Background: ALC1 is a macrodomain-containing SNF2-like ATPase activated to catalyze ATP-dependent nucleosome remodeling by PARP1 and its substrate NAD+.
Results: ALC1 is activated upon binding of its macrodomain to PAR in an ALC1·PARylated PARP1·nucleosome intermediate.
Conclusion: PAR on PARylated PARP1 acts as an allosteric effector of ALC1 activity.
Significance: Defining mechanisms underlying chromatin remodeling is important for understanding chromosome function.
Keywords: ATPases, Chromatin, Chromatin Remodeling, DNA Repair, Gene Regulation, SNF2-like ATPase
Abstract
The human ALC1/CHD1L oncogene encodes an SNF2 family ATPase with a macrodomain that binds poly(ADP-ribose) (PAR). We and others previously showed that ALC1 possesses a cryptic ATP-dependent nucleosome remodeling activity that is potently activated in the presence of PARP1 and NAD+, its substrate for PAR synthesis. In this work, we dissected the mechanism by which PARP1 and NAD+ activate ALC1 nucleosome remodeling. We demonstrate that ALC1 activation depends on the formation of a stable ALC1·PARylated PARP1·nucleosome intermediate. In addition, by exploiting a novel PAR footprinting assay, we obtained evidence that the ALC1 macrodomain remains stably associated with PAR on autoPARylated PARP1 during the course of nucleosome remodeling reactions. Taken together, our findings are consistent with the model that PAR present on PARylated PARP1 acts as an allosteric effector of ALC1 nucleosome remodeling activity.
Introduction
The human ALC1 (amplified in liver cancer 1; also known as CHD1L (chromodomain-helicase-DNA-binding protein 1-like) gene encodes a member of the SNF2 (sucrose non-fermenter 2) superfamily of ATPases. ALC1 was originally identified as a gene present on a short human chromosome 1q21 region that is amplified in hepatocellular carcinomas (1). Overexpression of the ALC1 protein was found to transform human cells and to be oncogenic in mice (1, 2). More recently, the ALC1 gene was found to be mutated in patients with congenital anomalies of the kidney and urinary tract (3). Although the mechanism of ALC1 action in these processes is not known, ALC1 has been reported to have roles in DNA repair (4) and in controlling the expression of several genes implicated in tumorigenesis and metastasis (1, 2, 5).
ALC1 is unique among SNF2 family members because it includes a macrodomain that is capable of binding selectively to poly(ADP-ribose) (PAR).3 Previous studies from our laboratory and elsewhere revealed that ALC1 possesses cryptic DNA-dependent ATPase and ATP-dependent nucleosome sliding activities that are strongly activated in the presence of the catalytically active poly(ADP-ribose) polymerase PARP1 and its substrate NAD+ (4, 6). Suggesting that binding of the macrodomain to PAR is essential for ALC1 activation, ALC1 macrodomain mutations that prevent PAR binding abolish ALC1 DNA-dependent ATPase and ATP-dependent nucleosome sliding. Nevertheless, we observed that binding of free PAR chains to the macrodomain is not sufficient to activate DNA-dependent ATPase and nucleosome remodeling activities, arguing that the ALC1 activation process is more complex (6).
In this study, we explored the mechanism by which PARP1 and NAD+ function together to activate ALC1 nucleosome remodeling and the role of PAR in this process. As described below, we demonstrate that ALC1 activation proceeds via formation of a stable ALC1·PARylated PARP1·nucleosome intermediate. In addition, through development and application of a novel PAR footprinting assay, we obtained evidence that ALC1 activation results from a direct and stable interaction of the ALC1 macrodomain with PAR conjugated to PARylated PARP1. Below, we present these findings, which are consistent with the model that PAR conjugated to PARP1 functions as an allosteric effector that activates ALC1 nucleosome remodeling activity.
