Background: Endocytic traffic, mediated partially by BAR proteins, is essential for proper growth factor function.
Results: Loss of PACSIN2 increases EGFR surface expression, receptor activation, and downstream signaling.
Conclusion: PACSIN2 acts as negative regulator of EGF receptor signaling.
Significance: This study identifies PACSIN2 as a key regulator of growth factor signaling.
Keywords: Epidermal Growth Factor (EGF), Epidermal Growth Factor Receptor (EGFR), ERK, Membrane Trafficking, Trafficking, BAR Domain, PACSIN2
Abstract
Signaling via growth factor receptors, including the epidermal growth factor (EGF) receptor, is key to various cellular processes, such as proliferation, cell survival, and cell migration. In a variety of human diseases such as cancer, aberrant expression and activation of growth factor receptors can lead to disturbed signaling. Intracellular trafficking is crucial for proper signaling of growth factor receptors. As a result, the level of cell surface expression of growth factor receptors is an important determinant for the outcome of downstream signaling. BAR domain-containing proteins represent an important family of proteins that regulate membrane dynamics. In this study, we identify a novel role for the F-BAR protein PACSIN2 in the regulation of EGF receptor signaling. We show that internalized EGF as well as the (activated) EGF receptor translocated to PACSIN2-positive endosomes. Furthermore, loss of PACSIN2 increased plasma membrane expression of the EGF receptor in resting cells and increased EGF-induced phosphorylation of the EGF receptor. As a consequence, EGF-induced activation of Erk and Akt as well as cell proliferation were enhanced in PACSIN2-depleted cells. In conclusion, this study identifies a novel role for the F-BAR-domain protein PACSIN2 in regulating EGF receptor surface levels and EGF-induced downstream signaling.
Introduction
Signaling via receptor tyrosine kinases is essential for many cellular processes, such as proliferation, differentiation, cell survival, and cell migration (1, 2). Aberrant expression and activation of receptor tyrosine kinases is causally related to human diseases such as cancer, inflammation, and angiogenesis. One of the best studied members of receptor tyrosine kinases is the epidermal growth factor receptor (EGF receptor, also known as ErbB1). The EGF receptor is ubiquitously expressed, and increased expression of the receptor is often observed in cancer (3). Inactive EGF receptor resides on the cell surface. Binding of EGF to the extracellular amino-terminal domain of the EGF receptor leads to receptor autophosphorylation and activation and subsequently to the activation of several downstream signaling pathways such as Erk and Akt signaling (4).
Proper signaling of the EGF receptor, followed by its down-regulation, depends on correct intracellular trafficking and localization to appropriate intracellular structures. The level of EGF receptor on the cell surface is an important determinant for the outcome of downstream signaling. In resting cells, ∼2% of the EGF receptor is constitutively internalized in the absence of ligand, and most of the internalized receptor recycles back to the plasma membrane (5). Ligand binding induces rapid internalization of the EGF receptor. This ensures efficient termination of signaling by targeting the receptor for lysosomal degradation or recycling. Upon internalization of the EGF receptor, signaling can continue from early endosomes as well. This has been shown to be important for certain signaling pathways, including the Erk pathway, as inhibition of endocytosis was found to impair pathway activation (6, 7). Depending on the dose of EGF, the EGF receptor can be internalized via clathrin-mediated endocytosis, which has been implicated in recycling of the receptor, and clathrin-independent endocytosis, which has been linked to receptor degradation (7, 8). It is now widely accepted that internalization and trafficking is an important mode to control EGF receptor signaling (9, 10).
Bin/amphiphysin/Rvs (BAR)3 domain-containing proteins represent an important family of proteins that regulate membrane dynamics. Via their conserved BAR domain, these proteins bind to, stabilize, and induce membrane curvature. As a consequence, BAR domains induce invaginations of the plasma membrane and, subsequently, vesicular-tubular structures that are involved in membrane dynamics, including receptor internalization (11, 12). Several of these BAR proteins have been implicated in controlling EGF receptor signaling. Cbl can form a complex with the adapter protein CIN85 and the BAR protein endophilin, which initiates EGF receptor internalization, thereby controlling receptor signaling and down-regulation. Preventing this complex formation inhibits EGF receptor internalization and delays receptor degradation resulting in increased signaling (13). Furthermore, the F-BAR (Fer-CIP4 homology-BAR; a subclass of the BAR domain family) protein CIP4 regulates late events in EGF receptor trafficking from endosomes toward lysosomes, which results in receptor degradation. Lack of CIP4 accumulated the EGF receptor on early endosomes with prolonged signaling as a result (14).
Here, we describe an additional F-BAR family member, PACSIN2 that controls EGF signaling. PACSIN2 associates to several proteins such as Rac1, dynamin, Neuronal Wiskott-Aldrich Syndrome Protein (N-WASP), and synaptojanin via its C-terminal Src homology 3 (SH3) domain (15–17). The PACSIN2 F-BAR domain, located in its N-terminal region, mediates membrane binding and is involved in homo- and hetero-oligomerization (18). Due to this oligomerization, PACSIN2 can associate to multiple proteins at once, linking the actin regulatory network with the endocytic machinery (17).
The current study shows that PACSIN2 is a negative regulator of EGF receptor activation and signaling. Initially, we found that EGF is internalized to PACSIN2-positive vesicles, and we could visualize accumulation of both total and activated EGF receptor on PACSIN2-positive endosomes upon EGF stimulation. We show that in PACSIN2 knockdown cells, as well as in cells expressing an SH3 or BAR domain mutant of PACSIN2 (inhibiting protein interactions and internalization, respectively), EGF receptor surface levels were increased. Furthermore, EGF-mediated activation and phosphorylation of the EGF receptor as well as of its downstream targets, Erk and Akt, is enhanced in PACSIN2 knockdown cells. As a result, loss of PACSIN2 enhanced EGF-induced cell growth. Finally, we show that these effects are not specific for EGF because signaling by hepatocyte growth factor (HGF), and in primary endothelial cells also by TNFα, is similarly regulated by PACSIN2. In summary, these data show that PACSIN2 is a key regulator of growth factor signaling in epithelial and in endothelial cells, regulating growth factor receptor surface levels and downstream signaling.
EXPERIMENTAL PROCEDURES
Antibodies, Reagents, and Expression Constructs
Antibodies
Anti-PACSIN2 (AP8088b) was from Abgent. Anti-Actin (A3853) was from Sigma. Anti-early endosomal antigen-1 (EEA1; 610457) was from BD Transduction Laboratories. Anti-pTyr (pTyr-20; 03-7720), anti-transferrin receptor (13-6800), and anti-c-Myc (13-2500) were from Invitrogen. Anti-HC10 was used to visualize MHC-I and was a kind gift from Dr. Mar Fernandez-Borja (Sanquin Research, University of Amsterdam, The Netherlands). Anti-β1-integrin (610468) and anti-RhoGDI (610255) were from BD Transduction Laboratories. Anti-EGFR (4267), anti-pEGFR-Y1068 (3777), anti-Akt (9272), and anti-pAkt-Ser-473 (3787S) were from Cell Signaling. For Western blot, anti-Erk (SC-153) and anti-pErk (SC-7383) were from Santa Cruz Biotechnology. For the NanoPro assays, anti-Erk1/2 (06-182) was from Millipore, and anti-pErk (9101) was from Cell Signaling. For detection in NanoPro, secondary HRP-labeled antibodies were from ProteinSimple. Secondary HRP-labeled antibodies for Western blot were from Pierce. Secondary Alexa Fluor-labeled antibodies for immunofluorescence were from Invitrogen. F-actin was detected using Texas Red- or Alexa Fluor 633-labeled phalloidin (Invitrogen). Nuclei were stained with Hoechst (H-3569; Invitrogen).
Reagents
Recombinant human epidermal growth factor (Cyt-217) and recombinant human hepatocyte growth factor (human HGF; Cyt-244) were obtained from Prospec and used at a concentration of 100 ng/ml for the indicated time points. EGF-Texas Red was obtained from Molecular Probes (E-3480) and used at a concentration of 100 ng/ml for the indicated time points. Recombinant human tumor necrosis factor-α (TNF-α) was obtained from Peprotech (300-01A). MG132 (C2211) and chloroquine (C6628) were obtained from Sigma (C2211).
Expression Constructs
pEYFP-PACSIN WT and Myc-tagged PACSIN2 R50D were described previously (16). Myc-tagged PACSIN2 WT and Myc-tagged PACSIN2 Y435E/P478L were a kind gift from Markus Plomann (University of Cologne, Cologne, Germany).
Cell Culture and Transfections
HeLa cells are maintained in a humidified atmosphere at 37 °C and 5% CO2 in Iscove's modified Dulbecco's medium (Biowhittaker) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen), 300 μg/ml glutamine, and 100 units/ml penicillin and streptomycin. Primary human umbilical vein endothelial cells (HUVEC) were purchased from Lonza (Baltimore, MD) and cultured in EGM2 medium, containing singlequots (Lonza). For ectopic expression, HeLa cells were transiently transfected with TransIT (Mirus) according to the manufacturers' recommendations.
Lentiviral shRNAi and siRNA Silencing
Lentiviral short hairpin RNA (shRNA) constructs for PACSIN2 from the TRC/Sigma Mission library were obtained from Sigma-Aldrich. The SHC002 scrambled shRNA construct (Sigma-Aldrich) was used as a negative control. All shRNA constructs were in the pLKO.1 vector backbone. shRNA-expressing lentiviral particles were prepared using HEK293T cells and virus was transduced as described previously (19).
The sequence for control siRNA was as follows: 5′-CGUACGCGGAAUACUUCGAtt-3′ (Eurogentec). The sequence for PACSIN2 siRNA was as follows: 5′-GGAGAAGCUGGCUAUCUCACGAGAAtt-3′ (Eurogentec). The sequence for PACSIN2 shRNA (TRCN0000037982) was as follows: CCGGAGTGCAAGCAAGATGTTCTTACTCGAGTAAGAACATCTTGCTTGCACTTTTTTG. Transfections of siRNA were performed with INTERFERin (Polyplus transfection) according to the manufacturers' recommendations.
SDS-PAGE and Western Blot Analysis
Proteins were separated on SDS-PAGE gels and transferred onto nitrocellulose transfer membrane using the iBlot Dry Blotting System (Invitrogen) according to the manufacturers' recommendations. Following blocking in 5% low fat milk in TBST (Tris-buffered saline Tween 20) for 30 min, the blots were incubated with the primary antibody overnight at 4 °C. Next, the blots were washed five times for 10 min in TBST and subsequently incubated with HRP-conjugated secondary antibodies (dilution 1:5000) in TBST for 1 h at room temperature. Finally, blots were washed three times with TBST for 30 min each and subsequently developed by ECL (GE Healthcare).
