Background: Microtubule effects on cell migration are poorly understood.
Results: Tubulin mutations or drug treatments that suppress microtubule dynamics impede cell locomotion. Depolymerizing microtubules does not inhibit movement, but it becomes random.
Conclusion: Microtubules act to restrain cell movement and specify directionality.
Significance: Drugs have dose-dependent effects on microtubule behavior, cell migration, and mitosis. Understanding these actions will lead to more effective drug use.
Keywords: Cell Migration, Cytoskeleton, Drug Action, Microtubules, Tubulin, Dynamic Instability, Locomotion, Mutations, Nocodazole, Paclitaxel
Abstract
Although microtubules have long been implicated in cell locomotion, the mechanism of their involvement remains controversial. Most studies have concluded that microtubules play a positive role by regulating actin polymerization, transporting membrane vesicles to the leading edge, and/or facilitating the turnover of adhesion plaques. Here we used wild-type and mutant CHO cell lines with alterations in tubulin to demonstrate that microtubules can also act to restrain cell motility. Tubulin mutations or low concentrations of drugs that suppress microtubule dynamics without affecting the amount of microtubule polymer inhibited the rate of migration by preventing microtubule reorganization in the trailing portion of the cells where the more dynamic microtubules are normally found. Under these conditions, cells along the edge of a wound still extended lamellipodia and elongated toward the wound but were inhibited in their ability to retract their tails, thus retarding forward progress. The idea that microtubules normally act to restrain cell locomotion was confirmed by treating cells with high concentrations of nocodazole to depolymerize the microtubule network. In the absence of microtubules, wild-type CHO and HeLa cells could still move at near normal speeds, but the movement became more random. We conclude that microtubules act both to restrain cell movement and to establish directionality.
Introduction
The ability of cells to migrate is critical for the development of multicellular organisms, as well as for immunity and repair of injury in adult animals. Similarly, the movement of vascular endothelial cells represents an important aspect of angiogenesis and is a target for therapies designed to limit the size of tumors in patients with cancer (1, 2). Cell migration can also be involved in pathological processes such as the metastasis of tumor cells to sites that are distant from the primary tumor (3). From both a fundamental biological perspective and to develop the capability to therapeutically intervene in disease processes, it is imperative to understand the basic mechanisms that control cell migration.
The involvement of actin in cell motility is well documented (4). Cells initiate movement by extending a lamellipodium, a protrusion of the membrane driven by actin polymerization, in the forward direction to establish the leading edge of the cell. This forward movement is consolidated by the establishment and maturation of adhesion plaques and leads to a cell that is directionally elongated. For cell migration to occur, however, contractile forces mediated by actin/myosin stress fibers must cause the trailing edge of the cell to retract and thus allow the cytoplasm and nucleus to translocate toward the leading edge. Although many of the details have yet to be worked out, the central role played by actin polymerization and actin/myosin contractile forces in causing cell movement is well established.
Less is known about the role of microtubules in this process. The potential involvement of microtubules in cell locomotion became clear following experiments in which movement was inhibited in cells that were treated with drugs known to interfere with microtubule assembly, implying that microtubules were needed for cell motility (5, 6). However, the picture became clouded by reports that microtubule-free cytoplasts from fish keratocytes were highly motile and that drug-treated neutrophils actually moved more rapidly than the nontreated cells (7, 8). The picture was further complicated by reports that very low concentrations of microtubule drugs that were insufficient to block mitosis or cause microtubule depolymerization were nonetheless effective at inhibiting cell migration (9–11). These low drug concentrations inhibit cell migration in parallel with a suppression of microtubule dynamics but have no effect on cell division or microtubule polymer levels (12–15). The tentative conclusion from these studies is that dynamic microtubules are required for cell motility, but the possibility remains that motility is affected by some other action of the drugs.
We now report the results of studies designed to further explore the mechanism by which microtubules influence cell migration. Cells expressing mutant forms of tubulin were used to eliminate the possibility that drug side effects were responsible for the inhibition of cell migration noted in earlier studies, and different drug concentrations were used to address the fundamental question of whether microtubules act to facilitate or restrain cell movement.
EXPERIMENTAL PROCEDURES
Maintenance of Cell Lines
Wild-type CHO cells (16) and colcemid-resistant mutant CV 2-8 CHO cells (17) were grown in α-minimum essential medium supplemented with 5% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37 °C and 5% CO2. Paclitaxel-dependent mutant Tax11–6 CHO cells (18) were maintained in the same medium supplemented with 200 nm paclitaxel. HeLa cells were grown in α-minimum essential medium containing 10% FBS.
Immunofluorescence
The cells were seeded onto sterile glass coverslips and grown for 24 h. They were then pre-extracted by incubating coverslips for 1 min at 4 °C in microtubule-stabilizing buffer (20 mm Tris-HCl, pH 6.8, 1 mm MgCl2, 2 mm EGTA, 0.5% Nonidet P-40) containing 4 μm paclitaxel to maintain microtubule integrity (19). The cells were then fixed in methanol at −20 °C for 10 min, rehydrated with PBS, incubated with a 1:100 dilution of mouse monoclonal antibody DM1A for 1 h at 37 °C, washed, and stained for 1 h with a 1:100 dilution of Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen) containing 1 μg/ml of DAPI. The cells were viewed using a fluorescence microscope equipped with a digital camera.
Transfection and Live Cell Microscopy
For live cell microscopy, parental CHO cells and CHO cell mutants were seeded onto 35-mm μ-dishes (Ibidi LLC, Verona, WI) and transfected with EGFP-MAP4 (20) using Lipofectamine 2000 as previously described (15, 21). After incubation at 37 °C and 5% CO2 for 24 h, images were captured every 5 s using a DeltaVision Core imaging system equipped with a CCD camera and softWorx image capture software (Applied Precision, Inc.).
Microtubule Dynamics
Microtubule life history plots were generated by measuring microtubule length from an arbitrary internal reference point as a function of time. Growth and shortening rates were calculated from the slopes of the plots using linear regression. Changes measuring less than 0.5 μm in a 5-s interval were not considered to be real episodes of growth or shortening and were disregarded in the calculations. Catastrophe was defined as the transition from either growth or pause (no change in length) to shortening, and its frequency was calculated as the number of transitions divided by the time spent in growth and pause. A transition from shortening to either growth or pause was recorded as rescue, and its frequency was calculated as the number of such events divided by the time spent shortening. Dynamicity, an overall measure of dynamic behavior, was calculated as the total change in length (growth plus shortening) divided by the total time the microtubule was under observation (22). Each parameter was calculated from microtubules persisting for at least 2 min and is expressed as the mean ± S.E.
Wound Healing Assay
Cells were grown to 80% of confluence in α-minimum essential medium containing 5% FBS (for CHO cells and its mutants) and 10% FBS (for HeLa cells). A scratch mark was introduced, the monolayer was washed with PBS to remove floating cells, and fresh medium was added with or without drugs at varying concentrations. The monolayer was allowed to recover for 0.5 h at 37 °C and 5% CO2. Cells migrating into the wound were then photographed every 15 min using phase contrast optics with a 4× objective, a CCD camera, and Velocity software (PerkinElmer Life Sciences). To better examine the morphology of the migrating cells, a similar experiment was performed using a 10× objective and recording images 1 min apart. To determine the rate at which cells moved into a wound (the wound healing rate), the average width of the wound, measured using five different microscopic fields, at the end of the experiment was subtracted from the average starting width, and the difference was divided by 2 (to account for the presence of two migrating edges) and by the total observation time.
Cell Tracking
Stacks of images showing the time course of cells moving into a wound were recorded by Velocity software and opened in ImageJ with the LOCI Bio-Formats importer plug in. The coordinates of the nucleus of a given cell were determined in each image using the manual tracking tool of ImageJ. These coordinates were plotted versus time to show the migration paths of individual cells. The rate at which the cells moved was calculated in two ways. Directional velocity was determined by calculating the difference between the starting and ending x coordinates and dividing that distance by the total observation time. In the second calculation, speed was determined by adding the distances cells moved irrespective of direction during each 15-min observation interval and dividing by the total time of observation. For the latter calculation, intervals during which there was no cell movement were considered to be “resting states” and were therefore excluded. The amount of time a cell spent in resting states was calculated by adding the number of intervals in which no movement was detected, multiplying by 15 min (the duration of the interval), and dividing by the number of hours the cell was under observation. At least five cells were averaged for each of these calculations.
