Background: Spermatozoa are excellent model cells to analyze the dynamics of SNARE assembly during exocytosis.
Results: Both an antibody that blocks Munc18-1 function and recombinant Munc18-1 prevent the assembly of trans-SNARE complexes.
Conclusion: Munc18-1 plays a key function in the dynamics of trans-SNARE complex assembly.
Significance: Unveiling the mechanism of acrosomal exocytosis will improve our understanding of fertilization and regulated secretion.
Keywords: Exocytosis, Membrane Fusion, Neurotoxin, SNARE Proteins, Spermatozoa
Abstract
The spermatozoon is a very specialized cell capable of carrying out a limited set of functions with high efficiency. Sperm are then excellent model cells to dissect fundamental processes such as regulated exocytosis. The secretion of the single dense-core granule of mammalian spermatozoa relies on the same highly conserved molecules and goes through the same stages as exocytosis in other types of cells. In this study, we describe the presence of Munc18-1 in human sperm and show that this protein has an essential role in acrosomal exocytosis. We observed that inactivation of endogenous Munc18-1 with a specific antibody precluded the stabilization of trans-SNARE complexes and inhibited acrosomal exocytosis. Addition of recombinant Munc18-1 blocked secretion by sequestering monomeric syntaxin, an effect that was rescued by α-soluble NSF attachment protein. By electron microscopy, we observed that both the anti-Munc18-1 antibody and recombinant Munc18-1 inhibited the docking of the acrosome to the plasma membrane. In conclusion, our results indicate that Munc18-1 plays a key role in the dynamics of trans-SNARE complex assembly and/or stabilization, a process that is necessary for the docking of the outer acrosomal membrane to the plasma membrane and subsequent fusion pore opening.
Introduction
Intracellular trafficking is an essential feature of all eukaryotic cells. Cargo molecules are transported among the different membrane-bound compartments by highly regulated membrane fusion events. In particular, exocytosis is the fusion of secretory granules with the plasma membrane. A complex molecular machinery, well conserved along evolution, is necessary to fuse intracellular compartments. Its basic components include Rab GTPases, Sec1/Munc18 (SM)2 proteins, and SNAREs (1, 2). In regulated exocytosis, fusion is exquisitely controlled, and a number of other factors are involved (3–5).
The SNARE superfamily comprises a large set of proteins carrying a characteristic ∼60-residue “SNARE motif” with heptad repeats (6). These motifs are assembled in four-helix bundles characteristic of SNARE complexes. SNAREs in opposite membranes assemble into trans-SNARE complexes (also known as SNAREpins) forcing membranes close together as they zip up to promote fusion (7). After fusion, the fully zippered SNAREs remaining in the fused organelle are in a cis configuration. Disassembly of these cis-SNARE complexes requires the concerted action of α-SNAP and NSF, whereby NSF uses energy from ATP hydrolysis to dissociate SNARE complexes (8). Exocytic SNAREs belong to the following three families: syntaxin and SNAP-23/25, present in the plasma membrane, and VAMP/synaptobrevin (VAMP/Syb) in the granule membrane (9, 10).
A requirement for SM proteins has been found in all the intracellular membrane fusion events that have been analyzed, from yeast to mammalian cells. In particular, Munc18-1 is involved in exocytosis in neuroendocrine cells. Distinct binding modes between Munc18-1 and SNARE proteins have been reported (11, 12). In vitro, Munc18-1 binds with high affinity to syntaxin 1 in its closed conformation. Syntaxin has a three-helix N-terminal “Habc” domain that folds back and binds the SNARE motif, precluding the formation of SNARE complexes. Considering that interactions between SNAREs are essential for exocytosis, one would expect that Munc18-1 serves as a negative regulator of exocytosis (13, 14). However, this view cannot be reconciled with the phenotype of Munc18-1-deficient animals and yeast (15–17). In fact, most studies strongly suggest that Munc18-1 has an activating rather than an inhibitory role in exocytosis (15, 18, 19). Moreover, Munc18-1 binds SNARE complexes and strongly accelerates the fusion reaction of reconstituted liposomes (12, 20–23).
The spermatozoon, the male gamete, is a highly differentiated cell capable of finding the female gamete, sorting all the physical barriers that protect this immobile cell, and fusing with the oocyte to generate a diploid zygote (24). To perform these complex tasks, the spermatozoon undergoes dramatic morphological and functional changes during spermiogenesis, resulting in a very specialized cell capable of carrying out a limited set of functions with high efficiency. Sperm are excellent model cells to dissect fundamental processes such as flagellum-mediated cell motility (25), chemotaxis (26–28), and regulated exocytosis (29, 30). The acrosome reaction is a regulated secretion event essential for fertilization. As in other exocytoses, the plasma membrane and granule membrane must fuse to release the acrosomal contents. However, acrosomal exocytosis is special in several aspects. In mammals, the acrosome is a very large and flat granule covering the nucleus. The membrane facing the plasma membrane is called the outer acrosomal membrane. Upon stimulation, multiple fusion pores form, connecting the interior of the acrosome with the extracellular medium. These fusion pores expand, causing the fenestration of both membranes and the release of the granule contents together with hybrid vesicles composed of patches of plasma membrane and outer acrosomal membrane (31). The molecular mechanism participating in the membrane fusion process necessary for acrosomal exocytosis has been intensively studied by us and other laboratories (Refs. 29, 30 and references therein). Acrosome release shares a common molecular mechanism with other calcium-dependent exocytoses reported in different cell types. Acrosomal exocytosis requires, among others, SNAREs (29, 32–34), NSF and α-SNAP (35–37), Rab3A (35, 38–40), complexin (34, 41, 42), RIM and Munc13 (43), and synaptotagmin (44–46). According to several pieces of evidence, we have proposed that, in resting sperm, SNAREs are assembled in stable cis complexes in both plasma and acrosomal membranes, probably because NSF is phosphorylated and inactivated (36, 47). Upon stimulation, calcium coming from the extracellular medium triggers Rab27/Rab3A activation (48) and SNARE complex disassembly (47). The disentanglement of cis complexes requires NSF activation, a process that is mediated by the phosphotyrosine phosphatase PTP1B (36). Free SNAREs are now able to re-assemble in trans. The process goes through a transient stage where SNAREs are assembled in partially zippered (loose) trans complexes stabilized by complexin and resistant to tetanus toxin (TeTx) but sensitive to botulinum neurotoxins (BoNT) (47). Electron transmission images show that the acrosomal and the plasma membranes are in tight apposition at this stage (49). We refer to this morphological (i.e. membranes at less than 8 nm) and biochemical (i.e. sensitive to BoNTs and resistant to TeTx) condition as the “docked” state of the acrosome. The final fusion step requires a local increase of calcium coming from the acrosome through inositol 1,4,5-trisphosphate-sensitive calcium channels (47, 50). Calcium activates the synaptotagmin-dependent relief of the complexin block, and acrosomal exocytosis is completed (42).
Here, we describe the presence of Munc18-1 in human sperm and show that this protein has an essential role in acrosomal exocytosis. We observed that inactivation of endogenous Munc18-1 with a specific antibody precluded the stabilization of trans-SNARE complexes and inhibited acrosomal exocytosis. Addition of recombinant Munc18-1 blocked secretion by sequestering syntaxin, an effect that was rescued by α-SNAP. By electron microscopy, we observed that both the anti-Munc18-1 antibody and recombinant Munc18-1 inhibited the docking of the outer acrosomal to the plasma membrane.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant streptolysin O (SLO) was obtained from Dr. Bhakdi (University of Mainz, Mainz, Germany). Spermatozoa were cultured in human tubal fluid media (as formulated by Irvine Scientific, Santa Ana, CA) supplemented with 0.5% bovine serum albumin. The Munc18-1 peptide and rabbit polyclonal anti-Munc18-1 (serum), the rabbit polyclonal anti-syntaxin1A (serum), and anti-VAMP2/syb2 (clone 69.1, purified IgG) antibodies were from Synaptic Systems (Göttingen, Germany). Rabbit polyclonal anti-syntaxin 1 and anti-Munc18 antibody from Abcam (Cambridge, MA) were used exclusively for the Western blot shown in Fig. 1B. Horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L), Cy3-conjugated goat anti-rabbit and goat anti-mouse IgG (H+L) were from Jackson ImmunoResearch (West Grove, PA). O-Nitrophenyl EGTA-acetoxymethyl ester (NP-EGTA-AM), N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), and BAPTA-AM were from Molecular Probes (Eugene, OR). Pre-stained molecular weight markers were from Boston BioProducts Inc. (Worcester, MA). Nickel-nitrilotriacetic acid-agarose was from GE Healthcare. All electron microscopy supplies were from Pelco (Ted Pella Inc.). All other chemicals were purchased from Sigma, Genbiotech, or Tecnolab (all from Buenos Aires, Argentina).
FIGURE 1.
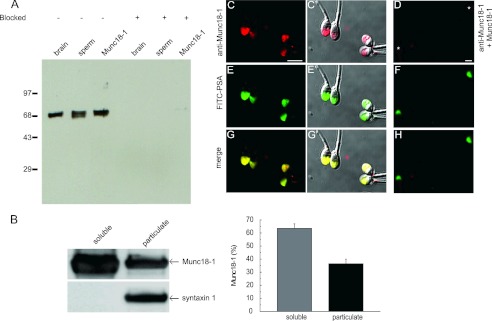
Munc18-1 is present in human sperm. A, postnuclear membrane pellet from rat brain (1 μg of protein, brain), a human sperm extract (5 × 106 cells corresponding to 5 μg of protein, sperm), and recombinant Munc18-1 (Munc18-1, 1 ng) were resolved in 10% Tricine gels, transferred to nitrocellulose membranes, and probed with an anti-Munc18-1 antibody from Synaptic Systems (left) or the antibody preblocked with the recombinant protein (4-fold molar excess, right). Molecular weight markers ( × 10−3) are indicated on the left. B, sperm disrupted by nitrogen cavitation and separated into particulate and soluble fractions by ultracentrifugation were loaded (5 × 106 cells) onto SDS-polyacrylamide gels and probed on blots with the anti-Munc18-1 (top) and anti-syntaxin 1 (bottom) antibodies from Abcam (left). The percentage in each fraction was quantified in three independent experiments; the mean ± S.E. is shown on the right. C–H, sperm were fixed on coverslips and labeled with the anti-Munc18-1 antibody from Synaptic Systems. The acrosomal region was stained with fluorescein isothiocyanate-coupled PSA-FITC that recognizes the intra-acrosomal content. Shown are epifluorescence micrographs of typically stained cells with the anti-Munc18-1 antibody (C) and anti-Munc18-1 antibody preblocked with 4-fold excess recombinant protein (D), PSA-FITC (E and F), and colocalization (G and H). In C′, E′, and G′, the fluorescence images were superimposed to differential interference contrast (DIC) images to depict the localization of the labels within the cells. Notice that Munc18-1 localized to the acrosomal region. Bar, 5 μm. Asterisks in D indicate cells with intact acrosomes but without Munc18-1 staining.
