Background: Whether neuronal nitric-oxide synthase (nNOS) plays a role in the endothelial NOS (eNOS)-dependent negative inotropic effect of β3-adrenergic stimulation remains to be established.
Results: nNOS knock-out or inhibition leads to increased superoxide production, eNOS uncoupling, and abrogation of β3-adrenergic responses.
Conclusion: Disabling nNOS disrupts eNOS function and downstream signaling.
Significance: nNOS plays a crucial role in preserving myocardial nitroso-redox balance and coupled eNOS activity.
Keywords: Adrenergic Receptor, Glutathionylation, Nitric Oxide, Nitric-oxide Synthase, Oxidative Stress, Myocardium
Abstract
Myocardial constitutive No production depends on the activity of both endothelial and neuronal NOS (eNOS and nNOS, respectively). Stimulation of myocardial β3-adrenergic receptor (β3-AR) produces a negative inotropic effect that is dependent on eNOS. We evaluated whether nNOS also plays a role in β3-AR signaling and found that the β3-AR-mediated reduction in cell shortening and [Ca2+]i transient amplitude was abolished both in eNOS−/− and nNOS−/− left ventricular (LV) myocytes and in wild type LV myocytes after nNOS inhibition with S-methyl-l-thiocitrulline. LV superoxide (O2˙̄) production was increased in nNOS−/− mice and reduced by l-Nω-nitroarginine methyl ester (l-NAME), indicating uncoupling of eNOS activity. eNOS S-glutathionylation and Ser-1177 phosphorylation were significantly increased in nNOS−/− myocytes, whereas myocardial tetrahydrobiopterin, eNOS Thr-495 phosphorylation, and arginase activity did not differ between genotypes. Although inhibitors of xanthine oxidoreductase (XOR) or NOX2 NADPH oxidase caused a similar reduction in myocardial O2˙̄, only XOR inhibition reduced eNOS S-glutathionylation and Ser-1177 phosphorylation and restored both eNOS coupled activity and the negative inotropic and [Ca2+]i transient response to β3-AR stimulation in nNOS−/− mice. In summary, our data show that increased O2˙̄ production by XOR selectively uncouples eNOS activity and abolishes the negative inotropic effect of β3-AR stimulation in nNOS−/− myocytes. These findings provide unequivocal evidence of a functional interaction between the myocardial constitutive NOS isoforms and indicate that aspects of the myocardial phenotype of nNOS−/− mice result from disruption of eNOS signaling.
Introduction
Myocardial constitutive nitric oxide (NO) production has been known to regulate the inotropic response to β1-AR3 stimulation, although the source of NO responsible for this effect is still debated (1). By contrast, it is generally accepted that the negative inotropic effect elicited by β3-AR stimulation depends on the myocardial release of NO by the endothelial NOS isoform (eNOS) (2–4). β3-ARs are thought to co-localize with eNOS in the sarcolemmal caveolar microdomains of left ventricular (LV) myocytes, and β3-AR stimulation has been shown to stimulate eNOS activity (3) and decrease contraction and the [Ca2+]i transient amplitude (5, 6).
A neuronal NOS (nNOS) isoform is also constitutively present in the myocardium where it plays an important role in the regulation of inotropy and Ca2+ fluxes, by affecting the S-nitrosylation and phosphorylation state of a number of proteins involved in excitation-contraction coupling (7–11). Whether nNOS is involved in β3-AR signaling remains unclear; however, observations that nNOS may shuttle between the sarcoplasmic reticulum and the sarcolemmal membrane (where it co-localizes with caveolin-3) (8, 12–14) suggest that nNOS-derived NO may also be released within the caveolar microdomain from where it may contribute to the negative inotropic effect of β3-AR stimulation. In addition, nNOS has been shown to regulate the activity of myocardial xanthine oxidoreductase (XOR) (15, 16), and O2˙̄ production is increased in the LV myocardium of nNOS knock-out mice (nNOS−/−) (15, 16). Excess O2˙̄ production may reduce the bioavailability of eNOS-derived NO both directly, by scavenging NO, and indirectly, by oxidizing the NOS co-factor tetrahydrobiopterin (BH4) (17), stimulating arginase activity (18), decreasing l-arginine transport across the sarcolemma (19), or increasing eNOS S-glutathionylation (20), all of which, in turn, may lead eNOS to “uncouple” and generate O2˙̄ instead of NO (17). To address these issues, we investigated the effect of disrupting nNOS on myocardial eNOS function and the β3-AR-mediated reduction in contraction and [Ca2+]i transients in murine LV myocytes.
EXPERIMENTAL PROCEDURES
All chemicals were purchased from Sigma-Aldrich unless specified. Mice (3–6 months old) homozygous for targeted disruption of nNOS (21) or eNOS gene (22) were compared with their wild type littermates (nNOS+/+ and eNOS+/+, respectively). The treatment of all animals was in accordance with the Home Office Guidance on the Operation of Animals (Scientific Procedures) Act, 1986 (H.M.S.O.).
LV myocytes were isolated, and cell shortening (by video edge detection; IonOptix Corp.) and [Ca2+]i transients (Fura-2, 5 μm; Molecular Probes) were measured in field-stimulated LV myocytes (1 Hz, 35 ± 1.5 °C) as described previously (7). Measurements from at least 10 steady state contractions were averaged in each cell for each stage of the experimental protocols. All of the experiments were carried out at 35 ± 1.5 °C.
Selective β3-AR stimulation was achieved by perfusing the myocytes with the β3-AR agonist BRL 37344 (BRL, 10 μm; i.e., the concentration at which we recorded the largest negative inotropic effect in isolated murine LV myocytes; Tocris Cookson Ltd.), and the β1- and β2-AR blocker nadolol (NAD, 10 μm). S-Methyl-l-thiocitrulline (SMTC, 0.1 μm), oxypurinol (100 μm), apocynin (100 μm), and the gp91 ds tat-peptide (10 μm) were used to pharmacologically inhibit nNOS, XOR, or NADPH oxidase, respectively.
O2˙̄ production was measured in intact LV myocytes and LV homogenates from nNOS−/− mice and their wild type littermates by using lucigenin (5 μm) enhanced chemiluminescence and HPLC detection of dihydroethidium conversion to 2-hydroxyethidium, respectively (23). The tiron-inhibitable fraction of O2˙̄ was expressed as relative light units/s per 100,000 myocytes or per mg of protein, as appropriate. Peroxynitrite production was measured in LV homogenates by luminol (100 μm) enhanced chemiluminescence; the final result was expressed as the urate (1 mm) inhibitable fraction in (relative light unit/s/mg of protein) BH4, and its oxidized products (7,8-dihydropterin (BH2) and biopterin) were evaluated in LV homogenates by using an isocratic HPLC system and sequential electrochemical (Coulochem III; ESA Inc.) and fluorescence (Jasco) detection (23). Myocardial NOS and arginase activity was evaluated by conversion of [14C]l-arginine to citrulline or ornithine, respectively, in the presence of Nω-hydroxy-nor-arginine, (Calbiochem) and DTT (for NOS activity only) by using a HPLC system, as described previously (23); the l-NAME- and Nω-hydroxy-nor-arginine inhibitable fractions were used to evaluate NOS and arginase activity, respectively.
l-Arginine transport was measured in freshly isolated LV myocytes after incubation with 14C l-arginine (5.0E−5 Ci/ml; Amersham Biosciences) at 37 °C for 30 min. The cell pellets were lysed, and following protein precipitation by the addition of 10% TCA, the supernatant was analyzed by HPLC. Chromatographic peaks were integrated and expressed as integrated units/mg of protein. Myocyte viability (Trypan blue) after l-arginine incubation did not differ between genotypes (∼60% for both).
