Abstract
The actual levels of steroid hormones in organs are vital for endocrine, reproductive and neuronal health and disorders. We developed an accurate method to determine the levels of steroid hormones and steroid conjugates in various organs by an efficient preparation using a solid-phase-extraction cartridge. Each steroid was identified by the precursor ion spectra using liquid chromatography–electrospray ionization time-of-flight mass spectrometry, and the respective steroids were quantitatively analysed in the selected reaction monitoring mode by liquid chromatograph-mass spectrometry/mass spectrometry (LC-MS/MS). The data showed that significant levels of testosterone, corticosterone and precursors of both hormones were detected in all organs except liver. The glucuronide conjugates of steroid hormones and the precursors were detected in all organs except liver, but sulfate conjugates of these steroids were observed only in the target organs of the hormones and kidney. Interestingly, these steroids and the conjugates were not observed in the liver except pregnenolone. In conclusion, an accurate determination of tissue steroids was developed using LC-MS analysis. Biosynthesis of steroid hormones from the precursors was estimated even in the target organs, and the delivery of these steroid conjugates was also suggested via the circulation without any significant hepatic participation.
Keywords: accurate determination, conjugation, LC-MS analysis, metabolism, tissue steroids
Steroid hormones are synthesized from cholesterol, and many are of great clinical importance (1). The measurement of steroid profiles indicates an important physiological state in endocrine systems, and it has been newly applied in the diagnoses and treatments of congenital adrenal hyperplasia, adrenal insufficiency, chronic pelvic pain and prostatitis, oncology (breast cancer) and athletic competition. Accurate assay methods for the determination of tissue steroid levels are required for the clinical evaluation of a number of common endocrine disorders. Radioimmunoassays (RIAs) are commonly used for the determination of the actual levels of testosterone (TS) (2). As RIA methods have the inherent limitations in specificity, it is difficult to determine accurately the low levels of TS present in females and children (3, 4). Gas chromatography-mass spectrometry is used for the determination of low levels of natural steroids with excellent specificity and sensitivity (5). However, this method usually requires extra steps for sample preparation and cleanup and is often encountered with the problems in the thermal stability of the steroid derivatives. Recently, several liquid chromatograph-mass spectrometry (LC-MS) methods have been developed for the simultaneous determination of low levels of steroids. The chemical derivatization using a number of reagents, such as dansyl chloride and 2-hydrazino-4-(trifluoromethyl)-pyrimidine enhance the sensitivity of the LC-MS determination of steroids in various biological samples (6, 7). However, for accurate determination of both steroid hormones and glucuronide and sulfate conjugates simultaneously in organs containing various matrixes, an analysis should be conducted without the removal of the matrix, which reduces the ionization efficiency of electrospray ionization (ESI) in LC-MS/MS analysis. A simultaneous analysis was performed on steroid hormones and the conjugate forms in urine and water containing trace amounts of matrix (8–11). It has generally been proposed that the presence of interference peaks co-existing in biological matrixes and co-eluting with the analyte of interest caused ionization suppression, reducing the detection sensitivity (12, 13). For the measurement of steroids in organs, LC-MS analysis has several advantages over immunoassays, including a better specificity and the ability to quantify numerous steroids in a single run (14, 15). Especially, liquid chromatography–electrospray ionization time-of-flight mass spectrometry (LC-TOFMS) made possible to perform the accurate mass determination of components containing matrixes. This method could be applied to drug screening using ESI to produce pseudomolecular ions, measuring their mass and comparing the results with a database containing the exact monoisotopic masses of the target analysis (16, 17). The difference between the theoretical and measured isotopic patterns provides the evaluation and numerical expression using the SigmaFit algorithm for exact identification. For example, it has been demonstrated to aid in the identification and to reduce the incidence of false-positive findings in human urine drug screening when combined with mass accuracies of <10 ppm using this method (18).
In this study, we developed accurate and highly sensitive assay methods for various steroids and their glucuronide and sulphate conjugates using LC-TOF MS and LC-MS/MS, and obtained the actual levels of steroid hormones and precursors in various organs. Based on our results, we discussed about metabolism and the possibility of steroid biosynthesis and conjugate reactions in each organ.
Materials and Methods
Chemicals and reagents
The following were purchased from Sigma-Aldrich (St. Louis, MO, USA): steroid hormones—TS, 17α and 17β-estradiol (17α, 17β-E2); precursors—pregnenolone (PGN), progesterone (PGT), 17-hydroxyprogesterone (HPGT), androstenedione (ADS), deoxycorticosterone (DCC) and corticosterone (CCS); conjugated metabolites—TS-17(β-d-glucuronide) (TS-17G), 17β-estradiol-3(β-d-glucuronide) (E2-3G) and 17β-estradiol-3-sulphate(E2-3S); and stable isotopes—TS-d3 and 17β-estradiol-d2 (E2-d2). Triethylamine was purchased from Wako Pure Chemical Industries (Osaka, Japan). Methanol, acetonitrile and hexane for pesticide residue analysis and citric acid were purchased from Kanto Chemical (Tokyo, Japan). LC-MS grade acetonitrile, formic acid and ammonium hydroxide solution were purchased from Supelco (Bellefonte, PA, USA). β-Glucuronidase (Type B-1) and sulphatase (Type H-1) were purchased from Sigma-Aldrich.