EXPERIMENTAL PROCEDURES
Enzymes
PARP1 (high specific activity, >95% pure) was obtained from Trevigen. FLAG-tagged ALC1 and ALC1 mutants were expressed in baculovirus-infected Sf9 cells as described (6) and purified by anti-FLAG-agarose immunoaffinity chromatography as follows. Lysates from 1 × 108 cells were incubated with 0.5 ml of anti-FLAG M2-agarose beads (Sigma) for at least 12 h at 4 °C. The beads were washed three times with buffer containing 10 mm HEPES-NaOH (pH 7.9), 1.5 mm MgCl2, 0.8 m NaCl, 10 mm KCl, 0.2% Triton X-100, and 1:500 protease inhibitor mixture (Sigma P8340), and bound proteins were eluted from the beads with elution buffer containing 10 mm HEPES-NaOH (pH 7.9), 0.1 m NaCl, 1.5 mm MgCl2, 0.05% Triton X-100, 1:500 protease inhibitor mixture, and 200 μg/ml FLAG peptide (Sigma). Protein concentrations were estimated by comparing the intensities of Coomassie Blue-stained bands with that of BSA in SDS-polyacrylamide gels scanned using an Odyssey® imaging system (LI-COR Biosciences).
Nucleosome Remodeling Assays
Mononucleosomes were reconstituted by dilution transfer from HeLa cell oligonucleosomes on a 32P-end-labeled 216-bp DNA fragment (601-lat Gal4) generated by PCR from pGEM3Z-601-Gal4 (7, 8). 1 pmol of ALC1 and 0.5 pmol of PARP1 were incubated at 32 °C for 30 min with mononucleosomes (0.01 pmol of labeled mononucleosome and 0.25 pmol of unlabeled oligonucleosomes) in 20 mm HEPES-NaOH (pH 7.9), 50 mm NaCl, 4.5 mm MgCl2, 2 mm DTT, 0.5 mm PMSF, 100 μg/ml BSA, 10% glycerol, 0.02% Triton X-100, and 0.02% Nonidet P-40. ATP, NAD+, and benzamide were included in reactions as indicated in Fig. 1. Reaction products were incubated for a further 30 min with 10 units of HhaI and resolved on gels containing 7% polyacrylamide (19:1 acrylamide:bisacrylamide), 7 m urea, 45 mm Tris borate, and 1 mm EDTA (pH 8.3).
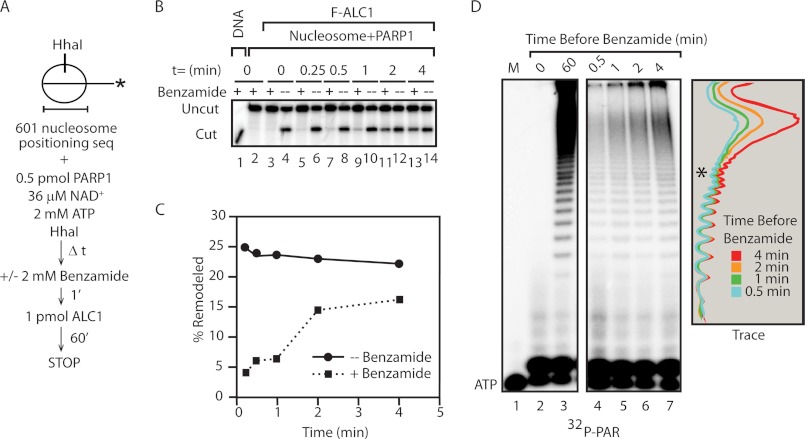
FIGURE 1.
Rapid formation of a benzamide-resistant intermediate in ALC1 activation. A, diagram describing the protocol and positioned nucleosome substrate with an HhaI site on 216-bp 601-lat Gal4 DNA fragment used for benzamide challenge nucleosome remodeling assay. The asterisk indicates 32P-labeled DNA end. B, Naked DNA (lane 1) or positioned nucleosomes (lanes 2–14) were incubated with HhaI in the absence (lane 1) or presence (lanes 2–14) of PARP1. Where indicated, PARP1 inhibitor benzamide was added to reactions at the times shown in the figure. One minute later, ALC1 was added, and remodeling reactions proceeded for an additional 60 min, and DNA or nucleosomes were monitored for HhaI restriction enzyme accessibility. C, quantitation of experiment shown in B. D, kinetics of PAR synthesis. Reactions with [32P]NAD+ were performed according to the protocol shown in A, except that ALC1 was omitted. PAR synthesized during the reactions was purified, analyzed on denaturing polyacrylamide gels, and visualized on a phosphorimager (left panels). Marker lane (M) contains [γ-32P]ATP. The graph on the right shows traces of lanes 4–7 generated using ImageQuant TL software.