Myocardial β3-AR expression was evaluated by using real time RT-PCR as previously described (24). The following primer sequences were used: sense, 5′-CTCCCCTGGTTCCATTCCTT-3′; and antisense, 5′-TGGTCTTTTCTACCCTGCTGC-3′. PCR conditions were 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The results were expressed as ΔCt, corresponding to the difference between the Ct of the gene of interest and the Ct of the housekeeping gene, hypoxanthine-guanine phosphoribosyltransferase.
eNOS protein level and the eNOS Thr-495 and Ser-1177 phosphorylated fractions were determined in LV myocytes lysates by using a mouse monoclonal anti-eNOS antibody (BD Transduction Laboratories) and antibodies against the eNOS Thr-495 and Ser-1177 phosphorylation sites (Cell Signaling) after stripping the membrane. S-Glutathionylation of eNOS (20) was evaluated by using an anti-glutathione monoclonal antibody (Virogen) in eNOS immunoprecipitates (Santa Cruz Biotechnologies) from nNOS−/− and wild type LV myocytes incubated with oxypurinol, apocynin (100 μm), DTT (100 mm), or vehicle. In some experiments, LV myocytes were incubated with DTT (100 μm) for 20 min before immunoprecipitation and immunoblotting (20). Co-localization of XOR with eNOS (BD Transduction Laboratories), NOX2 (Abcam), and nNOS (Santa Cruz Biotechnologies) was carried out in LV homogenates.
All of the data are expressed as the means ± S.E. Comparisons between genotypes were carried out using an unpaired t test. Comparisons of the effects of β3-AR stimulation between genotypes or groups were carried out using analysis of variance and the Scheffe's post hoc test. The null hypothesis was rejected at p < 0.05.
RESULTS
The Effect of β3-AR Stimulation Is Abolished in the Presence of nNOS Inhibition or Gene Deletion
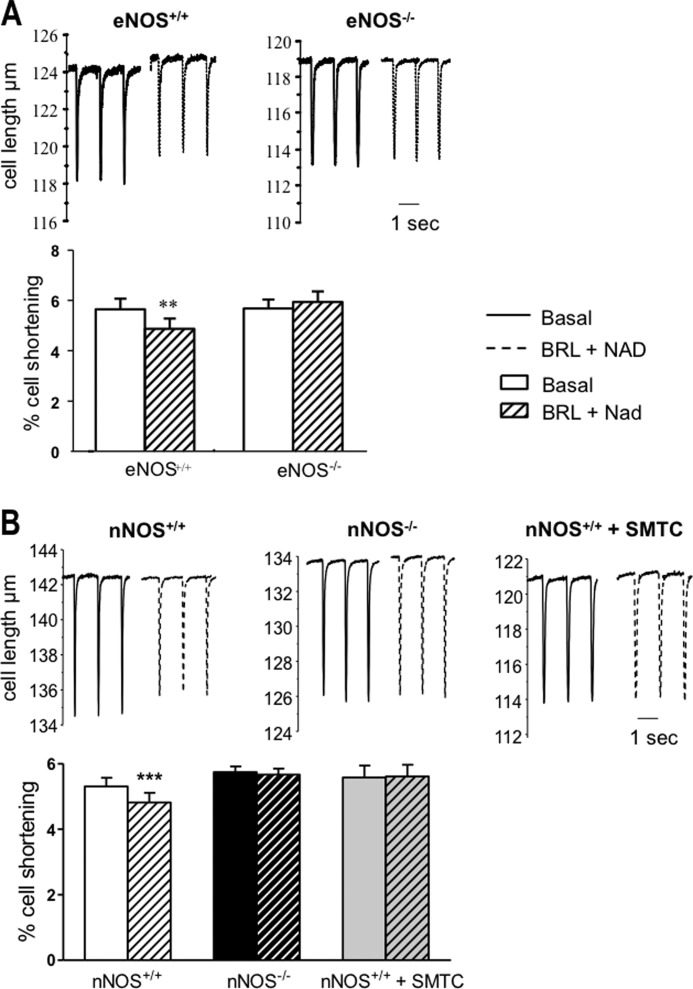
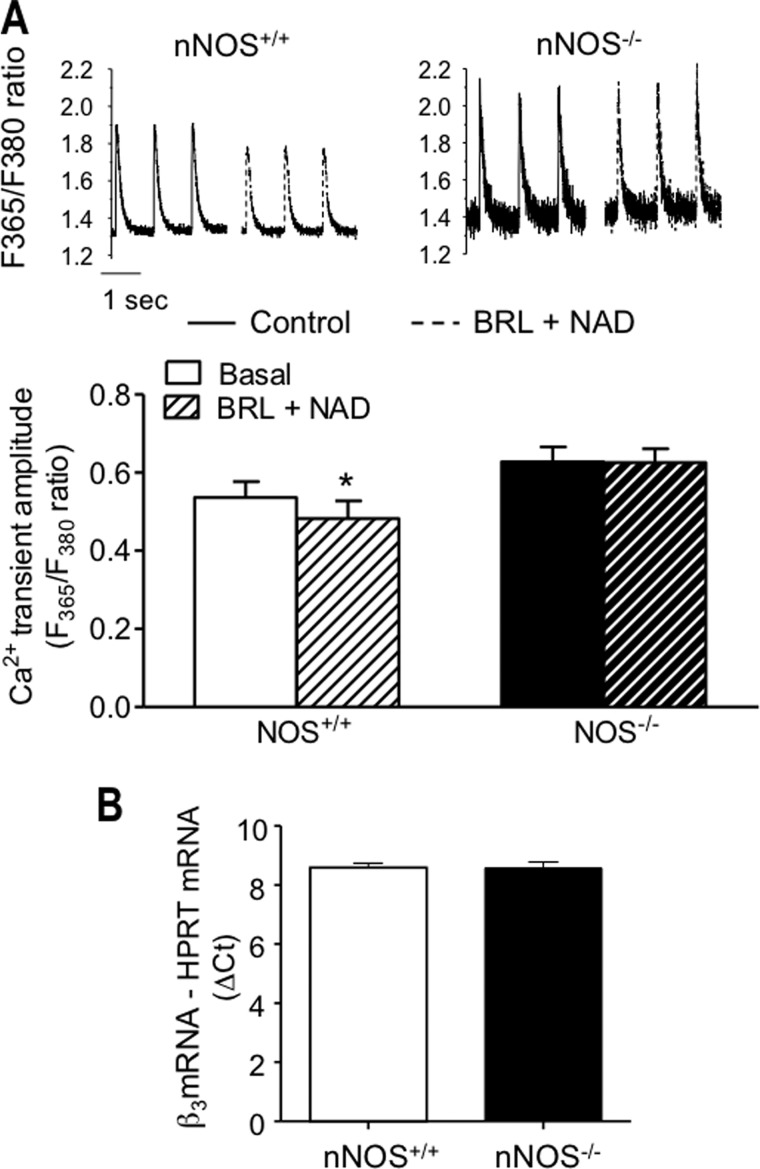
β3-AR stimulation with BRL+NAD resulted in a small but significant reduction in cell shortening in LV myocytes from both eNOS+/+ and nNOS+/+ mice (Fig. 1). As expected, BRL+NAD had no effect on contraction in myocytes from eNOS−/− mice (Fig. 1A); however, the negative inotropic effect of β3-AR stimulation was also abolished in nNOS−/− myocytes and in nNOS+/+ myocytes pretreated with the nNOS-specific inhibitor, SMTC (100 μm; Fig. 1B). Similarly, BRL+NAD decreased the amplitude of the [Ca2+]i transient in LV myocytes from both eNOS+/+ (in F365/F380, from 0.47 ± 0.04 to 0.43 ± 0.04 in the presence of BRL+NAD, n = 16, p = 0.0006) and nNOS+/+ mice (Fig. 2A) but had no effect in eNOS−/− myocytes (in F365/F380, from 0.41 ± 0.03 to 0.44 ± 0.04 in the presence of BRL+NAD, n = 10, p = 0.09) or in the presence of nNOS gene deletion (Fig. 2A) or inhibition with SMTC (in F365/F380, from 0.60 ± 0.07 under control conditions to 0.62 ± 0.07 in the presence of BRL+NAD, n = 14, p = 0.39). Real time RT-PCR showed that myocardial β3-AR gene expression did not differ between nNOS1−/− mice and their wild type littermates (Fig. 2B). Similarly, there were no differences in eNOS protein level in isolated LV myocytes (see Fig. 6B) and LV homogenates from nNOS−/− mice (data not shown). As reported previously (7, 25), basal cell shortening and Ca2+ transient amplitude (Figs. 1B and 2A) were significantly greater in nNOS−/− myocytes than in their wild type littermates, whereas no difference was found between eNOS+/+ and eNOS−/− myocytes (Fig. 1A).