Recombinant Proteins
A pQE9 (Qiagen GmbH, Hilden, Germany) construct encoding full-length wild type α-SNAP was a kind gift from Dr. S. Whiteheart (University of Kentucky, Lexington). The N-terminal truncated mutant α-SNAP(160–295) in pQE30 (Qiagen) was generously provided by Dr. A. Morgan, and the full-length protein bearing the point mutation L294A and cloned in pQE30 (Qiagen) was a kind gift from Dr. R. Burgoyne (both from the University of Liverpool, Liverpool, UK). Plasmids encoding Munc18-1, NSF, the cytosolic domains of syntaxin 1(1–262), syntaxin 1(25–262), and syntaxin 1(1–262, I233A) in pET28a (Stratagene, La Jolla, CA) were generously provided by Dr. D. Fasshauer (Max-Planck Institute for Biophysical Chemistry, Göttingen, Germany). Expression plasmids encoding the light chain of wild type TeTx and BoNT/C and BoNT/C-E230A fused to His6 (pQE3, Qiagen) were generously provided by Dr. T. Binz (Medizinische Hochschule Hannover, Hannover, Germany), and the enzymatically inactive mutant (TeTx-E234Q) was generously provided by Dr. R. Jahn (Max-Planck Institute for Biophysical Chemistry, Göttingen, Germany). The expression plasmid encoding amino acids 1–321 of wild type PTP1B fused to His6 in pET21b (Stratagene) was kindly provided by Dr. N. Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). An expression plasmid pQE-80L containing the cDNA-encoding human Rab3A was generously provided by Dr. C. López (Cuyo University, Mendoza, Argentina). Purification of His6-tagged recombinant proteins was carried out under native conditions according to instructions (Qiagen) except that the purification buffers contained 20 mm Tris-HCl, pH 7.4, instead of 50 mm phosphate, pH 8; NaCl was 200 mm for NSF and 500 mm for the rest; lysis buffer contained 2 mm imidazole; washing buffer contained 8 mm imidazole; and elution buffer contained 400 mm imidazole. 0.5 mm ATP, 5 mm MgCl2, 5% glycerol, and 2 mm β-mercaptoethanol were added to all buffers involved in the purification of His6-NSF. The His6 tag was cleaved from Munc18-1 by incubation with thrombin during dialysis. Thrombin activity was stopped with 2 mm PMSF. Syntaxins were extracted from bacterial pellets under denaturing conditions (6 m urea) because most of the proteins accumulated in inclusion bodies. Rab3A was prenylated and loaded with GTPγS as described previously (48). According to a Triton X-114 partition assay (48), the prenylation efficiency was about 90%,3 Recombinant protein concentrations were determined by the protein assay (Bio-Rad) in 96-well microplates. BSA was used as a standard, and the results were quantified on a 3550 microplate reader (Bio-Rad).
Human Sperm Preparation and Acrosomal Exocytosis Assay
Human semen samples were obtained from normal healthy donors. Semen was allowed to liquefy for 30–60 min at 37 °C. We used a swim-up protocol to isolate highly motile sperm. Sperm concentrations were adjusted to 7–10 × 106/ml before incubating for at least 2 h under capacitating conditions (human tubal fluid-0.5% BSA, 37 °C, 5% CO2, 95% air). Sperm were washed once with PBS and incubated in cold PBS containing 2.1 units/ml SLO for 15 min at 4 °C. Cells were washed as before and resuspended in ice-cold sucrose buffer (250 mm sucrose, 0.5 mm EGTA, 20 mm Hepes-K, pH 7) containing 2 mm DTT. We added inhibitors and stimulants sequentially as indicated in the figure keys and incubated for 10–15 min at 37 °C after each addition. When indicated, we preloaded SLO-permeabilized sperm with photo-inhibitable NP-EGTA-AM before incubating in the presence of inhibitors and/or calcium, carrying out all procedures in the dark. Photolysis was induced after the last incubation by exposing twice (1 min each time) to a UV transilluminator (FBTIV-614, Fisher), mixing, and incubating for 5 min at 37 °C. Sperm were spotted on Teflon-printed slides, air-dried, and fixed/permeabilized in ice-cold methanol for 1 min. Acrosomal status was evaluated by staining with FITC-coupled Pisum sativum (PSA-FITC) according to Ref. 51. At least 200 cells were scored using an upright Nikon microscope equipped with epifluorescence optics. Basal (“control,” no stimulation) and positive (“calcium,” 0.5 mm CaCl2 corresponding to 10 μm free calcium estimated by Maxchelator (Chris Patton, Stanford University, CA) controls were included in all experiments. Acrosomal exocytosis indexes were calculated by subtracting the number of spontaneously reacted spermatozoa from all values and expressing the results as a percentage of the acrosome reaction observed in the positive control. Data were evaluated using one-way analysis of variance. The Tukey-Kramer post hoc test was used for pairwise comparisons. Differences were considered significant at the p < 0.05 level.
Indirect Immunofluorescence for Munc18-1
Sperm were spotted on polylysine-coated 9-mm round coverslips before fixing/permeabilizing in 2% paraformaldehyde, 0.1% Triton X-100 in PBS for 10 min at room temperature. Sperm were incubated in PBS containing 100 mm glycine overnight at 4 °C and washed twice with PBS containing 0.4% polyvinylpyrrolidone (PVP; 40,000 average molecular weight; ICN) (PBS/PVP). Nonspecific staining was blocked by incubation in 5% bovine serum albumin in PBS/PVP for 1 h at room temperature. Cells were labeled with an anti-Munc18-1 antibody (1 h at 37 °C, 1:200 in 3% BSA-PBS/PVP), followed by Cy3-conjugated goat anti-rabbit IgG (H+L) (1 h at 37 °C, 5 μg/ml in 1% BSA PBS/PVP). Coverslips were washed (three times) with PBS/PVP between incubations. Samples were mounted with Gelvatol and stored at 4 °C in the dark. Slides were examined with an Eclipse TE2000 Nikon microscope equipped with a Plan Apo 60×/1.40 oil objective and a Hamamatsu Orca 100 camera (Hamamatsu Corp., Bridgewater, NJ) operated with MetaMorph 6.1 software (Universal Imaging, Downingtown, PA). Images were processed using Image J (freeware from National Institutes of Health).
Indirect Immunofluorescence for VAMP2/syb2 and Syntaxin 1
We processed permeabilized human sperm as described for acrosome reaction assays, adding inhibitors and calcium (stimulant) sequentially as indicated in the figure keys and incubating for 8–10 min at 37 °C after each addition. Subsequently, we attached 7–10 × 105 sperm to polylysine-coated 9-mm round coverslips and processed as explained above. Nonspecific staining was blocked by incubation in 5% BSA in PBS/PVP either with (anti-syntaxin 1A immunostaining) or without (anti-VAMP2/syb2 immunostaining) 0.2% SDS for 1 h at 37 °C. Anti-syntaxin 1A (1:50) or anti-VAMP2/syb2 (20 μg/ml) antibodies were diluted in 3% bovine serum albumin in PBS/PVP (incubation for 1 h at 37 °C). After washing twice for 10 min with 2% saponin in PBS, Cy3-conjugated goat anti-rabbit or anti-mouse IgGs (5 μg/ml in 1% bovine serum albumin in PBS/PVP) were added and incubated for 1 h at room temperature protected from light. Coverslips were washed six times for 6 min with PBS/PVP. Cells were subsequently stained for acrosomal contents as described before, mounted with 1% propyl gallate, 50% glycerol in PBS, and stored at 4 °C. The presence of immunostaining in the acrosomal region was scored in digital images from at least 10 fields containing ≥200 cells in total.
Human Sperm Subcellular Fractionation
We used two different cell-disruption methods. For hypo-osmotic shock, we followed the protocol described previously (52). Briefly, capacitated sperm were washed twice with PBS and diluted 1:9 in hypo-osmotic swelling buffer; after 2 h at 37 °C in a water bath, at least 80% of the cells were swollen. We transferred the suspensions to ice, disrupted sperm by sonication, and centrifuged at 4,000 × g (4 °C, 15 min) to remove cell debris. We centrifuged the supernatant at 10,000 × g (4 °C, 10 min) and the resultant supernatant at 208,000 × g (4 °C, 2 h). The final pellets containing the particulate fraction as well as the final supernatants containing the soluble fraction were stored at −70 °C until use. For nitrogen cavitation, we followed the protocol described previously (34) with slight modifications. Briefly, 1 × 109 Percoll-washed human sperm cells were resuspended in 5 mm Tris, 250 mm sucrose, pH 7.4, and cavitated using a cell disruption device (Parr Instruments, Moline, IL). The cavitate fraction was centrifuged twice at 1,000 × g for 10 min at 4 °C. Supernatants were combined and centrifuged at 4 °C for 10 min at 6,000 × g. The supernatant was pelleted at 285,000 × g at 4 °C for 70 min. The final membrane pellet and supernatant were frozen in liquid nitrogen and stored at −70 °C for later use. When required, proteins were precipitated with CCl3H-CH3OH and dissolved in sample buffer by heating once at 60 °C for 10 min and once at 95 °C for 3 min.
SDS-PAGE and Western Blots
Proteins were separated on 8% Tris/Tricine-SDS gels or 8% Tris-glycine SDS gels and transferred to 0.22-μm nitrocellulose membranes (Hybond, GE Healthcare). Nonspecific reactivity was blocked by incubation for 1 h at room temperature with 5% skim milk dissolved in washing buffer (PBS, pH 7.6, 0.2% Tween 20). Blots were incubated with the anti-Munc18-1 antibody (1:10,000 in blocking solution for 1 h at room temperature). Horseradish peroxidase-conjugated goat anti-rabbit IgG was used as secondary antibody (0.25 μg/ml, 1 h of incubation, room temperature). Excess first and second antibodies were removed by washing five times for 10 min in washing buffer. Detection was accomplished with chemiluminescence systems from either PerkinElmer Life Sciences or Millipore on a Luminescent Image Analyzer LAS-4000 (Fujifilm, Tokyo, Japan) or exposure to Pierce CL-XPosure Film (Tecnolab). Quantification of signal intensities was carried out with ImageJ.
Transmission Electron Microscopy
We processed permeabilized human sperm as described for acrosomal exocytosis assays except that after incubation with calcium to initiate exocytosis, we added 1.6 mm tannic acid and incubated for 5 min. Afterward, we fixed sperm suspensions in 2.5% glutaraldehyde overnight at 4 °C in PBS/calcium (PBS containing 2 mm CaCl2, pH 7.4). Fixed sperm samples were embedded in 1.5% low melting point agarose, postfixed in 1% osmium tetroxide in PBS for 2 h, washed three times with PBS, and dehydrated sequentially with increasing concentrations of ice-cold acetone. Cells were infiltrated in 1:1 acetone/Epon for 2 h at room temperature and finally embedded in fresh resin. We cut thin sections (60–80 nm) with a diamond knife (Diatome, Washington, D. C.) on a Leica Ultracut R ultramicrotome, collected them on 200-mesh copper grids, and stained them with saturated uranyl acetate in methanol and lead citrate. Grids were observed and photographed in a Zeiss EM 902 at 50 kV. We included negative (not stimulated) and positive (stimulated with calcium in the presence of 2-aminoethoxydiphenyl borate (2-APB) to inhibit the final stages of the AR) controls in all experiments.