Nucleosome and DNA Binding Assays
40 pmol of 5′-biotinylated 601-lat Gal4 DNA fragment or mononucleosomes assembled on the same DNA fragment were bound to 400 μl of streptavidin-coupled Dynabeads® (Invitrogen), washed, and resuspended in a final volume of 400 μl (100 fmol of DNA or mononucleosome/μl of beads). 0.5 pmol of PARP1, with or without 1 pmol of ALC1, was incubated with 0.5 pmol of immobilized nucleosomes or DNA in 40 μl of buffer containing 20 mm HEPES (pH 7.9), 50 mm NaCl, 0.5% Nonidet P-40, 10% glycerol, 5 mm MgCl2, 1 mm DTT, 0.5 mm PMSF, 1 mm ATP, 100 μg/ml BSA, and NAD+ as indicated in the figures. Nucleosome- or DNA-bound intermediates on Dynabeads® were collected using DynaMagTM magnets (Invitrogen), washed with 200 μl of the same buffer, and transferred to a fresh microcentrifuge tube, and bound proteins were eluted with 1× SDS sample buffer. Template-bound intermediates were transferred to PVDF membranes and visualized using a Typhoon PhosphorImager (Molecular Dynamics) to detect 32P-PARylated proteins or by Western blotting using an Odyssey® imaging system.
Isolation and Analysis of PAR
ALC1·PARylated PARP1·nucleosome or PARylated PARP1·nucleosome intermediates were assembled in the presence of [32P]NAD+ (PerkinElmer Life Sciences) as described above. To isolate radiolabeled PAR, reaction products were digested in 60 μl of 20 mm EDTA, 0.1% SDS, 200 mm NaCl, 20 μg of GlycoBlue (Ambion), and 10 μg of proteinase K (Sigma) at 60 °C for 12 h. Following proteinase K digestion, reactions were brought to 0.08 m KOH and incubated for a further 3 h at 60 °C to remove any remaining peptides bound to the PAR species. PAR was precipitated with 70% ethanol; resuspended in loading buffer consisting of 6 m urea, 0.02% bromphenol blue, and 0.02% xylene cyanol; and analyzed on gels containing 20 or 25% polyacrylamide (19:1 acrylamide:bisacrylamide), 7 m urea, 45 mm Tris borate, and 1 mm EDTA (pH 8.3). Reaction products were visualized using a Typhoon PhosphorImager and analyzed using ImageQuant TL software (Molecular Dynamics).
RESULTS
ALC1 Activation Is Preceded by Rapid Formation of a Benzamide-resistant Intermediate
To begin to investigate the mechanism by which PARP1 and NAD+ function together to activate ALC1, we sought to isolate and define intermediates in the process. Previous studies using the PARP1 inhibitor benzamide, which prevents PAR synthesis by PARP1 (9), have provided information about the nature of the essential PARP1- and NAD+-dependent step(s) in ALC1 activation. In these experiments, benzamide was found to block ALC1 DNA-dependent ATPase and ATP-dependent nucleosome remodeling activities when added at the beginning of reactions (4, 6). This observation, together with evidence that free PAR is not sufficient to activate ALC1, argued that ALC1 activation depends on PARylation of one or more components of the nucleosome remodeling reaction. Indicating that ALC1 PARylation is not essential for ALC1 activation, preincubation of PARP1 and NAD+ with nucleosomes or with naked DNA prior to addition of benzamide and ALC1 rendered reactions resistant to benzamide (4, 6). Arguing that histone PARylation does not play an essential role in formation of this benzamide-resistant intermediate, we observed that either nucleosomes or naked DNA can support PARP1- and NAD+-dependent ALC1 DNA-dependent ATPase activity (6). Taken together, these findings indicate that a benzamide-resistant intermediate capable of supporting ALC1 activation and containing PARylated PARP1 can form in the absence of ALC1.