FIGURE 1.
The negative inotropic effect of β3-AR stimulation with BRL (10 μm, in the presence of the β1- and β2-AR blocker NAD 10 μm) in murine LV myocytes is abolished in the presence of eNOS gene deletion (A, n = 18 LV myocytes) or nNOS disruption (B, n = 72 nNOS−/− myocytes and n = 20 nNOS+/+myocytes incubated with the nNOS inhibitor, SMTC). **, p < 0.01 for the effect of β3-AR stimulation in n = 15 eNOS+/+ myocytes; ***, p < 0.0001 for the effect of β3-AR stimulation in n = 39 nNOS+/+ myocytes.
FIGURE 2.
The reduction in the amplitude of the [Ca2+]i transient in response to β3-AR stimulation is abolished in LV myocytes from nNOS−/− mice (A; *, p < 0. 05 for the effect of β3-AR stimulation, n = 21 nNOS+/+ myocytes, and n = 19 nNOS−/− myocytes), in the absence of differences in β3-AR expression (B, n = 9 measurements from 3 hearts/genotype).
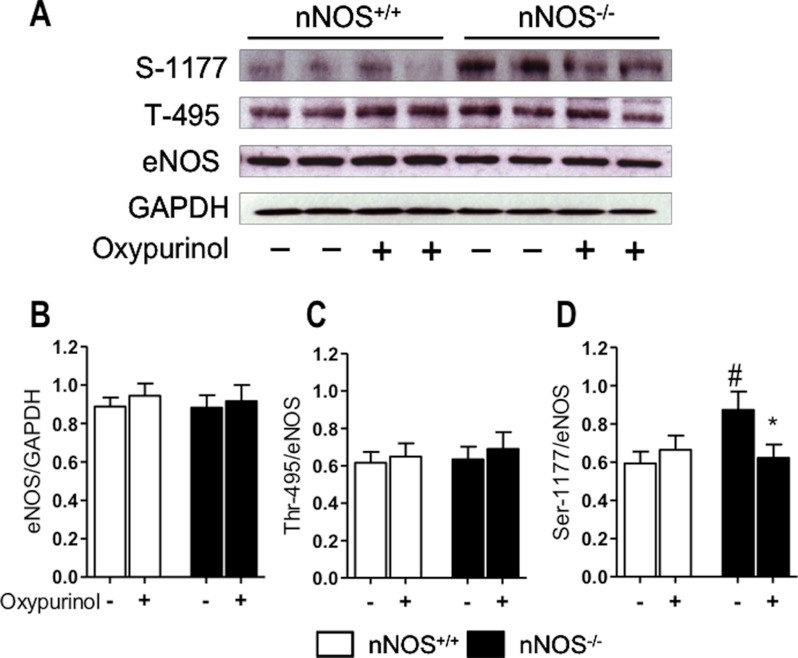
FIGURE 6.
Immunoblots show no difference in eNOS protein in LV myocytes from nNOS−/− and nNOS+/+ mice (A and B). eNOS phosphorylation at Ser-1177 is significantly greater in nNOS−/− mice, but this difference is abolished by oxypurinol (A and C). By contrast, eNOS phosphorylation at Thr-495 does not differ between genotypes or after oxypurinol (A and D). #, p < 0.05 for comparisons between genotypes; *, p < 0.05 for the effect of oxypurinol; n = 12 hearts/genotype.
XOR Inhibition Restores the Negative Inotropic Effect of β3-AR Stimulation in nNOS−/− Myocytes
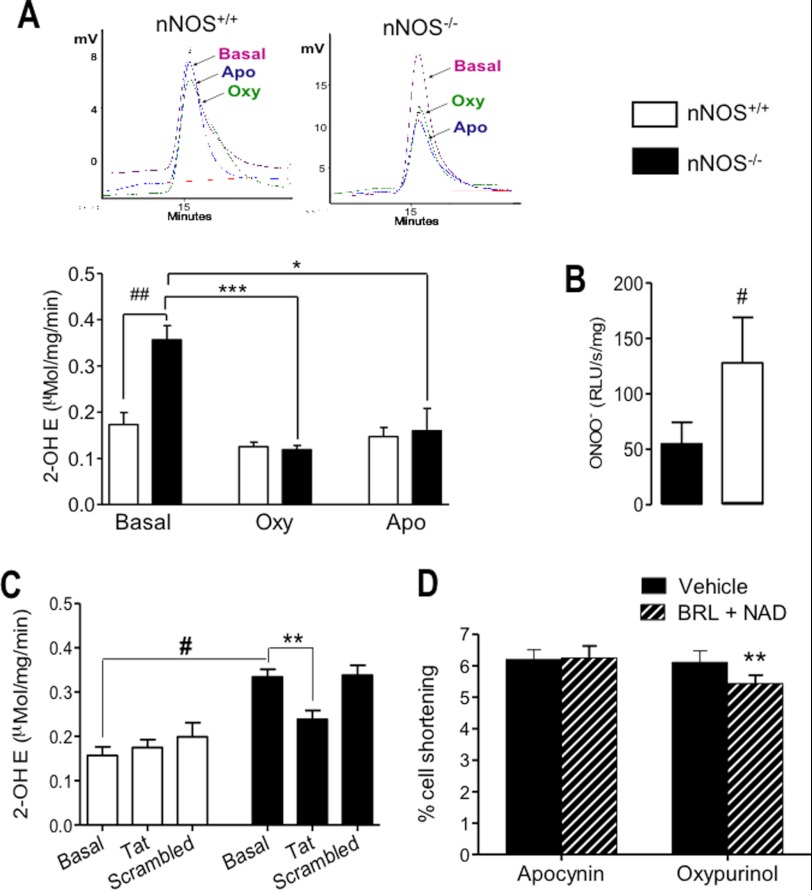
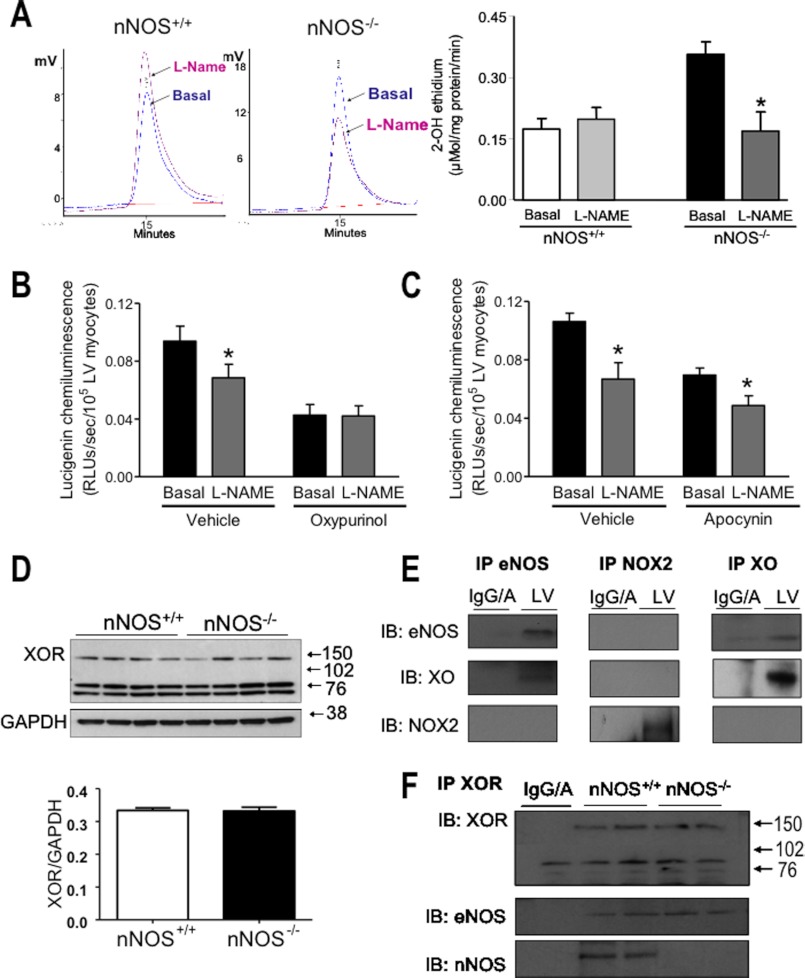
O2˙̄ production has been reported to be increased in LV homogenates and tissue chunks from nNOS−/− mice (15, 16). In agreement with these data, we found a significant increase in O2˙̄ in intact nNOS−/− LV myocytes using lucigenin-enhanced chemiluminescence (not shown) and confirmed these findings by an independent measurement of O2˙̄ production (2-hydroxyethidium detection by HPLC) in LV homogenates (Fig. 3A). Peroxynitrite release (by luminol-enhanced chemiluminescence) was also increased in LV homogenates from nNOS−/− mice (Fig. 3B). Inhibition of XOR by oxypurinol or NADPH oxidase by apocynin (Fig. 3A) or the gp91 ds tat peptide (Fig. 3C) significantly decreased O2˙̄ production in the nNOS−/− myocardium but had no significant effects in nNOS+/+ hearts. Following the application of either inhibitor, myocardial O2˙̄ production in nNOS−/− mice was no longer different from that recorded in wild type littermates.
FIGURE 3.
O2˙̄ (A) and peroxynitrite (B) production is increased in LV homogenates from nNOS−/− mice (A, top panels, typical 2-hydroxyethidium (2-OH E) chromatograms in nNOS+/+ and nNOS−/− LV homogenates; bottom panel, average data from n = 5 hearts/genotype). Both XOR inhibition with oxypurinol (Oxy) and NOX2 NADPH oxidase inhibition with apocynin (Apo) (A) or the gp91 ds tat-peptide (C) reduce O2˙̄ production in nNOS−/− mice, but only oxypurinol restores the negative inotropic effect of β3-AR stimulation (D). ##, p < 0.005; #, p < 0.05 versus nNOS+/+. ***, p < 0.001 for the effect of oxypurinol; *, p < 0.05 for the effect of apocynin; **, p < 0.01 for the effect of the gp91 ds tat-peptide (Tat versus samples treated with the scrambled peptide, Scrambled) or BRL in the presence of oxypurinol in nNOS−/− LV myocytes.