Transwell Assay
For Transwell assays, ∼1000 cells were plated in 24-well cell culture inserts with an 8-μm pore size (BD Biosciences, San Jose, CA). The cells were then incubated in the presence or absence of drugs for 5 h at 37 °C and 5% CO2 to allow cells to migrate to the bottom side of the membrane. At the end of the incubation, residual cells in the upper chamber were removed with a cotton swab, and the membrane was excised, fixed in methanol, and stained with DAPI. Fluorescence microscopy was used to count the total number of cells in 15 randomly chosen fields on the lower side of the membrane, and the results were normalized by dividing by the number of cells counted in the absence of drug treatment. Each experiment was repeated at least three times.
Statistics
Each experiment was performed a minimum of three times, and differences between groups were determined using either a Student's t test (two groups) or analysis of variance with an appropriate post-test (more than two groups). A p value of less than 0.05 was considered significant. All of the data are expressed as the means ± S.D. or means ± S.E.
RESULTS
Altered Migration in Cell Lines with Mutant Tubulin
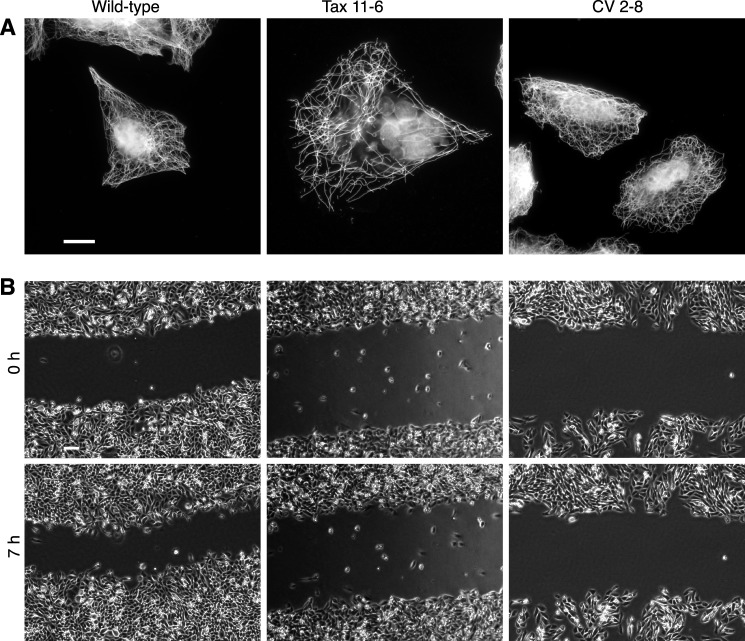
Previous work from our laboratory and others showed that tubulin mutations are able to confer altered sensitivity to microtubule-targeted drugs (23–25). In this study, we investigated whether tubulin mutations are also able to affect cell movement in a wound healing assay. Two mutants were tested: Tax 11-6, a paclitaxel-resistant and dependent CHO cell line with increased sensitivity to colcemid (18), and CV 2-8, a colcemid-resistant CHO cell line with increased sensitivity to paclitaxel (26). Originally, these cell lines were isolated using single-step selections in the presence of a minimally toxic concentration of each drug. Tubulin mutations were confirmed by two-dimensional gel electrophoresis and mRNA sequencing. The results revealed that Tax 11-6 contains an E77K mutation in α-tubulin that reduces microtubule assembly by ∼50% when the cells are grown without paclitaxel (21). On the other hand, CV 2-8 has an E55K mutation in α-tubulin that increases microtubule assembly by 20% (17). Tubulin immunofluorescence confirmed a partially disrupted microtubule cytoskeleton in Tax 11-6 when the cells were grown without paclitaxel but a robust cytoskeleton in CV 2-8 (Fig. 1A). To quantify migration, mutant and wild-type cells were grown to 80% confluence, a scratch was introduced in the monolayer, and the rate at which the wound closed was monitored by time lapse microscopy (Fig. 1B). The analysis revealed that the rate of wound healing for mutant cell lines was only 40–50% of the rate exhibited by wild-type cells (Table 1). These findings indicated that tubulin mutations can change the migratory behavior of a cell, but the changes in movement do not appear to correlate with the drug resistance phenotype or with effects of the mutations on the level of microtubule polymer.
FIGURE 1.
Wound healing ability of wild-type and mutant CHO cells. A, immunofluorescence. Wild-type CHO cells, paclitaxel-resistant (and dependent) mutant Tax 11-6, and colcemid-resistant mutant CV 2-8 were stained with an antibody to α-tubulin and with DAPI to compare their microtubule cytoskeletons and cell morphologies. Scale bar, 10 μm. B, wound healing assay. Nearly confluent cultures of each of the three cell lines were scratched to form cell-free zones, which were photographed with a 4× objective at various times to measure how quickly the wound closed. Time points at 0 and 7 h are shown. Scale bar, 100 μm.
TABLE 1.
CHO cell migration
The values shown represent the means ± S.D. The numbers in parentheses represent values normalized to the wild-type control.
| Cell line | Wound healing rate | Cell tracking data |
||
|---|---|---|---|---|
| X-velocitya | Speedb | Resting statec | ||
| μm/h | μm/h | μm/h | min/h | |
| Wild-type | 9.37 ± 1.10 (100) | 9.85 ± 1.35 (100) | 14.81 ± 5.19 (100) | 3.6 ± 3.3 (1) |
| Tax 11-6 | 4.50 ± 0.87 (48) | 3.92 ± 0.91 (40) | 10.45 ± 1.54 (71) | 22.8 ± 9.6 (6.3) |
| CV 2-8 | 3.67 ± 1.27 (39) | 3.20 ± 1.73 (32) | 10.30 ± 1.69 (70) | 29.4 ± 6.8 (8.2) |
a The average velocity towards the wound (X-direction) was calculated as the difference between the x coordinates at the start and end of the experiment divided by the time of observation.
b The average speed of cell movement was calculated by adding the distances moved (irrespective of direction) during each 15-min interval and dividing by the total time of observation. Intervals in which there was no cell displacement were not included in this calculation.
c A cell was determined to be in a resting state if it failed to move during one or more 15-min intervals. The average number of such intervals/cell was calculated for each cell line. To convert to min/h, the number of rest intervals was divided by 5 h (the time of observation) and multiplied by 15 min (the duration of one interval).