RESULTS
Presence and Subcellular Localization of Munc18-1 in Human Sperm
Members of the SM family of proteins are essential for intracellular membrane fusion events. Given that neuronal SNAREs are present in human sperm (32), we investigated the possible participation of Munc18-1 in the secretion of the acrosomal granule. We searched for the presence of Munc18-1 in human sperm by Western blot using an anti-Munc18-1 antibody. Analysis of whole human sperm extracts showed the presence of a single protein band with apparent molecular mass of 68 kDa that co-migrated with the positive controls (rat brain and recombinant rat Munc18-1, Fig. 1A, left lanes). Bands were not observed when the antibody was preincubated with recombinant Munc18-1 (Fig. 1A, right lanes), confirming the specificity of the antibody. To determine the subcellular localization of Munc18-1 in human sperm, cells were broken by nitrogen cavitation or hypo-osmotic shock. Soluble and particulate fractions were separated by centrifugation and probed by Western blot. Densitometric analysis revealed that about 60% of Munc18-1 was soluble when cells were disrupted by nitrogen cavitation (Fig. 1B). Similar results were observed when cells were broken by hypo-osmotic shock (70% of Munc18-1 was recovered in the soluble fraction, see supplemental Fig. 1). Indirect immunofluorescence was used to localize Munc18-1 on fixed sperm. The anti-Munc18-1 antibody co-localized with PSA-FITC, a bona fide acrosomal marker (Fig. 1G). This fluorescence pattern was specific because it was not observed when the antibody was previously blocked with recombinant Munc18-1 (Fig. 1D). Taken together, these observations indicate that Munc18-1 is present in human sperm and localizes to the acrosomal region, consistent with a function in acrosomal exocytosis.
Munc18-1 Participates in Acrosomal Exocytosis
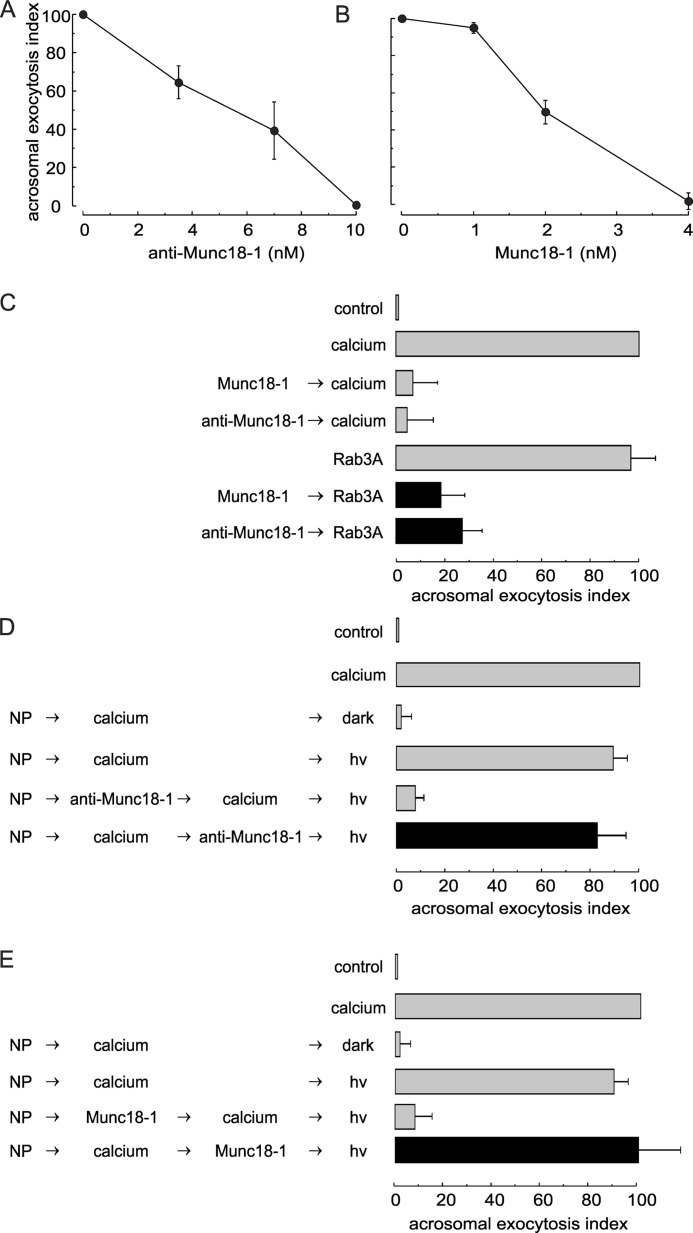
To test whether Munc18-1 has a role in the membrane fusion process leading to acrosomal exocytosis, the anti-Munc18-1 antibody was introduced into SLO-permeabilized human sperm before challenging with calcium to trigger secretion. Exocytosis was strongly inhibited by the antibody in a dose-response fashion with an EC50 of about 6 nm (Fig. 2A). In other exocytic models, both genetic depletion and overexpression of Munc18 inhibit exocytosis (15, 16, 53–56). Therefore, we tested the effect of loading permeabilized sperm with recombinant rat Munc18-1 on acrosomal exocytosis. Munc18-1 inhibited calcium-triggered exocytosis with an EC50 of about 2 nm (Fig. 2B). Almost complete inhibition was observed with 4 nm Munc18-1. We have estimated the molar excess of recombinant Munc18-1 over the endogenous protein by comparing the Western blot signals in nitrocellulose membranes loaded with known concentrations of recombinant protein and sperm extracts (e.g. Western blot in Fig. 1A). From these calculations, we estimate that 4 nm recombinant Munc18-1 represents a 200-fold excess over the endogenous protein present in the 350,000 cells included in each functional assay. An early event during acrosomal exocytosis is the activation of Rab3A. In fact, prenylated and GTP-loaded Rab3A induces acrosomal exocytosis (57). The anti-Munc18-1 antibody and recombinant Munc18-1 inhibited secretion triggered by this small GTPase, showing that Rab3A-stimulated exocytosis also depends on Munc18-1 (Fig. 2C).
FIGURE 2.
Munc18-1 is essential for human sperm acrosomal exocytosis. A and B, SLO-permeabilized human sperm were treated for 15 min at 37 °C with increasing concentrations of anti-Munc18-1 antibody (A) or recombinant Munc18-1 (B). Exocytosis was initiated with 0.5 mm CaCl2, and cells were incubated for a further 15 min. The values represent mean ± S.E. of at least three independent experiments. C, permeabilized sperm were treated with 4 nm Munc18-1 (Munc18-1) or 10 nm anti-Munc18-1 antibody (anti-Munc18-1) before initiating acrosomal exocytosis with 300 nm prenylated and GTPγS-loaded Rab3A (Rab3A, black bars). Controls (gray bars) included the following: background exocytosis in the absence of any stimulation (control); exocytosis stimulated by 0.5 mm CaCl2 or 300 nm GTPγS-bound Rab3A, and the effect of 4 nm Munc18-1 or 10 nm anti-Munc18-1 on calcium-stimulated exocytosis. D and E, SLO-permeabilized sperm were loaded with 10 μm NP-EGTA-AM (NP) for 10 min at 37 °C to chelate intravesicular calcium. Next, acrosomal exocytosis was initiated by adding 0.5 mm CaCl2 (calcium), and samples were incubated for further 10 min at 37 °C. This protocol allows exocytosis to proceed up to the intra-acrosomal calcium-sensitive step. At this stage, sperm were treated for 10 min with 10 nm anti-Munc18-1 (anti-Munc18-1; D) or 4 nm Munc18-1 (Munc18-1; E). All these procedures were carried out in the dark. UV photolysis of the chelator was induced at the end of the incubation period (hv), and the samples were incubated for 5 min to promote exocytosis (black bars). Several controls were included (gray bars) as follows: background exocytosis in the absence of any stimulation (control); exocytosis stimulated by 0.5 mm CaCl2; inhibitory effect of NP-EGTA-AM in the dark and recovery upon illumination, and the inhibitory effect of anti-Munc18-1 and Munc18-1 when present throughout the experiment. For all panels, acrosomal exocytosis was evaluated using PSA-FITC, and data were normalized as described under “Experimental Procedures.” The values represent mean ± S.E. of at least three independent experiments. The anti-Munc18-1 antibody used for these experiments was from Synaptic Systems.
Next, we explored at which step of the exocytic cascade Munc18-1 is required. Several pieces of evidence indicate that calcium coming from the extracellular medium is not sufficient for triggering acrosomal exocytosis (47, 50). A local increase in calcium supplied by intracellular stores (most likely the acrosome) is necessary to promote the interplay among SNARE complexes, complexin, and synaptotagmin that leads to the opening of fusion pores. We speculate that a local calcium increase is required to provide the necessary concentration in the scarce and very compartmentalized cytoplasm located between the outer acrosomal and the plasma membranes. By using a membrane-permeable, light-sensitive calcium chelator (NP-EGTA-AM) that accumulates intra-acrosomally in SLO-permeabilized sperm, we have been able to distinguish factors that act upstream or downstream of the acrosomal calcium release (47). When permeabilized sperm with NP-EGTA-AM-loaded acrosomes are stimulated in the dark, exocytosis proceeds up to a stage that requires the release of calcium from inside the acrosome. If an inhibitor affecting a factor that is necessary before the acrosomal calcium release is added at this point, exocytosis will be completed when NP-EGTA-AM is photoinactivated, because the factor is not longer required. If exocytosis is not completed after inactivating the chelator, it means that the factor acts downstream from the acrosomal calcium release. When the anti-Munc18-1 antibody was tested in the NP-EGTA-AM assay, it was not inhibitory when added after stimulating with calcium (Fig. 2D, black bar). This observation indicates that endogenous Munc18-1 participates at a stage before the acrosomal calcium efflux. The same result was obtained with recombinant Munc18-1 (Fig. 2E, black bar), indicating that addition of this protein affects a step upstream from the release of calcium from the acrosome. In conclusion, according to these observations, Munc18-1 has an active role in acrosomal exocytosis at a stage before the intra-acrosomal calcium efflux that triggers membrane fusion.