To extend our understanding of ALC1 activation, we carried out similar benzamide challenge experiments to explore the kinetics of formation of the benzamide-resistant intermediate. To do so, we preincubated nucleosomes with PARP1 and NAD+ for varying lengths of time before adding benzamide and ALC1 and assayed for nucleosome remodeling by monitoring deprotection of an HhaI site located near the nucleosome dyad (diagrammed in Fig. 1A). Consistent with our previous results, addition of benzamide at the beginning of the reaction completely blocked ALC1 activity (Fig. 1B, lane 3). Under the conditions of these assays, a benzamide-resistant intermediate that supports ALC1-dependent nucleosome remodeling formed very rapidly in the presence of PARP1 and NAD+, reaching near maximal levels within 2–4 min (Fig. 1B, compare lanes 5, 7, 9, 11, and 13 with lanes 6, 8, 10, 12, and 14, respectively, and Fig. 1C, which shows quantitation of the data from the experiment of Fig. 1B).
To estimate the length of PAR chains synthesized during formation of the benzamide-resistant intermediate in the experiment of Fig. 1B, we performed a parallel experiment in which nucleosomes and PARP1 were incubated for varying lengths of time in the presence of [32P]NAD+. Reaction mixtures were then treated with proteinase K, and PAR chains were cleaved from the remaining PARP1 protein fragments by base treatment, ethanol-precipitated, and analyzed on denaturing polyacrylamide gels. As shown in Fig. 1D, similar amounts of PAR species migrating more rapidly than the position of the asterisk (estimated length of 12–15 ADP-ribose units) were synthesized after 0.5 min, a time point at which only a small amount of benzamide-resistant intermediate had formed, and after 4 min, a time sufficient for formation of enough intermediate to support near maximal nucleosome remodeling (compare lanes 4 and 7). In contrast, longer species continued to accumulate during this time period (lanes 4–7), suggesting that formation of the benzamide-resistant intermediate that supports ALC1 activation requires synthesis by PARP1 of these longer PAR chains.
Cooperative Binding of PARP1 and ALC1 to Nucleosomes
We previously showed that that the binding of ALC1 to immobilized DNA or nucleosomes depends on PARP1 and NAD+ (6). These experiments did not, however, address the fate of PARP1 during ALC1 activation. If PARylated PARP1 contributes to both ALC1 activation and subsequent ATP-dependent nucleosome remodeling or DNA-dependent ATP hydrolysis, PARylated PARP1 might be predicted to remain as an integral component of an activated ALC1·nucleosome or ALC1·DNA intermediate throughout reactions. Alternatively, PARylated PARP1 might be required for the initial activation of ALC1 but then be displaced upon the binding of ALC1 to its substrates and/or hydrolysis of ATP.
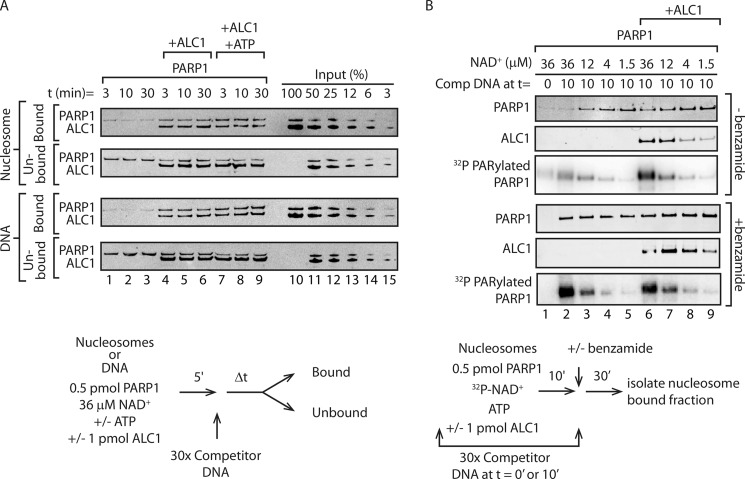
To address this issue, immobilized nucleosomes or DNA was incubated with PARP1 and NAD+ with or without ALC1 and ATP. Excess competitor DNA was added as a sink for unbound or disassociated PARP1 and ALC1 after a time sufficient for formation of the benzamide-resistant intermediate. At varying times after addition of competitor, the amounts of PARP1 and ALC1 remaining bound to immobilized nucleosomes and DNA or released were measured by Western blotting. As shown in Fig. 2A, PARP1 could be detected in the bound fraction after incubation with immobilized nucleosomes or DNA. Notably, retention of PARP1 on nucleosomes or DNA increased significantly in the presence of ALC1 (compare lanes 1–3 with lanes 4–6), suggesting that the binding of PARP1 and ALC1 to nucleosomes or DNA is cooperative. ALC1·PARP1·nucleosome or ALC1·PARP1·DNA complexes were stable, as they exhibited no apparent dissociation during a 30-min incubation following addition of competitor. Furthermore, the amount of bound PARP1 was not decreased when binding assays included ATP (compare lanes 4–6 with lanes 7–9), suggesting that PARP1 is not displaced from ALC1·PARP1·nucleosome or ALC1·PARP1·DNA intermediates upon ATP hydrolysis.