To evaluate whether reduced myocardial bioavailability of eNOS-derived NO secondary to increased O2˙̄ production may account for the lack of response to β3-AR stimulation in nNOS−/− myocytes, we examined the inotropic effect of BRL+NAD after inhibiting XOR or NADPH oxidases with oxypurinol or apocynin, respectively. Although both inhibitors reduced O2˙̄ release from nNOS−/− myocytes (Fig. 3C), oxypurinol restored the negative inotropic action of β3-AR stimulation in nNOS−/− myocytes, whereas apocynin had no effect (Fig. 3D). Neither apocynin nor oxypurinol restored the negative inotropic response to β3-AR stimulation in LV myocytes isolated from eNOS−/− mice (e.g., cell shortening was 5.57 ± 0.41% in the presence of oxypurinol and 5.49 ± 0.41% in the presence of oxypurinol and BRL + NAD, n = 15, p = 0.61). These data indicate that nNOS disruption is associated with an increase in myocardial O2˙̄ production from both XOR and NOX2 NADPH oxidases; however, only XOR inhibition restores the negative inotropic response to β3-AR stimulation in LV myocytes from nNOS−/− mice.
eNOS Activity Is Uncoupled in the LV Myocardium of nNOS−/− Mice
A XOR-dependent reduction in the myocardial bioavailability of eNOS derived-NO in nNOS−/− mice may be due to direct scavenging of NO by O2˙̄ and/or to eNOS uncoupling, a phenomenon whereby the catalytic electron flow within the enzyme is uncoupled from NO synthesis and diverted to molecular oxygen to yield O2˙̄ (17). Consistent with the latter, NOS inhibition with l-NAME caused a significant reduction in O2˙̄ production in LV homogenates from nNOS−/− mice (2-hydroxyethidium detection by HPLC; Fig. 4A). Similar results were obtained in isolated LV myocytes by using lucigenin-enhanced chemiluminescence (not shown). By contrast, l-NAME had no significant effect on O2˙̄ release in LV homogenates from wild type mice (Fig. 4A). The effect of l-NAME on O2˙̄ production in the nNOS−/− myocardium was abolished after XOR inhibition with oxypurinol (Fig. 4B) but not after apocynin (Fig. 4C).
FIGURE 4.
l-NAME decreases superoxide production in nNOS−/− LV homogenates but not in their wild type littermates. A, examples of 2-hydroxyethidium chromatograms with or without l-NAME (on the left) and average data from n = 4 hearts/genotype (on the right) indicate that cardiac eNOS activity is uncoupled in nNOS−/− mice (*, p < 0.05). B and C, both oxypurinol (B) and apocynin (C) decrease superoxide release in nNOS−/− LV myocytes but only oxypurinol restores coupled eNOS activity. *, p < 0.05 for the effect of l-NAME in nNOS−/− myocytes. D, XOR protein level (∼140 kDa) in LV homogenates did not differ between nNOS−/− and wild type mice (nNOS+/+); n = 12 hearts/genotype. E, in LV homogenates from wild type mice, eNOS co-localizes with XOR but not with NOX2 NADPH oxidases. F, XOR co-localizes with both eNOS and nNOS in the wild type LV myocardium and remains co-localized with eNOS in the presence of nNOS gene deletion. Note that the bands at ∼80 kDa are also visible in the IgA (negative) control; thus they do not represent XOR products. IP, immunoprecipitates; IB, immunoblots; IgG/A, IgG and IgA negative control.
XOR protein abundance (∼140 kDa) did not differ in the nNOS−/− myocardium (Fig. 4D). eNOS co-immunoprecipitated with XOR but not with NOX2 NADPH oxidases (Fig. 4E). nNOS was also detectable in XOR immunoprecipitates (Fig. 4F), as reported previously (15). These findings suggest a functional interaction between the constitutive NOS isoforms and XOR in murine LV myocytes and indicate that an increase in XOR-mediated O2˙̄ production selectively accounts for eNOS uncoupling in the myocardium of nNOS−/− mice.