Depletion of Munc18-1 Blocks Acrosomal Exocytosis at a Stage Prior to SNARE Assembly in trans Complexes
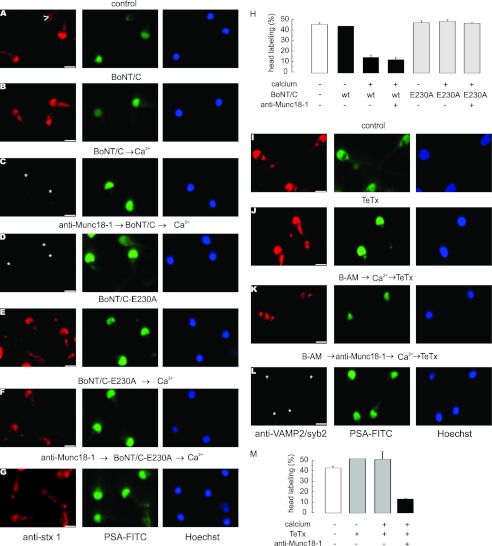
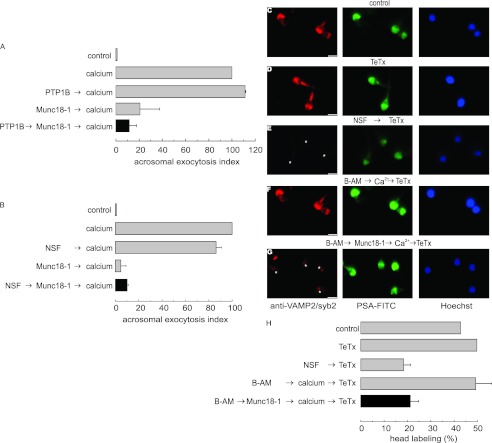
We have previously reported that SNAREs are assembled in neurotoxin-resistant cis complexes in resting sperm (47). Upon calcium stimulation, cis complexes are disentangled, releasing monomeric proteins sensitive to all neurotoxins. The acrosome swells, and the outer acrosomal membrane contacts the plasma membrane promoting the formation of loose trans-SNARE complexes sensitive to BoNTs but resistant to TeTx. At this “docking” stage, calcium released from the acrosome is required to trigger membrane fusion. Therefore, the results shown in Fig. 2 indicate that Munc18-1 is involved in exocytosis at stages where SNAREs pass from cis to loose trans complexes. It was then relevant to assess the effect of Munc18-1 depletion on SNARE toxin sensitivity during exocytosis. We initially investigated whether the anti-Munc18-1 antibody interferes with SNARE complex disassembly. In a first approach, we tested syntaxin sensitivity to the light chain of BoNT/C by indirect immunofluorescence. The rationale of this assay is that when this protein is cleaved by the toxin, the anti-syntaxin antibody does not recognize the fragments and the immunolabel is lost (47). Syntaxin has a distinct acrosomal labeling pattern in about 50% of the cells, which was similar in unstimulated sperm in the absence (Fig. 3A) or presence (Fig. 3B) of BoNT/C, reinforcing the notion that SNAREs are toxin-resistant in resting sperm. Upon stimulation with calcium, the acrosomal labeling was lost in most cells, indicating that syntaxin became toxin-sensitive (Fig. 3C). The presence of the anti-Munc18-1 antibody did not prevent this calcium-dependent sensitization, indicating that the depletion of active endogenous Munc18-1 did not impair the disassembly of cis complexes (Fig. 3D). To show that the diminished syntaxin labeling was due to proteolysis, similar experiments were carried out with BoNT/C-E230A, a mutant without proteolytic activity. This protein did not affect syntaxin immunofluorescence in any condition (Fig. 3, E–G). Immunofluorescence quantitations are shown in Fig. 3H.
FIGURE 3.
Anti-Munc18-1 antibody does not interfere with cis-SNARE complex disassembly but prevents trans-SNARE complex assembly. A–G, permeabilized spermatozoa were loaded, when indicated, with 10 nm anti-Munc18-1 (anti-Munc18-1, D and G), subsequently treated, when indicated, with 100 nm BoNT/C (BoNT/C, B–D) or the catalytically dead BoNT/C (BoNT/C-E230A, E–G), and finally stimulated, when indicated, with 0.5 mm CaCl2 (Ca2+, C, D, F, and G) or left untreated (A, B, and E). The cells were then fixed and triple stained with the rabbit polyclonal anti-syntaxin 1A antibody (red, left panels), PSA-FITC (to differentiate between reacted and intact sperm; green, central panels), and Hoechst 33342 (to visualize all cells in the field; blue, right panels). Asterisks indicate cells with intact acrosomes but without syntaxin 1 immunostaining due to toxin cleavage. Arrowhead in A shows a spermatozoon that has lost the acrosome and the syntaxin 1 immunolabel. Bars, 5 μm. H, quantification of the percentage of cells treated as in A–G exhibiting syntaxin 1 acrosomal staining from three independent experiments (mean ± S.E.). I–L, permeabilized spermatozoa were loaded, when indicated, with 10 μm BAPTA-AM (B-AM, K and L) and with 10 nm anti-Munc18-1 (L), subsequently stimulated, when required, with 0.5 mm CaCl2 (K and L), and finally incubated (except the control) with 100 nm TeTx (J–L). The cells were then fixed and triple stained with the mouse monoclonal anti-VAMP2/syb2 antibody (red, left panels), PSA-FITC (green, central panels), and Hoechst 33342 (blue, right panels). Asterisks indicate cells with intact acrosomes but without VAMP2/syb2 immunostaining due to toxin cleavage. M, quantification of the percentage of cells treated as in I–L exhibiting VAMP2/syb2 acrosomal staining from three independent experiments (mean ± S.E.). The anti-Munc18-1 and anti-syntaxin 1 antibodies used for these experiments were from Synaptic Systems.
The previous results are compatible with two possibilities as follows: (i) in the absence of active Munc18-1, cis SNARE complexes are disassembled, and the formation of loose trans complexes is prevented, leaving the proteins as monomers, which are sensitive to BoNT/C; or (ii) in the absence of active Munc18-1, cis SNARE complexes are disassembled but the individual proteins reassemble in loose trans complexes, which are also sensitive to BoNT/C. To distinguish between these two scenarios, we used TeTx, a toxin that cleaves monomeric VAMP2/syb2 but does not hydrolyze this protein when assembled in complexes. In agreement with previous results, the percentage of VAMP2/syb2 labeling was similar in unstimulated sperm in the absence (Fig. 3I) or presence (Fig. 3J) of TeTx. When permeabilized sperm were stimulated in the presence of BAPTA-AM, to deplete intra-acrosomal calcium and prevent membrane loss due to exocytosis, the fusion process progressed to a stage where loose trans complexes, insensitive to TeTx, were formed (Fig. 3K). In contrast, in the presence of anti-Munc18-1, VAMP2/syb2 was TeTx-sensitive (Fig. 3L), suggesting that the formation of ternary trans complexes had been prevented. Immunofluorescence quantitations are shown in Fig. 3M. Taken together, these data suggest that Munc18-1 is required for the assembly of trans-SNARE complexes.
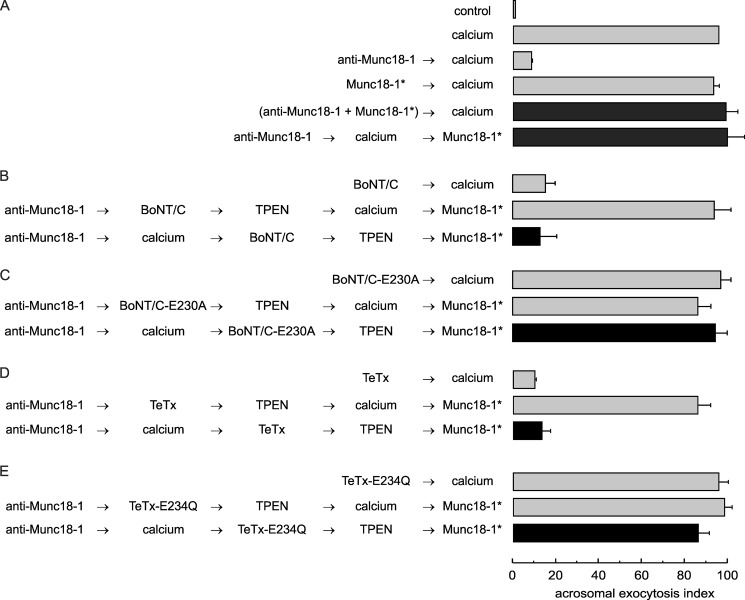
We performed the next series of experiments to investigate whether the findings depicted in Fig. 3 have a functional correlation in exocytosis. For this, we needed to set up conditions to reverse the inhibitory effect of the anti-Munc18-1 antibody. This was accomplished by adding the peptide (Munc18-1*) used to generate this antibody either together with the antibody or at the end of the incubation (Fig. 4A, black bars). To stop neurotoxin action, we chelated Zn2+, an ion essential for the toxin proteolytic activity, by adding TPEN (47). SLO-permeabilized sperm were loaded with anti-Munc18-1 and incubated with calcium to initiate exocytosis. BoNT/C was added, and the mixture was incubated under conditions in which the toxin could cleave syntaxin; subsequently, TPEN was added to inactivate the toxin and the peptide to relieve the exocytotic block from anti-Munc18-1. Calcium failed to elicit exocytosis under these conditions (Fig. 4B, black bar). A BoNT/C mutant, lacking protease activity, was used as control. This protein did not block exocytosis under any condition (Fig. 4C).
FIGURE 4.
Functional assay confirms that the anti-Munc18-1 antibody inhibits acrosomal exocytosis by preventing trans-SNARE complex assembly. A, SLO-permeabilized sperm were treated for 15 min at 37 °C with 10 nm anti-Munc18-1 antibody alone or preincubated with 40 nm of the peptide used to generate the antibody (Munc18-1*). Acrosomal exocytosis was initiated with 0.5 mm CaCl2 (37 °C for 15 min). When indicated, Munc18-1* was added at the end of the incubation to rescue the exocytotic block (37 °C for 10 min). B–E, permeabilized sperm were incubated with 10 nm anti-Munc18-1 antibody for 10 min at 37 °C followed by 0.5 mm CaCl2 to initiate exocytosis. After 10 min, 100 nm of the light chain of wild type BoNT/C (B), catalytically dead BoNT/C-E230A (C), wild type TeTx (D), or catalytically dead TeTx-E234Q (E) was added and incubated for a further 10 min to assess toxin sensitivity at the step blocked by the antibody. We stopped toxin activity with 2.5 μm TPEN for 10 min and released the block with 40 nm Munc18-1* for 10 min at 37 °C (black bars). Controls (gray bars) included the following: background exocytosis in the absence of any stimulation (control), exocytosis stimulated by 0.5 mm CaCl2, and the effect of 10 nm anti-Munc18-1 or 40 nm Munc18-1* on calcium-stimulated exocytosis. Controls also included the inhibitory effect of 100 nm wild type BoNT/C and wild type TeTx, and the lack of effect of the inactive neurotoxins. Finally, another set of controls showed that the toxins cannot affect exocytosis if cells are not stimulated and that TPEN efficiently inactivates neurotoxins. Sperm were fixed, and acrosomal exocytosis was evaluated using PSA-FITC, and data were normalized as described under “Experimental Procedures.” The values represent mean ± S.E. of at least three independent experiments. The anti-Munc18-1 antibody used for these experiments was from Synaptic Systems.
The same experiments were repeated substituting BoNT/C by TeTx to test whether VAMP2/syb2 was protected in SNARE complexes. This toxin blocked secretion, indicating that stable SNARE complexes were not formed in the presence of the anti-Munc18-1 antibody (Fig. 4D, black bar). In contrast, when we used the inactive toxin, no inhibition of the exocytosis was observed (Fig. 4E). In conclusion, immunofluorescence and functional assays indicate that inactivation of Munc18-1 does not prevent cis-SNARE disassembly but blocks the assembly of trans complexes.