FIGURE 2.
Cooperative binding of PARP1 and ALC1 to nucleosomes. A, biotinylated DNA or mononucleosomes reconstituted with HeLa cell histones were immobilized on streptavidin beads and incubated for 5 min with PARP1 and NAD+, with or without ALC1 and ATP. After addition of competitor DNA, reactions were incubated for the indicated times. PARP1 and ALC1 in bound and unbound fractions was detected by Western blotting. B, nucleosome binding reactions were performed with the indicated concentrations of NAD+ and with or without benzamide as diagrammed in the figure. PARP1 and ALC1 in bound and unbound fractions was detected by Western blotting, and 32P-PARylated PARP1 was detected by phosphorimaging of the same membrane used in the Western blots.
PARylation of PARP1 has been shown to decrease its affinity for DNA or nucleosomes (reviewed in Refs. 10 and 11). To investigate the relationship between PAR synthesis and formation of the stable ALC1·PARP1·nucleosome intermediate, we manipulated the extent of PARP1 PARylation by varying the concentration of NAD+ included in the binding assays. Consistent with previous evidence that the binding of ALC1 to DNA or nucleosomes depends on PARP1 and NAD+, the amount of ALC1 binding depended on NAD+ concentration; at the lowest concentration of NAD+, less ALC1 remained associated with immobilized nucleosomes than when NAD+ was present at the highest concentrations used (Fig. 2B, lanes 6–9). There was little NAD+-dependent change in the amount of bound PARP1 when the binding reactions included ALC1 (lanes 6–9); however, when ALC1 was not included in the binding reactions, substantially less PARP1 remained associated with immobilized nucleosomes at high NAD+ concentrations, where the extent of [32P]NAD+ incorporation into PARylated PARP1 was greater (Fig. 2B, upper panels, compare lanes 2–5 with lanes 6–9). The NAD+-dependent decrease in PARP1 bound to nucleosomes depended on PAR synthesis because it was mitigated when benzamide was added to reactions along with competitor DNA (Fig. 2B, lower panels). We similarly observed that enhanced binding of PARP1 to DNA in the presence of ALC1 was most pronounced when reactions contained NAD+ (data not shown). Taken together, our findings are consistent with the model that ALC1 and PARylated PARP1 bind cooperatively to nucleosomes or DNA to form an intermediate that is stable in the presence or absence of ATP.
ALC1 Protects PAR Chains with ∼3 to >20 ADP-ribose Units from Digestion by Poly(ADP-ribose) Glycohydrolase
We previously observed that the DNA-dependent ATPase and nucleosome remodeling activities of ALC1 were abolished by an ALC1 macrodomain point mutation (D723A) that interferes with its binding to free PAR, suggesting that binding of PAR to the ALC1 macrodomain is likely to play a role in ALC1 activation. To investigate potential ALC1-PAR interactions under nucleosome remodeling conditions, we developed a novel PAR footprinting assay in which we exploited the ability of poly(ADP-ribose) glycohydrolase (PARG) to degrade PAR. PARG catalyzes hydrolysis of α(1″,2′) O-glycosidic linkages in PAR and can act as both an endo- and exoglycosidase (12, 13).