Microtubule Dynamics Are Reduced in Cell Lines with Mutant Tubulin
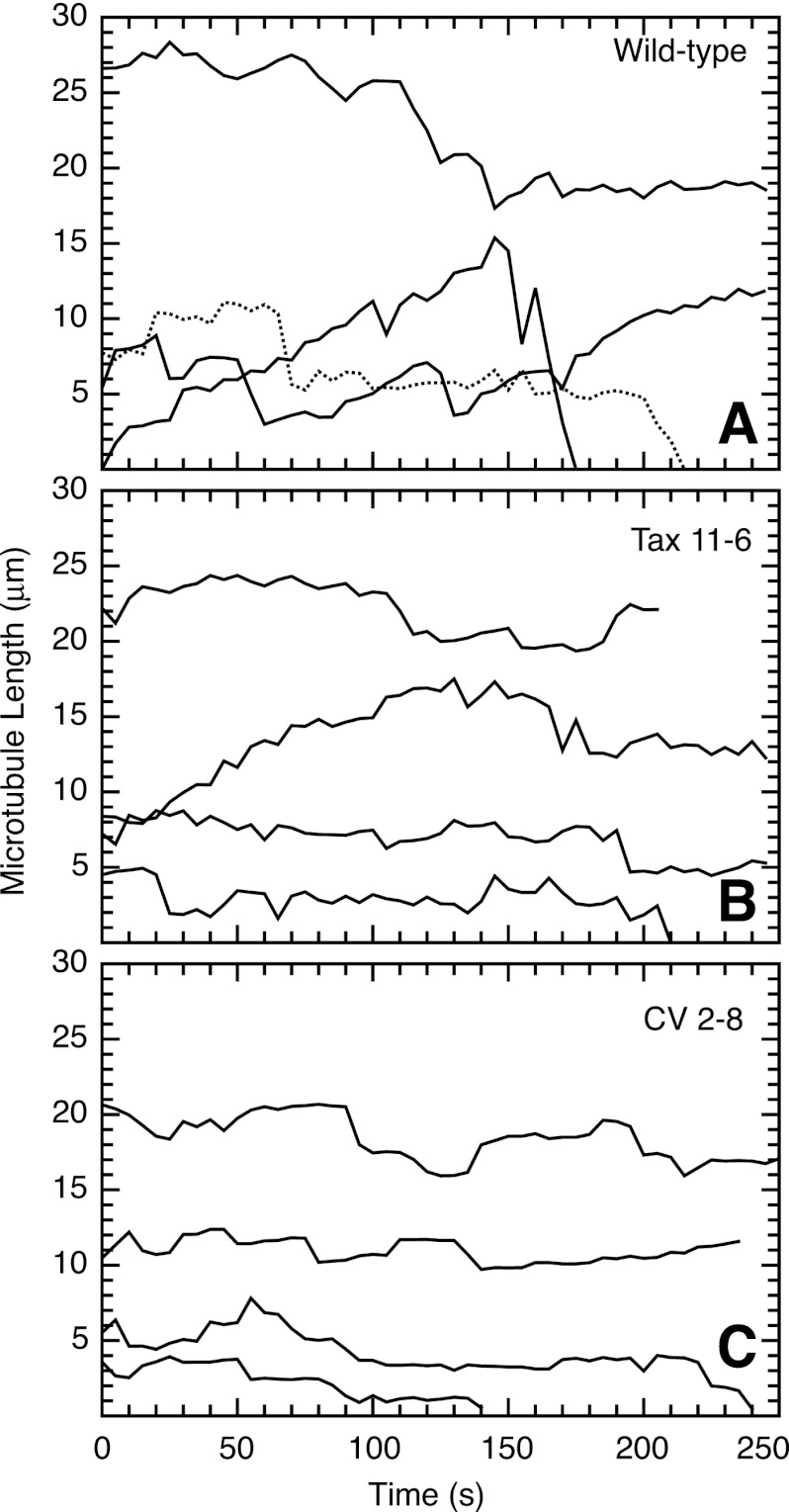
Our earlier studies showed that microtubule-destabilizing drugs at concentrations that are insufficient to cause a change in microtubule polymer levels or block cells in mitosis can nonetheless efficiently inhibit cell migration (15). Dose-response measurements further showed that the low drug concentrations inhibited microtubule dynamics in parallel with the reduction in cell migration. These findings led to the tentative conclusion that dynamic microtubules are needed for cell migration. To test whether tubulin mutations that affect cell migration also suppress microtubule dynamics, we transfected wild-type and mutant cell lines with EGFP-MAP4 and used live cell imaging to directly measure microtubule plus-end excursions. MAP4 is a microtubule-associated protein that decorates microtubules but has no effect on microtubule assembly or dynamics when overexpressed (15, 27, 28). By measuring microtubule length as a function of time, life history plots were generated and used to calculate various dynamic parameters including growth rates and durations, shortening rates and durations, transitions from growth or pause to shortening (catastrophe), transitions from shortening to growth or pause (rescue), and the total distance of growth plus shortening per unit time (dynamicity). The results summarized in supplemental Table S1 indicated that there was significant microtubule growth and shortening interspersed with pauses (periods during which length remained relatively constant) in the wild-type cells (Fig. 2A). Both mutant cell lines, on the other hand, exhibited reduced microtubule dynamics, even though they had opposing drug resistance profiles and an increase or a decrease in microtubule polymer levels (Fig. 2, B and C). The suppressed dynamics included reductions in both the growth and shortening rates, as well as in the dynamicity of the microtubules. In addition, microtubules in both mutants spent a significantly greater amount of time in a paused state compared with wild-type cells (supplemental Table S1). The results indicated that tubulin mutation-induced inhibition of cell migration is associated with suppression of microtubule dynamics. A similar conclusion was previously reached using drug treatment experiments.
FIGURE 2.
Microtubule life history plots of wild-type and mutant CHO cells. Wild-type CHO (A), mutant Tax 11–6 (B), and mutant CV 2–8 (C) cells were transfected with EGFP-MAP4 to label the microtubules, and the cells were photographed every 5 s to observe microtubule growth and shortening events. The contour distance from a microtubule end to an arbitrary fixed internal reference point was plotted versus the time of observation. Note that each line represents a single microtubule, and the starting position of the line along the y axis is arbitrary. Plots such as these were used to calculate microtubule growth and shrinkage rates, pauses (periods of no growth or shrinkage), transitions from growth or pause to shrinkage (catastrophes), transitions from shrinkage to growth or pause (rescues), and dynamicity (total of growth and shrinkage distances per unit time). These parameters are summarized in supplemental Table S1.
Mutants Have Increased Rest Intervals during Migration
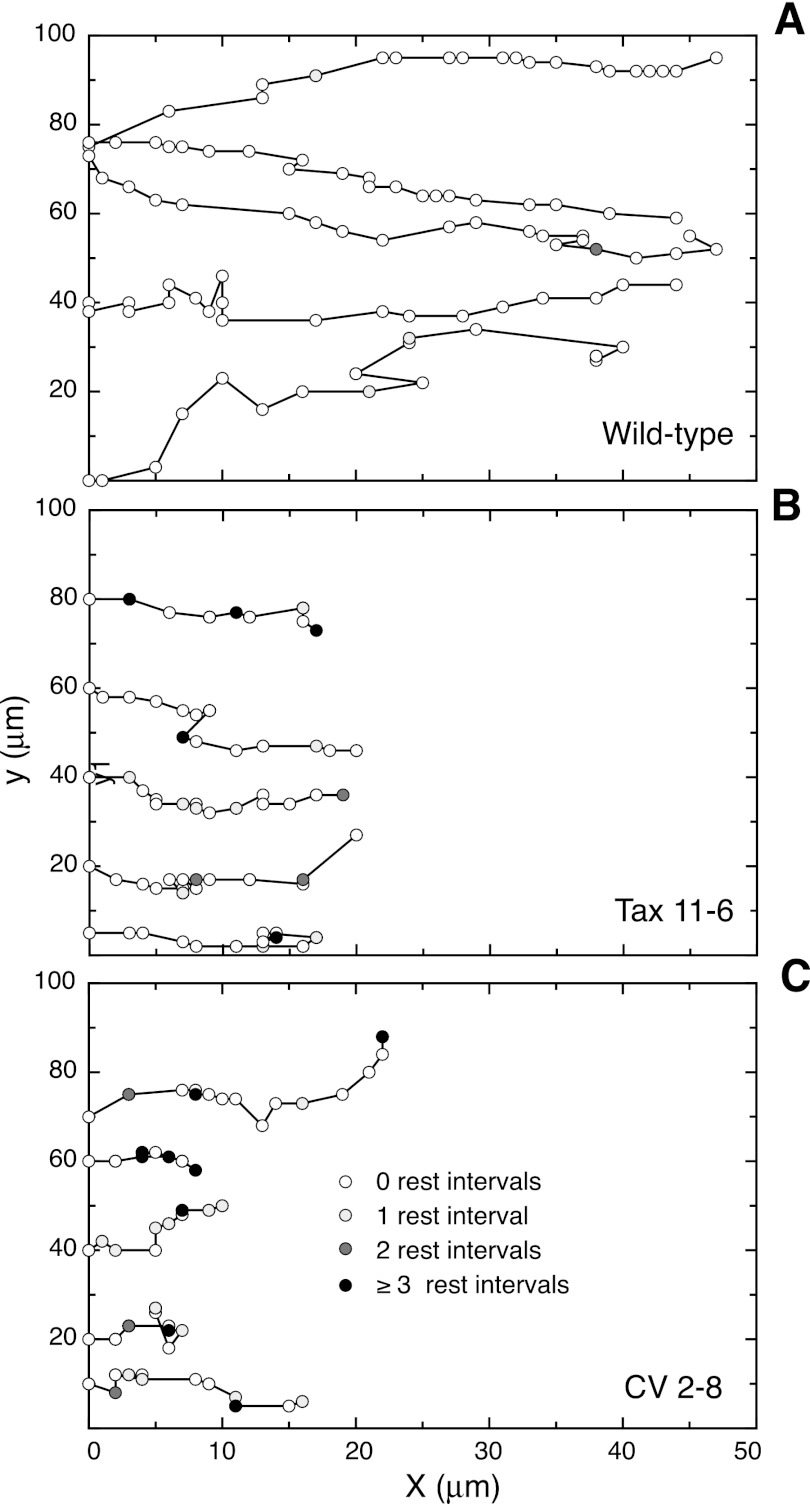
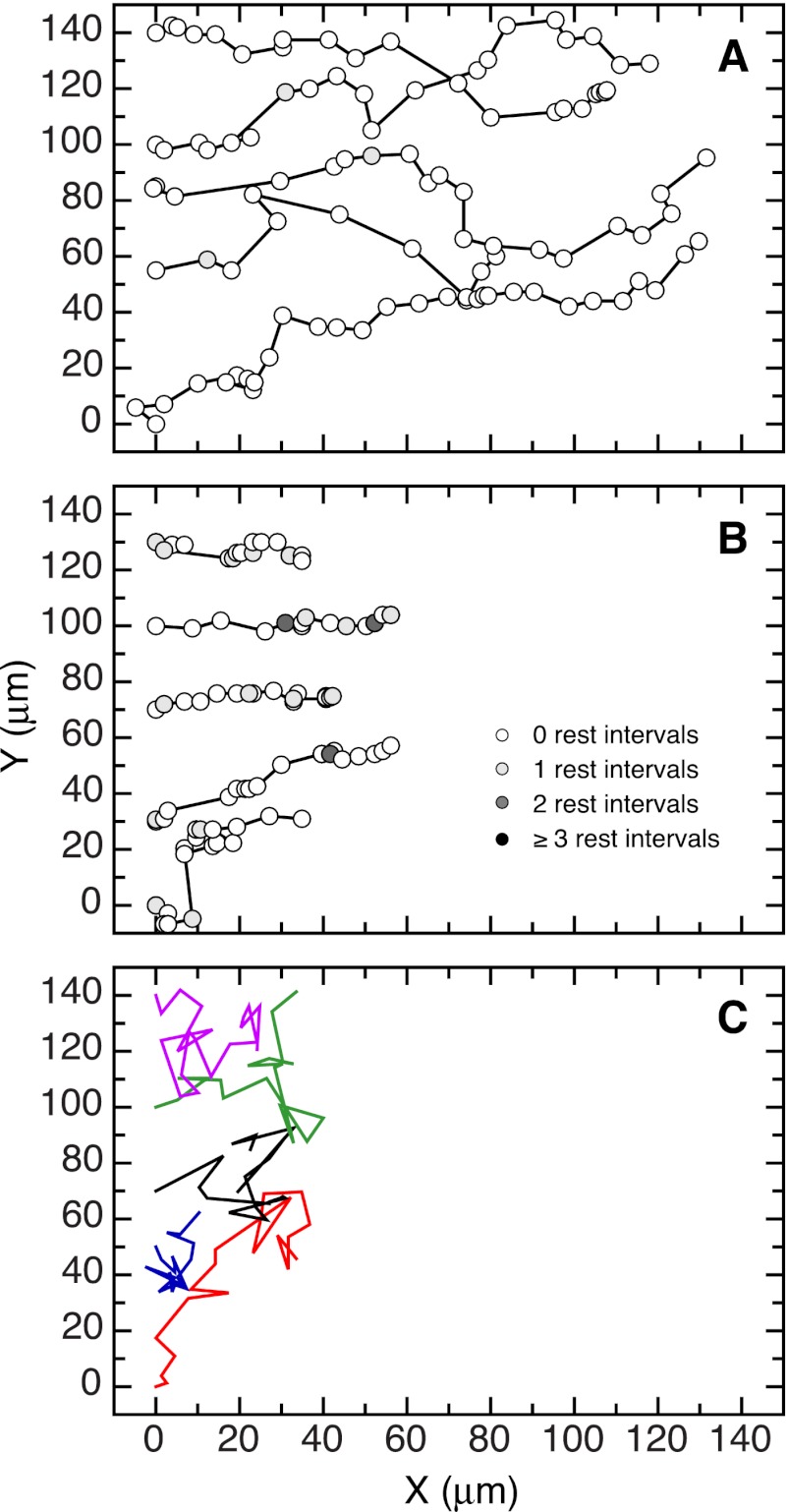