Preparation of rat organs
Male Sprague–Dawley rats (weight: 280 ± 20 g; age: 8–10 weeks) were fed, housed and allowed to adapt to their environments for 1 week before the experiments. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The blood and organs were prepared from the animals by exsanguination under isoflurane anaesthesia. After dissection, the organ samples excised post-mortem were weighed, immediately frozen and stored at −25°C until use.
Preparation of samples for MS analysis
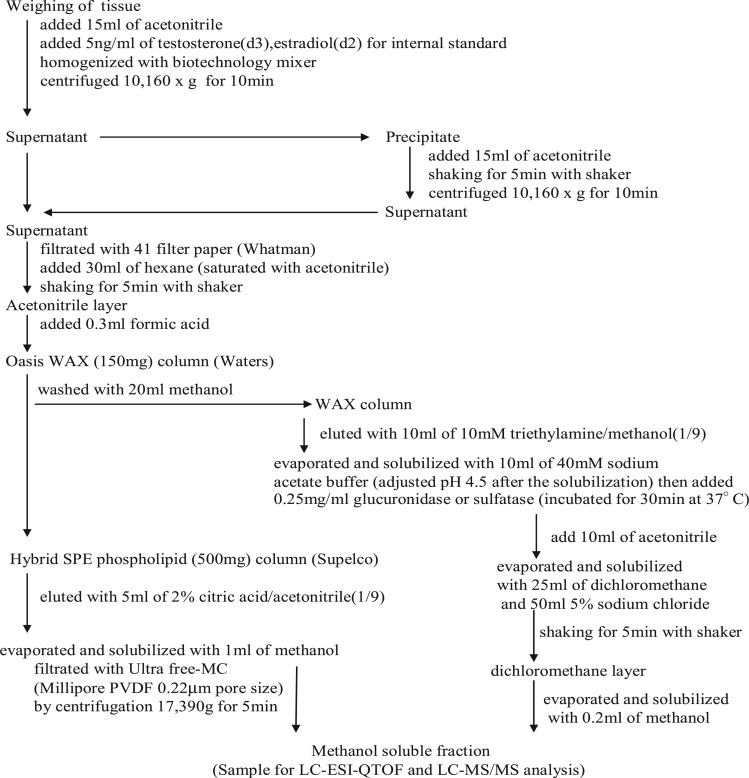
The samples were weighed in 50 ml centrifuge tubes. Then, 5.0 ng/ml TS and E2 of the internal standard were added to the tube. A 15-ml aliquot of acetonitrile was added, and the samples were homogenized for 1 min using a biotechnology mixer. The mixture was centrifuged at 10,160 × g for 10 min at 4°C, then 30 ml of hexane was added to the supernatant and the tube was shaken for 5 min at high speed. The acetonitrile layers were collected, and 0.3 ml formic acid was added. An Oasis Weak Anion Exchange (WAX) 150 mg solid-phase-extraction (SPE) cartridge (Waters Midford, MA, USA) was conditioned using 5 ml methanol, followed by acetonitrile containing 2% formic acid. The sample was loaded onto the WAX SPE cartridge at ∼3–5 ml/min (Sample 1). After loading, the waste sample collected via the Oasis WAX SPE cartridge was linked in-series to a Hybrid SPE Phospholipid cartridge (Supelco) (Sample 2). Sample 1 was collected in another bottle via the Oasis WAX SPE cartridge, which was loaded with 20 ml methanol, washed and then immediately loaded with 10 ml 10 mM triethylamine. The liquid was evaporated and solubilized with 10 ml 40 mM sodium acetate buffer (pH adjusted to 4.5 after the solubilization); 0.1 ml 2.5 mg/ml β-glucuronidase or sulphatase was added (incubation for 30 min at 37°C), and 10 ml acetonitrile was added, evaporated and solubilized with 25 ml of dichloromethane and 50 ml 5% sodium chloride. The dichloromethane layer was evaporated and solubilized with 0.2 ml methanol, and the steroids and conjugates were then assayed by LC-TOF MS and LC-MS/MS analyses. Sample 2 was collected for loading onto the Hybrid SPE Phospholipid cartridge, with 5 ml methanol and 5 ml acetonitrile with 2% citric acid then added. The collected extract was subsequently evaporated, and the leftover residue was dissolved in 1 ml methanol and then centrifuged at 17,390 × g for 5 min using PVDF 0.22 µm Ultrafree-MC (Millipore, Billerica, MA, USA). The supernatant was collected for the LC-TOF MS and LC-MS/MS analysis as shown in Fig. 1.