Confocal Laser Scanning Microscopy
Cells, seeded on fibronectin-coated glass coverslips, were transfected with the indicated plasmids or siRNA, and after 24–48 h, cells were fixed by 3.7% formaldehyde (Merck) in PBS for 10 min followed by permeabilization with 0.5% Triton X-100 in PBS (5 min at room temperature). Coverslips were then incubated for 15 min with 2% BSA in PBS at 37 °C to block aspecific binding. Immunostainings were performed at room temperature for 1 h with the indicated antibodies. Fluorescent imaging was performed with a confocal laser scanning microscope (LSM510/Meta; Carl Zeiss MicroImaging, Inc.) using a 63X/NA 1.40 (Carl Zeiss MicroImaging, Inc.). Image acquisition was performed with Zen 2009 software (Carl Zeiss MicroImaging, Inc.). For live-cell imaging, cells, seeded on fibronectin-coated glass coverslips, were transfected with the indicated plasmids. After 24 h, fluorescent imaging was performed. Colocalization was analyzed using Zen 2009 software (Carl Zeiss MicroImaging, Inc.). For quantification of colocalization, at least five images of at least five cells from at least two independent experiments were used for each condition.
Surface Biotinylation Pulldown Assay
Surface protein labeling studies were performed as follows. 24 hours after seeding, cells were transfected with siRNA as indicated. 48 hours after siRNA transfection, resting cells or cells, treated with human EGF for the indicated time periods were washed three times with cold PBS supplemented with 0.5 mm MgCl2 and 1 mm CaCl2. Cells were then incubated with 0.5 mg/ml sulfo-NHS-LC-biotin (21335, Thermo Scientific) in PBS for 30 min at 4 °C. After biotinylation all unbound biotin was removed by quenching with PBS containing 100 mm glycine for 15 min at 4 °C and subsequently washed three times with cold PBS supplemented with 0.5 mm MgCl2 and 1 mm CaCl2. Cells were then lysed in Nonidet P-40 lysis buffer (50 mm Tris/HCl, pH 7.5, 100 mm NaCl, 10 mm MgCl2, 10% glycerol, and 1% Nonidet P-40) supplemented with protease inhibitors (Complete mini EDTA, Roche Applied Science) and centrifuged at 20.000 × g for 10 min at 4 °C. The supernatant, containing biotinylated surface proteins, was then incubated in the presence of streptavidin-coated beads (Sigma) at 4 °C for 1 h while rotating. Surface protein levels were assayed by Western blot analysis.
Ubiquitylation Assay
To detect endogenous ubiquitylated EGF receptor cells, treated as indicated, were transfected with His6-Myc-tagged ubiquitin. 24 h after transfection, cells were washed with PBS (containing Mg2+ and Ca2+) at room temperature and lysed for 5 min in urea buffer (20 mm Tris-HCl, pH 7.5, 200 mm NaCl, 10 mm imidazole, 0.1% Triton X-100 in 8 m urea). Cells were scraped, collected, and incubated for 5 min at 37 °C and centrifuged for 5 min at 11,000 × g, after which the supernatant was incubated with 25 μl of prewashed, blocked (1 h at room temperature with 200 μg/ml BSA) TALON beads (Clontech) at room temperature for 1 h while rotating. Beads were washed five times with urea buffer and resuspended in SDS sample buffer. Endogenous, ubiquitylated EGF receptor was detected by Western blotting.
NanoPro Assay
Erk and Akt phosphorylation were measured by the NanoPro 1000 System (Protein Simple) according to the manufacturer's instructions. In short, 48 h after transfection with the indicated siRNA oligonucleotides, cells were stimulated with human EGF (100 ng/ml) and subsequently lysed in Bicine/CHAPS lysis buffer (ProteinSimple; 040-327) supplemented with 1× dimethyl sulfoxide inhibitor mix (ProteinSimple; 040-510) and 1× aqueous inhibitor mix (ProteinSimple; 040-482). Lysates were centrifuged at 20.000 × g for 10 min at 4 °C. Supernatant was loaded in small capillaries (ProteinSimple) together with Ampholyte premix G2 (ProteinSimple; 040-973) and pI standard ladder 3 (ProteinSimple; 040-646). Isoelectric focusing of proteins was performed by applying 21,000 microwatts for 40 min. After focusing, UV light was used to cross-link proteins to the inner capillary wall. After that, the capillary was washed and immunoprobed for the indicated proteins followed by washing to remove unbound antibodies. Finally, luminol and peroxide were added to generate chemiluminescence, which was captured by a CCD camera. Results were analyzed by software (Compass; ProteinSimple).
Peaks, generated using a total anti-Erk antibody, representing phospho-Erk isforms were validated with phospho-specific antibodies against Erk. Percentage phosphorylation of Erk, using a total anti-Erk antibody, was measured by calculating the phospho-peak area as a percentage of total phospho- and non-phospho-peak areas.
RESULTS
The Activated EGF Receptor and Internalized EGF Localize to PACSIN2-positive Early Endosomes
In resting cells, YFP-PACSIN2 dynamically shuttles from peripheral membrane ruffles to intracellular vesiculotubular structures (16). We found that following EGF treatment, YFP-PACSIN2 transiently accumulates on peripheral structures, small tubules as well as vesicles, with a concomitant reduction of PACSIN2 localization at the peripheral membrane (Fig. 1A and supplemental Movie 1). This suggests a functional link between EGF signaling and PACSIN2.
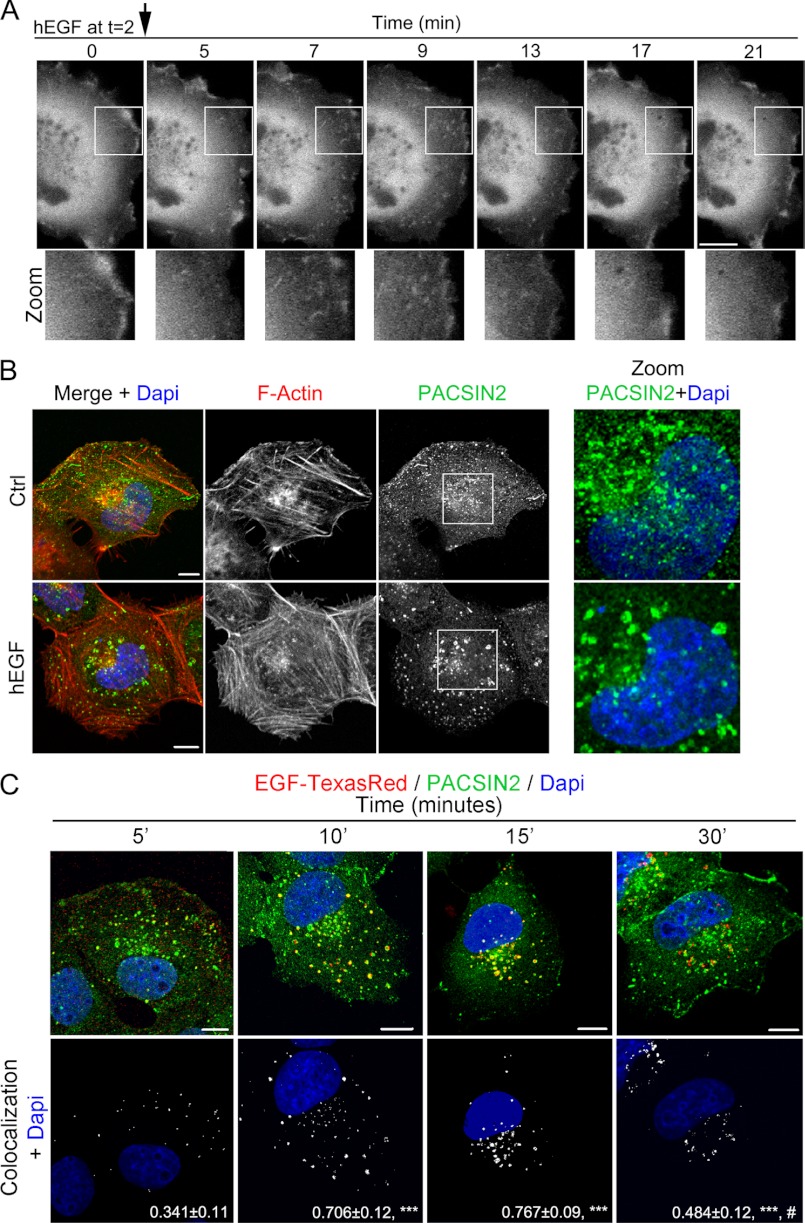
FIGURE 1.
EGF stimulation alters PACSIN2 distribution and is internalized to PACSIN2-positive endosomes. A, live-cell imaging of HeLa cells, transfected with YFP-PACSIN2, shows that PACSIN2 is associated with vesicular-tubular structures upon constitutive internalization in unstimulated cells. EGF (100 ng/ml) stimulation transiently accumulated internalized PACSIN2 in peripheral structures, both small tubules as well as vesicles. (See also supplemental Movie 1). B, intracellular localization of endogenous PACSIN2 in response to EGF (5 min (5'); 100 ng/ml) was studied by confocal microscopy using HeLa cells. In resting cells, PACSIN2 is localized at membrane ruffles partially on vesicular-tubular structures and on perinuclear vesicles. Upon EGF stimulation, a reduction of peripheral tubules was observed together with enlarged PACSIN2-positive perinuclear vesicles. C, EGF-Texas Red (100 ng/ml) transiently translocates upon internalization to PACSIN2-positive endosomes with a peak at 10–15 min. At 30 min (30'), some EGF-Texas Red can still be observed on PACSIN2-positive vesicles. Colocalization images show the amount of EGF-Texas Red colocalized to PACSIN2 structures in white. Numbers depict mean colocalization ± S.E. measured using Zen 2009 software. Scale bars, 10 μm. ***, p < 0.001 in comparison with 5-min time point; #, p < 0.01 in comparison to 15-min time point. Ctrl, control.
To analyze the effects of EGF on PACSIN2 distribution in more detail, we documented endogenous PACSIN2 localization by confocal microscopy. Similar to the data in Fig. 1A, PACSIN2 is in resting cells partially localized on vesiculotubular structures in the cell periphery as well as on perinuclear vesicles, which we previously identified as early endosomes (Fig. 1B, upper panels) (16). Upon EGF stimulation, PACSIN2-positive perinuclear vesicles become slightly enlarged (Fig. 1B, bottom panels and enlarged images). To test whether EGF localizes to the PACSIN2-positive compartment, we analyzed the distribution of internalized Texas Red-labeled EGF (EGF-TR) by confocal microscopy. Five min after its addition, a fraction of internalized EGF-TR localized to PACSIN2-positive endosomes, and this fraction was significantly increased (from 34 to 76%) after 10 or 15 min of EGF-TR internalization (Fig. 1C). At 30 min after addition, we could still find EGF-TR localizing to PACSIN2-positive endosomes, although less than in earlier time points suggesting that after 30 min, EGF-TR leaves the PACSIN2-positive compartment (Fig. 1C). This is in line with the notion that internalized EGF traffics through the early endosomal compartment (where PACSIN2 is present) toward the recycling or late endosomal compartment (10, 20).