PAR footprinting assays were performed as diagrammed in Fig. 3A. Bead-bound nucleosomes were incubated with PARP1 and [32P]NAD+ in the presence or absence of ALC1 for a time sufficient to allow formation of stable benzamide-resistant intermediates, and benzamide was added to block further PAR synthesis. After treatment of reaction mixtures with increasing concentrations of PARG, the beads were washed, and residual PAR associated with ALC1·PARylated PARP1·nucleosome or PARylated PARP1·nucleosome complexes was isolated and analyzed by denaturing gel electrophoresis. As shown in Fig. 3B, [32P]PAR chains in the PARylated PARP1·nucleosome complex were digested to near completion by PARG. In contrast, a nearly constant fraction of [32P]PAR chains in the ALC1·PARylated PARP1·nucleosome complex was resistant to digestion at all PARG concentrations used; notably, the size distribution of these protected digestion products (from ∼3 to >20 ADP-ribose units) was very similar over a 10-fold range of PARG concentrations. Whereas ALC1 carrying a mutation in its ATP-binding site behaved very similarly to the wild-type enzyme, PAR was not protected from digestion by ALC1 containing a macrodomain point mutation that prevents it from binding free PAR (Fig. 3C). These results argue that PAR binding by the ALC1 macrodomain is required for the observed PAR protection.
FIGURE 3.
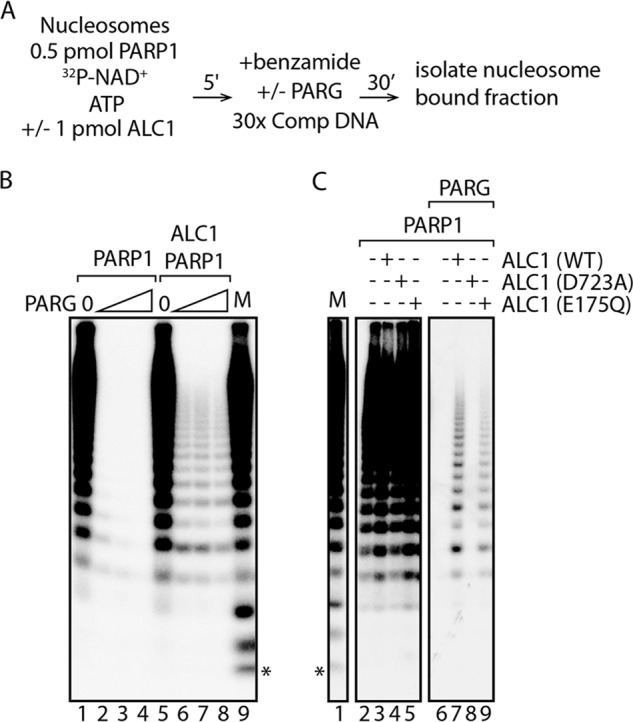
The ALC1 macrodomain protects PAR chains from ∼ 3 to more than 20 ADP-ribose units from digestion by PARG. A, diagram showing the protocol used for PAR footprinting assays. B, reactions performed as diagrammed in A contained no PARG (lanes 1 and 5) or 1.5 ng (lanes 2 and 6), 5 ng (lanes 3 and 7), and 15 ng (lanes 4 and 8) PARG. C, reactions performed as diagrammed in A contained PARP1 and wild-type or mutant ALC1 without or with 5 ng of PARG. Marker lanes (M) show the total reaction products synthesized in a reaction containing nucleosomes and PARP1. Free ATP runs at the position indicated by the asterisk.
DISCUSSION
ALC1 is an SNF2 family ATPase with a macrodomain. We and others previously demonstrated that ALC1 possesses cryptic DNA-dependent ATPase and ATP-dependent nucleosome remodeling activities that are activated in the presence of PARP1 and NAD+, its substrate for PAR synthesis (4, 6). Several lines of evidence argued that autoPARylation of PARP1, rather than PARylation of nucleosomal histones or ALC1, is the essential NAD+-dependent step in ALC1 activation. First, either nucleosomes or naked DNA can support PARP1- and NAD+-dependent ALC1 DNA-dependent ATPase activity, indicating that histone PARylation is not essential (6). Second, an intermediate that activates ALC1 and that is resistant to the PARP inhibitor benzamide can be formed by preincubation of PARP1 and NAD+ with nucleosomes or with naked DNA in the absence of ALC1 (4, 6).