Mechanism of eNOS Uncoupling in the Myocardium of nNOS−/− Mice
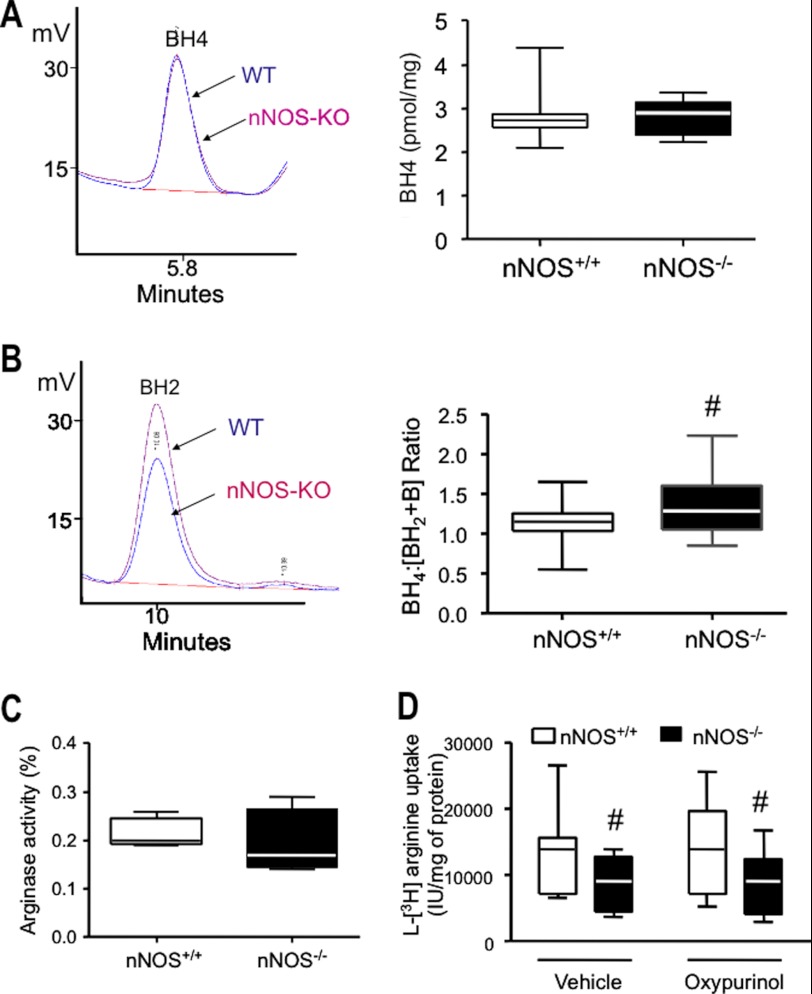
eNOS uncoupling may result from oxidation of the enzyme co-factor BH4 (17), decreased availability of l-arginine (26), and increased eNOS S-glutathionylation (20) or Thr-495 phosphorylation (27, 28). eNOS phosphorylation at Ser-1177 has been reported to increase both coupled and uncoupled eNOS activity (29). As shown in Fig. 5A, the BH4 content was virtually identical in LV myocytes from nNOS−/− and nNOS+/+ mice, whereas the ratio of BH4 to its oxidized products [BH2 + biopterin] was significantly increased in nNOS−/− mice (Fig. 5B), suggesting that the pool of O2˙̄ produced by XOR is not involved in myocardial BH4 oxidation.
FIGURE 5.
A and B, there are no differences in myocardial BH4 between genotypes (A, left panel, typical BH4 chromatogram; right panel, average data), whereas BH2 is significantly reduced in nNOS−/− mice (B, left panel, representative chromatogram), resulting in an increase in the ratio between BH4 and its oxidized products (B, right panel; #, p < 0.05 versus nNOS+/+ mice; n = 24 versus n = 23 hearts from nNOS−/− mice). C, myocardial arginase activity is not different in nNOS−/− mice (n = 6 hearts/genotype). D, arginine transport is decreased in LV myocytes from nNOS−/− mice and unchanged by oxypurinol (n = 9 hearts/genotype; #, p < 0.05 versus nNOS+/+ mice).
l-Arginine availability to eNOS depends on the active transport of the amino acid through the cell membrane via the cationic amino acid transporter and on its rate of utilization by competing tissue arginases (26). Arginase activity was unchanged in the LV myocardium of nNOS−/− mice (Fig. 5C). By contrast, the rate of l-arginine transport was lower in LV myocytes from nNOS−/− mice; however, incubation with oxypurinol had no effect on this measurement (Fig. 5D).
There was no difference in eNOS Thr-495 phosphorylation in LV myocytes isolated from nNOS−/− mice and their wild type littermates (Fig. 6C); however, phosphorylation of eNOS Ser-1177 was significantly increased in nNOS−/− myocytes (Fig. 6D). XOR inhibition with oxypurinol selectively reduced eNOS Ser-1177 phosphorylation in nNOS−/− myocytes and abolished the difference between genotypes (Fig. 6D).
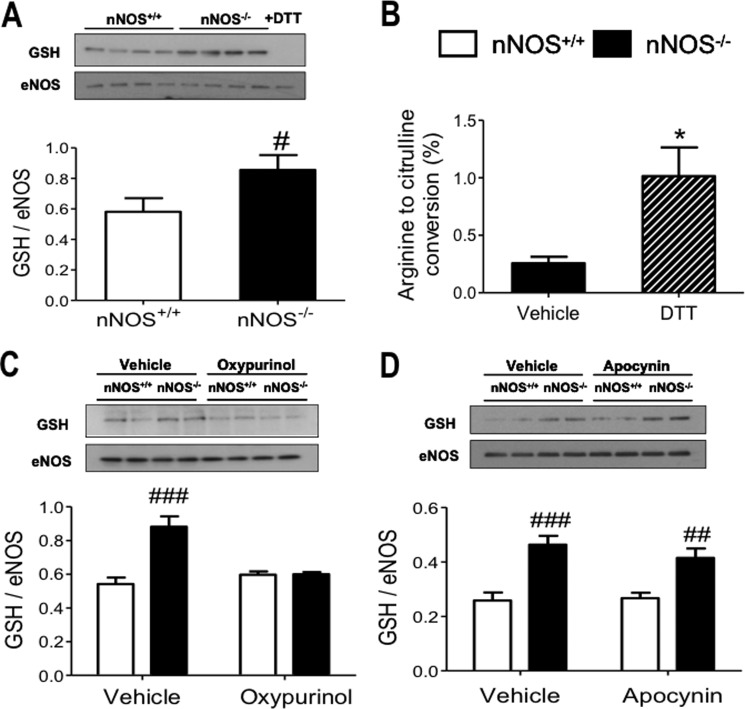
An increase in myocardial oxidative stress can lead to eNOS uncoupling by inducing S-glutathionylation of the enzyme (20). Fig. 7A shows that eNOS S-glutathionylation is increased in LV myocytes of nNOS−/− mice compared with wild type and that the reducing agent DTT abolished S-glutathionylation and greatly increased coupled eNOS activity in the nNOS−/− myocardium (Fig. 7B), consistent with a role of S-glutathionylation in suppressing the eNOS synthesis of NO in these mice. The difference in eNOS S-glutathionylation between genotypes was also abolished by incubation with oxypurinol (Fig. 7C, p < 0.0005 for the interaction between genotype and the effect of oxypurinol) but not by apocynin (Fig. 7D). These findings suggest that O2˙̄ production by XOR selectively increases eNOS S-glutathionylation and Ser-1177 phosphorylation of eNOS in LV myocytes from nNOS−/− mice.
FIGURE 7.
Immunoblots of eNOS immunoprecipitates with an anti-GSH antibody show that eNOS S-glutathionylation is increased in nNOS−/− LV myocytes (A; #, p < 0. 05 versus nNOS+/+ mice; n = 16 hearts/genotype) and abolished by the thiol-reducing agent DTT (100 μm). Incubation with DTT significantly increases eNOS activity in nNOS−/− LV myocardium (B; *, p < 0.05 for the effect of DTT). Incubation with oxypurinol abolishes the difference in eNOS S-glutathionylation between genotypes (C), whereas apocynin has no effect (D). ###, p < 0.001; ##, p < 0.01 versus nNOS+/+; n = 4–6 hearts/genotype.