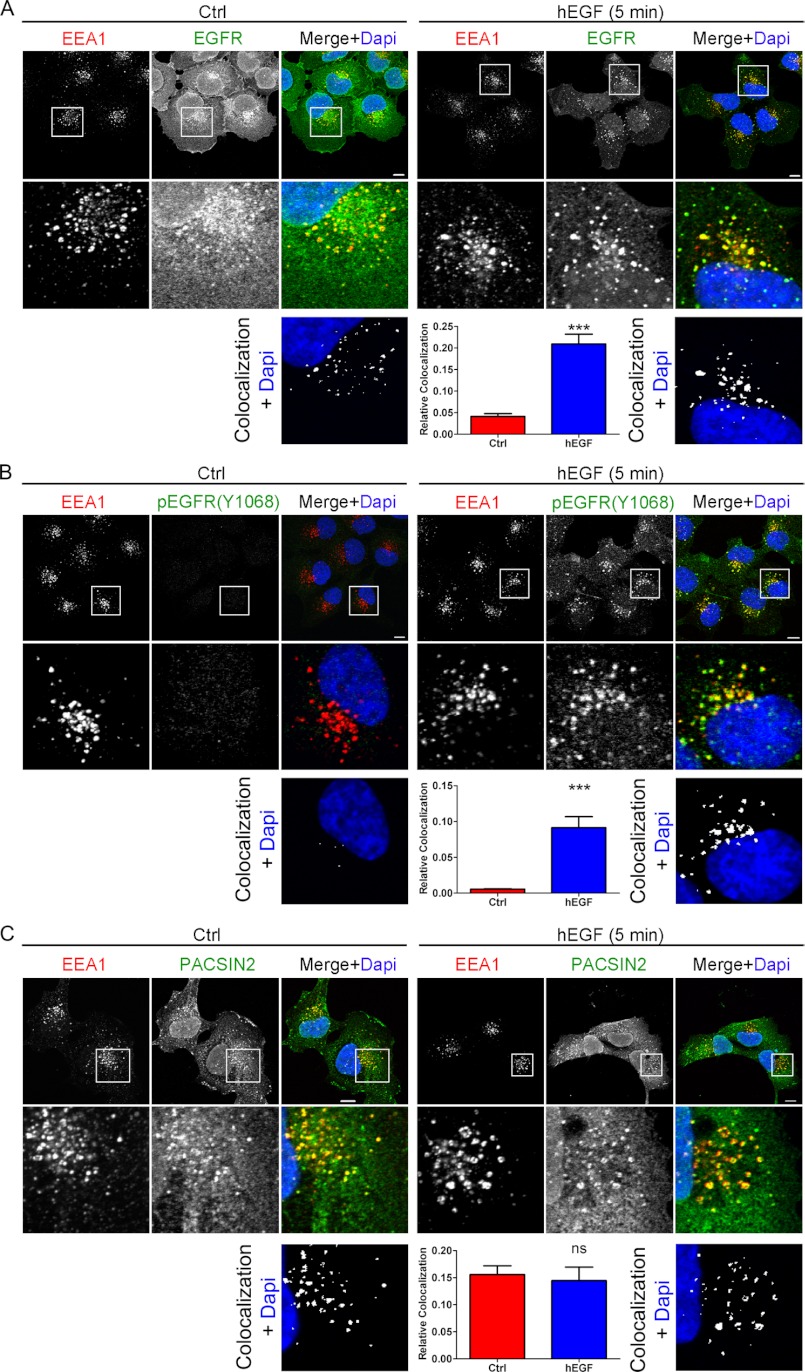
To establish whether receptor activation results in translocation of the receptor to PACSIN2-posive endosomes, we stained unstimulated or EGF-TR stimulated HeLa cells for endogenous PACSIN2. In addition, we visualized tyrosine-phosphorylated proteins using an antibody against phosphotyrosine (pTyr). Stimulation of cells with EGF-TR caused a distinct accumulation of tyrosine-phosphorylated proteins on PACSIN2-positive early endosomes (supplemental Fig. S1). This suggests that the activated EGF receptor is targeted to PACSIN2-positive endosomes. To confirm this, we immunostained control or EGF-stimulated HeLa cells for the EGF receptor as well as specifically for the activated EGF receptor using a phospho-EGFR-specific (Tyr-1068) antibody (21). We could not immunostain simultaneously for the endogenous EGF receptor and endogenous PACSIN2 because the antibodies for detection were from the same species. Because PACSIN2 colocalizes with EEA1 on early endosomes (Fig. 2C) (16), we used EEA1 as an intermediate to analyze colocalization of the (activated) EGF receptor with PACSIN2. EGF stimulation induces accumulation of the (activated) EGF receptor on early endosomes (Fig. 2, A and B) where also PACSIN2 is localized (Fig. 2C). This data is in good agreement with what we showed for internalized EGF-TR (Fig. 1C). Together, these data show that both internalized EGF-TR and the activated EGF receptor accumulate on PACSIN2-positive early endosomes upon EGF stimulation.
FIGURE 2.
The activated EGF receptor accumulates on PACSIN2-positive early endosomes upon EGF stimulation. A–C, using confocal microscopy, colocalization of the EGF receptor (A), phosphorylated EGF receptor at Tyr-1068 (B), and PACSIN2 (C) with EEA1 was analyzed. Endogenous proteins were detected by immunostaining. EGF (5 min; 100 ng/ml) stimulation accumulates the (phosphorylated) EGF receptor on early endosomes (A and B) where PACSIN2 is also present (C). Colocalization plots show the amount of EGF receptor (A), phospho-EGF receptor (B), and PACSIN2 (C) localized to EEA1-positive endosomes in white. Bar diagrams show mean colocalization ± S.E. Scale bars, 10 μm. ***, p < 0.001. Ctrl, control; ns, not significant.
PACSIN2 Regulates EGF Receptor Surface Expression Levels
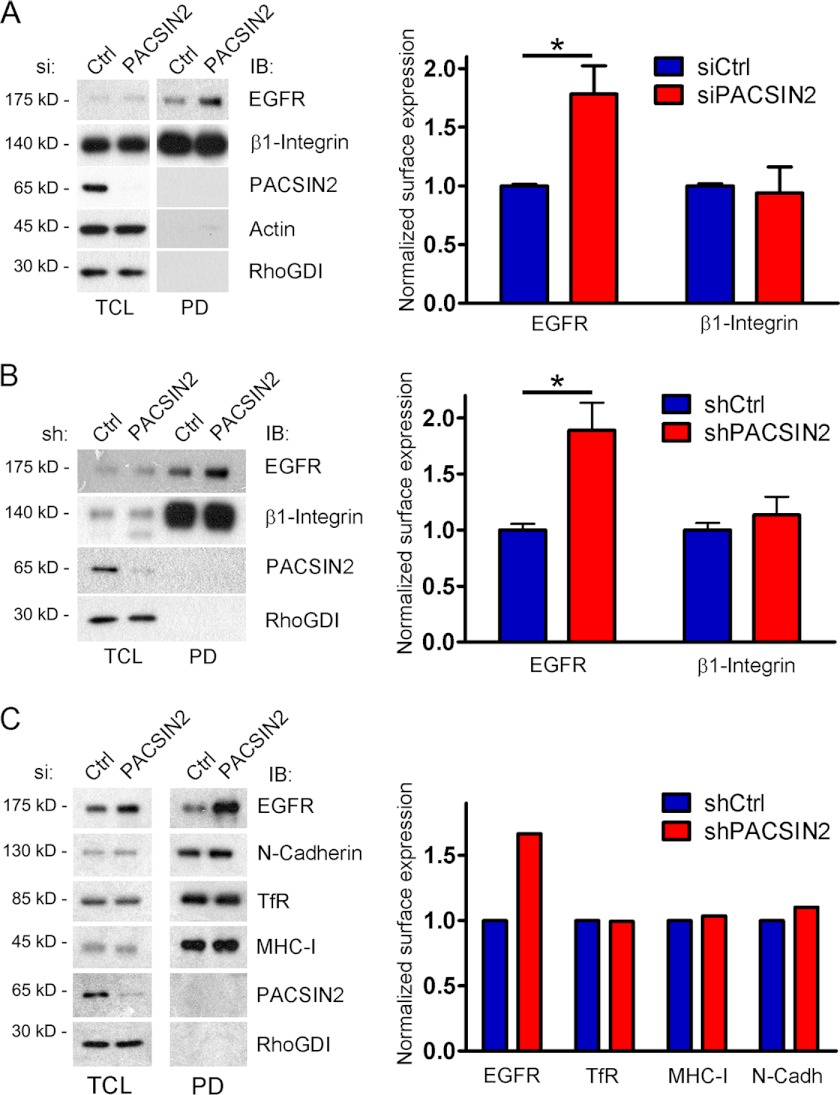
As demonstrated above, both internalized EGF and the EGF receptor localize to a PACSIN2-positive endocytic compartment (Figs. 1 and 2). Because PACSIN2 is an important regulator of membrane dynamics, PACSIN2 may control endocytic traffic of the EGF receptor. We therefore tested whether PACSIN2 regulates surface levels of the EGF receptor. To study this, we performed surface biotinylation experiments (see “Experimental Procedures”). HeLa cells, treated with either siRNA or lentiviral shRNA constructs to reduce PACSIN2 expression, were incubated with sulfo-NHS-LC-biotin at 4 °C to block internalization. Following streptavidin-based pulldowns of cell lysates, we analyzed protein surface expression by Western blotting. To ensure that only surface proteins were isolated, we stained our blots for RhoGDI, which is known to be localized to the cytosol (22). As expected, RhoGDI was not detected in the pulldown fraction (Fig. 3). Interestingly, EGF receptor surface expression showed a marked increase in HeLa cells treated with either PACSIN2-specific siRNA (Fig. 3A) or lentiviral shRNA directed against PACSIN2 (Fig. 3B). Surface levels of β1-integrin, transferrin receptor, MHC-I, and N-cadherin were comparable in PACSIN2 knockdown cells versus control cells (Fig. 3, A–C), indicating that the loss of PACSIN2 does not cause a general increase in surface expression of membrane proteins. These experiments indicate that, in the absence of EGF, PACSIN2 controls the surface levels of the EGF receptor.
FIGURE 3.
Knockdown of PACSIN2 increases EGF receptor surface levels in resting cells. A–C, left panels: surface biotinylation experiments, using HeLa cells, were performed to isolate all surface proteins. Endogenous PACSIN2, EGF receptor, transferrin receptor, N-cadherin (N-Cadh), MHC-I, and β1-integrin were detected by immunoblotting (IB). To ensure that only surface proteins are isolated, RhoGDI, known to be localized to the cytosol, was detected by immunoblotting in conjunction with actin, which, together with RhoGDI, served as a loading control for the PACSIN2 knockdown samples. EGF receptor surface expression showed a marked increase in HeLa cells treated with either PACSIN2-specific siRNA (A and C) or lentiviral shRNA directed against PACSIN2 (B). Surface levels of β1-integrin (A and B), transferrin receptor, N-cadherin, and MHC-I (C) were comparable in PACSIN2 knockdown cells versus control cells. Right panels: quantification of protein surface expression. Values are normalized to the controls. Data are mean values ± S.E. of three independent experiments (A and B) or mean values of two independent experiments (C; variation was <20%). *, p < 0.05. TCL, total cell lysate. PD, pulldown; Ctrl, control.