In this work, we have applied a combination of approaches to dissect in more detail the mechanism by which ALC1 nucleosome remodeling activity is activated by PARP1 and NAD+. In the course of our investigation, we considered two possible models for the mechanism of ALC1 activation. First, ALC1 activation might occur via a direct physical interaction between ALC1 and autoPARylated PARP1, leading to formation of a stable intermediate capable of catalyzing ATP-dependent nucleosome remodeling. Alternatively, given that PARylation of PARP1 has been shown to reduce its affinity for DNA or nucleosomes (10, 11, 14), it seemed possible that ALC1 might be activated by a different mechanism, perhaps by interacting transiently with autoPARylated PARP1 in a way that renders ALC1 competent to bind and remodel nucleosomes.
Our findings are most consistent with the model that activation of ALC1 occurs through direct and stable binding of ALC1 to autoPARylated PARP1. Our biochemical dissection of the mechanism of ALC1 activation argues that it proceeds with the formation of multiple intermediates: an initial complex of PARP1 bound to a nucleosome; a benzamide-resistant intermediate that contains nucleosome-bound PARylated PARP1; and a stable ternary intermediate that is composed of ALC1, autoPARylated PARP1, and nucleosome and that is capable of catalyzing nucleosome remodeling (Fig. 4). Importantly, the results of PAR footprinting assays argue that in the ternary intermediate, the ALC1 macrodomain is bound to PAR. Taken together with our evidence that ALC1 containing a macrodomain point mutation that abolishes its binding to PAR is inactive in nucleosome remodeling, our findings argue that PAR on PARylated PARP1 plays a unique role as an allosteric effector of ALC1 DNA-dependent ATPase and ATP-dependent chromatin remodeling activities.
FIGURE 4.
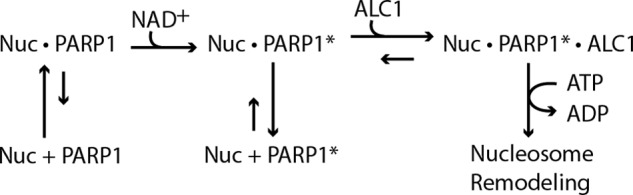
Proposed pathway for ALC1 activation by PARP1 and NAD+. Unmodified PARP1 binds nucleosomes with high affinity. NAD+-dependent PARylation of PARP1 decreases its affinity for nucleosomes or DNA; thus, in the absence of ALC1 the nucleosome-PARylated PARP1 intermediate tends to dissociate. ALC1 and PARylated PARP1 bind cooperatively to nucleosomes, leading to formation of a stable, activated intermediate that can catalyze ATP-dependent nucleosome remodeling. PARP1*, PARylated PARP1; Nuc, nucleosome.
Although a structure of the ALC1 macrodomain is not yet available, the structure of the macroH2A1.1 macrodomain in complex with ADP-ribose predicts that macrodomains bind at the distal ends of PAR chains and could, in principle, bind stably to polymers containing just two or three ADP-ribose units (15). Our data suggest, however, that such short PAR chains may not be sufficient to recruit ALC1 to PARylated PARP1 and support ALC1 activation, because we observe that formation of the benzamide-resistant intermediate correlates with synthesis of PAR chains longer than ∼12–15 ADP-ribose units. In the ALC1·PARP1·nucleosome intermediates, PAR chains ranging in size from ∼3 to more than 20 ADP-ribose units are protected from endo- and exo-glycolytic digestion by PARG. Although the size of the smallest of these corresponds with what might be expected based on the model for PAR binding by macroH2A1.1, the largest are considerably longer, likely reflecting interactions between PAR and ALC1 that fall outside of the ADP-ribose binding pocket in its macrodomain and that contribute to ALC1 activation.
This work was supported, in whole or in part, by National Institutes of Health Grant GM41628 from NIGMS (to J. W. C. and R. C. C.). This work was also supported by a grant to the Stowers Institute from the Helen Nelson Medical Research Fund at the Greater Kansas City Community Foundation.
