibodies binding to oxidized low-density lipoprotein and to decreased subclinical atherosclerosis. J. Am. Coll. Cardiol. 52, 1370–1378 [DOI] [PubMed] [Google Scholar]
- 40. Lasser E. C., Berry C., Kortman K. (1987) Diminished atherosclerotic arterial calcifications in asthma. A possible role for elevated endogenous heparin-like material. Allergy 42, 549–552 [DOI] [PubMed] [Google Scholar]
- 41. Naora H., van Leeuwen B. H., Bourke P. F., Young I. G. (1994) Functional role and signal-induced modulation of proteins recognizing the conserved TCATTT-containing promoter elements in the murine IL-5 and GM-CSF genes in T lymphocytes. J. Immunol. 153, 3466–3475 [PubMed] [Google Scholar]
- 42. Masuda E. S., Tokumitsu H., Tsuboi A., Shlomai J., Hung P., Arai K., Arai N. (1993) The granulocyte-macrophage colony-stimulating factor promoter cis-acting element CLE0 mediates induction signals in T cells and is recognized by factors related to AP1 and NFAT. Mol. Cell. Biol. 13, 7399–7407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim K. H., Yoon J. M., Choi A. H., Kim W. S., Lee G. Y., Kim J. B. (2009) Liver X receptor ligands suppress ubiquitination and degradation of LXRα by displacing BARD1/BRCA1. Mol. Endocrinol. 23, 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]