We next questioned whether PACSIN2 can regulate EGF-mediated internalization of the EGF receptor. HeLa cells treated with control and PACSIN2 siRNA oligonucleotides were incubated with EGF for the indicated time points. We then performed a surface biotinylation experiment as described above and analyzed EGF receptor surface expression by Western blotting. As expected, EGF stimulation down-regulated EGF receptor surface levels in control cells (Fig. 4A; upper and middle panel). Although we observed higher EGF receptor surface levels in PACSIN2 knockdown cells upon EGF stimulation compared with control cells, an EGF-mediated down-regulation of the receptor was still observed (Fig. 4A, upper and middle panels). This could be explained by the fact that under resting conditions, EGF receptor surface levels are already higher in PACSIN2 knockdown cells compared with control cells. These experiments indicate that, although PACSIN2 knockdown cells consistently show higher EGF receptor surface expression, EGF-mediated internalization of the receptor is not impaired.
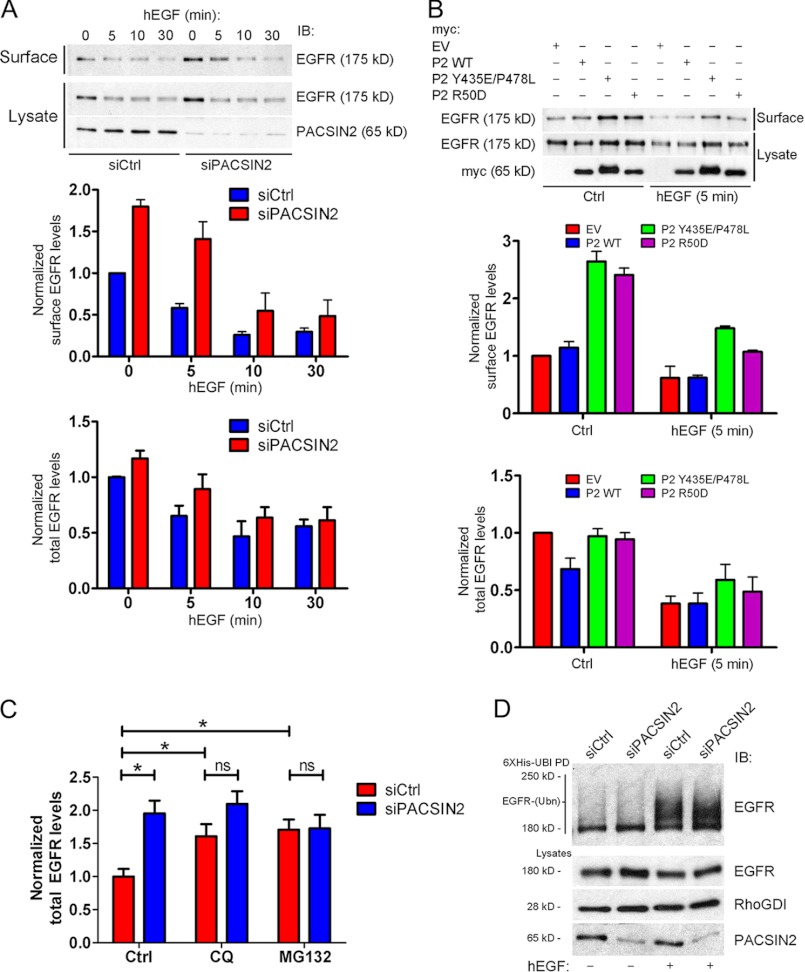
FIGURE 4.
Knockdown of PACSIN2 or expression of dominant-negative PACSIN2 mutants increases EGF receptor surface levels but not EGF-induced internalization. A, upper panel: surface biotinylation experiments, using HeLa cells, were performed to isolate all surface proteins. Endogenous PACSIN2 and EGF receptor were detected by immunoblotting (IB). In non-stimulated cells, knockdown of PACSIN2 increased EGF receptor surface levels. In HeLa cells stimulated with EGF for the indicated time points, depletion of PACSIN2 increased EGF receptor surface levels compared with control cells. However, EGF-mediated internalization is not impaired. A slight increase in total EGF receptor expression was observed as well in PACSIN2 knockdown cells versus control cells. Middle and bottom panels: quantification of EGF receptor surface (middle panel) and total EGF receptor (bottom panel) expression levels. Values are normalized to non-stimulated control cells. Data are mean values ± S.E. of three independent experiments. B, upper panel: surface biotinylation experiments, using HeLa cells transfected as indicated, were performed to isolate all surface proteins. Endogenous EGF receptor and Myc-tagged constructs were detected by immunoblotting. Cells expressing wild-type PACSIN2 (P2-WT) did not show altered surface or total EGF receptor levels. However, an SH3 domain mutant (P2 Y435E/P478L) or a BAR domain mutant (P2 R50D) increased EGF receptor surface levels compared with control cells (EV). In addition, although surface levels of the EGF receptor were increased in cells expressing either mutants compared with control cells (EV), EGF-induced internalization of the EGF receptor was observed. Middle and bottom panel: quantification of EGF receptor surface (middle panel) and total EGF receptor (bottom panel) expression levels. Values are relative to non-stimulated control cells. Data are mean values ± S.E. of three independent experiments. C, siRNA-mediated knockdown of PACSIN2 in HeLa cells results in significantly increased levels of EGF receptor. Inhibition of protein degradation by MG132 (25 μm) or chloroquine (CQ; 80 μm) increases levels of EGF receptor in control cells but not in PACSIN2-depleted cells. As a consequence, upon inhibition of protein degradation, no significant difference in the levels of EGF receptor is observed between control cells and PACSIN2-depleted cells. Values are normalized to those of untreated controls. Data are mean values ± S.E. of three independent experiments. *, p < 0.05; ns, not significant. D, endogenous ubiquitylated EGF receptor was isolated by a cobalt bead-based pulldown assay (see “Experimental Procedures”) from lysates of control cells and HeLa cells transfected with PACSIN2 siRNA co-transfected with His6-Myc-tagged ubiquitin and treated as indicated. Ubiquitylated (EGFR-Ubn) EGF receptor was detected by immunoblot (IB). PACSIN2 depletion does not affect EGF receptor ubiquitylation both in resting conditions and upon EGF stimulation.
We then questioned what the effects were on EGF receptor surface expression of the ectopic expression of wild-type PACSIN2 (P2-WT). In addition, we also expressed an SH3-domain mutant of PACSIN2 (P2-Y435E/P478L), which is impaired in binding to several proteins regulating membrane and cytoskeletal dynamics such as dynamin and Neuronal Wiskott-Aldrich Syndrome Protein (N-WASP) (15, 17). Finally, we also expressed a BAR domain mutant (P2-R50D), which, because of an arginine to aspartic acid mutation at position 50, can no longer bind to membranes and induce vesicular-tubular structures required for proper PACSIN2-mediated membrane dynamics (23, 24). Compared with control cells, expression of wild-type PACSIN2 did not affect EGF receptor surface expression, neither in resting nor in EGF-stimulated cells (Fig. 4B, upper and middle panels). Interestingly, similar to the knockdown experiments, ectopic expression of both the SH3 domain and the BAR domain mutants of PACSIN2 increased the EGF receptor surface levels in unstimulated cells. In addition, although higher surface levels of the EGF receptor were observed after EGF stimulation in cells expressing both PACSIN2 mutants, EGF still induced internalization of EGF receptor (Fig. 4B, upper and middle panels). Despite several attempts, we could find no evidence for complex formation between PACSIN2 and the EGF receptor suggesting that PACSIN2 plays a more generic role in regulating receptor traffic.
We observed a slight increase in total EGF receptor levels in PACSIN2 knockdown cells (Fig. 4, A and C), suggesting that functional inhibition of PACSIN2 results in reduced receptor degradation. As expected, inhibition of protein degradation via the proteasomal pathway (using MG132) or the lysosomal pathway (using chloroquine) resulted in increased levels of EGF receptor in control cells (Fig. 4C). Interestingly, inhibition of protein degradation by MG132 or chloroquine did not affect EGF receptor levels in PACSIN2 knockdown cells (Fig. 4C), suggesting that loss of PACSIN2 already blocks protein degradation. As a consequence, no significant difference in levels of EGF receptor in control cells versus PACSIN2 knockdown cells is observed when protein degradation is inhibited (Fig. 4C).
Next, we analyzed internalization of the EGF receptor upon stimulation with EGF-TR by confocal microscopy. We allowed EGF-TR to internalize in HeLa cells transfected with control or PACSIN2 siRNA for several time points and analyzed colocalization of EGF-TR as well as the EGF receptor itself with early endosomes visualized by EEA1 staining. After 15 min of internalization, we did not find a statistically significant reduction in internalized EGF-TR and EGF receptor to early endosomes in PACSIN2 knockdown cells (supplemental Fig. S2). In addition, at 30 or 90 min following addition of EGF-TR, no major difference was observed, with the exception of a small difference at t = 60 min, indicating that internalization of the EGF receptor upon stimulation with EGF-TR is not impaired in PACSIN2 knockdown cells (supplemental Fig. S2).
Next, we investigated whether PACSIN2-depletion alters ubiquitylation of the EGF receptor in resting conditions and upon EGF stimulation. Little is known about ubiquitylation of the receptor in resting cells. Using a ubiquitin pulldown assay (see “Experimental Procedures”), we showed that PACSIN2 depletion does not alter the ubiquitylation of the EGF receptor in the absence of EGF (Fig. 4D; left two lanes). It is widely accepted that EGF stimulation leads to ubiquitylation of the EGF receptor (9, 10). As expected, we clearly observed increased ubiquitylation of the EGF receptor upon EGF stimulation (Fig. 4D, lane 3). However, as in resting conditions, we did not observe altered ubiquitylation of the EGF receptor in PACSIN2-depleted cells upon EGF stimulation (Fig. 4D, lane 3 and 4). This suggests that PACSIN2 does not alter EGF receptor surface levels by altering the ubiquitylation of the receptor.
In summary, our data indicate that inhibiting PACSIN2 function either by knockdown or by ectoptic expression of dominant-negative mutants, results in increased EGF receptor expression at the plasma membrane. Under these conditions, ligand-induced loss of surface EGF receptor levels remains largely unaffected. Moreover, internalization of EGF-TR is similar in control cells versus PACSIN2-depleted cells, suggesting that PACSIN2 is not required for ligand-induced EGF receptor internalization but regulates ligand-independent receptor internalization. Interestingly, PACSIN2 does not alter ubiquitylation of the EGF receptor. However, as a consequence of the inhibition of ligand-independent internalization of the EGF receptor, loss of PACSIN2 also prevents degradation of the EGF receptor in unstimulated cells.