The reduced wound healing rates observed in the mutant cell lines could potentially be due to a reduction in the speed of cell movement, a loss of directionality of cell movement, or an increase in time during which the cells cease to move. We refer to the cessation of movement as “rest intervals” or resting states. To distinguish among these possibilities, individual cells moving into a scratch wound were tracked at 15-min intervals, and five representative tracks from each of the three cell lines are plotted in Fig. 3. All of the cells exhibited movement that was primarily directed toward the wound, but the progress of the mutant cells clearly lagged that of the wild-type control. To quantify the movement, we averaged the distance between the starting and final positions of individual cells in the X-direction (i.e., toward the wound) and divided by the total observation time. As expected, the velocities of cell movement calculated in this way were similar to the rates of wound healing determined directly from the closure of the wound (Table 1). In contrast, the speeds with which cells moved were higher when we included all directions of migration. This was expected because these measurements included movements that were in a direction other than just toward the wound. Unexpectedly, however, the decreases in motility of the mutant cells compared with wild-type cells were much smaller when calculated in this way. The reason for the discrepancy could be traced to the fact that mutant cells spent much more time in rest intervals, during which there was no net movement, and speed was only calculated using intervals in which the cells actually moved. Rest intervals are shown by the shaded circles in Fig. 3. Wild-type cells displayed few 15-min intervals in which no movement was detected, whereas mutant cells entered resting states more frequently, and the resting states were often of longer duration (indicated by the intensity of the shading in Fig. 3). These data were used to calculate the average amount of time per hour that each cell line was in a resting state. As summarized in Table 1, wild-type cells moved almost constantly and spent only about 3–4 min/h in the inactive state. In contrast, the mutant cells spent about one-third to one-half of their time unable to move, indicating that these nonmotile states are a major factor in the reduced ability of the cells to move into a wound. We conclude that tubulin mutations that suppress microtubule dynamics cause a decrease in the speed with which cells move, but in addition, they cause a dramatic increase in the amount of time cells spend in a resting state without cell movement.
FIGURE 3.
Tracking cells during wound healing. Conditions similar to those described in Fig. 1B were used to monitor the movement of individual wild-type (A) and mutant (B and C) CHO cells at the edge of a scratch wound. The x (toward the wound) and y coordinates of the nuclei are plotted. Open circles indicate the position of the nucleus at successive 15-min intervals. Shaded circles indicate that multiple 15-min intervals (designated as rest intervals) were needed before movement was seen. Each line represents the path of a different cell.
Suppressed microtubule dynamics in the mutant cells did not appear to have a significant effect on the directionality of cell movement. This conclusion came from the observation that migration-impaired mutant cells continued to move predominantly in the direction of the wound (Fig. 3) and suggested that suppression of microtubule dynamics does not affect cell polarization. To further test this possibility, we made use of reports showing that the centrosome of migrating cells often positions itself in front of the nucleus (i.e., toward the leading edge). This orientation is now commonly used as a marker for cell polarity (29). When we examined wild-type cells at the edge of a wound, we found that the centrosome was predominantly oriented in the direction of the leading edge (supplemental Fig. S1). No significant differences from the wild-type results were found in mutant cells or in wild-type cells treated with paclitaxel, further indicating that suppressing microtubule dynamics does not affect cell polarity.
