Abstract
The activity of biological molecules is often affected by their phosphorylation state. Regulatory phosphorylation operates as a binary switch and is usually controlled by counteracting kinases and phosphatases. However, phosphatidylinositol (PtdIns) has three phosphorylation sites on its inositol ring. The phosphorylation status of PtdIns is controlled by multiple kinases and phosphatases with distinct substrate specificities, serving as a ‘lipid code’ or ‘phosphoinositide code’. Class I phosphoinositide 3-kinase (PI3K) converts PtdIns(4,5)P2 to PtdIns(3,4,5)P3, which plays a pivotal role in signals controlling glucose uptake, cytoskeletal reorganization, cell proliferation and apoptosis. PI3K is pro-oncogenic, whereas phosphoinositide phosphatases that degrade PtdIns(3,4,5)P3 are not always anti-oncogenic. Recent studies have revealed the unique characteristics of phosphoinositide 5-phosphatases.
Keywords: lipid phosphatase, phosphoinositide, tumour suppressor
The Phosphoinositide System
The activities of biological molecules are often altered by reversible phosphorylation. Protein phosphorylation is regulated by protein kinases and protein phosphatases, and induces conformational changes in proteins and/or provides specific docking sites for inter- as well as intra-molecular interactions. Phosphorylated carbohydrates are sometimes used as metabolic regulators. Fructose-2,6-bis-phosphate (Fru-2,6-BP) plays a crucial role in regulating the glycolytic pathway as an allosteric activator of phosphofructokinase, a key enzyme in glycolysis. Intracellular Fru-2,6-BP levels are regulated by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, a bifunctional enzyme that possesses counteracting enzymatic activities on a single polypeptide (1). In contrast to the simple binary nature of these regulatory systems, phosphatidylinositol derivatives (phosphoinositides) have greater complexity.
Phosphatidylinositol (PtdIns) is a minor phospholipid in the cell membrane, where it exclusively localizes in the cytoplasmic leaflet. PtdIns can be phosphorylated at the 3, 4 and 5 positions of the inositol ring. Phosphoinositides play important roles in regulating cell proliferation, survival, cytoskeletal reorganization and vesicular transport, depending on their phosphorylation status (called a ‘lipid code’ or ‘phosphoinositide code’) (2). They primarily impact cellular behaviours by recruiting signalling molecules to specific intracellular locations. Several characteristic protein domains, including PH (pleckstrin homology), PX (Phox homology), ENTH (epsin NH2-terminal homology), FYVE (Fab-1, YGL023, Vps27 and EEA1), etc., are involved in specific interactions with various phosphoinositides (2).
PI3K and PTEN
The phosphorylation status of phosphoinositides is regulated by kinases and phosphatases with distinct specificities. Conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 by class I phosphoinositide 3-kinases (PI3Ks) is one of the key events in membrane receptor-mediated signal transduction (3). PtdIns(3,4,5)P3 associates with Akt (protein kinase B) as well as PDK1 (phosphoinositide-dependent kinase 1) through their PH domains and mediates the co-localization of these kinases to a membrane region adjacent to activated receptors. Importantly, Akt is phosphorylated by PDK1, and thus this co-localization permits efficient signalling via Akt. After secondary phosphorylation by mammalian target of rapamycin complex 2 (mTORC2), activated Akt regulates a wide variety of cellular responses, i.e., glucose uptake, cell proliferation, inhibition of apoptosis and protein translation, by phosphorylating downstream effectors, including kinases and transcription factors. PtdIns(3,4,5)P3 also recruits Tiam 1 and Vav1, guanine nucleotide exchange factors, to induce activation of Cdc42 and Rac, thus affecting cell motility and membrane ruffling. Deregulated PI3K signalling is oncogenic; overexpression or missense mutations in PI3K or its downstream kinase, Akt, have been detected in human ovarian, breast, thyroid and colon carcinomas (4). The action of PI3K is primarily counteracted by PTEN (phosphatase and tensin homologue deleted on chromosome 10), a phosphatase that dephosphorylates the 3-position of PtdIns(3,4,5)P3, PtdIns(3,4)P2 and PtdIns(3)P (5). PTEN is a representative tumour suppressor gene product, and deletion or loss-of-function mutations are frequently observed in a wide variety of malignant tumours.