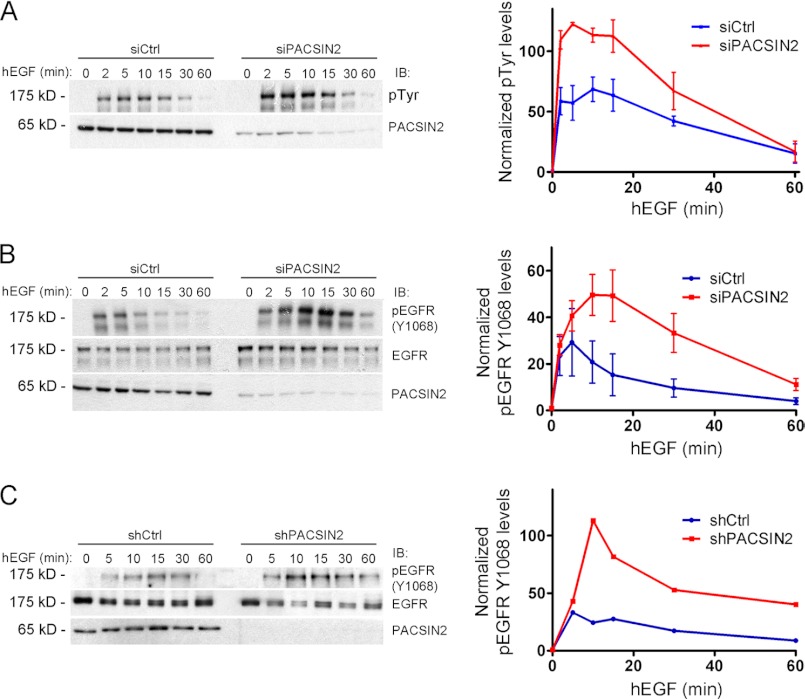
Knockdown of PACSIN2 Increases EGF-mediated Phosphorylation of the EGF Receptor
To determine whether the increased surface levels of the EGF receptor upon loss of PACSIN2 affects cellular responses to EGF, we first examined EGF receptor phosphorylation in control or PACSIN2 knockdown cells. Initially, to visualize EGF receptor phosphorylation, we used an antibody against tyrosine phosphorylated proteins and focused on the 175-kDa activated EGF receptor band. siRNA-mediated knockdown of PACSIN2 clearly increased EGF-mediated phosphorylation of the 175-kDa protein (Fig. 5A). Subsequently, we visualized EGF receptor phosphorylation with an antibody specific for phosphorylation on Tyr-1068. Similar to what we observed in Fig. 5A, siRNA-mediated knockdown of PACSIN2 increased EGF receptor phosphorylation on Tyr-1068 in response to EGF (Fig. 5B). To exclude off-target effects of the PACSIN2 siRNA, we examined EGF receptor phosphorylation in HeLa cells treated with a lentiviral shRNA targeting PACSIN2. In line with the results in Fig. 5B, lentiviral knockdown of PACSIN2 results in increased phosphorylation of the EGF receptor upon EGF stimulation (Fig. 5C). Interestingly, the effect of loss of PACSIN2 on EGF receptor phosphorylation is apparent mainly in the first 20 min because at later time points, EGF receptor phosphorylation declined to control levels (Fig. 5). In contrast, HeLa cells that ectopically express PACSIN2 show a slight reduction in EGF receptor phosphorylation in response to EGF (data not shown). However, this effect is limited, compared with the effects seen in the siRNA experiments, indicating that levels of endogenous PACSIN2 in HeLa cells are sufficient to regulate EGF receptor activation and phosphorylation.
FIGURE 5.
PACSIN2 negatively regulates EGF receptor phosphorylation and activation. A, left panel: HeLa cells, transfected with control (siCtrl) or PACSIN2 (siPACSIN2) siRNA were stimulated with EGF for the indicated time points. Endogenous PACSIN2 and tyrosine-phosphorylated proteins were detected by immunoblotting (IB). Knockdown of PACSIN2 enhanced EGF-mediated phosphorylation of the 175-kDa band, representing the EGF receptor, within the first 20 min. After 30–60 min, pTyr (at 175 kDa) levels declined to control levels. Right panel: graph shows the increase in phosphorylation relative to resting cells. Data are mean values of three independent experiments. Error bars indicate S.E. (B and C). Left panels: HeLa cells treated with either PACSIN2-specific siRNA (B) or lentiviral shRNA directed against PACSIN2 (C) were stimulated with EGF for the indicated time points. Endogenous PACSIN2, total EGF receptor, and phospho-EGF receptor (Tyr-1068) were detected by immunoblotting (IB). Knockdown of PACSIN2 enhanced EGF-mediated phosphorylation of the EGF receptor at Tyr-1068 within the initial 20 min. After 30–60 min, phospho-EGF (Tyr-1068) receptor levels declined to control levels. Right panels: Graph shows increase in phosphorylation relative to resting cells and normalized to total EGF receptor levels. Data are mean values of two (C) or three (B) independent experiments. Error bars indicate S.E. hEGF, human EGF.
These data show that PACSIN2, by controlling EGF receptor surface levels, regulates receptor activation and phosphorylation. The marked increase in EGF-mediated receptor phosphorylation observed in PACSIN2 knockdown cells reveals an important, previously unnoticed, role for PACSIN2 in regulating EGF receptor activation.
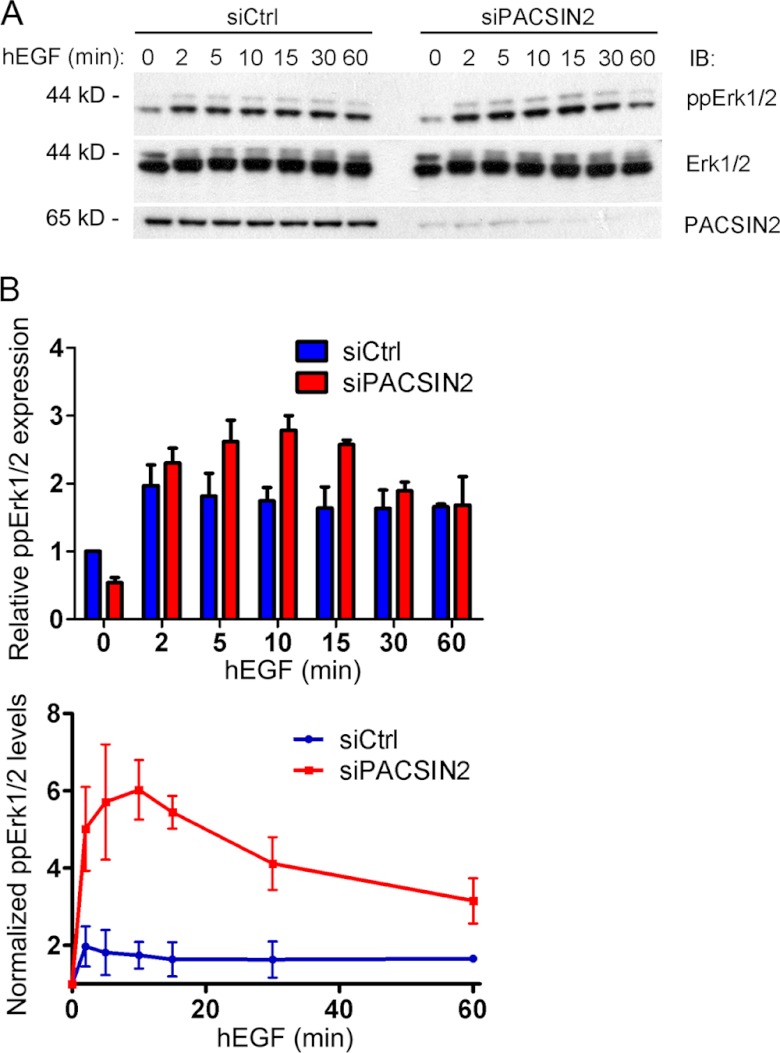
PACSIN2 Negatively Regulates Signaling Downstream of the EGF Receptor
EGF engagement leads to activation of the EGF receptor and of several downstream intracellular signaling pathways, such as the Erk and Akt pathways (25, 26). As we observed a marked increase in EGF receptor phosphorylation in PACSIN2 knockdown cells, we decided to study whether loss of PACSIN2 affects signaling downstream of the EGF receptor as well. Therefore, we analyzed EGF-mediated Erk phosphorylation in HeLa cells transfected with the PACSIN2 siRNA. Interestingly, in resting cells, loss of PACSIN2 results in a slight decrease in the levels of phosphorylated Erk1/2. In contrast, upon EGF stimulation, we observed an increase in Erk phosphorylation in PACSIN2 knockdown cells compared with control cells (Fig. 6, A and B). In combination, these changes result in enhanced Erk1/2 phosphorylation in response to EGF in PACSIN2 knockdown cells (Fig. 6B, bottom panel).
FIGURE 6.
PACSIN2 negatively regulates Erk phosphorylation downstream of EGF. A, HeLa cells treated with control or PACSIN2-specific siRNA were stimulated with EGF (100 ng/ml) for the indicated time points. Endogenous PACSIN2, Erk1/2, and phospho-Erk1/2 were detected by immunoblotting (IB). PACSIN2 depletion enhances EGF-mediated Erk1/2 phosphorylation compared with control cells within the initial 20 min. After 30–60 min, phospho-Erk1/2 levels declined to control (Ctrl) levels. B, upper panel: phospho-Erk1/2 levels relative to total Erk for each time point are shown. Data are normalized to basal levels of phospho-Erk1/2 in control cells. Mean values of three independent experiments are shown. Error bars indicate S.E. Bottom panel: EGF-mediated induction of Erk1/2 phosphorylation in control versus PACSIN2-depleted cells is shown. Data are relative to total Erk1/2 levels and normalized to basal levels of phospho-Erk1/2. Mean values of three independent experiments. Error bars indicate S.E. hEGF, human EGF.
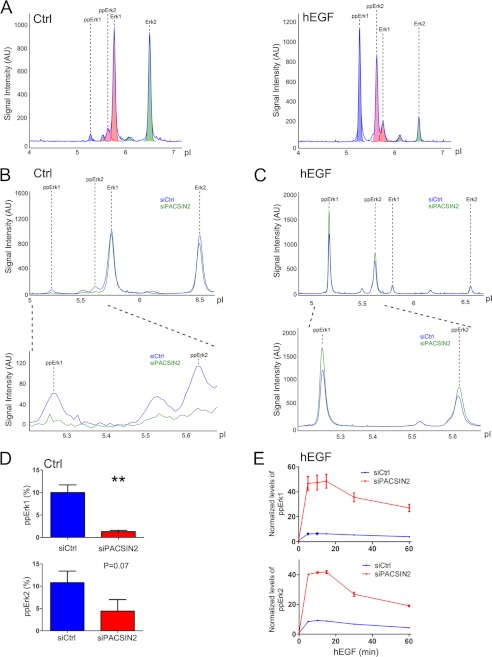
To study Erk phosphorylation in a more quantitative manner, we used a NanoPro Assay (see “Experimental Procedures”). Here, levels of (un)phosphorylated Erk are quantitatively measured by separation of proteins based on their pI, followed by immunodetection. As shown in Fig. 7A, for each Erk isoform, both the unphosphorylated and phosphorylated species, a peak is generated and the size of the peak (measured by the area under the peak) represents the (phospho)-protein levels. Using this approach, it is possible to study in a quantitative manner the amount of Erk phosphorylation for both isoforms separately but in the same samples. Fig. 7A (left panel) shows that in resting cells, the majority of Erk is in its unphosphorylated state. However, upon EGF stimulation, a shift toward the phosphorylated state can be clearly observed (Fig. 7A; right panel). Using this technique, we analyzed the effects of loss of PACSIN2 on EGF-mediated Erk phosphorylation. Similar to the Western blotting results, we observed decreased levels of Erk phosphorylation in resting, PACSIN2-depleted cells (Fig. 7, B and D). In contrast, upon EGF stimulation, Erk phosphorylation was increased in cells treated with PACSIN2 siRNA (Fig. 7C), resulting in enhanced Erk phosphorylation in response to EGF in PACSIN2-depleted cells compared with control cells (Fig. 7E).