Microtubule Dynamics Are Required for Tail Retraction
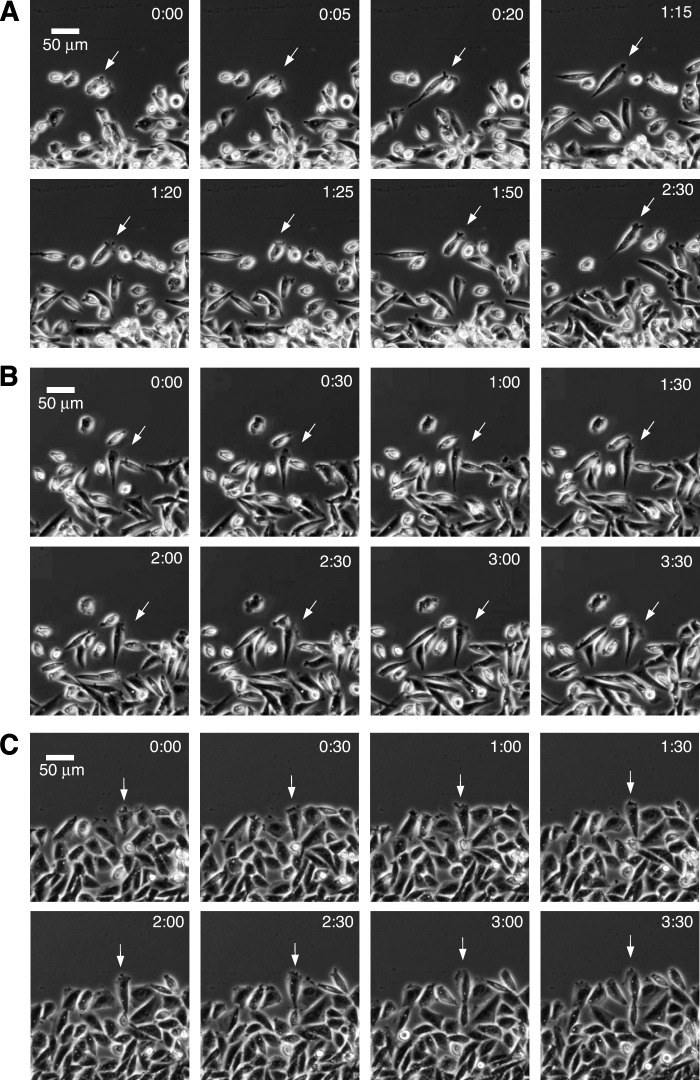
To obtain a clearer picture of how suppression of dynamics might prolong the resting state and inhibit cell motility, we studied morphological changes in cells moving toward a wound. Wild-type cells moved in a typical manner by extending lamellipodia, elongating in the direction of movement, and translocating forward by retracting their tails (Fig. 4A). Wild-type cells treated with low concentrations of paclitaxel sufficient to suppress microtubule dynamics also extended lamellipodia and elongated, but much more time was required for tail retraction, leaving many cells in the elongated state for as long as 4 h (Fig. 4B). Mutant cells exhibited behavior similar to drug-treated wild-type cells (Fig. 4C). The results indicate that suppression of microtubule dynamics by drug treatment or expression of mutant tubulin inhibits cell locomotion by interfering with the ability of migrating cells to retract their tails.
FIGURE 4.
Time-lapse video of cells moving into a wound. Wild-type (A), wild-type treated with 5 nm paclitaxel (B), and mutant CV 2-8 (C) are shown. The cells were photographed with a 10× objective every 1 min using phase contrast optics. The time in h:min from the start of the sequence is indicated. Scale bar, 50 μm. The arrows were added to aid in tracking individual cells through the time lapse sequences.
Dynamic Microtubules Are Enriched in the Tail of Migrating Cells
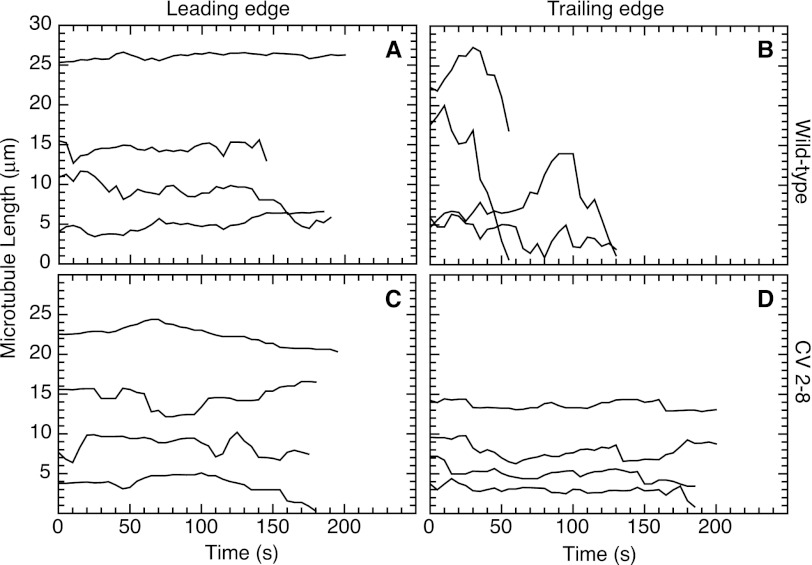
The observation that suppressing microtubule dynamics inhibited tail retraction suggested that the tail region should be enriched in dynamic microtubules. To test this prediction, measurements were made at the leading and trailing edges of cells moving into a scratch wound. As previously reported by others (30), we found that microtubules oriented toward the leading edge of wild-type CHO cells had suppressed dynamics with few and very short excursions of growth and shrinkage (Fig. 5A and supplemental Table S2), whereas microtubules at the trailing edge were very dynamic and exhibited particularly long shrinkage events that were seldom seen at the leading edge (Fig. 5B and supplemental Table S2). In fact, the long shrinkage distances are underrepresented in Fig. 5, because we routinely omit data for microtubules that do not persist for at least 2 min, and this failure to persist was frequent in the trailing but not in the leading edges. In contrast to the highly dynamic microtubules found near the trailing edge of wild-type cells, mutant CV 2-8 exhibited suppressed microtubule dynamics at both the leading (Fig. 5C and supplemental Table S2) and trailing edges (Fig. 5D and supplemental Table S2). The observation that dynamics are suppressed in the trailing edge of mutant cells is consistent with a decreased ability of the microtubules to remodel and could explain the relative inability of the cells to retract their tails during cell migration.
FIGURE 5.
Microtubule dynamics at the leading (A and C) and trailing edges (B and D) of migrating cells. Wild-type CHO cells (A and B) and mutant CV 2-8 (C and D) were transfected with EGFP-MAP4 and grown to near confluence. A scratch was introduced into the monolayer, and the lengths of individual microtubules in cells moving into the wound were measured every 5 s. The lengths were plotted against time to generate life history plots. Note that lengths were measured relative to a fixed internal reference point and are not meant to indicate the actual total length of a microtubule. Also, the position on the y axis is arbitrary, and a decrease to zero does not mean that the microtubule has completely disappeared. Some observation times were shorter than others because a microtubule depolymerized past the reference point or went out of focus. Each line represents a single microtubule.
Microtubule Disruption Restores Cell Motility
We next questioned why microtubule dynamics are needed for optimal cell motility. Although most models for microtubule involvement in locomotion propose that they play a positive role, we considered the alternative possibility that microtubules normally act to inhibit and control cell movement. To test this idea, we measured cell migration in CHO cells treated with increasing concentrations of nocodazole, a drug that suppresses dynamics at low concentrations but causes microtubule disassembly at higher concentrations (supplemental Fig. S2). Initially, we tried a wound healing assay but found that high drug concentrations caused many cells to lose adhesion to the culture dish resulting in loss of confluence and disruption of the edge of the wound.