Recombinant Munc18-1 Blocks Acrosomal Exocytosis by Sequestering Syntaxin
In some exocytic models, overexpression of Munc18 blocks secretion (55, 56). In Fig. 2, we have shown that recombinant Munc18-1 inhibits exocytosis initiated by calcium or Rab3A and prior to the calcium efflux from the acrosome. We wondered whether this protein, similar to the anti-Munc18-1 antibody, would affect the cis-to-trans-SNARE rearrangement occurring during this window of time. To assess whether Munc18-1 inhibits exocytosis by preventing cis-SNARE disassembly, these complexes were disentangled by adding recombinant PTP1B, which activates endogenous NSF, or by adding recombinant NSF (the effect of both treatments has been validated in previous reports (36, 37); see also Fig. 5E); afterward, Munc18-1 was added, and secretion was stimulated with calcium. As shown in Fig. 5, A and B, the protein was still inhibitory (black bars), indicating that it blocks secretion at a step downstream of cis-SNARE complex disassembly. To assess whether Munc18-1 inhibits the stabilization of trans complexes, we used the indirect immunofluorescence approach described earlier. Intra-acrosomal calcium was depleted in permeabilized sperm by BAPTA-AM. Exocytosis was then stimulated by calcium in the absence or presence of Munc18-1. Next, TeTx was added to cleave monomeric VAMP2/syb2. In the presence of BAPTA-AM, calcium promoted the formation of a TeTx-insensitive state (Fig. 5F). In contrast, VAMP2/syb2 remained toxin-sensitive when Munc18-1 was included in the assay (Fig. 5G). As a control, we tested that recombinant NSF was able to sensitize VAMP2/syb2 to the toxin (Fig. 5E). Immunofluorescence quantitations are shown in Fig. 5H. In conclusion, these results indicate that an excess of Munc18-1 blocks secretion at a step downstream from the disassembly of cis-SNARE complexes but upstream of the formation of TeTx-resistant trans-SNARE complexes.
FIGURE 5.
Recombinant Munc18-1 halts acrosomal exocytosis at a stage when SNAREs are monomeric. A and B, SLO-permeabilized spermatozoa were loaded with 27 nm PTP1B (A) or 300 nm NSF (B) and incubated for 15 min at 37 °C to disassemble cis-SNARE complexes. Next, we added 4 nm Munc18-1 and incubated for a further 15 min at 37 °C. Finally, we added 0.5 mm CaCl2 and incubated for an additional 15 min to stimulate exocytosis (black bars). Controls (gray bars) included the following: background exocytosis in the absence of any stimulation (control); exocytosis stimulated by 0.5 mm CaCl2 (calcium); exocytosis inhibited by 4 nm Munc18-1 and unperturbed by 27 nm PTP1B or 300 nm NSF. Sperm were fixed, and acrosomal exocytosis was evaluated using PSA-FITC, and data normalized as described under “Experimental Procedures.” The values represent mean ± S.E. of at least three independent experiments. C–G, we incubated SLO-permeabilized sperm under resting conditions (C and D) or with 300 nm NSF to promote cis-SNARE complex disassembly (E). At the end of the incubation, we added 100 nm light chain of TeTx to test VAMP2/syb2 neurotoxin sensitivity (D and E). Other aliquots were incubated with 10 μm BAPTA-AM (B-AM, F and G). When indicated, 4 nm Munc18-1 was added to the assay (G). Next, we added 0.5 mm CaCl2 to stimulate exocytosis. At the end of the incubation, we added 100 nm light chain of TeTx. We then fixed and triple-stained the cells with an anti-VAMP2/syb2 antibody (red, left panels), PSA-FITC (to differentiate between reacted and intact sperm; green, central panels), and Hoechst 33342 (to visualize all cells in the field; blue, right panels). Asterisks indicate cells with intact acrosomes but without VAMP2/syb2 immunostaining due to toxin cleavage. Bars, 5 μm. H, quantification of the percentage of cells treated as in C–G exhibiting VAMP2/syb2 acrosomal staining from three independent experiments (mean ± S.E.).
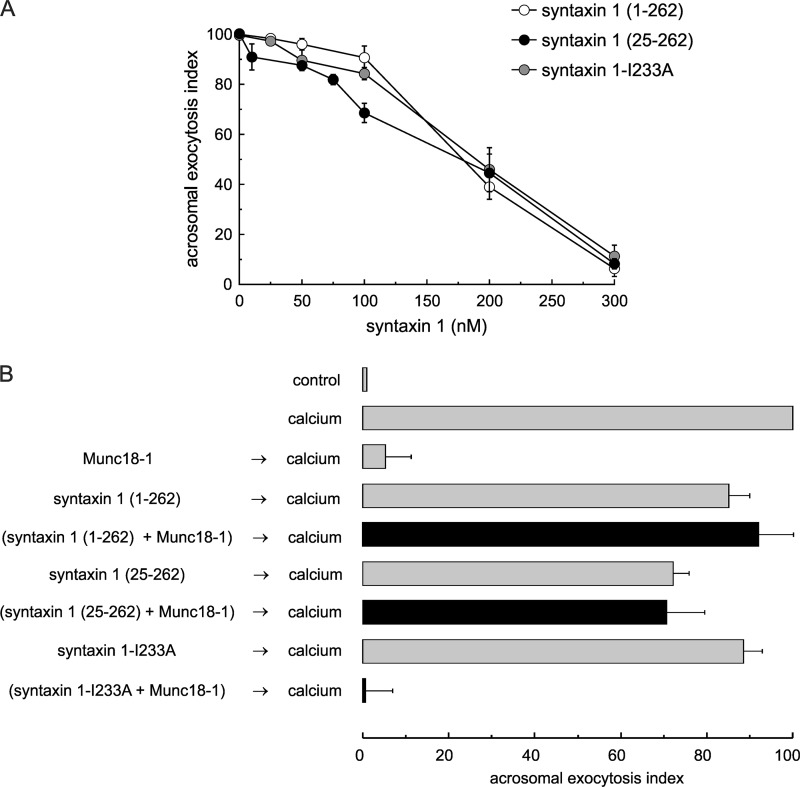
In vitro, Munc18-1 binds the closed conformation of monomeric syntaxin 1 (13, 14); hence, it may inhibit secretion by sequestering endogenous syntaxin. We speculated that if Munc18-1 inhibits exocytosis by forming complexes with the endogenous protein, preincubation with a soluble recombinant syntaxin fragment would impair the ability of Munc18-1 to block acrosomal exocytosis. To perform these experiments, several soluble syntaxin 1 constructs were selected as follows: the entire cytoplasmic domain (residues 1–262); a mutant lacking the N-terminal peptide (residues 25–262), and a cytoplasmic domain (residues 1–262) carrying a point mutation that abrogates its binding to Munc18-1 (I233A) (58–60). These proteins were tested in the assay and proved to be inhibitory at relatively high concentrations, likely by competing with endogenous syntaxin 1 (Fig. 6A) (37). Munc18-1 was preincubated with subinhibitory concentrations of the syntaxin constructs and added to permeabilized sperm; afterward, exocytosis was stimulated with calcium. As shown in Fig. 6B, the syntaxin constructs, at the concentration tested, had a mild effect on exocytosis. However, both the cytoplasmic domain of syntaxin 1 and the N-terminal mutant that bind Munc18-1 abrogated the inhibitory effect of Munc18-1. In contrast, the I233A mutant was unable to prevent the inhibitory effect of Munc18-1. These observations support the idea that recombinant Munc18-1 inhibits acrosomal exocytosis by sequestering endogenous monomeric syntaxin.
FIGURE 6.
Recombinant Munc18-1 inhibits acrosomal exocytosis by sequestering monomeric syntaxin. A, SLO-permeabilized human sperm were treated for 15 min at 37 °C with increasing concentrations of syntaxin 1(1–262, white circles), syntaxin 1(25–262, black circles), or syntaxin 1-I233A (gray circles). Next, we added 0.5 mm CaCl2 and incubated for an additional 15 min to stimulate exocytosis. B, SLO-permeabilized sperm were treated for 15 min at 37 °C with 4 nm Munc18-1 pre-mixed with 100 nm syntaxin 1(1–262), 100 nm syntaxin 1(25–262), or 100 nm syntaxin 1-I233A. Next, we added 0.5 mm CaCl2 and incubated for an additional 15 min to stimulate exocytosis (black bars). Several controls were included (gray bars) as follows: background exocytosis in the absence of any stimulation (control); exocytosis stimulated by 0.5 mm CaCl2 (calcium); Munc18-1 effect on calcium-stimulated exocytosis; lack of effect of low concentration of syntaxin 1(1–262), syntaxin 1(25–262), and syntaxin 1-I233A. A and B, cells were fixed; acrosomal exocytosis was evaluated using PSA-FITC, and data were normalized as described under “Experimental Procedures.” The values represent mean ± S.E. of at least three independent experiments.
α-SNAP Rescues the Block Imposed by Munc18-1
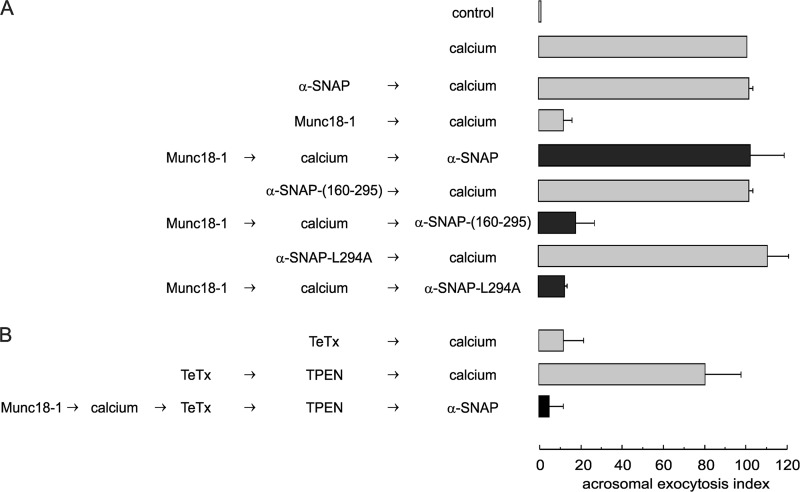
α-SNAP assists NSF in the disentangling of SNARE complexes. In addition, this protein binds the SNARE domain of monomeric syntaxin 1 (61). We have reported that recombinant α-SNAP can inhibit acrosomal exocytosis by forming complexes with syntaxin, an effect that is reversed by NSF (37). We then hypothesized that if Munc18-1 inhibits acrosomal exocytosis by holding syntaxin in a closed, inactive conformation, recombinant α-SNAP (at sub-inhibitory concentrations) in combination with endogenous NSF could promote the destabilization of the syntaxin/Munc18-1 dimer and restore fusion.
We first tested if sub-inhibitory concentrations of α-SNAP could restore fusion blocked by Munc18-1. As shown in Fig. 7A, exocytosis was resumed when full-length α-SNAP was added to Munc18-1-inhibited assays, consistent with the idea that α-SNAP can promote the release of syntaxin from the closed conformation stabilized by Munc18-1. This conclusion was supported by the fact that a truncated α-SNAP(160–295) that can interact with NSF but not with syntaxin (62) was ineffective. Moreover, the complete protein with a point mutation that binds both syntaxin and NSF, but that does not stimulate the ATPase activity of this chaperone (α-SNAP-L294A (62)), was unable to reverse the Munc18-1 block, indicating that NSF plays an essential role in the rescue promoted by α-SNAP.