FIGURE 7.
PACSIN2 negatively regulates EGF-mediated signaling. A, the NanoPro Assay was used to quantitatively measure Erk phosphorylation. Plot shows (un)phosphorylated ERK species as as indicated. Peak area is a measure for protein levels. In resting cells, unphosphorylated Erk1 and Erk2 are dominant (left panel). EGF stimulation induces Erk phosphorylation, which is visualized as increased levels of validated phospho-ERK peaks, focused at more acidic pI (right panel). B, visualization of levels of Erk1/2 and phospho-Erk1/2 in unstimulated cells transfected with control siRNA (siCtrl, blue) and PACSIN2 siRNA (siPACSIN2, green). C, levels of Erk1/2 and phospho-Erk1/2 in EGF-stimulated (5 min) cells transfected with control siRNA (siCtrl, blue) and PACSIN2 siRNA (siPACSIN2, green). In response to EGF, PACSIN2 depletion increased Erk1/2 phosphorylation compared with control cells. D, ppErk1/2 levels in unstimulated cells, transfected with control siRNA (siCtrl, blue) and PACSIN2 siRNA (siPACSIN2, red) were quantified. Data are mean values of three independent experiments. Error bars indicate S.E. **, p < 0.01. E, PACSIN2 knockdown enhanced EGF-mediated phosphorylation of phospho-Erk1/2 compared with control cells. Data are normalized to basal phospho-Erk1/2 levels. Graphs shown are representative for three independent experiments. Error bars indicate S.E. hEGF, human EGF. AU, Arbitrary Unit.
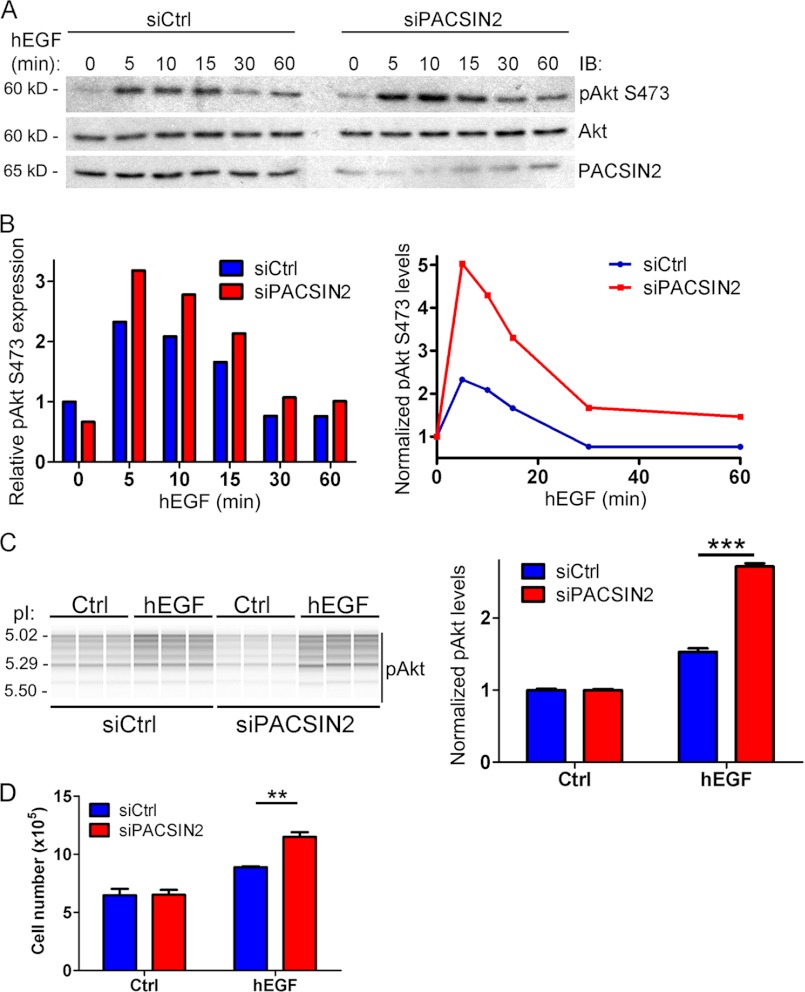
In addition to Erk phosphorylation, we analyzed whether loss of PACSIN2 affects EGF-induced Akt phosphorylation as well. In unstimulated cells, levels of phosphorylated Akt, relative to those of total Akt in those samples are slightly reduced as deduced from the quantification of the Western blots (Fig. 8, A and B). This effect can be more clearly seen in the Nanopro analysis (Fig. 8C, left panel). However, upon stimulation with EGF, Akt phosphorylation is increased in PACSIN2 knockdown cells compared with control cells (Fig. 8, A and B), resulting in enhanced EGF-mediated Akt phosphorylation in PACSIN2 knockdown cells. These findings are similar to what we observed for Erk phosphorylation.
FIGURE 8.
PACSIN2 negatively regulates Akt phosphorylation downstream of EGF. A, HeLa cells treated with control or PACSIN2-specific siRNA were stimulated with EGF (100 ng/ml) for the indicated time points. Endogenous PACSIN2, Akt, and phospho-Akt (Ser-473) were detected by immunoblotting (IB). Knockdown of PACSIN2 increased Akt phoshporylation downstream of EGF compared with control cells within the initial 20 min. After 30–60 min, phospho-Akt levels declined to control levels. B, left panel: phospho-Akt (Ser-473) levels relative to total Akt for each time point are shown. Data are normalized to basal levels of phospho-Akt in control cells. Mean values of two independent experiments are shown. Right panel: EGF-mediated induction of Akt phosphorylation in control versus PACSIN2-depleted cells is shown. Data are relative to total Akt levels and normalized to basal levels of phospho-Akt (Ser-473). Mean values of two independent experiments are shown. C, Akt phosphorylation measured by NanoPro Assay. Left panel: control (Ctrl) or PACSIN2 siRNA treated HeLa cells were analyzed in triplicate for Akt phosphorylation using the NanoPro Assay. Peaks generated are transformed in a Western blot-like representation of the data (Compass Software; ProteinSimple). All peaks are represented as bands, and band intensity indicates phospho-Akt protein levels. PACSIN2 depletion decreased basal phospho-Akt levels. Upon EGF stimulation, enhanced Akt phosphorylation is observed in PACSIN2-depleted cells compared with control cells. Right panel: quantification of Akt phosphorylation shows increased phospho-Akt levels in PACSIN2-depleted cells in response to EGF compared with control cells. Bar diagram shows levels of phospho-Akt normalized to levels in unstimulated cells. Mean values of Akt phosphorylation are shown. Error bars indicate S.E. ***, p < 0.001. D, EGF-mediated cell growth was analyzed in control cells (siCtrl) compared with PACSIN2-depleted cells (siPACSIN2). 48 h after siRNA transfection, HeLa cells were incubated with EGF (100 ng/ml). After 24 h, cells were counted. Cell growth was significantly enhanced in PACSIN2-depleted cells in response to EGF but not in resting cells. Data are mean values of three independent experiments. Error bars indicate S.E. **, p < 0.01. hEGF, human EGF.
Next, we analyzed Akt phosphorylation using the NanoPro Assay. In contrast to Erk, the peak pattern for Akt phosphorylation comprises a series of different Akt phospho-species. Therefore, we determined Akt phosphorylation based on the combined signal generated upon detection with a phospho-specific Akt antibody. The NanoPro 1000 system is capable of generating a Western blot-like representation of the data (Fig. 8C, left panel), which gives an indication of the level of Akt phosphorylation. Similar to our Western blotting results, it is clear that loss of PACSIN2 results in increased Akt phosphorylation in response to EGF (Fig. 8C, right panel). These experiments show that PACSIN2 is an important regulator of EGF-mediated signaling as knockdown of PACSIN2 results in enhanced phosphorylation of Erk and Akt in response to EGF.
EGF receptor signaling is involved in various processes, including cell growth. Therefore, we analyzed the role of PACSIN2 in EGF-mediated cell growth. HeLa cells treated with control or PACSIN2-specific siRNA oligonucleotides were stimulated with EGF. 24 h after addition of EGF, cell numbers were determined in the different conditions. Loss of PACSIN2 has no effect on proliferation of cells that were not stimulated with EGF. However, EGF stimulation did lead to a significant increase in cell number in PACSIN2 knockdown cells compared with control cells (Fig. 8D).
Together, these data show that, by regulating EGF receptor traffic/internalization, PACSIN2 plays a key role in the activation and downstream signaling of the EGF receptor. EGF-mediated activation and phosphorylation of the EGF receptor as well as of its downstream targets, Erk and Akt, is enhanced in PACSIN2 knockdown cells. As a result, loss of PACSIN2 promotes EGF-induced cell growth.
PACSIN2 Regulates Signaling Downstream of HGF and TNFα
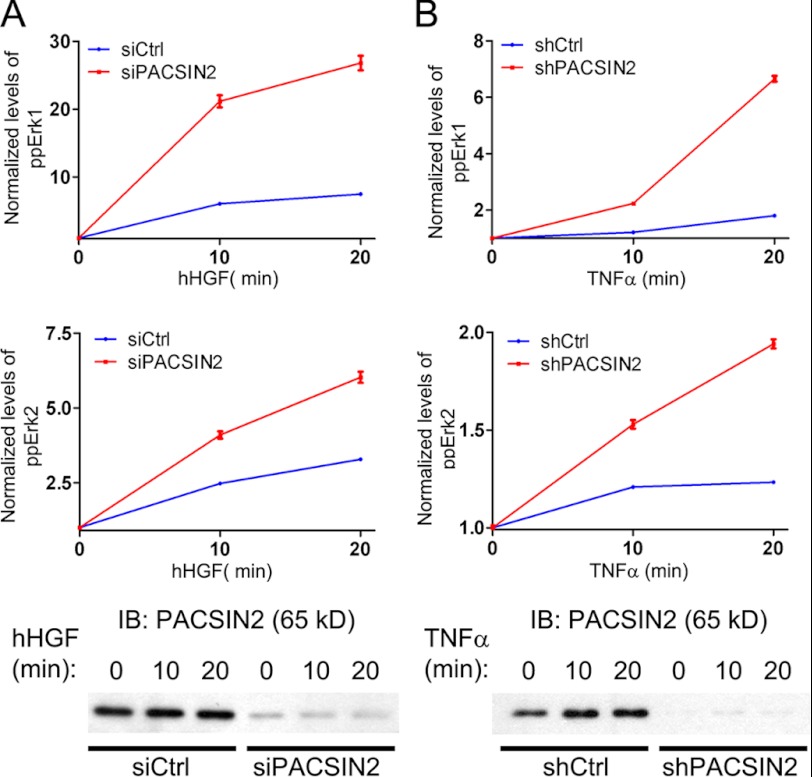
So far, we have shown that PACSIN2 is an important regulator of EGF receptor signaling. We questioned whether the regulatory role of PACSIN2 is specific for the EGF receptor or that PACSIN2 regulates signaling downstream of other growth factor receptors as well. To study this, we investigated whether PACSIN2 can regulate signaling downstream of the HGF receptor, which is known to activate the MAPK pathway (27). HeLa cells, treated with control or PACSIN2-specific siRNA, were stimulated with HGF. Using the NanoPro Assay, we analyzed Erk phosphorylation and observed that similar to what we have shown for EGF, enhanced phosphorylation of Erk in response to HGF (Fig. 9A). Next, we questioned whether the role for PACSIN2 is cell type-specific. Therefore, we analyzed Erk phosphorylation in primary human endothelial cells in response to TNFα, known to activate the MAPK pathway (28). HUVEC, treated with control or PACSIN2-specific lentiviral shRNA constructs, were stimulated with TNFα. Subsequently, Erk phosphorylation was measured using the NanoPro Assay. Phosphorylation of Erk, induced by TNFα (Fig. 9B), showed a marked increase in cells transduced with the PACSIN2 shRNA, compared with control cells. A similar trend was observed for HUVEC treated with VEGF (data not shown).