DISCUSSION
The novel findings emerging from this work are as follow: 1) the eNOS-dependent negative inotropic effect of β3-AR stimulation and the associated reduction in the amplitude of the [Ca2+]i transient are abolished in LV myocytes from nNOS−/− mice, in the absence of differences in LV β3-AR mRNA expression and eNOS protein level between nNOS−/− mice and their wild type littermates. 2) Acute inhibition of nNOS mimics the effects of nNOS gene deletion, confirming that the abolition of the inotropic response to β3-AR stimulation is NO-dependent and does not reflect a secondary adaptation to chronic nNOS gene deletion. 3) Myocardial O2˙̄ production is significantly increased in nNOS−/− mice. Inhibition of XOR and NOX2 NADPH oxidase activity caused a similar reduction in O2˙̄ release; however, only XOR inhibition rescued the negative inotropic effect of β3-AR stimulation in nNOS−/− LV myocytes. Immunoprecipitation showed co-localization of eNOS with XOR but not with NOX2 NADPH oxidase. 4) eNOS activity is uncoupled in LV myocytes from nNOS−/− mice. XOR inhibition reversed eNOS uncoupling in nNOS−/− myocytes, whereas NOX2 NADPH oxidase inhibition did not. 5) arginase activity, eNOS Thr-495 phosphorylation, and the myocardial level of BH4 do not differ between nNOS−/− mice and their wild type littermates. Sarcolemmal l-arginine transport was reduced in nNOS−/− LV myocytes but remained unchanged after incubation with oxypurinol. By contrast, eNOS Ser-1177 phosphorylation and S-glutathionylation of eNOS were significantly higher in LV myocytes of nNOS−/− mice. These differences were abolished by DTT or oxypurinol but not by apocynin. DTT significantly increased eNOS coupled activity in the nNOS−/− LV myocardium.
In summary, enhanced XOR-mediated O2˙̄ release in nNOS−/− LV myocytes leads to reversible eNOS uncoupling and further O2˙̄ production by increasing the enzyme S-glutathionylation (20) and Ser-1177 phosphorylation (29) and abolishes the negative inotropic effects of β3-AR stimulation, indicating that aspects of the functional myocardial phenotype of nNOS−/− mice result from disruption of eNOS signaling. Inhibitors of XOR or NOX2 NADPH oxidases cause a similar reduction in myocardial O2˙̄ in the nNOS−/− myocardium, but only XOR inhibition reduces eNOS S-glutathionylation and Ser-1177 phosphorylation and restores both eNOS coupling and the inotropic response to β3-AR stimulation in nNOS−/− LV myocytes, suggesting that the subcellular localization of O2˙̄ release may account for the diverse and specialized actions of reactive oxygen species in the heart.
Constitutive NOS Activity and the β3-AR-mediated Regulation of Myocardial Inotropy
A negative inotropic response to β3-AR stimulation has been previously demonstrated in several (but not all) species and experimental models, including human endomyocardial biopsies (24, 30) and murine LV myocytes (4). Further work indicated that the signaling cascade downstream of β3-AR stimulation involves the production of NO, most likely through activation of eNOS (2, 3). In our study, both eNOS gene deletion and non-isoform-specific NOS inhibition with nitro-l-arginine, (data not shown) abolished the negative inotropic response to β3-AR stimulation in field stimulated LV myocytes, confirming an obligatory role for eNOS-derived NO in mediating this response. However, we found that the inotropic and [Ca2+]i transient response to β3-AR stimulation was also abolished in LV myocytes from nNOS−/− mice and in nNOS+/+ myocytes after pharmacological inhibition of nNOS with SMTC. Although the precise localization of β3-ARs in the myocyte sarcolemmal membrane remains to be established, their functional connection with eNOS suggests that they may be placed in proximity with caveolar microdomains. In contrast, in normal hearts, myocardial nNOS is mostly localized to the sarcoplasmic reticulum (15), implying that involvement of nNOS-derived NO in β3-AR signaling may be indirect. Khan et al. (15) and Kinugawa et al. (16) first showed an increased O2˙̄ production by XOR in the myocardium of nNOS−/− mice. XOR was also found to be located to the sarcoplasmic reticulum and to co-immunoprecipitate with nNOS, suggesting that the latter may inhibit XOR activity or play an important role in scavenging O2˙̄ produced by this oxidase system; excess O2˙̄ release by XOR may, in turn, disrupt eNOS signaling and regulatory function. Our findings are in keeping with this interpretation and provide further insights into the mechanism responsible for the reduced availability of eNOS-derived NO in the myocardium of nNOS−/− mice. The fact that a similar reduction in O2˙̄ production could be achieved by inhibiting XOR or NOX2 NADPH oxidases but that only XOR inhibition rescued the inotropic effect of β3-AR stimulation in nNOS−/− myocytes suggests that myocytes might have evolved mechanisms for localizing O2˙̄ release that are of key importance for achieving specificity of action (similar to the localization of NO signaling afforded by the spatial distribution of NO synthase isoforms) (1). Indeed, our findings indicate that eNOS co-localizes with XOR but not with NOX2 NADPH oxidases. The mechanism by which nNOS-derived NO regulates XOR activity remains uncertain. Decreased S-nitrosylation of XOR cysteine thiols may promote the oxidative conversion of xanthine dehydrogenase to xanthine oxidase (32); the latter preferentially utilizes oxygen (rather than NAD+) as an electron acceptor, leading to increased O2˙̄ generation (33). Similarly, reduced eNOS S-nitrosylation in the myocardium of nNOS−/− mice may make cysteine thiols in the eNOS reductase domain more prone to S-glutathionylation. Whether these findings in LV myocytes are mitigated by the paracrine effect of endothelial NO production or by the application of NO donors remains to be established.
Mechanisms of eNOS Uncoupling in the LV Myocardium of nNOS−/− Mice
The reduction in O2˙̄ observed after NOS inhibition with l-NAME indicates that myocardial eNOS activity is uncoupled in nNOS−/− mice, thereby contributing to the total pool of O2˙̄ release rather than synthesizing NO. XOR inhibition recovered both coupled eNOS function and the negative inotropic response to β3-AR stimulation. In the cardiovascular system, oxidation of the NOS critical co-factor BH4 (to BH2 and other biopterins) has been the most commonly reported mechanism responsible for myocardial eNOS uncoupling in the presence of oxidative stress (23, 34–36); however, we did not detect any difference in BH4 content; indeed, the ratio between BH4 and its oxidized products was increased in the myocardium of nNOS−/− mice, implying that increased O2˙̄ production by XOR does not lead to BH4 oxidation under these conditions, presumably either because of compartmentalization of the BH4 pool away from XOR or secondary to an adaptive induction of the BH4 “salvage” pathway, as observed in diabetes (37). l-Arginine deficiency has also been implicated in NOS uncoupling. Insufficient l-arginine availability to NOS can result from up-regulation of myocardial arginase activity or insufficient entry of l-arginine via the membrane transporter. We found that l-arginine transport was decreased in LV myocytes from nNOS−/− mice; however, XOR inhibition with oxypurinol (which restores both eNOS coupling and the response to β3-AR stimulation in nNOS−/− myocytes) did not affect the rate of l-arginine transport in nNOS−/− myocytes, implying that decreased l-arginine bioavailability to eNOS via this mechanism is unlikely to account for eNOS uncoupling and suppressed β3-AR responses in nNOS−/− myocytes. More recently, Chen et al. (20) reported that S-glutathionylation of cysteine residues in the reductase domain of eNOS results in reversible enzyme uncoupling and O2˙̄ production, implying that, together with BH4 oxidation, this mechanism could account for eNOS dysfunction in response to redox stress (e.g., in the presence of diabetes mellitus (37)). Our findings suggest that XOR-dependent O2˙̄ production can uncouple eNOS activity by increasing the enzyme S-glutathionylation in the absence of changes in BH4 availability and BH4 oxidation products. Because BH4 depletion in the myocardium has only been documented in the presence of severe or long standing myocardial stress (e.g., left ventricular pressure overload, prolonged global ischemia, or persistent atrial fibrillation (23, 34–36)), it is possible that S-glutathionylation plays a more dynamic role in the regulation of eNOS function in response to subtler changes in the myocardial redox state. Although eNOS uncoupling in the nNOS−/− mouse does not cause LV dysfunction per se, a contribution of this mechanism to the more severe adverse LV remodeling observed in nNOS−/− mice after a myocardial infarction (38–40) cannot be excluded.