FIGURE 7.
α-SNAP rescues the block imposed by Munc18-1. A, SLO-permeabilized spermatozoa were loaded with 4 nm Munc18-1 for 10 min at 37 °C and subsequently challenged with 0.5 mm CaCl2 for 10 min at 37 °C. We then added 100 nm wild type α-SNAP, 100 nm α-SNAP(160–295), or 100 nm α-SNAP-L294A and incubated at 37 °C for 15 min (black bars). B, SLO-permeabilized spermatozoa were loaded with 4 nm Munc18-1 for 10 min at 37 °C and subsequently challenged with 0.5 mm CaCl2 for 10 min at 37 °C. After this incubation, we added 100 nm wild type TeTx and incubated for 10 min at 37 °C. We stopped toxin activity with 2.5 μm TPEN for 10 min. At the end of the incubation, we released the block with 100 nm wild type α-SNAP for 10 min at 37 °C (black bar). Controls in A and B (gray bars) included the following: background acrosomal exocytosis in the absence of any stimulation (control); acrosomal exocytosis stimulated by 0.5 mm CaCl2 (calcium); inhibitory effect of 4 nm Munc18-1 or 100 nm wild type TeTx, and lack of effect of 100 nm α-SNAPs. Cells were fixed; acrosomal exocytosis was evaluated using PSA-FITC, and data were normalized as described under “Experimental Procedures.” The values represent mean ± S.E. of at least three independent experiments.
We next tested how Munc18-1 influences SNARE configuration in our functional assay, taking advantage of the reversibility of its effect by α-SNAP. For these experiments, exocytosis was stimulated with calcium in the presence of recombinant Munc18-1; TeTx was then added, and the mixture was incubated to allow toxin action. Afterward, the activity of TeTx was blocked with TPEN, and α-SNAP was added to reverse the Munc18-1 block. Exocytosis was not rescued in this experiment (Fig. 7B, black bar), indicating that VAMP2/syb2 was sensitive to the toxin at the stage where the process was halted by Munc18-1. These results indicate that recombinant Munc18-1 prevents the formation of trans-SNARE complexes, reinforcing the conclusions of the immunofluorescence experiments shown in Fig. 5, C–H.
Inactivation of Endogenous Munc18-1 and an Excess of the Recombinant Protein Inhibit Docking of the Acrosome to the Plasma Membrane
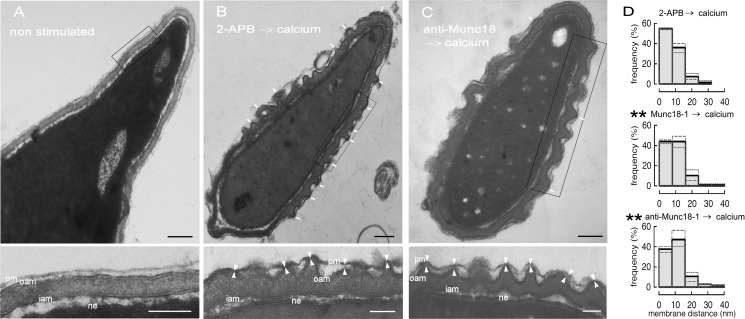
Docking, a strong and spatially tight interaction between the two membranes that are going to fuse, is a mandatory step that precedes bilayer mixing (63). Munc18-1 has been implicated in docking via an interaction with syntaxin 1 in adult bovine chromaffin cells and other models (64, 65). In the acrosome exocytosis model, depletion of intra-acrosomal calcium halts exocytosis at a stage where SNAREs are assembled in loose trans complexes (47). SNAREs cannot interact at distances longer than 8 nm (66). Hence, we define morphological membrane docking as regions where the distance between the acrosomal and the plasma membrane is 8 nm or shorter. These tight membrane appositions are observed at the protruding edges of deep invaginations of the outer acrosomal membrane (49). According to the results shown above, inactivation of Munc18-1 by a specific antibody or addition of an excess of the recombinant protein before challenging with calcium prevents the formation of trans-SNARE complexes. Hence, we would expect that SNARE-dependent docking should be altered. To investigate whether Munc18-1 affects docking, we loaded permeabilized human sperm with the anti-Munc18-1 antibody or the recombinant protein, initiated acrosomal exocytosis with calcium, and scrutinized the docking status of the acrosomes by transmission electron microscopy. In resting sperm, the plasma and outer acrosomal membranes were parallel, maintaining a distance of about 10–20 nm (Fig. 8A). Upon stimulation, the acrosome swelled, and the outer acrosomal membrane formed invaginations. The percentage of swollen acrosomes was around 25% for all conditions tested, indicating that the treatments did not affect acrosomal swelling, a process that is required before membrane docking in human sperm. As reported previously, we found a high frequency of close appositions when acrosomal exocytosis was stimulated in the presence of 2-APB, an inositol 1,4,5-trisphosphate-sensitive calcium channel inhibitor that blocks intra-acrosomal calcium efflux (Fig. 8B) (50). In contrast, when the anti-Munc18-1 antibody (Fig. 8C) or recombinant Munc18-1 (data not shown) was added prior to the calcium challenge, the frequency of close contacts diminished. We measured the distances between the outer acrosomal membrane and the plasma membrane at the edge of the invaginations and plotted them as histograms (Fig. 8D). The histograms showed that the presence of both Munc18-1 and the anti-Munc18-1 antibody blocked exocytosis at a stage where the distance distribution was significantly different from that observed when the process was stopped by an inhibitor that does not affect the formation of trans-SNARE complexes (2-APB (49)).
FIGURE 8.
Anti-Munc18-1 and recombinant Munc18-1 prevent docking of the acrosome to the plasma membrane. SLO-permeabilized human sperm were treated for 15 min at 37 °C with 100 μm 2-APB, 4 nm Munc18-1, or 10 nm anti-Munc18-1 antibody from Synaptic Systems, before challenging with 0.5 mm CaCl2 for 15 min at 37 °C. A, untreated cell showing the morphology of resting sperm (non-swollen, undocked acrosome). B, sperm treated with 2-APB and stimulated with calcium, showing the morphology of swollen, docked acrosomes (a transient stage only visible in unreacted cells, hence the choice of 2-APB, a late acting acrosomal exocytosis blocker). C, sperm treated with anti-Munc18-1 and stimulated with calcium. White arrows in B and C point to regions of tight appositions between the plasma and outer acrosomal membranes. Notice the decrease of tight appositions in the anti-Munc18-1-treated sperm. Lower panels in A–C show enlarged boxed regions in the corresponding upper panel. pm, plasma membrane; oam, outer acrosomal membrane; iam, inner acrosomal membrane; ne, nuclear envelope. White arrowheads in B and C (lower panels) point to the outer acrosomal and the plasma membranes at the edges of acrosomal invaginations. Black bars, 200 nm; white bars, 100 nm. D, distance between the outer acrosomal and plasma membranes was measured at the edge of acrosomal invaginations (as indicated with white arrowheads in B and C, lower panels). Only images where the membrane bilayers were clearly distinguished were analyzed. Upper panel, calcium-stimulated sperm pretreated with 2-APB. Middle panel, calcium-stimulated sperm pretreated with recombinant Munc18-1. Bottom panel, calcium-stimulated sperm pretreated anti-Munc18-1. At least 100 distances in 10 different cells from two independent experiments were measured for each condition. Distance distributions are plotted as histograms (gray dashed lines correspond to experiment 1; gray dotted lines to experiment 2, and black solid lines to the distance distribution of both experiments). The histograms corresponding to the two experiments were compared using the Kolmogorov-Smirnov test (**, significantly different form the 2-APB histogram, p < 0.01).
It is reasonable to assume that after acrosomal swelling, the outer acrosomal membrane can wave and move randomly, approaching and departing from the plasma membrane in a dynamic way. If SNAREs in both membranes are active, when the distance is close enough they will form permanent trans-SNARE complexes, preventing new movements of the membrane and stabilizing areas of tight apposition. In a previous report, we had modeled the membrane distance distribution under these assumptions (supplemental Fig. 2A) (49). In this model, a parameter measures the probability of stable association between membranes (SAP). We hypothesize that an excess of recombinant Munc18-1 or the anti-Munc18-1 antibody, which prevent the formation of trans-SNARE complexes, must affect SAP. Simulations of the model were performed using different SAP values, and the membrane distance for each simulation was recorded. We compared the distance distribution in 20,000 simulations at each SAP value with the distance distribution observed experimentally by using a least square criterion (supplemental Fig. 2B). For the control condition (2-APB), the best fit was observed for SAP at about 0.1, and the best fit for the distance distribution in the presence of Munc18-1 and anti-Munc18-1 was observed for SAP >0.03 and >0.01%, respectively (supplemental Fig. 2, B and C). A 3- or 10-fold decrease in the probability of stable membrane association can explain the strong effect of the protein and the antibody on acrosomal exocytosis. These data indicate that both sequestration of Munc18-1 and an excess of the protein alter membrane docking, probably by preventing the formation of trans-SNARE complexes.
DISCUSSION
Acrosomal exocytosis is a regulated secretion that uses a molecular machinery similar to that described for many other secretory cells. However, the process has several remarkable differences with secretion observed, for instance, in neurons and chromaffin cells. NSF is inactive in resting sperm (36), and SNAREs are assembled in neurotoxin-resistant cis complexes (47). It is unclear whether these complexes are the result of previous membrane fusion processes. During acrosome formation, there is an intense vesicular traffic between the Golgi and the nascent acrosomal vesicle (67). The acrosome is catalogued as a lysosome-related granule that interacts with the endocytic pathway during its biogenesis (68). The cis complexes observed in resting sperm may be the remnant of these early transport events. Upon challenging with acrosomal exocytosis inducers, cis-SNARE complexes are disassembled, releasing monomeric proteins sensitive to all neurotoxins. Simultaneously, the acrosome swells and the outer acrosomal membrane forms invaginations. The protruding ring-shaped edges of these invaginations contact the plasma membrane; these contact areas are stabilized by the assembly of loose trans-SNARE complexes sensitive to BoNTs but resistant to TeTx. At this docking stage, calcium released from the acrosome is required to trigger membrane fusion. Fusion pores open and expand, releasing hybrid vesicles consisting of patches of outer acrosomal and plasma membranes. This complex secretory event occurs in a single granule large enough to be observed by fluorescence microscopy; hence, the process of SNARE switching from inactive cis to fusion-competent trans complexes can be studied at a detailed level that is hard to achieve in other secretory cells.
In this study we have focused on the role of an SM protein in acrosomal exocytosis. These proteins play a central role in most membrane fusion events, from yeast to mammals; however, their function in acrosomal exocytosis had not been studied before. The only previous report in the literature is the identification of Munc18-2 in boar spermatozoa (34). Because of the similarity in the fusion machinery among neurons and sperm, we searched for Munc18-1, a well characterized SM protein that regulates exocytosis in numerous neuroendocrine cells. We found that the protein is present and localizes to the acrosomal region of human sperm. A large proportion of Munc18-1 was not associated with membranes. However, when sperm were permeabilized with SLO, most of the protein remained cell-associated (data not shown), indicating that Munc18-1 is not totally free in the sperm cytoplasm.