FIGURE 9.
PACSIN2 is a generic regulator of growth factor signaling. A and B, using the NanoPro 1000 Assay, Erk phosphorylation was measured downstream of HGF in HeLa cells and downstream of TNFα in HUVECs. Upper and middle panels: knockdown of PACSIN2 enhanced Erk1 (upper panels) and Erk2 (middle panels) phosphorylation downstream of human HGF (hHGF; A), TNFα (B) compared with control cells. Bottom panels: immunoblotting (IB) was performed to assess siRNA-mediated knockdown of PACSIN2 in HeLa cells (A) and shRNA-mediated knockdown of PACSIN2 in HUVECs (B). Graphs are representative for three independent experiments. Data are normalized to basal phospho-Erk levels. Error bars indicate S.E.
Collectively, these data show that PACSIN2 regulates signaling downstream of EGF and HGF in epithelial cells and downstream of TNFα in endothelial cells. Loss of PACSIN2 results in an increase in Erk activation downstream of both EGF and HGF in epithelial cells and of TNFα in endothelial cells suggesting that the regulatory role of PACSIN2 in growth factor signaling is more general. However, further studies are needed to determine whether PACSIN2 regulates signaling downstream of HGF and TNFα in a similar fashion as to what we have shown for EGF.
DISCUSSION
BAR domains are found in proteins that are important regulators of membrane dynamics and vesicular traffic. Several BAR domain proteins have been implicated in regulating growth factor signaling. The BAR domain protein endophilin regulates, in conjunction with Cbl and CIN85, EGF receptor internalization. Inhibition of this complex, e.g. via Alix/AIP1, impairs proper receptor endocytosis thereby preventing signal termination with increased signaling as a result (13, 29). These studies underscored the importance of receptor internalization in controlling signaling output. In addition to internalization, receptor sorting toward the lysosomal pathway with receptor degradation as a consequence represents an alternative mechanism to terminate growth factor signaling. Several BAR domain proteins have been shown to regulate the sorting of the EGF receptor. SNXs (sorting nexins), of which several comprise a BAR domain, have emerged as a group of proteins, associated with endosomal compartments, that regulate receptor trafficking (30, 31). SNX1 and SNX5 associate with each other via their BAR domain. Interestingly, whereas SNX5 prevents EGF receptor degradation, SNX1 promotes degradation of the EGF receptor and attenuates the effect of SNX5 (32, 33).
To avoid aberrant growth factor signaling, with severe diseases as a potential outcome, efficient signal termination is of great importance. Upon growth factor receptor activation, the receptor is internalized, and subsequent post-endocytic traffic targets the receptor either for degradation or for recycling back to the plasma membrane. The increased surface EGF receptor levels in unstimulated PACSIN2 knockdown cells could be due to decreased internalization or increased receptor recycling. Although we could not find clear evidence that PACSIN2 regulates ligand-dependent internalization of the EGF receptor, our data do suggest that PACSIN2 is involved in ligand-independent internalization of the EGF receptor. The data in Figs. 1 and 2 of this work show that EGF stimulation affects the localization of PACSIN2 and that ligand-induced, internalized EGFR shows a partial colocalization with PACSIN2. Moreover, the data in Fig. 2A show that also in unstimulated cells, there is limited but detectable colocalization of PACSIN2 with the EGFR. This supports our subsequent findings that loss of PACSIN2 affects EGFR surface levels and traffic in a ligand-independent fashion.
The mechanisms that control ligand-independent, constitutive receptor internalization is not well understood, as most studies focused on ligand-induced down-regulation of growth factor receptors. However, it was shown that expression of dominant active Rab5 (Q79L) caused ligand-independent internalization of the EGF receptor thereby decreasing surface EGF receptor levels. As a result, upon EGF stimulation, less receptor activation and downstream signaling was observed (34). Interestingly, we have previously shown that PACSIN2 is localized to Rab5-positive endosomes (16). Because Rab5Q79L accumulates PACSIN2 on enlarged Rab5Q79L-positive endosomes (supplemental Fig. S3), it could well be that PACSIN2 functions in the same pathway regulating ligand-independent internalization of the EGF receptor. In accordance, we show that loss of PACSIN2 results in increased EGF receptor surface levels and subsequently increased EGF-mediated signaling.
A related aspect that remains to be addressed concerns the effects of PACSIN2 on expression and surface levels of other members of the HER family, i.e. HER2–4. These can form homodimers as well as heterodimers, the composition of which affects receptor internalization and recycling. Several studies have shown that expression of HER2 increases the surface expression of the EGFR, resulting in enhanced EGF-induced signaling (35–37). This is reminiscent of our current data and warrants further research into a link between PACSIN2, the expression of HER2, and a potential role in PACSIN2-mediated regulation of heterodimerization with consequent effects on EGFR traffic. In contrast to the EGFR, however, there is currently no published literature on regulation of HER2 by BAR domain proteins.
Recent studies have revealed distinct internalization pathways depending on EGF concentrations. High (100 ng/ml) concentrations of EGF lead to clathrin-independent/lipid raft-dependent internalization of the EGF receptor, whereas low (1 ng/ml) EGF concentrations lead to clathrin-dependent internalization (8). Interestingly, some studies showed that raft-mediated internalization is associated with EGF receptor degradation, whereas clathrin-mediated internalization is associated with sustained signaling (7). We previously found evidence that PACSIN2 could function in raft-mediated endocytosis as we found cholera toxin B to be internalized via PACSIN2-positive tubular structures (16). Thus, it could well be that depletion of PACSIN2 shifts internalization of the EGF receptor to a clathrin-dependent pathway with increased signaling as a result. Moreover, reduced internalization via a raft-dependent pathway would also result in less receptor degradation, which is in line with what we observed in PACSIN2-depleted cells. However, this topic requires further investigation as some studies have implicated PACSIN proteins in clathrin-mediated internalization as well (38, 39).
In addition to internalization, an alternative mechanism that controls receptor surface levels is recycling. Similar to the F-BAR protein CIP4 (14), PACSIN2 could regulate receptor surface expression by regulating receptor sorting. Upon ligand-(in)dependent internalization, part of the receptor is targeted for degradation, whereas the remainder recycles back to the plasma membrane (9, 10). Receptors destined for degradation travel via early endosomes to multivesicular endosomes/bodies and are eventually degraded in lysosomes. The Endosomal Sorting Complex Required for Transport (ESCRT) protein Tsg101 is involved in the formation of these multivesicular endosomes/bodies (40). Upon depletion of Tsg101, EGF receptor degradation was impaired, and consequently, increased EGF-mediated receptor phosphorylation was observed (21). Similarly, preventing EGF receptor degradation by the pharmacological inhibitor monensin, which blocks acidification of early endosomes and thereby formation of lysosomes (41), resulted in accumulation of the EGF receptor on early endosomes and enhanced receptor phosphorylation (21). In line with these studies, we observe less degradation and increased ERK activation when PACSIN2 is depleted. However, in these published studies, the EGF receptor is retained on early endosomes, causing increased signaling. In contrast, we did not find clear evidence that PACSIN2 depletion retains the EGF receptor on early endosomes. Thus, our data show that PACSIN2 regulates EGF receptor internalization in resting cells and acts upstream of proteasomal or lysosomal degradation pathways.
Although our data suggest PACSIN2 to be most relevant for ligand-independent traffic of the EGFR, it cannot be excluded that PACSIN2 acts by modulating any of the previously proposed pathways or regulatory proteins that control EGFR internalization and endosomal traffic. These include several protein kinases, such as PKC (42), ERK (4, 43), and the leucine-rich repeat kinase LRRK1 (44). The PACSIN-binding protein SPIN90 is regulated by ERK and colocalizes with internalized EGF on early endosomes (45). Whether SPIN90, in conjunction with PACSIN2, also is involved in ligand-independent traffic of the EGF receptor remains to be investigated. In addition, several protein phosphatases, including SH2-containing 5′-inositol phosphatase, SHIP2, have been implicated in EGFR internalization (46, 47). Establishing a functional connection between these kinase and phosphatase pathways, PACSIN2, and the EGF receptor warrants future research.
An interesting additional finding of this study was that the regulatory role of PACSIN2 is not specific for the EGF receptor. Depletion of PACSIN2 enhanced Erk activation downstream of EGF and HGF in epithelial cells but also downstream TNFα in endothelial cells, suggesting a generic role for PACSIN2 in growth factor receptor signaling.
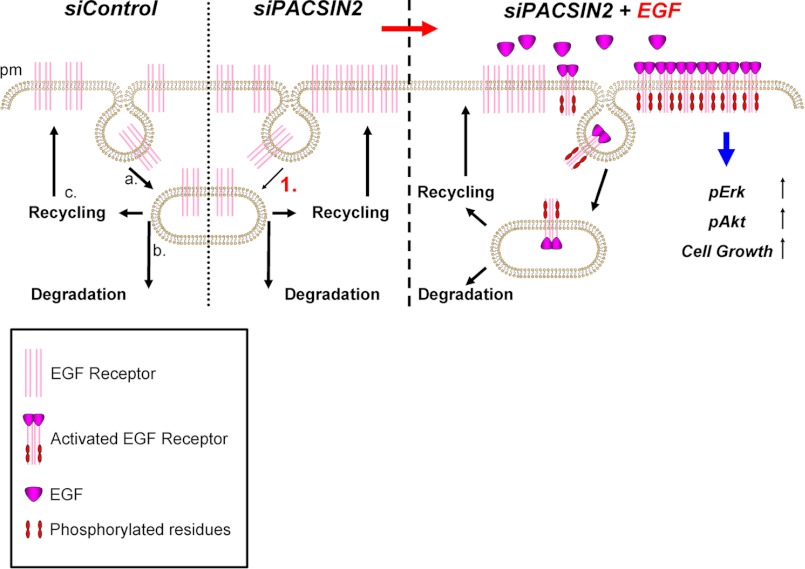
In conclusion, our data suggest the following model (Fig. 10). In unstimulated cells, constitutive internalization of the growth factor receptor takes place (Fig. 10a). Subsequently, the receptor becomes partially degraded (Fig. 10b) but the main fraction recycles back to the plasma membrane (Fig. 10c). When PACSIN2 is depleted, growth factor receptors at the surface accumulate by inhibition of ligand-independent internalization (1). As a result of increased surface receptor levels in PACSIN2-depleted cells, ligand-induced growth factor stimulation increases activation of the receptor and consequent downstream signaling toward ERK, Akt and, ultimately, cell growth.
FIGURE 10.