There was no difference in eNOS Thr-495 phosphorylation between genotypes or after oxypurinol, suggesting that an increase in protein kinase C-mediated phosphorylation of eNOS at this site (27, 28) is unlikely to contribute to eNOS uncoupling in the myocardium of nNOS−/− mice. By contrast, we observed an oxypurinol-reversible increase in eNOS phosphorylation at Ser-1177 in LV myocytes from nNOS−/− mice. Phosphorylation at Ser-1177 has recently been shown to increase both coupled and uncoupled eNOS activity, thereby stimulating both NO and O2˙̄ production from the enzyme (29). This implies that, in addition to causing eNOS uncoupling by increasing the enzyme S-glutathionylation (20), XOR-derived O2˙̄ induces changes in eNOS phosphorylation that are expected to increase the rate of O2˙̄ production from the uncoupled enzyme, further increasing myocardial oxidative stress following the disruption of nNOS signaling. An increase in eNOS Ser-1177 phosphorylation in the presence of oxidative stress is unusual but not unique (41). Whereas Akt-mediated Ser-1177 phosphorylation of endothelial eNOS has been consistently found to be inhibited in oxidative conditions (42, 43), PKA-mediated phosphorylation of myocardial eNOS at the same residue could increase, because oxidation of thiol residues in type I PKA (by H2O2 (44) or xanthine)4 can directly activate the kinase (44).
Conclusions
Our findings provide unequivocal evidence of a functional interaction between the myocardial constitutive NOS isoforms and indicate that aspects of the myocardial phenotype of nNOS−/− mice result from disruption of eNOS signaling. Loss of sarcolemmal nNOS leads to functional ischemia and muscle damage in patients with Duchenne muscular dystrophy (31, 45, 46); our findings suggest that oxidative stress and disruption of eNOS signaling resulting from loss of nNOS may also contribute to the impaired vasodilatation and ischemic damage observed in the skeletal and cardiac muscle of patients with dystrophinopathies and constitute a possible target for therapeutic intervention. By the same token, attenuation of XOR-mediated myocardial oxidative stress and preservation of myocardial eNOS function may contribute to the beneficial effect of nNOS up-regulation in the failing myocardium (12, 13).
This work was supported by a Wellcome Trust Prize Studentship (to W. O. I.) and funds from the British Heart Foundation (to Y. H. Z., M. H. Z., R. C., M. J. C., and B. C.), the Oxford Biomedical Research Centre (to R. J. and B. C.), and the Fondation Leducq (to B. C., S. R., and J.-L. B.).
X. Sun, M. H. Zhang, and B. Casadei, unpublished results.
- AR
- adrenergic receptor
- NOS
- nitric-oxide synthase
- nNOS
- neuronal NOS
- eNOS
- endothelial NOS
- LV
- left ventricular
- SMTC
- S-methyl-l-thiocitrulline
- l-NAME
- l-Nω-nitroarginine methyl ester
- BH4
- tetrahydrobiopterin
- XOR
- xanthine oxidoreductase
- NAD
- nadolol
- BH2
- 7,8-dihydropterin
- BRL
- BRL 37344.
REFERENCES
- 1. Zhang Y. H., Casadei B. (2012) Sub-cellular targeting of constitutive NOS in health and disease. J. Mol. Cell Cardiol. 52, 341–350 [DOI] [PubMed] [Google Scholar]
- 2. Gauthier C., Leblais V., Kobzik L., Trochu J. N., Khandoudi N., Bril A., Balligand J. L., Le Marec H. (1998) The negative inotropic effect of β3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Invest. 102, 1377–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brixius K., Bloch W., Pott C., Napp A., Krahwinkel A., Ziskoven C., Koriller M., Mehlhorn U., Hescheler J., Fleischmann B., Schwinger R. H. (2004) Mechanisms of β3-adrenoceptor-induced eNOS activation in right atrial and left ventricular human myocardium. Br. J. Pharmacol. 143, 1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barouch L. A., Harrison R. W., Skaf M. W., Rosas G. O., Cappola T. P., Kobeissi Z. A., Hobai I. A., Lemmon C. A., Burnett A. L., O'Rourke B., Rodriguez E. R., Huang P. L., Lima J. A., Berkowitz D. E., Hare J. M. (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416, 337–339 [DOI] [PubMed] [Google Scholar]
- 5. Cheng H. J., Zhang Z. S., Onishi K., Ukai T., Sane D. C., Cheng C. P. (2001) Upregulation of functional β3-adrenergic receptor in the failing canine myocardium. Circ. Res. 89, 599–606 [DOI] [PubMed] [Google Scholar]
- 6. Au A. L., Kwan Y. W. (2002) Modulation of L-type Ca2+ channels by β3-adrenoceptor activation and the involvement of nitric oxide. J. Card. Surg. 17, 465–469 [DOI] [PubMed] [Google Scholar]
- 7. Sears C. E., Bryant S. M., Ashley E. A., Lygate C. A., Rakovic S., Wallis H. L., Neubauer S., Terrar D. A., Casadei B. (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ. Res. 92, e52–e59 [DOI] [PubMed] [Google Scholar]
- 8. Sun J., Picht E., Ginsburg K. S., Bers D. M., Steenbergen C., Murphy E. (2006) Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ. Res. 98, 403–411 [DOI] [PubMed] [Google Scholar]
- 9. Wang H., Kohr M. J., Traynham C. J., Wheeler D. G., Janssen P. M., Ziolo M. T. (2008) Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am. J. Physiol. Cell Physiol. 294, C1566–C1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y. H., Zhang M. H., Sears C. E., Emanuel K., Redwood C., El-Armouche A., Kranias E. G., Casadei B. (2008) Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ. Res. 102, 242–249 [DOI] [PubMed] [Google Scholar]
- 11. Carnicer R., Hale A. B., Suffredini S., Liu X., Reilly S., Zhang M. H., Surdo N. C., Bendall J. K., Crabtree M. J., Lim G. B., Alp N. J., Channon K. M., Casadei B. (2012) Cardiomyocyte GTP cyclohydrolase 1 and tetrahydrobiopterin increase NOS1 activity and accelerate myocardial relaxation. Circ. Res. 111, 718–727 [DOI] [PubMed] [Google Scholar]
- 12. Bendall J. K., Damy T., Ratajczak P., Loyer X., Monceau V., Marty I., Milliez P., Robidel E., Marotte F., Samuel J. L., Heymes C. (2004) Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in β-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 110, 2368–2375 [DOI] [PubMed] [Google Scholar]
- 13. Damy T., Ratajczak P., Shah A. M., Camors E., Marty I., Hasenfuss G., Marotte F., Samuel J.-L., Heymes C. (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363, 1365–1367 [DOI] [PubMed] [Google Scholar]
- 14. Mohamed T. M., Oceandy D., Prehar S., Alatwi N., Hegab Z., Baudoin F. M., Pickard A., Zaki A. O., Nadif R., Cartwright E. J., Neyses L. (2009) Specific role of neuronal nitric-oxide synthase when tethered to the plasma membrane calcium pump in regulating the β-adrenergic signal in the myocardium. J. Biol. Chem. 284, 12091–12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan S. A., Lee K., Minhas K. M., Gonzalez D. R., Raju S. V., Tejani A. D., Li D., Berkowitz D. E., Hare J. M. (2004) Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. U.S.A. 101, 15944–15948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinugawa S., Huang H., Wang Z., Kaminski P. M., Wolin M. S., Hintze T. H. (2005) A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ. Res. 96, 355–362 [DOI] [PubMed] [Google Scholar]