4-Phosphatases as Tumour Suppressors
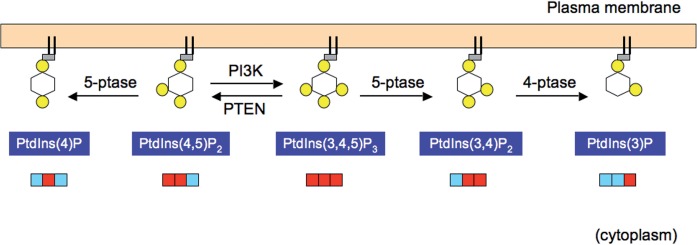
PtdIns(3,4,5)P3 is alternatively down-regulated by phosphatases that dephosphorylate the 5-position to yield PtdIns(3,4)P2 (Fig. 1). Two 4-phosphatases, inositol polyphosphate 4-phosphatase-type I and II (also termed INPP4A and INPP4B, respectively) in turn dephosphorylate PtdIns(3,4)P2 to produce PtdIns(3)P (6). Thus, these 4-phosphatases appear to be terminating enzymes of PI3K signalling in the alternative pathway of PtdIns(3,4,5)P3 inactivation (7). INPP4A and INPP4B were shown to antagonize PI3K/Akt signalling in vitro, and cells lacking INPP4A or INPP4B exhibited transformed phenotypes (8–10). In addition, loss of INPP4B was associated with a higher tumour grade (9, 10). These reports are consistent with the notion that enzymes counteracting PI3K are tumour suppressors.
Fig. 1.
Two pathways that terminate PI3K signalling. The phosphorylation status of each phosphoinositide is schematically presented (top). Yellow circles denote phosphorylated positions on the inositol ring. They are also presented as a triple-digit binary code (bottom). Red and light blue denote phosphorylated and unphosphorylated states, respectively. PI3K is counteracted by phosphoinositide 3-phosphatase PTEN, or the successive action of 5-phosphatase (5-ptase) and 4-phosphatase (4-ptase). PTEN and 4-phosphatases have tumour suppressor functions, whereas 5-phosphatases exhibit negative or positive effects on tumourigenesis.
Phosphoinositide 5-Pohosphatases—Anti-oncogenic or Pro-oncogenic?
Phosphoinositide 5-phosphatases may also counteract PI3K signalling because they are required for signal termination by 4-phosphatases. To date, nine enzymes are known to have phosphoinositide 5-phosphatase activity. INPP5E/type IV 5-phosphatase in humans is an orthologue of pharbin (5-phosphatase that induces arborization) in rats and a 72-kDa 5-phosphatase in mice (7). INPP5E is widely expressed and reportedly the most potent enzyme that dephosphorylates the 5-position of PtdIns(3,4,5)P3 (11) while it also hydrolyses PtdIns(4,5)P2. INPP5E was shown to negatively regulate insulin-like growth factor 1-mediated Akt phosphorylation, membrane ruffling and protein synthesis in knock-down (12) and overexpression studies (11, 12), indicating that INPP5E counteracts PI3K signalling. However, the role of INPP5E in tumourigenesis remains unclear. INPP5E is overexpressed in cervical cancer, non-Hodgkin’s lymphoma, and uterine leiomyosarcoma (7). Recently, mutations in human INPP5E were found in Joubert and MORM syndromes that accompany cilia destabilization (13, 14). Fibroblasts derived from Joubert patients, in which INPP5E enzymatic activity is impaired, entered the cell cycle less efficiently after serum stimulation (13). These findings appear to contradict the potential tumour suppressor functions of INPP5E.
SHIP2 (SH2-domain containing inositol phosphatase 2) has an in vitro substrate specificity similar to that of INPP5E (12). In SHIP2-null mice, insulin-induced Akt phosphorylation was enhanced in the liver and muscle, indicating that SHIP2 counteracts PI3K signalling (15). However, SHIP2 is overexpressed in breast carcinoma cell lines, and SHIP2 knock-down in MDA-MB-231 cells unexpectedly suppressed cell proliferation, in vivo tumourigenicity, and metastatic potential (16). Although this action might be cell-type specific, these findings suggest that SHIP2 promotes tumourigenesis and tumour progression in mammary epithelial cells. In contrast, SHIP1, a homologue of SHIP2 predominantly expressed in haematopoietic cells, appears to have tumour suppressor functions in vitro and in vivo. Ship1−/− mice develop a myeloproliferative disease (17). SHIP1 is down-regulated in T-cell leukemia cell lines, and restoration of SHIP1 expression attenuates PI3K signalling and cell proliferation (18). Loss-of-function mutations in the SHIP1 gene have been identified in leukemia (19).
The mechanisms underlying these divergent effects of phosphoinositide 5-phosphatases on tumourigenesis remain to be elucidated. It is possible that 5-phosphatases differently affect tumourigenesis depending on co-existing 4-phosphatase activity. In the presence of high 4-phosphatase activity, PtdIns(3,4)P2 generated by 5-phosphatases would be immediately converted to PtdIns(3)P. 5-phosphatases appear to be anti-oncogenic in this situation. In the presence of low 4-phosphatase activity, however, 5-phosphatase would induce accumulation of PtdIns(3,4)P2. Recent findings suggested that PtdIns(3,4)P2 and PtdIns(3,4,5)P3 can have distinct functions (20). PtdIns(3,4)P2 may activate Akt more efficiently than PtdIns(3,4,5)P3 (21) and possibly other target proteins. Thus 5-phosphatase can be pro-oncogenic in this context.
There is increasing information on the behaviour and function of 5-phosphatases (22). Additional work will lead to a further understanding of the context-dependent nature of 5-phosphatase functions in cell regulation.