PACSIN2 regulates growth factor receptor surface expression, thereby controlling receptor output. In control cells, constitutive internalization (a) of the growth factor receptor occurs. Upon internalization, a fraction of the receptor is targeted for degradation (b) while the remainder recycles back to the plasma membrane (c). In PACSIN2-depleted cells, increased growth factor receptor surface levels are observed as a consequence of reduced ligand-independent endocytosis (1). As a result of increased surface receptor levels, in cells depleted for PACSIN2, growth factor stimulation promotes receptor activation and downstream signaling (indicated by the blue arrow).
Acknowledgments
The NanoPro 1000 System (ProteinSimple) in the Hordijk laboratory was enabled through a “middelgroot” investment grant (40-00506-98-10013) from the Netherlands Organization for Scientific Research. We thank Dr. M. Fernandez-Borja for stimulating discussions.

This article contains supplemental Figs. S1–S3 and Movie 1.
- BAR
- Bin/amphiphysin/Rvs
- SH3
- Src homology 3
- EGFR
- EGF receptor
- CIP4
- Cdc42-interacting protein 4
- EEA1
- early endosomal antigen 1
- EGF-TR
- EGF-Texas Red
- HGF
- hepatocyte growth factor
- HUVEC
- human umbilical vein endothelial cell(s)
- PACSIN2
- protein kinase and casein kinase substrate in neurons 2
- Bicine
- N,N-bis(2-hydroxyethyl)glycine.
REFERENCES
- 1. van der Geer P., Hunter T., Lindberg R. A. (1994) Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell Biol. 10, 251–337 [DOI] [PubMed] [Google Scholar]
- 2. Blume-Jensen P., Hunter T. (2001) Oncogenic kinase signalling. Nature 411, 355–365 [DOI] [PubMed] [Google Scholar]
- 3. Bublil E. M., Yarden Y. (2007) The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr. Opin. Cell Biol. 19, 124–134 [DOI] [PubMed] [Google Scholar]
- 4. Gan Y., Shi C., Inge L., Hibner M., Balducci J., Huang Y. (2010) Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29, 4947–4958 [DOI] [PubMed] [Google Scholar]
- 5. Herbst J. J., Opresko L. K., Walsh B. J., Lauffenburger D. A., Wiley H. S. (1994) Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J. Biol. Chem. 269, 12865–12873 [PubMed] [Google Scholar]
- 6. Vieira A. V., Lamaze C., Schmid S. L. (1996) Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086–2089 [DOI] [PubMed] [Google Scholar]
- 7. Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P. P. (2008) Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell 15, 209–219 [DOI] [PubMed] [Google Scholar]
- 8. Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiley H. S. (2003) Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 284, 78–88 [DOI] [PubMed] [Google Scholar]
- 10. Sorkin A., Goh L. K. (2008) Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 314, 3093–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsujita K., Suetsugu S., Sasaki N., Furutani M., Oikawa T., Takenawa T. (2006) Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 172, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frost A., Unger V. M., De Camilli P. (2009) The BAR domain superfamily: membrane-molding macromolecules. Cell 137, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soubeyran P., Kowanetz K., Szymkiewicz I., Langdon W. Y., Dikic I. (2002) Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183–187 [DOI] [PubMed] [Google Scholar]
- 14. Hu J., Troglio F., Mukhopadhyay A., Everingham S., Kwok E., Scita G., Craig A. W. (2009) F-BAR-containing adaptor CIP4 localizes to early endosomes and regulates Epidermal Growth Factor Receptor trafficking and downregulation. Cell Signal. 21, 1686–1697 [DOI] [PubMed] [Google Scholar]
- 15. Chitu V., Stanley E. R. (2007) Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol. 17, 145–156 [DOI] [PubMed] [Google Scholar]
- 16. de Kreuk B. J., Nethe M., Fernandez-Borja M., Anthony E. C., Hensbergen P. J., Deelder A. M., Plomann M., Hordijk P. L. (2011) The F-BAR domain protein PACSIN2 associates with Rac1 and regulates cell spreading and migration. J. Cell Sci. 124, 2375–2388 [DOI] [PubMed] [Google Scholar]
- 17. Kessels M. M., Qualmann B. (2004) The syndapin protein family: linking membrane trafficking with the cytoskeleton. J. Cell Sci. 117, 3077–3086 [DOI] [PubMed] [Google Scholar]
- 18. Kessels M. M., Qualmann B. (2006) Syndapin oligomers interconnect the machineries for endocytic vesicle formation and actin polymerization. J. Biol. Chem. 281, 13285–13299 [DOI] [PubMed] [Google Scholar]
- 19. Nethe M., Anthony E. C., Fernandez-Borja M., Dee R., Geerts D., Hensbergen P. J., Deelder A. M., Schmidt G., Hordijk P. L. (2010) Focal-adhesion targeting links caveolin-1 to a Rac1-degradation pathway. J. Cell Sci. 123, 1948–1958 [DOI] [PubMed] [Google Scholar]
- 20. Haglund K., Dikic I. (2012) The role of ubiquitylation in receptor endocytosis and endosomal sorting. J. Cell Sci. 125, 265–275 [DOI] [PubMed] [Google Scholar]
- 21. Rush J. S., Quinalty L. M., Engelman L., Sherry D. M., Ceresa B. P. (2012) Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J. Biol. Chem. 287, 712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olofsson B. (1999) Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 11, 545–554 [DOI] [PubMed] [Google Scholar]
- 23. Shimada A., Takano K., Shirouzu M., Hanawa-Suetsugu K., Terada T., Toyooka K., Umehara T., Yamamoto M., Yokoyama S., Suetsugu S. (2010) Mapping of the basic amino-acid residues responsible for tubulation and cellular protrusion by the EFC/F-BAR domain of pacsin2/Syndapin II. FEBS Lett. 584, 1111–1118 [DOI] [PubMed] [Google Scholar]
- 24. Wang Q., Navarro M. V., Peng G., Molinelli E., Goh S. L., Judson B. L., Rajashankar K. R., Sondermann H. (2009) Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc. Natl. Acad. Sci. U.S.A. 106, 12700–12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grant S. L., Hammacher A., Douglas A. M., Goss G. A., Mansfield R. K., Heath J. K., Begley C. G. (2002) An unexpected biochemical and functional interaction between gp130 and the EGF receptor family in breast cancer cells. Oncogene 21, 460–474 [DOI] [PubMed] [Google Scholar]
- 26. Wells A. (1999) EGF receptor. Int. J. Biochem. Cell Biol. 31, 637–643 [DOI] [PubMed] [Google Scholar]
- 27. Liu Z. X., Yu C. F., Nickel C., Thomas S., Cantley L. G. (2002) Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin-focal adhesion kinase association. J. Biol. Chem. 277, 10452–10458 [DOI] [PubMed] [Google Scholar]
- 28. Mechtcheriakova D., Schabbauer G., Lucerna M., Clauss M., De Martin R., Binder B. R., Hofer E. (2001) Specificity, diversity, and convergence in VEGF and TNF-α signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 15, 230–242 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt M. H., Hoeller D., Yu J., Furnari F. B., Cavenee W. K., Dikic I., Bögler O. (2004) Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol. Cell Biol. 24, 8981–8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Weering J. R., Verkade P., Cullen P. J. (2010) SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin. Cell Dev. Biol. 21, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Worby C. A., Dixon J. E. (2002) Sorting out the cellular functions of sorting nexins. Nat. Rev. Mol. Cell Biol. 3, 919–931 [DOI] [PubMed] [Google Scholar]
- 32. Kurten R. C., Cadena D. L., Gill G. N. (1996) Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272, 1008–1010 [DOI] [PubMed] [Google Scholar]
- 33. Liu H., Liu Z. Q., Chen C. X., Magill S., Jiang Y., Liu Y. J. (2006) Inhibitory regulation of EGF receptor degradation by sorting nexin 5. Biochem. Biophys. Res. Commun. 342, 537–546 [DOI] [PubMed] [Google Scholar]
- 34. Dinneen J. L., Ceresa B. P. (2004) Continual expression of Rab5(Q79L) causes a ligand-independent EGFR internalization and diminishes EGFR activity. Traffic 5, 606–615 [DOI] [PubMed] [Google Scholar]
- 35. Hendriks B. S., Opresko L. K., Wiley H. S., Lauffenburger D. (2003) Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J. Biol. Chem. 278, 23343–23351 [DOI] [PubMed] [Google Scholar]
- 36. Hendriks B. S., Opresko L. K., Wiley H. S., Lauffenburger D. (2003) Coregulation of epidermal growth factor receptor/human epidermal growth factor receptor 2 (HER2) levels and locations: quantitative analysis of HER2 overexpression effects. Cancer Res. 63, 1130–1137 [PubMed] [Google Scholar]
- 37. Shankaran H., Wiley H. S., Resat H. (2006) Modeling the effects of HER/ErbB1–3 coexpression on receptor dimerization and biological response. Biophys. J. 90, 3993–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Modregger J., Ritter B., Witter B., Paulsson M., Plomann M. (2000) All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J. Cell Sci. 113, 4511–4521 [DOI] [PubMed] [Google Scholar]
- 39. Qualmann B., Kelly R. B. (2000) Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 148, 1047–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Razi M., Futter C. E. (2006) Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell 17, 3469–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. King A. C. (1984) Monensin, like methylamine, prevents degradation of 125I-epidermal growth factor, causes intracellular accumulation of receptors and blocks the mitogenic response. Biochem. Biophys. Res. Commun. 124, 585–591 [DOI] [PubMed] [Google Scholar]
- 42. Bao J., Alroy I., Waterman H., Schejter E. D., Brodie C., Gruenberg J., Yarden Y. (2000) Threonine phosphorylation diverts internalized epidermal growth factor receptors from a degradative pathway to the recycling endosome. J. Biol. Chem. 275, 26178–26186 [DOI] [PubMed] [Google Scholar]
- 43. Huang Y., Li X., Jiang J., Frank S. J. (2006) Prolactin modulates phosphorylation, signaling and trafficking of epidermal growth factor receptor in human T47D breast cancer cells. Oncogene 25, 7565–7576 [DOI] [PubMed] [Google Scholar]
- 44. Hanafusa H., Ishikawa K., Kedashiro S., Saigo T., Iemura S., Natsume T., Komada M., Shibuya H., Nara A., Matsumoto K. (2011) Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nat. Commun. 2, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim S. H., Choi H. J., Lee K. W., Hong N. H., Sung B. H., Choi K. Y., Kim S. M., Chang S., Eom S. H., Song W. K. (2006) Interaction of SPIN90 with syndapin is implicated in clathrin-mediated endocytic pathway in fibroblasts. Genes Cells 11, 1197–1211 [DOI] [PubMed] [Google Scholar]
- 46. Prasad N. K., Decker S. J. (2005) SH2-containing 5′-inositol phosphatase, SHIP2, regulates cytoskeleton organization and ligand-dependent down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 280, 13129–13136 [DOI] [PubMed] [Google Scholar]
- 47. Prasad N. K., Tandon M., Badve S., Snyder P. W., Nakshatri H. (2008) Phosphoinositol phosphatase SHIP2 promotes cancer development and metastasis coupled with alterations in EGF receptor turnover. Carcinogenesis 29, 25–34 [DOI] [PubMed] [Google Scholar]