- 17. Vásquez-Vivar J., Kalyanaraman B., Martásek P., Hogg N., Masters B. S., Karoui H., Tordo P., Pritchard K. A., Jr. (1998) Superoxide generation by endothelial nitric oxide synthase. The influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 95, 9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyamu E. W., Perdew H., Woods G. M. (2008) Cysteine-iron promotes arginase activity by driving the Fenton reaction. Biochem. Biophys. Res. Commun. 376, 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Venardos K., Zhang W.-Z., Lang C., Kaye D. M. (2009) Effect of peroxynitrite on endothelial l-arginine transport and metabolism. Int. J. Biochem. Cell Biol. 41, 2522–2527 [DOI] [PubMed] [Google Scholar]
- 20. Chen C.-A., Wang T.-Y., Varadharaj S., Reyes L. A., Hemann C., Talukder M. A., Chen Y.-R., Druhan L. J., Zweier J. L. (2010) S-Glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468, 1115–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang P. L., Dawson T. M., Bredt D. S., Snyder S. H., Fishman M. C. (1993) Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273–1286 [DOI] [PubMed] [Google Scholar]
- 22. Shesely E. G., Maeda N., Kim H. S., Desai K. M., Krege J. H., Laubach V. E., Sherman P. A., Sessa W. C., Smithies O. (1996) Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 93, 13176–13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reilly S. N., Jayaram R., Nahar K., Antoniades C., Verheule S., Channon K. M., Alp N. J., Schotten U., Casadei B. (2011) Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation. Implications for the antiarrhythmic effect of statins. Circulation 124, 1107–1117 [DOI] [PubMed] [Google Scholar]
- 24. Moniotte S., Kobzik L., Feron O., Trochu J. N., Gauthier C., Balligand J. L. (2001) Upregulation of β3-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103, 1649–1655 [DOI] [PubMed] [Google Scholar]
- 25. Ashley E. A., Sears C. E., Bryant S. M., Watkins H. C., Casadei B. (2002) Cardiac nitric oxide synthase 1 regulates basal and β-adrenergic contractility in murine ventricular myocytes. Circulation 105, 3011–3016 [DOI] [PubMed] [Google Scholar]
- 26. Berkowitz D. E., White R., Li D., Minhas K. M., Cernetich A., Kim S., Burke S., Shoukas A. A., Nyhan D., Champion H. C., Hare J. M. (2003) Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108, 2000–2006 [DOI] [PubMed] [Google Scholar]
- 27. Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001) Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 88, e68–e75 [DOI] [PubMed] [Google Scholar]
- 28. Lin M. I., Fulton D., Babbitt R., Fleming I., Busse R., Pritchard K. A., Jr., Sessa W. C. (2003) Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of l-arginine metabolism to efficient nitric oxide production. J. Biol. Chem. 278, 44719–44726 [DOI] [PubMed] [Google Scholar]
- 29. Chen C. A., Druhan L. J., Varadharaj S., Chen Y. R., Zweier J. L. (2008) Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J. Biol. Chem. 283, 27038–27047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gauthier C., Tavernier G., Charpentier F., Langin D., Le Marec H. (1996) Functional β3-adrenoceptor in the human heart. J. Clin. Invest. 98, 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai Y., Thomas G. D., Yue Y., Yang H. T., Li D., Long C., Judge L., Bostick B., Chamberlain J. S., Terjung R. L., Duan D. (2009) Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 119, 624–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishino T., Nishino T. (1997) The conversion from the dehydrogenase type to the oxidase type of rat liver xanthine dehydrogenase by modification of cysteine residues with fluorodinitrobenzene. J. Biol. Chem. 272, 29859–29864 [DOI] [PubMed] [Google Scholar]
- 33. McManaman J. L., Neville M. C., Wright R. M. (1999) Mouse mammary gland xanthine oxidoreductase. Purification, characterization, and regulation. Arch. Biochem. Biophys. 371, 308–316 [DOI] [PubMed] [Google Scholar]
- 34. Takimoto E., Champion H. C., Li M., Ren S., Rodriguez E. R., Tavazzi B., Lazzarino G., Paolocci N., Gabrielson K. L., Wang Y., Kass D. A. (2005) Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Invest. 115, 1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dumitrescu C., Biondi R., Xia Y., Cardounel A. J., Druhan L. J., Ambrosio G., Zweier J. L. (2007) Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc. Natl. Acad. Sci. U.S.A. 104, 15081–15086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silberman G. A., Fan T.-H., Liu H., Jiao Z., Xiao H. D., Lovelock J. D., Boulden B. M., Widder J., Fredd S., Bernstein K. E., Wolska B. M., Dikalov S., Harrison D. G., Dudley S. C., Jr. (2010) Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation 121, 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuhmacher S., Oelze M., Bollmann F., Kleinert H., Otto C., Heeren T., Steven S., Hausding M., Knorr M., Pautz A., Reifenberg K., Schulz E., Gori T., Wenzel P., Münzel T., Daiber A. (2011) Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes 60, 2608–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dawson D., Lygate C. A., Zhang M. H., Hulbert K., Neubauer S., Casadei B. (2005) nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation 112, 3729–3737 [DOI] [PubMed] [Google Scholar]
- 39. Saraiva R. M., Minhas K. M., Raju S. V., Barouch L. A., Pitz E., Schuleri K. H., Vandegaer K., Li D., Hare J. M. (2005) Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction. Role of nitroso-redox equilibrium. Circulation 112, 3415–3422 [DOI] [PubMed] [Google Scholar]
- 40. Burger D. E., Lu X., Lei M., Xiang F.-L., Hammoud L., Jiang M., Wang H., Jones D. L., Sims S. M., Feng Q. (2009) Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation 120, 1345–1354 [DOI] [PubMed] [Google Scholar]
- 41. Hoshino S., Kikuchi Y., Nakajima M., Kimura H., Tsuyama S., Uemura K., Yoshida K. (2005) Endothelial NO synthase (eNOS) phosphorylation regulates coronary diameter during ischemia-reperfusion in association with oxidative stress. Free Radic. Res. 39, 481–489 [DOI] [PubMed] [Google Scholar]
- 42. Du X. L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Invest. 108, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zou M. H., Hou X. Y., Shi C. M., Nagata D., Walsh K., Cohen R. A. (2002) Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J. Biol. Chem. 277, 32552–32557 [DOI] [PubMed] [Google Scholar]
- 44. Brennan J. P., Bardswell S. C., Burgoyne J. R., Fuller W., Schröder E., Wait R., Begum S., Kentish J. C., Eaton P. (2006) Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J. Biol. Chem. 281, 21827–21836 [DOI] [PubMed] [Google Scholar]
- 45. Thomas G. D., Sander M., Lau K. S., Huang P. L., Stull J. T., Victor R. G. (1998) Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 95, 15090–15095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sander M., Chavoshan B., Harris S. A., Iannaccone S. T., Stull J. T., Thomas G. D., Victor R. G. (2000) Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U.S.A. 97, 13818–13823 [DOI] [PMC free article] [PubMed] [Google Scholar]