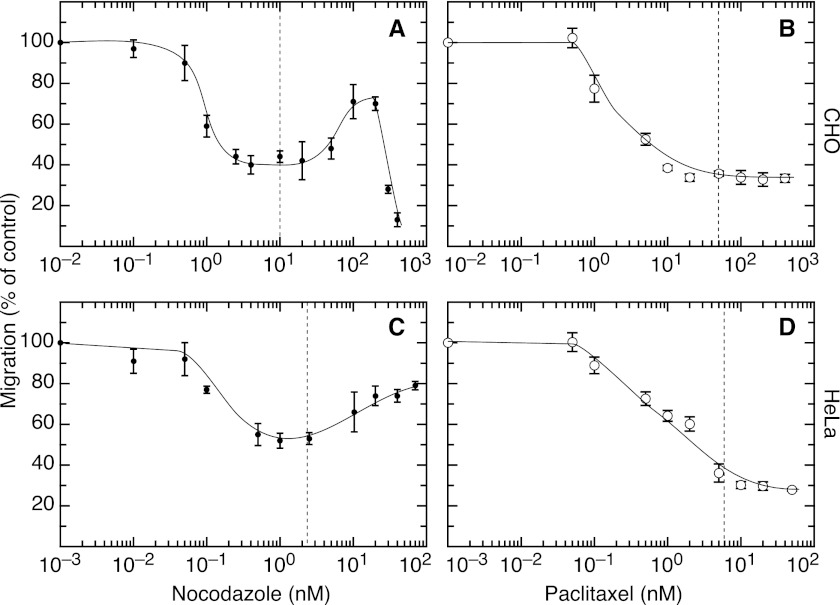
We then turned to a Transwell assay that measures the ability of cells added to the top of a porous membrane to move to the bottom surface. As shown in Fig. 6 (A and C), CHO and HeLa cells had a reduced ability to migrate through the membrane in the presence of low, nontoxic concentrations of nocodazole that suppress microtubule dynamics (15). Motility remained inhibited at higher drug concentrations that inhibit cell division (IC50 concentrations for inhibition of cell division are indicated by the vertical dashed lines in Fig. 6) (15). At still higher drug concentrations that eliminate most of the microtubules (supplemental Fig. S2), however, motility was partially restored. Increasing the drug beyond this point caused the motility to fall precipitously in CHO cells, presumably because of other unknown toxic effects. A similar drop was not seen in the case of HeLa cells because they are much more sensitive to antimitotic drugs, and thus we did not need such high concentrations to completely depolymerize the microtubules. The inability of high nocodazole concentrations to completely restore normal motility is likely due to the fact that many of the cells accumulate in mitosis, a stage during which the cells are nonadherent and do not migrate.
FIGURE 6.
Cell migration as a function of drug concentration. A Transwell assay was used to measure the number of CHO (A and B) and HeLa (C and D) cells that migrated through the membrane during a 5-h incubation in the presence of varying concentrations of nocodazole (A and C) and paclitaxel (B and D). The values for each graph were normalized by dividing by the number obtained in the absence of drug. Each point represents the average from three independent experiments with its associated standard deviation. The vertical dotted lines indicate the IC50 concentration of drug needed for inhibition of cell division.
In contrast to the recovery of motility with high concentrations of nocodazole, motility was not restored by high concentrations of paclitaxel (Fig. 6, B and D). These different outcomes are consistent with the actions of the drugs in causing microtubule depolymerization (nocodazole) as opposed to enhancing microtubule assembly (paclitaxel). Thus, the recovery of cell motility with increasing concentrations of nocodazole is likely due to the loss of microtubules rather than some other drug effect.
Cells without Microtubules Move at Normal Speed but Lose Their Directionality
Although the CHO wound healing assay was compromised by cells detaching at high nocodazole concentrations, it was possible to follow HeLa cells because they remained much more adherent. This allowed us to test the effects of eliminating microtubules on cell motility using a second assay and had the added advantage of eliminating mitotic and any other nonadherent cells from the calculations. Tracks following the movement of five representative HeLa cells in differing nocodazole concentrations are shown in Fig. 7 and supplemental Movies S1–S3. In the absence of drug (Fig. 7A and supplemental Movie S1), HeLa cells moved in a manner that was similar to CHO cells (Fig. 3), i.e., they moved predominately in the direction of the wound but at a much faster rate (Table 2). Also similar to the experiment with CHO cells, the addition of 1 nm nocodazole, a concentration that suppresses microtubule dynamics, slowed migration and increased the frequency and duration of resting states, but the directionality of the cells was maintained (Fig. 7B and supplemental Movie S2). In contrast to the effects of the low drug concentration, the addition of 70 nm nocodazole, a concentration that eliminates the microtubule network (supplemental Fig. S2), caused cells to move much more randomly, i.e., the directionality of the cells toward the wound was lost (Fig. 7C and supplemental Movie S3). Despite causing more erratic movement, the loss of microtubules completely reversed the speed inhibition that was seen at lower nocodazole concentrations and also reversed the drug-induced increase in time cells spent in a resting state (Table 2). These results indicated that microtubules are required to provide directionality for cell movement, but they are not required to drive the movement.
FIGURE 7.
Tracks of HeLa cells moving into a wound. A scratch assay was used to follow the paths of individual HeLa cells moving into a wound in the absence (A) or presence of 1 nm (B) and 70 nm (C) nocodazole. The cells were photographed at 15-min intervals, and the nuclear coordinates are plotted as circles. Shaded circles indicate that multiple intervals (rest intervals) passed before movement was detected. Circles were omitted, and the paths were indicated with different colors in C because of extensive overlap.
TABLE 2.
HeLa cell migration
The values shown represent the means ± S.D. The numbers in parentheses represent the values normalized to the no-drug control.
| Nocodazole | Wound healing rate | Cell tracking data |
||
|---|---|---|---|---|
| X-velocitya | Speedb | Resting statec | ||
| μm/h | μm/h | μm/h | min/h | |
| 0 nm | 23.9 ± 3.4 (100) | 25.3 ± 2.6 (100) | 36.4 ± 5.3 (100) | 1.8 ± 1.6 (1) |
| 1 nm | 9.1 ± 1.7 (38) | 10.3 ± 2.2 (41) | 18.4 ± 4.6 (51) | 13.2 ± 5.0 (7.3) |
| 70 nm | 8.3 ± 1.2 (35) | 7.6 ± 3.8 (30) | 39.0 ± 8.8 (107) | 1.8 ± 1.7 (1) |
a The average velocity towards the wound (X-direction) was calculated as the difference between the x coordinates at the start and end of the experiment divided by the time of observation.
b The average speed of cell movement was calculated by adding the distances moved (irrespective of direction) during each 15-min interval and dividing by the total time of observation. Intervals in which there was no cell displacement were not included in this calculation.
c A cell was determined to be in a resting state if it failed to move during one or more 15-min intervals. The average number of such intervals/cell was calculated for each cell line. To convert to min/h, the number of rest intervals was divided by 5 h (the time of observation) and multiplied by 15 min (the duration of one interval).
DISCUSSION
There is little consensus on the role of microtubules in cell locomotion. Studies using small molecule inhibitors have concluded that microtubules are required (5, 6), and proposals for their involvement include transporting membrane vesicles to the leading edge of motile cells, facilitating the turnover of adhesion plaques, and regulating the assembly or contractility of the actin cytoskeleton (31, 32). On the other hand, the use of the same inhibitors in hematopoietic and glial cells produced similar or even more rapid cell locomotion compared with untreated controls (7, 33–35). Of particular note, Euteneuer and Schliwa (8) reported that fish epidermal keratocytes as well as cytoplasmic fragments of those cells are capable of cell locomotion in the absence of microtubules. Some of the reported discrepancies are likely due to differences in drug concentrations or motility assays that were used in the experiments, but the most common explanation that has emerged in the literature attributes the different findings to the cell lines that were studied. According to this view, leukocytes, neutrophils, and other small immune cells use a form of movement that does not require microtubules, whereas larger cells such as fibroblasts use a different form of motility that is microtubule-dependent (reviewed in Ref. 32). Although this explanation is certainly possible, we searched for a single mechanism that would apply to all vertebrate cells regardless of size.
Some of the first clues that drug concentrations could be playing a role in the disparate results from different laboratories came from observations that low drug concentrations insufficient to depolymerize microtubules or block cells in mitosis were nonetheless capable of inhibiting cell migration (9, 11). A similar experiment led to speculation that drugs were inhibiting cell motility by interfering with microtubule dynamics (10), and later direct measurements of dynamics confirmed that prediction (12, 15). At the other end of the spectrum, it is not very surprising that some studies reported inhibition of locomotion with high drug concentrations, because we have found that very high concentrations can produce toxicity that is independent of their effects on microtubules. In addition, we found that intermediate concentrations sufficient to depolymerize microtubules may have little or no effect on motility depending on the assay that is used. Thus, the use of different concentrations of the same drug can produce very different and seemingly conflicting outcomes.