- PAR
- poly(ADP-ribose)
- PARG
- poly(ADP-ribose) glycohydrolase.
REFERENCES
- 1. Ma N. F., Hu L., Fung J. M., Xie D., Zheng B. J., Chen L., Tang D. J., Fu L., Wu Z., Chen M., Fang Y., Guan X. Y. (2008) Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology 47, 503–510 [DOI] [PubMed] [Google Scholar]
- 2. Chen M., Huang J. D., Hu L., Zheng B. J., Chen L., Tsang S. L., Guan X. Y. (2009) Transgenic CHD1L expression in mouse induces spontaneous tumors. PLoS ONE 4, e6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brockschmidt A., Chung B., Weber S., Fischer D. C., Kolatsi-Joannou M., Christ L., Heimbach A., Shtiza D., Klaus G., Simonetti G. D., Konrad M., Winyard P., Haffner D., Schaefer F., Weber R. G. (2012) CHD1L: a new candidate gene for congenital anomalies of the kidneys and urinary tract (CAKUT). Nephrol. Dial. Transplant. 27, 2355–2364 [DOI] [PubMed] [Google Scholar]
- 4. Ahel D., Horejsí Z., Wiechens N., Polo S. E., Garcia-Wilson E., Ahel I., Flynn H., Skehel M., West S. C., Jackson S. P., Owen-Hughes T., Boulton S. J. (2009) Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325, 1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen L., Chan T. H., Yuan Y. F., Hu L., Huang J., Ma S., Wang J., Dong S. S., Tang K. H., Xie D., Li Y., Guan X. Y. (2010) CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J. Clin. Invest. 120, 1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottschalk A. J., Timinszky G., Kong S. E., Jin J., Cai Y., Swanson S. K., Washburn M. P., Florens L., Ladurner A. G., Conaway J. W., Conaway R. C. (2009) Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl. Acad. Sci. U.S.A. 106, 13770–13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Owen-Hughes T., Utley R. T., Steger D. J., West J. M., John S., Côté J., Havas K. M., Workman J. L. (1999) Analysis of nucleosome disruption by ATP-driven chromatin remodeling complexes. Methods Mol. Biol. 119, 319–331 [DOI] [PubMed] [Google Scholar]
- 8. Gutiérrez J. L., Chandy M., Carrozza M. J., Workman J. L. (2007) Activation domains drive nucleosome eviction by SWI/SNF. EMBO J. 26, 730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Purnell M. R., Whish W. J. (1980) Novel inhibitors of poly(ADP-ribose) synthetase. Biochem. J. 185, 775–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Amours D., Desnoyers S., D'Silva I., Poirier G. G. (1999) Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 342, 249–268 [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M. Y., Zhang T., Kraus W. L. (2005) Poly(ADP-ribosyl)ation by PARP-1: 'PAR-laying' NAD+ into a nuclear signal. Genes Dev. 19, 1951–1967 [DOI] [PubMed] [Google Scholar]
- 12. Miwa M., Tanaka M., Matsushima T., Sugimura T. (1974) Purification and properties of glycohydrolase from calf thymus splitting ribose-ribose linkages of poly(adenosine diphosphate ribose). J. Biol. Chem. 249, 3475–3482 [PubMed] [Google Scholar]
- 13. Kim I. K., Kiefer J. R., Ho C. M., Stegeman R. A., Classen S., Tainer J. A., Ellenberger T. (2012) Structure of mammalian poly(ADP-ribose) glycohydrolase reveals a flexible tyrosine clasp as a substrate-binding element. Nat. Struct. Mol. Biol. 19, 653–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim M. Y., Mauro S., Gévry N., Lis J. T., Kraus W. L. (2004) NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell 119, 803–814 [DOI] [PubMed] [Google Scholar]
- 15. Timinszky G., Till S., Hassa P. O., Hothorn M., Kustatscher G., Nijmeijer B., Colombelli J., Altmeyer M., Stelzer E. H., Scheffzek K., Hottiger M. O., Ladurner A. G. (2009) A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat. Struct. Mol. Biol. 16, 923–929 [DOI] [PubMed] [Google Scholar]