Sequestration of endogenous Munc18-1 by a specific antibody abrogated calcium and Rab3A-triggered acrosomal exocytosis, indicating that the protein was not only present but also important for secretion. When we monitored the stage at which the anti-Munc18-1 antibody blocked exocytosis, we observed that the SNAREs syntaxin 1 and VAMP2/syb2 were sensitive to neurotoxins. This indicates that Munc18-1 is necessary for the formation or stabilization of trans-SNARE complexes. There is evidence that Munc18-1 not only binds the closed conformation of monomeric syntaxin 1 but also open syntaxin assembled in SNARE complexes of different compositions (12). Consistent with these interactions, it has been shown that Munc18-1 accelerates the fusion rate between liposomes carrying cognate SNARE proteins (20). SM proteins may also influence the sensitivity of SNARE complexes to α-SNAP/NSF action (69, 70). In yeast, the protein-sorting complex HOPS, which includes the Munc18-1 homolog Vps33p, prevents trans-SNARE disentangling by Sec17/Sec18 (α-SNAP/NSF homologs). In contrast, HOPS does not prevent the action of Sec17/Sec18 on cis complexes (69). In sperm, we have shown that cis-SNARE complexes can be disassembled by α-SNAP/NSF (36, 37, 47), whereas TeTx-resistant trans complexes are resistant to NSF/α-SNAP action (47). In the absence of active Munc18-1, trans complexes may not form, or alternatively, they may remain sensitive to α-SNAP/NSF allowing the SNAREs to cycle through monomeric, toxin-sensitive states.
Sequestration of Munc18-1 by the specific antibody halts the secretory process at the first step where the protein is required but does not rule out the possibility that it may be necessary for later steps (22, 71, 72). Curiously, the antibody has no effect after the system has arrived at the step where an intra-acrosomal calcium efflux is required, an observation in conflict with a function of Munc18-1 downstream the assembly of trans-SNARE complexes. Antibodies are large proteins; hence, the tight membrane appositions characteristic of the docked stage may prevent the Munc18-1/antibody interaction. Alternatively, at this step, Munc18-1 may be engaged in a multimeric complex hiding the epitope recognized by the antibody. The lack of effect of the antibody at late stages is consistent with recent observations supporting a prevailing role of Munc18-1 before/during SNARE-complex assembly (60).
Binding of recombinant Munc18-1 to the closed inactive conformation of endogenous syntaxin 1 is likely responsible for the inhibition of acrosomal exocytosis. This hypothesis is supported by the observation that syntaxin remains toxin-sensitive in the presence of recombinant Munc18-1. It is also supported by the neutralization of the Munc18-1 inhibition by syntaxin constructs able to bind Munc18-1. Our results are in line with the observation that Munc18 overexpression blocks secretion in other models (55, 56) and with a recently published model of SNARE/SM networks that shows that SM inhibits membrane fusion at high SM/syntaxin ratios (73).
We made the novel observation that α-SNAP was able to relieve the block imposed by Munc18-1. This effect depends on the syntaxin binding properties of α-SNAP and requires NSF. α-SNAP functions as a co-chaperone for NSF-mediated disassembly of cis-SNARE complexes. We hypothesize that binding of α-SNAP to any complex containing syntaxin will recruit NSF and promote the release of free syntaxin. It has been reported that α-SNAP can release syntaxin from other protein complexes (37, 74).
When spermatozoa are activated in the presence of inhibitors of intra-acrosomal calcium release, the acrosome morphology changes dramatically; the granule swells, and the outer acrosomal membrane forms deep invaginations. At the protruding edge of these invaginations, tight appositions between the granule and the plasma membrane are observed (49). We refer to these appositions as docking regions. Interestingly, at this stage, SNAREs are assembled in BoNT-sensitive, TeTx-resistant complexes, suggesting that the molecular mechanism for docking relies on partially assembled trans-SNARE complexes (47). Consistent with this idea, when monomeric SNAREs are cleaved by neurotoxins, the frequency of docked membranes decreases dramatically (49). A similar effect is observed when free syntaxin is sequestered by an excess of recombinant α-SNAP (37). Immunofluorescence and functional data show that both an anti-Munc18-1 antibody and an excess of recombinant Munc18-1 prevent the stabilization of TeTx-resistant SNARE complexes. In consequence, they should affect docking. Consistent with this corollary, we observed a significant decrease in the frequency of docking when acrosomal exocytosis was stimulated in the presence of recombinant Munc18-1 or the specific antibody. These observations are consistent with results in other models where an active role of SM proteins in vesicle docking has been proposed (63, 75, 76).
In conclusion, our results indicate that Munc18-1 plays a key function in the dynamics of trans-SNARE complex assembly and/or stabilization, a process necessary for membrane docking before the opening of fusion pores.
Acknowledgments
We are grateful to Dr. James Rothman for reagents and to Iris Douglas, Jeff Coleman, Graciela Gutierrez, Alejandra Medero and Marcelo Furlan for excellent technical assistance.
This work was supported by Grants 06/J388 and 06/J353 SeCTyP from National University of Cuyo, Argentina, Grant PIP 112-200801-02277 from CONICET, Argentina, and Grants PICT-2008-1114, PICT-2011-2310, and PICT-2006-01036 from ANPCyT, Argentina.

This article contains supplemental Figs. 1 and 2 and additional references.
M. A. Bustos and C. N. Tomes, unpublished results.
- SM
- Sec1/Munc18
- 2-APB
- 2-aminoethoxydiphenyl borate
- PSA-FITC
- fluorescein isothiocyanate-coupled P. sativum agglutinin
- NSF
- N-ethylmaleimide-sensitive factor
- SNAP
- soluble NSF attachment protein
- NP-EGTA-AM
- O-nitrophenyl EGTA acetoxymethyl ester
- PTP1B
- protein-tyrosine phosphatase 1B
- SLO
- streptolysin O
- GTPγS
- guanosine 5–3-O-(thio)triphosphate
- BoNT
- botulinum neurotoxin
- TeTx
- tetanus toxin
- TPEN
- N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- PVP
- polyvinylpyrrolidone
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- SAP
- stable association probability.
REFERENCES
- 1. Südhof T. C., Rothman J. E. (2009) Membrane fusion. Grappling with SNARE and SM proteins. Science 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wickner W., Schekman R. (2008) Membrane fusion. Nat. Struct. Mol. Biol. 15, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgoyne R. D., Morgan A. (2003) Secretory granule exocytosis. Physiol. Rev. 83, 581–632 [DOI] [PubMed] [Google Scholar]
- 4. Lang T., Jahn R. (2008) Core proteins of the secretory machinery. Handb. Exp. Pharmacol. 184, 107–127 [DOI] [PubMed] [Google Scholar]
- 5. Rizo J., Rosenmund C. (2008) Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 15, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunger A. T. (2005) Structure and function of SNARE and SNARE-interacting proteins. Q. Rev. Biophys. 38, 1–47 [DOI] [PubMed] [Google Scholar]
- 7. Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) SNAREpins. Minimal machinery for membrane fusion. Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 8. May A. P., Whiteheart S. W., Weis W. I. (2001) Unraveling the mechanism of the vesicle transport ATPase NSF, the N-ethylmaleimide-sensitive factor. J. Biol. Chem. 276, 21991–21994 [DOI] [PubMed] [Google Scholar]
- 9. Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. (1993) A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 [DOI] [PubMed] [Google Scholar]
- 10. Fasshauer D., Sutton R. B., Brunger A. T., Jahn R. (1998) Conserved structural features of the synaptic fusion complex. SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. U.S.A. 95, 15781–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christie M. P., Whitten A. E., King G. J., Hu S. H., Jarrott R. J., Chen K. E., Duff A. P., Callow P., Collins B. M., James D. E., Martin J. L. (2012) Low-resolution solution structures of Munc18-syntaxin protein complexes indicate an open binding mode driven by the syntaxin N-peptide. Proc. Natl. Acad. Sci. U.S.A. 109, 9816–9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dulubova I., Khvotchev M., Liu S., Huryeva I., Südhof T. C., Rizo J. (2007) Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. U.S.A. 104, 2697–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Südhof T. C., Rizo J. (1999) A conformational switch in syntaxin during exocytosis. Role of munc18. EMBO J. 18, 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Misura K. M., Scheller R. H., Weis W. I. (2000) Three-dimensional structure of the neuronal Sec1-syntaxin 1a complex. Nature 404, 355–362 [DOI] [PubMed] [Google Scholar]
- 15. Verhage M., Maia A. S., Plomp J. J., Brussaard A. B., Heeroma J. H., Vermeer H., Toonen R. F., Hammer R. E., van den Berg T. K., Missler M., Geuze H. J., Südhof T. C. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869 [DOI] [PubMed] [Google Scholar]
- 16. Harrison S. D., Broadie K., van de Goor J., Rubin G. M. (1994) Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron 13, 555–566 [DOI] [PubMed] [Google Scholar]
- 17. Novick P., Field C., Schekman R. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 [DOI] [PubMed] [Google Scholar]
- 18. Wu M. N., Littleton J. T., Bhat M. A., Prokop A., Bellen H. J. (1998) ROP, the Drosophila Sec1 homolog, interacts with syntaxin and regulates neurotransmitter release in a dosage-dependent manner. EMBO J. 17, 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weimer R. M., Richmond J. E., Davis W. S., Hadwiger G., Nonet M. L., Jorgensen E. M. (2003) Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 6, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen J., Tareste D. C., Paumet F., Rothman J. E., Melia T. J. (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 [DOI] [PubMed] [Google Scholar]
- 21. Rathore S. S., Bend E. G., Yu H., Hammarlund M., Jorgensen E. M., Shen J. (2010) Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proc. Natl. Acad. Sci. U.S.A. 107, 22399–22406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen J., Rathore S. S., Khandan L., Rothman J. E. (2010) SNARE bundle and syntaxin N-peptide constitute a minimal complement for Munc18-1 activation of membrane fusion. J. Cell Biol. 190, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi L., Kümmel D., Coleman J., Melia T. J., Giraudo C. G. (2011) Dual roles of Munc18-1 rely on distinct binding modes of the central cavity with Stx1A and SNARE complex. Mol. Biol. Cell 22, 4150–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yanagimachi R. (1994) in The Physiology of Reproduction (Knobil E., Neill J. D., eds) pp. 189–281, Raven Press, Ltd., New York [Google Scholar]