The results from the studies described here further strengthen and extend the hypothesis that microtubule dynamics, as opposed to the presence of microtubules per se, influence the rate at which cells migrate. The need for rapid microtubule dynamics was established using two mutant cell lines with differing mutations in α-tubulin. Although we cannot completely rule out the possibility that the cells contain additional mutations that affect cell motility, the fact that they were independently isolated in a single-step selection from a cloned cell population argues that they contain a minimum number of mutations relative to the parental cells and are unlikely to share any random mutations that may be present in addition to the known mutations in α-tubulin. The fact that the α-tubulin mutations had opposing effects on drug resistance and microtubule polymer levels yet both inhibited cell movement suggested that factors regulating the extent of microtubule assembly can produce changes in sensitivity to drugs that affect mitosis, but the steady state microtubule level is not a critical factor for determining the rate of cell migration. The microtubule-related factor that did affect motility appeared to be dynamics, an activity that was suppressed in both mutants.
A similar conclusion was previously reached by treating various cell lines with low drug concentrations that do not affect mitosis yet inhibit motility (15), as well as by experiments showing that β3-tubulin expression caused cells to strongly resist the suppressive effects of low paclitaxel concentrations on both microtubule dynamics and cell migration, yet produced only a small change in sensitivity to the higher concentrations of paclitaxel needed to inhibit cell division (14). The results obtained with β3 expression and with the mutant cell lines described here demonstrate unequivocally that inhibition of cell migration by antimitotic drugs is due to their suppression of microtubule dynamics rather than to some other drug effect. The fact that Tax 11-6 has some problems in cell division in the absence of paclitaxel (18) but CV 2-8 has normal growth also shows that the mechanism of microtubule involvement in cell migration is divorced from microtubule effects on mitosis.
To further explore the need for microtubule dynamics, we examined the movement of individual mutant cells and found that suppression of microtubule dynamics caused a relatively small reduction in the speed at which cells move but caused a large increase in resting states, during which cells failed to translocate. Drug-treated cells were still able to extend lamellipodia and elongate in the direction of migration, but they spent a long time in the elongated state unable to retract their tails. Consistent with this observation, dynamic microtubules in wild-type cells were largely restricted to the tail as previously noted (30), but trailing microtubules were far less dynamic in migration-impaired mutant cells. It thus appears that suppression of microtubule dynamics “freezes” the tail into a configuration that is not easily remodeled.
To explain this observation, we considered many of the existing theories for how microtubules influence cell motility (e.g., remodeling cell adhesion plaques or influencing actin assembly) but found that they could not adequately account for our data, nor could they explain the studies reporting that some cells such as neutrophils and fish keratocytes move efficiently without microtubules. What all the current theories have in common is that they assume a positive role for microtubules such that inhibition of their activity would result in a slowing of cell motility. We considered the alternate possibility that microtubules act to inhibit cell movement (13). In other words, we tested the idea that microtubules act as a brake to modulate (suppressed dynamics) or prevent (frozen dynamics) locomotion. In agreement with this hypothesis, the studies presented here show that tubulin mutations or low drug concentrations that suppress microtubule dynamics inhibit cell motility, presumably by making the microtubules more stable, but higher drug concentrations that depolymerize the microtubules allow cell movement to recover. These results were further strengthened by the observation that paclitaxel, a drug that promotes rather than inhibits microtubule assembly, does not allow cell mobility to recover at any concentration. The experiments further showed that microtubules are not needed for cells to move, but they are required to specify their direction of movement.
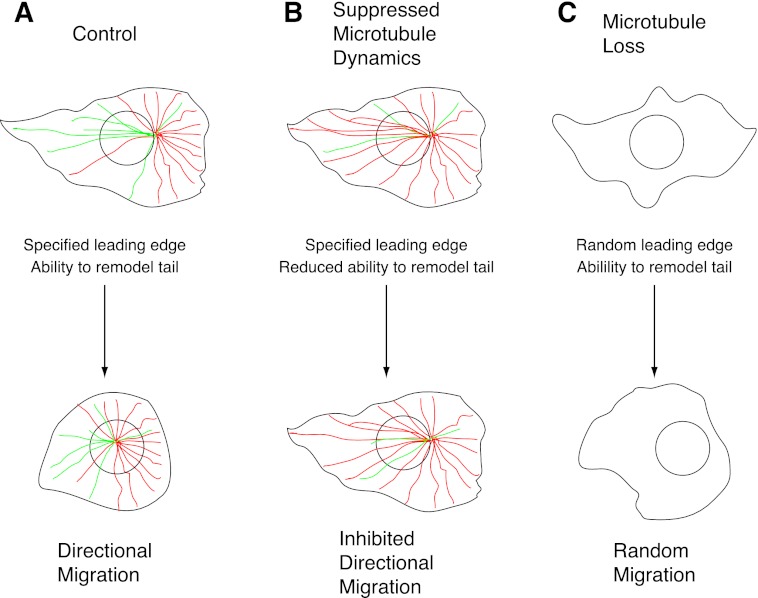
Based on these results, we propose the following model for microtubule involvement in cell migration (Fig. 8). The model is based on the premise that microtubules are not needed for cell motility, but when present, they act to restrain regional remodeling of cell morphology and thereby specify the direction in which cells migrate. In a normal migrating cell (Fig. 8A), lamellipodia extend in numerous directions, but chemotactic or other signals suppress microtubule dynamics in one area of the cell, resulting in microtubule stabilization, inhibition of lamellipodium retraction in that region, and therefore prioritization of the lamellipodium to establish the leading edge. The process is facilitated by the redistribution of microtubules to this region resulting from their polarized stabilization (little turnover at the front of the cell but continued high turnover at the back). The more numerous forward directed microtubules then may aid cell elongation by delivering vesicles that help to feed the growth of the leading edge. Cell stretching is counteracted by actin/myosin contractile forces that seek to return the cell to a more rounded morphology, but the contractile forces are in turn inhibited by the presence of microtubules that act as “struts” to maintain cell elongation as envisioned in the tensegrity model of cell structure (36, 37). Microtubules have directly been shown to resist contractile forces in cultured cells, as well as in smooth and cardiac muscle (38–40). Microtubule resistance is especially strong at the leading edge of migrating cells because of the higher density of microtubules and their relatively low dynamicity. At the trailing edge, however, dynamic microtubules are rapidly remodeled, allowing contractile forces to retract the tail, thereby permitting the cell to move forward. Thus, by the selective stabilization of microtubules at the leading edge, cell polarity is established, and cells can move directionally up a chemical gradient or toward an open wound. In the latter assay, it is unclear whether selective stabilization occurs at the leading edge because of molecules released by cells damaged during the formation of the wound or whether there is selective destabilization (increased dynamicity) at the rear of the cell caused by cell-to-cell contact as has been reported in other studies (41).
FIGURE 8.
Model for microtubule involvement in cell motility. See text for details. Dynamic microtubules are green; microtubules with suppressed dynamics are red. Cells with normal microtubule dynamics (A), suppressed dynamics (B), or lacking microtubules (C) are depicted.
When microtubule dynamics are globally suppressed by low drug concentrations or by tubulin mutations (Fig. 8B), a similar sequence of events takes place, and the difference between front to back microtubule stability still exists but is reduced. As a result, cells are still able to polarize, but there may be relatively more microtubules directed to the rear than before, and they are less dynamic and less able to remodel. The cells now spend more time in the elongated resting state unable to retract their tails, thereby inhibiting forward movement. At high drug concentrations sufficient to eliminate microtubules (Fig. 8C), lamellipodia are still able to extend, but they are relatively short-lived because microtubules are not present to prevent their retraction. One of the lamellipodia can still temporarily become predominant by chance, and so cells can still move. However, the movement becomes randomized because there is no longer selective microtubule stabilization to prioritize lamellipodia in any specific direction. Because microtubules are not present to counteract contractile forces, the speed with which these cells move can be similar to or even greater than the speed of untreated control cells.
This relatively simple description of cell motility can potentially explain many of the confusing and often contradictory conclusions that have appeared in the literature concerning the role of microtubules in cell locomotion. The idea that microtubules act to retard motility is consistent with observations from a variety of systems, indicating that microtubules can resist actin/myosin contractility, and is also consistent with the inhibition of tail retraction that we see when microtubule dynamics are suppressed. We propose that selective suppression of microtubule dynamics by chemotactic factors may be responsible for reorganization of microtubules toward the leading edge, resulting in polarization of the cell and directional motility.
Acknowledgments
We thank Dr. Joanna Olmsted for providing us with EGFP-MAP4. We are also grateful to the staff of the Live Cell Imaging Facility of the Snyder Institute for Chronic Disease at the University of Calgary for assistance with imaging.