Conflict of interest
None declared.
Glossary
Abbreviations
- ENTH
epsin NH2-terminal homology
- FYVE
Fab-1/YGL023/Vps27/EEA1
- mTORC2
mammalian target of rapamycin complex 2
- PH
pleckstrin homology
- PtdIns
phosphatidylinositol
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- PX
Phox homology
References
- 1.Pilkis SJ, Claus TH, Kurland IJ, Lange AJ. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annu. Rev. Biochem. 1993;64:799–835. doi: 10.1146/annurev.bi.64.070195.004055. [DOI] [PubMed] [Google Scholar]
- 2.Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat. Chem. Biol. 2010;6:507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 5.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipids phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 6.Majerus PW, York JD. Phosphoinositide phosphatases and disease. J. Lipid Res. 2009;50:S249–S254. doi: 10.1194/jlr.R800072-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooms LM, Horan KA, Rahman P, Seaton G, Gurung R, Kethesparan DS, Mitchell CA. The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem. J. 2009;419:29–49. doi: 10.1042/BJ20081673. [DOI] [PubMed] [Google Scholar]
- 8.Ivetac I, Gurung R, Hakim S, Horan KA, Sheffield DA, Binge LC, Majerus PW, Tiganis T, Mitchell CA. Regulation of PI(3)K/Akt signalling and cellular transformation by inositol polyphosphate 4-phosphatase-1. EMBO Rep. 2009;10:487–493. doi: 10.1038/embor.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin WM, Rameh L, Salmena L, Pandolfi PP, Cantley LC. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedele CG, Ooms LM, Ho M, Vieusseux J, O'Toole SA, Millar EK, Lopez-Knowles E, Sriratana A, Gurung R, Baglietto L, Giles GG, Bailey CG, Rasko JE, Shields BJ, Price JT, Majerus PW, Sutherland RL, Tiganis T, McLean CA, Mitchell CA. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc. Natl Acad. Sci. USA. 2010;107:22231–22236. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kisseleva MV, Cao L, Majerus PW. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J. Biol. Chem. 2002;277:6266–6272. doi: 10.1074/jbc.M105969200. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Ijuin T, Itoh T, Takenawa T. Regulation of IGF-1/PI3K/Akt signalling by the phosphoinositide phosphatase pharbin. J. Biochem. 2011;150:83–93. doi: 10.1093/jb/mvr037. [DOI] [PubMed] [Google Scholar]
- 13.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compère P, Schiffmann SN, Gergely F, Riley JH, Pérez-Morga D, Woods CG, Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 15.Sleeman MW, Wortley KE, Lai KM, Gowen LC, Kintner J, Kline WO, Garcia K, Stitt TN, Yancopoulos GD, Wiegand SJ, Glass DJ. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat. Med. 2005;11:199–205. doi: 10.1038/nm1178. [DOI] [PubMed] [Google Scholar]
- 16.Prasad NK, Tandon M, Badve S, Snyder PW, Nakshatri H. Phosphoinositol phosphatase SHIP2 promotes cancer development and metastasis coupled with alterations in EGF receptor turnover. Carcinogenesis. 2008;29:25–34. doi: 10.1093/carcin/bgm213. [DOI] [PubMed] [Google Scholar]
- 17.Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn S, Endl E, Fehse B, Weck MM, Mayr GW, Jücker M. Restoration of SHIP activity in a human leukemia cell line downregulates constitutively activated phosphatidylinositol 3-kinase/Akt/GSK-3β signaling and leads to an increased transit time through the G1 phase of the cell cycle. Leukemia. 2004;18:1839–1849. doi: 10.1038/sj.leu.2403529. [DOI] [PubMed] [Google Scholar]
- 19.Luo JM, Yoshida H, Komura S, Ohishi N, Pan L, Shigeno K, Hanamura I, Miura K, Iida S, Ueda R, Naoe T, Akao Y, Ohno R, Ohnishi K. Possible dominant-negative mutation of the SHIP gene in acute myeloid leukemia. Leukemia. 2003;17:1–8. doi: 10.1038/sj.leu.2402725. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki J, Kofuji S, Itoh R, Momiyama T, Takayama K, Murakami H, Chida S, Tsuya Y, Takasuga S, Eguchi S, Asanuma K, Horie Y, Miura K, Davis EM, Mitchell C, Yamasaki M, Hirai H, Takenawa T, Suzuki A, Sasaki T. The PtdIns(3,4)P2 phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature. 2010;465:497–501. doi: 10.1038/nature09023. [DOI] [PubMed] [Google Scholar]
- 21.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 22.Conduit SE, Dyson JM, Mitchell CA. Inositol polyphosphate 5-phosphatases; new players in the regulation of cilia and ciliopathies. FEBS Lett. 2012;586:2846–2857. doi: 10.1016/j.febslet.2012.07.037. [DOI] [PubMed] [Google Scholar]