- 25. Lesich K. A., Pelle D. W., Lindemann C. B. (2008) Insights into the mechanism of ADP action on flagellar motility derived from studies on bull sperm. Biophys. J. 95, 472–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerrero A., Nishigaki T., Carneiro J., Yoshiro Tatsu, Wood C. D., Darszon A. (2010) Tuning sperm chemotaxis by calcium burst timing. Dev. Biol. 344, 52–65 [DOI] [PubMed] [Google Scholar]
- 27. Alvarez L., Dai L., Friedrich B. M., Kashikar N. D., Gregor I., Pascal R., Kaupp U. B. (2012) The rate of change in Ca2+ concentration controls sperm chemotaxis. J. Cell Biol. 196, 653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blengini C. S., Teves M. E., Uñates D. R., Guidobaldi H. A., Gatica L. V., Giojalas L. C. (2011) Human sperm pattern of movement during chemotactic re-orientation toward a progesterone source. Asian J. Androl. 13, 769–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayorga L. S., Tomes C. N., Belmonte S. A. (2007) Acrosomal exocytosis, a special type of regulated secretion. IUBMB. Life 59, 286–292 [DOI] [PubMed] [Google Scholar]
- 30. Tomes C. (2007) in Molecular Mechanisms of Exocytosis (Regazzi R., ed) pp. 117–147, Landes Bioscience, Georgetown, TX [Google Scholar]
- 31. Barros C., Bedford J. M., Franklin L. E., Austin C. R. (1967) Membrane vesiculation as a feature of the mammalian acrosome reaction. J. Cell Biol. 34, C1–C5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomes C. N., Michaut M., De Blas G., Visconti P., Matti U., Mayorga L. S. (2002) SNARE complex assembly is required for human sperm acrosome reaction. Dev. Biol. 243, 326–338 [DOI] [PubMed] [Google Scholar]
- 33. Ramalho-Santos J., Moreno R. D., Sutovsky P., Chan A. W., Hewitson L., Wessel G. M., Simerly C. R., Schatten G. (2000) SNAREs in mammalian sperm. Possible implications for fertilization. Dev. Biol. 223, 54–69 [DOI] [PubMed] [Google Scholar]
- 34. Tsai P. S., Brewis I. A., van Maaren J., Gadella B. M. (2012) Involvement of complexin 2 in docking, locking, and unlocking of different SNARE complexes during sperm capacitation and induced acrosomal exocytosis. PLoS ONE 7, e32603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michaut M., Tomes C. N., De Blas G., Yunes R., Mayorga L. S. (2000) Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc. Natl. Acad. Sci. U.S.A. 97, 9996–10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zarelli V. E., Ruete M. C., Roggero C. M., Mayorga L. S., Tomes C. N. (2009) PTP1B dephosphorylates N-ethylmaleimide-sensitive factor and elicits SNARE complex disassembly during human sperm exocytosis. J. Biol. Chem. 284, 10491–10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodríguez F., Bustos M. A., Zanetti M. N., Ruete M. C., Mayorga L. S., Tomes C. N. (2011) α-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin. PLoS ONE 6, e21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopez C. I., Belmonte S. A., De Blas G. A., Mayorga L. S. (2007) Membrane-permeant Rab3A triggers acrosomal exocytosis in living human sperm. FASEB J. 21, 4121–4130 [DOI] [PubMed] [Google Scholar]
- 39. Iida H., Yoshinaga Y., Tanaka S., Toshimori K., Mori T. (1999) Identification of Rab3A GTPase as an acrosome-associated small GTP-binding protein in rat sperm. Dev. Biol. 211, 144–155 [DOI] [PubMed] [Google Scholar]
- 40. Ward C. R., Faundes D., Foster J. A. (1999) The monomeric GTP-binding protein, Rab3A, is associated with the acrosome in mouse sperm. Mol. Reprod. Dev. 53, 413–421 [DOI] [PubMed] [Google Scholar]
- 41. Zhao L., Burkin H. R., Shi X., Li L., Reim K., Miller D. J. (2007) Complexin I is required for mammalian sperm acrosomal exocytosis. Dev. Biol. 309, 236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roggero C. M., De Blas G. A., Dai H., Tomes C. N., Rizo J., Mayorga L. S. (2007) Complexin/synaptotagmin interplay controls acrosomal exocytosis. J. Biol. Chem. 282, 26335–26343 [DOI] [PubMed] [Google Scholar]
- 43. Bello O. D., Zanetti M. N., Mayorga L. S., Michaut M. A. (2012) RIM, Munc13, and Rab3A interplay in acrosomal exocytosis. Exp. Cell Res. 318, 478–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roggero C. M., Tomes C. N., De Blas G. A., Castillo J., Michaut M. A., Fukuda M., Mayorga L. S. (2005) Protein kinase C-mediated phosphorylation of the two polybasic regions of synaptotagmin VI regulates their function in acrosomal exocytosis. Dev. Biol. 285, 422–435 [DOI] [PubMed] [Google Scholar]
- 45. Michaut M., De Blas G., Tomes C. N., Yunes R., Fukuda M., Mayorga L. S. (2001) Synaptotagmin VI participates in the acrosome reaction of human spermatozoa. Dev. Biol. 235, 521–529 [DOI] [PubMed] [Google Scholar]
- 46. Hutt D. M., Baltz J. M., Ngsee J. K. (2005) Synaptotagmin VI and VIII and syntaxin 2 are essential for the mouse sperm acrosome reaction. J. Biol. Chem. 280, 20197–20203 [DOI] [PubMed] [Google Scholar]
- 47. De Blas G. A., Roggero C. M., Tomes C. N., Mayorga L. S. (2005) Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 3, e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bustos M. A., Lucchesi O., Ruete M. C., Mayorga L. S., Tomes C. N. (2012) Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc. Natl. Acad. Sci. U.S.A. 109, E2057–E2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zanetti N., Mayorga L. S. (2009) Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol. Reprod. 81, 396–405 [DOI] [PubMed] [Google Scholar]
- 50. De Blas G., Michaut M., Treviño C. L., Tomes C. N., Yunes R., Darszon A., Mayorga L. S. (2002) The intra-acrosomal calcium pool plays a direct role in acrosomal exocytosis. J. Biol. Chem. 277, 49326–49331 [DOI] [PubMed] [Google Scholar]
- 51. Mendoza C., Carreras A., Moos J., Tesarik J. (1992) Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J. Reprod. Fertil. 95, 755–763 [DOI] [PubMed] [Google Scholar]
- 52. Tomes C. N., De Blas G. A., Michaut M. A., Farré E. V., Cherhitin O., Visconti P. E., Mayorga L. S. (2005) α-SNAP and NSF are required in a priming step during the human sperm acrosome reaction. Mol. Hum. Reprod. 11, 43–51 [DOI] [PubMed] [Google Scholar]
- 53. Voets T., Toonen R. F., Brian E. C., de Wit H., Moser T., Rettig J., Südhof T. C., Neher E., Verhage M. (2001) Munc18-1 promotes large dense-core vesicle docking. Neuron 31, 581–591 [DOI] [PubMed] [Google Scholar]
- 54. Weimer R. M., Richmond J. E. (2005) Synaptic vesicle docking. A putative role for the Munc18/Sec1 protein family. Curr. Top. Dev. Biol. 65, 83–113 [DOI] [PubMed] [Google Scholar]
- 55. Dresbach T., Burns M. E., O'Connor V., DeBello W. M., Betz H., Augustine G. J. (1998) A neuronal Sec1 homolog regulates neurotransmitter release at the squid giant synapse. J. Neurosci. 18, 2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martin-Verdeaux S., Pombo I., Iannascoli B., Roa M., Varin-Blank N., Rivera J., Blank U. (2003) Evidence of a role for Munc18-2 and microtubules in mast cell granule exocytosis. J. Cell Sci. 116, 325–334 [DOI] [PubMed] [Google Scholar]
- 57. Yunes R., Michaut M., Tomes C., Mayorga L. S. (2000) Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol. Reprod. 62, 1084–1089 [DOI] [PubMed] [Google Scholar]
- 58. Wu M. N., Fergestad T., Lloyd T. E., He Y., Broadie K., Bellen H. J. (1999) Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron 23, 593–605 [DOI] [PubMed] [Google Scholar]
- 59. Burkhardt P., Hattendorf D. A., Weis W. I., Fasshauer D. (2008) Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 27, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meijer M., Burkhardt P., de Wit H., Toonen R. F., Fasshauer D., Verhage M. (2012) Munc18-1 mutations that strongly impair SNARE-complex binding support normal synaptic transmission. EMBO J. 31, 2156–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanson P. I., Otto H., Barton N., Jahn R. (1995) The N-ethylmaleimide-sensitive fusion protein and α-SNAP induce a conformational change in syntaxin. J. Biol. Chem. 270, 16955–16961 [DOI] [PubMed] [Google Scholar]
- 62. Barnard R. J., Morgan A., Burgoyne R. D. (1997) Stimulation of NSF ATPase activity by α-SNAP is required for SNARE complex disassembly and exocytosis. J. Cell Biol. 139, 875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verhage M., Sørensen J. B. (2008) Vesicle docking in regulated exocytosis. Traffic 9, 1414–1424 [DOI] [PubMed] [Google Scholar]
- 64. de Wit H., Cornelisse L. N., Toonen R. F., Verhage M. (2006) Docking of secretory vesicles is syntaxin dependent. PLoS ONE 1, e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Han G. A., Malintan N. T., Saw N. M., Li L., Han L., Meunier F. A., Collins B. M., Sugita S. (2011) Munc18-1 domain-1 controls vesicle docking and secretion by interacting with syntaxin-1 and chaperoning it to the plasma membrane. Mol. Biol. Cell 22, 4134–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li F., Pincet F., Perez E., Eng W. S., Melia T. J., Rothman J. E., Tareste D. (2007) Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 14, 890–896 [DOI] [PubMed] [Google Scholar]
- 67. Burgos M. H., Fawcett D. W. (1955) Studies on the fine structure of the mammalian testis. I. Differentiation of the spermatids in the cat (Felis domestica) J. Biophys. Biochem. Cytol. 1, 287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berruti G., Paiardi C. (2011) Acrosome biogenesis. Revisiting old questions to yield new insights. Spermatogenesis 1, 95–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu H., Jun Y., Thompson J., Yates J., Wickner W. (2010) HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 29, 1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alpadi K., Kulkarni A., Comte V., Reinhardt M., Schmidt A., Namjoshi S., Mayer A., Peters C. (2012) Sequential analysis of trans-SNARE formation in intracellular membrane fusion. PLoS Biol. 10, e1001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jorgacevski J., Potokar M., Grilc S., Kreft M., Liu W., Barclay J. W., Bückers J., Medda R., Hell S. W., Parpura V., Burgoyne R. D., Zorec R. (2011) Munc18-1 tuning of vesicle merger and fusion pore properties. J. Neurosci. 31, 9055–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Graham M. E., Barclay J. W., Burgoyne R. D. (2004) Syntaxin/Munc18 interactions in the late events during vesicle fusion and release in exocytosis. J. Biol. Chem. 279, 32751–32760 [DOI] [PubMed] [Google Scholar]
- 73. Xia T., Tong J., Rathore S. S., Gu X., Dickerson J. A. (2012) Network motif comparison rationalizes Sec1/Munc18-SNARE regulation mechanism in exocytosis. BMC Syst. Biol. 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McMahon H. T., Missler M., Li C., Südhof T. C. (1995) Complexins. Cytosolic proteins that regulate SNAP receptor function. Cell 83, 111–119 [DOI] [PubMed] [Google Scholar]
- 75. Gulyás-Kovács A., de Wit H., Milosevic I., Kochubey O., Toonen R., Klingauf J., Verhage M., Sørensen J. B. (2007) Munc18-1. Sequential interactions with the fusion machinery stimulate vesicle docking and priming. J. Neurosci. 27, 8676–8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hickey C. M., Wickner W. (2010) HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol. Biol. Cell 21, 2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]