Footnotes
This work was supported in part by National Institutes of Health Grant CA 85935 (to F. C.) and Canadian Institute for Health Research Grant MOP-14180 (to K. P.).

This article contains supplemental Tables S1 and S2, Figs. S1 and S2, and Movies S1–S3.
REFERENCES
- 1. Samant R. S., Shevde L. A. (2011) Recent advances in anti-angiogenic therapy of cancer. Oncotarget 2, 122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz E. L. (2009) Antivascular actions of microtubule-binding drugs. Clin. Cancer Res. 15, 2594–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmer T. D., Ashby W. J., Lewis J. D., Zijlstra A. (2011) Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 63, 568–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration. Integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 5. Goldman R. D. (1971) The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J. Cell Biol. 51, 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasiliev J. M., Gelfand I. M., Domnina L. V., Ivanova O. Y., Komm S. G., Olshevskaja L. V. (1970) Effect of colcemid on the locomotory behaviour of fibroblasts. J. Embryol. Exp. Morphol. 24, 625–640 [PubMed] [Google Scholar]
- 7. Dziezanowski M. A., DeStefano M. J., Rabinovitch M. (1980) Effect of antitubulins on spontaneous and chemotactic migration of neutrophils under agarose. J. Cell Sci. 42, 379–388 [DOI] [PubMed] [Google Scholar]
- 8. Euteneuer U., Schliwa M. (1984) Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature 310, 58–61 [DOI] [PubMed] [Google Scholar]
- 9. Belotti D., Vergani V., Drudis T., Borsotti P., Pitelli M. R., Viale G., Giavazzi R., Taraboletti G. (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin. Cancer Res. 2, 1843–1849 [PubMed] [Google Scholar]
- 10. Liao G., Nagasaki T., Gundersen G. G. (1995) Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level. Implications for the role of dynamic microtubules in cell locomotion. J. Cell Sci. 108, 3473–3483 [DOI] [PubMed] [Google Scholar]
- 11. Vacca A., Iurlaro M., Ribatti D., Minischetti M., Nico B., Ria R., Pellegrino A., Dammacco F. (1999) Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood 94, 4143–4155 [PubMed] [Google Scholar]
- 12. Mikhailov A., Gundersen G. G. (1998) Relationship between microtubule dynamics and lamellipodium formation revealed by direct imaging of microtubules in cells treated with nocodazole or taxol. Cell Motil. Cytoskeleton 41, 325–340 [DOI] [PubMed] [Google Scholar]
- 13. Ganguly A., Cabral F. (2011) The arresting action of microtubules in cell motility. Cell Cycle 10, 2614–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganguly A., Yang H., Cabral F. (2011) Class III β-tubulin counteracts the ability of paclitaxel to inhibit cell migration. Oncotarget 2, 368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang H., Ganguly A., Cabral F. (2010) Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. J. Biol. Chem. 285, 32242–32250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cabral F., Sobel M. E., Gottesman M. M. (1980) CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered β-tubulin. Cell 20, 29–36 [DOI] [PubMed] [Google Scholar]
- 17. Hari M., Wang Y., Veeraraghavan S., Cabral F. (2003) Mutations in α- and β-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol. Cancer Ther. 2, 597–605 [PubMed] [Google Scholar]
- 18. Schibler M. J., Cabral F. (1986) Taxol-dependent mutants of Chinese hamster ovary cells with alterations in α- and β-tubulin. J. Cell Biol. 102, 1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minotti A. M., Barlow S. B., Cabral F. (1991) Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J. Biol. Chem. 266, 3987–3994 [PubMed] [Google Scholar]
- 20. Olson K. R., McIntosh J. R., Olmsted J. B. (1995) Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J. Cell Biol. 130, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganguly A., Yang H., Cabral F. (2010) Paclitaxel-dependent cell lines reveal a novel drug activity. Mol. Cancer Ther. 9, 2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan M. A., Wilson L. (1998) Use of drugs to study role of microtubule assembly dynamics in living cells. Methods Enzymol. 298, 252–276 [DOI] [PubMed] [Google Scholar]
- 23. Cabral F. (2000) Editorial. Drug Resistance Updates 3, 1–6 [DOI] [PubMed] [Google Scholar]
- 24. Huzil J. T., Chen K., Kurgan L., Tuszynski J. A. (2007) The roles of β-tubulin mutations and isotype expression in acquired drug resistance. Cancer Inform. 3, 159–181 [PMC free article] [PubMed] [Google Scholar]
- 25. Yin S., Zeng C., Hari M., Cabral F. (2012) Random mutagenesis of β-tubulin defines a set of dispersed mutations that confer paclitaxel resistance. Pharm. Res. 29, 2994–3006 [DOI] [PubMed] [Google Scholar]
- 26. Schibler M. J., Barlow S. B., Cabral F. (1989) Elimination of permeability mutants from selections for drug resistance in mammalian cells. FASEB J. 3, 163–168 [DOI] [PubMed] [Google Scholar]
- 27. Barlow S., Gonzalez-Garay M. L., West R. R., Olmsted J. B., Cabral F. (1994) Stable expression of heterologous microtubule-associated proteins (MAPs) in Chinese hamster ovary cells. Evidence for differing roles of MAPs in microtubule organization. J. Cell Biol. 126, 1017–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X. M., Peloquin J. G., Zhai Y., Bulinski J. C., Borisy G. G. (1996) Removal of MAP4 from microtubules in vivo produces no observable phenotype at the cellular level. J. Cell Biol. 132, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueda M., Gräf R., MacWilliams H. K., Schliwa M., Euteneuer U. (1997) Centrosome positioning and directionality of cell movements. Proc. Natl. Acad. Sci. U.S.A. 94, 9674–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salaycik K. J., Fagerstrom C. J., Murthy K., Tulu U. S., Wadsworth P. (2005) Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J. Cell Sci. 118, 4113–4122 [DOI] [PubMed] [Google Scholar]
- 31. Kaverina I., Straube A. (2011) Regulation of cell migration by dynamic microtubules. Semin. Cell Dev. Biol. 22, 968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Small J. V., Geiger B., Kaverina I., Bershadsky A. (2002) How do microtubules guide migrating cells? Nat. Rev. Mol. Cell Biol. 3, 957–964 [DOI] [PubMed] [Google Scholar]
- 33. Edelson P. J., Fudenberg H. F. (1973) Effect of vinblastine on the chemotactic responsiveness of normal human neutrophils. Infect. Immun. 8, 127–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keller H. U., Naef A., Zimmermann A. (1984) Effects of colchicine, vinblastine and nocodazole on polarity, motility, chemotaxis and cAMP levels of human polymorphonuclear leukocytes. Exp. Cell Res. 153, 173–185 [DOI] [PubMed] [Google Scholar]
- 35. Spooner B. S., Yamada K. M., Wessells N. K. (1971) Microfilaments and cell locomotion. J. Cell Biol. 49, 595–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brangwynne C. P., MacKintosh F. C., Kumar S., Geisse N. A., Talbot J., Mahadevan L., Parker K. K., Ingber D. E., Weitz D. A. (2006) Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 173, 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ingber D. E. (2003) Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 116, 1157–1173 [DOI] [PubMed] [Google Scholar]
- 38. Brown R. A., Talas G., Porter R. A., McGrouther D. A., Eastwood M. (1996) Balanced mechanical forces and microtubule contribution to fibroblast contraction. J. Cell Physiol. 169, 439–447 [DOI] [PubMed] [Google Scholar]
- 39. Zhang D., Jin N., Rhoades R. A., Yancey K. W., Swartz D. R. (2000) Influence of microtubules on vascular smooth muscle contraction. J. Muscle Res. Cell Motil. 21, 293–300 [DOI] [PubMed] [Google Scholar]
- 40. Cheng G., Zile M. R., Takahashi M., Baicu C. F., Bonnema D. D., Cabral F., Menick D. R., Cooper G. (2008) A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am. J. Physiol. Heart Circ. Physiol. 294, H2231–H2241 [DOI] [PubMed] [Google Scholar]
- 41. Kadir S., Astin J. W., Tahtamouni L., Martin P., Nobes C. D. (2011) Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J. Cell Sci. 124, 2642–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]