Fig. 1.
Preparation of lipophilic steroids and hydrophilic conjugate forms of the steroids from various organs for MS analysis.
Determination of steroids expressed in the tissues
The HPLC system was a UFLC Nexera (Shimadzu, Japan) instrument, comprising a vacuum degasser, autosampler, binary pump and column oven. The separation was achieved using an L-column 2 [C18 2.1 × 150 mm 2-μm particle size, Chemicals Evaluation and Research Institute (CERI), Japan] at a 200 µl/min flow rate at 40°C. A guard column with the pre-column filter (L-column pre-column filter 0.5 µm, CERI, Japan) was used for the analysis. A 10-µl aliquot was used for the autosampler injection. The positive ion mode scanning of a gradient mobile phase consisting of (A) 0.1% formic acid solution and (B) acetonitrile with 0.1% formic acid solution was used. For the gradient elution, (A)/(B) ratios were used from 95/5 to 40/60 and 40/60 to 5/95 for between 0 and 3 min and between 3 and 9 min, respectively, followed by a 2 min hold at 95% (B) and a final return to 95% (A) within 4 min. The negative ion mode scanning of a gradient mobile phase consisting of (A) 0.03% ammonium hydroxide solution and (B) acetonitrile with 0.03% ammonium hydroxide solution was also used. The same positive ion mode was used for the gradient pattern. The mass analyser was a microTOF-QII time-of-flight mass spectrometer (Bruker Daltonics, Bremen, Germany), equipped with an orthogonal ESI source and a 6-port diverter valve. The instrument was operated in positive ion or negative ion mode using a range of 50–1,000 m/z. The capillary voltage of the ion source was set to 4,500 V, then the nebulizer gas flow was 1.6 bar and the dry gas flow was 8 l/min. The dry temperature was set to 180°C. Instrument calibration was performed prior to each sequence using 10 mM sodium formate/2-propanol (1:1, v/v). The post-run internal mass scale calibration for the individual samples was performed using data acquired during a calibration injection at the beginning of the run via a 6-port diverter valve equipped with a 20-µl loop. The calibrant was also injected at the end of each run for the verification of the calibration stability. The instrument calibration and post-run internal mass scale calibration were performed using sodium formate ions Na (NaCOOH) 1–14 ranging from 90.9766 to 974.8132 m/z with an accuracy of 5 ppm. The data processing was performed using Data Analysis software (version 4.0, Bruker Daltonics). The base mass peak (after background subtraction) was measured after the proton subtraction in the compounds (10 ppm tolerance). For each retrieved chemical formula, the mass error (difference between the measured and theoretical masses) and SigmaFit [a parameter, calculated by the Bruker software, accounting for the difference between the theoretical and measured isotonic pattern; the lower is the sigma value (usually <0.05), the better the matching is] were calculated.
Quantification of steroids expressed in tissues
For the quantification of the steroid hormones and conjugated metabolites, a TSQ Quantum Ultra triple-stage quadrupole mass spectrometer connected to an Ultimate 3,000 (Thermo Fisher Scientific, San Jose, CA, USA) and an ESI ion source device was constructed (LC-MS/MS). Separation was achieved using an L-column 2 [C18 2.1 × 150 mm 3-μm particle size (CERI)] at a 200-μl/min flow rate at 40°C. A guard column with packing material was used as the analytical column. A 10-μl aliquot was used for the autosampler injection. A gradient mobile phase consisting of (A) 0.03% ammonium hydroxide solution and (B) acetonitrile with 0.03% ammonium hydroxide solution was used. In particular, the positive ion microanalysis of original TS and CCS conjugate were used with a mobile phase consisting of (A) 0.1% acetic acid and (B) acetonitrile with 0.1% acetic acid solution. The gradient pattern was the same as for the LC-TOF MS described earlier. The total run time for each sample analysis was 15 min, and the data were collected between 3 and 13 min; the column effluents before 3 min and after 13 min were diverted as waste. The MS system and data were operated and analysed using Xcalliber and LCquan 2.6 software, respectively. The mass spectrometer was operated with positive and negative ionization mode switching. The instrumental parameters were optimized during the direct infusion of the compounds with solvent consisting of 50% (0.1% acetic acid in water/acetonitrile at 1:1 [v/v]) at a flow rate of 5 μl/min. The [M+H]+, [M-H2O]+ and [M−H]− ions of the compounds were identified by LC-MS, with Q1 operated in the full scanning mode in the range of 50–800 m/z. A product ion spectrum was obtained for each compound. Selected reaction monitoring (SRM) was used for the quantitative analysis (Fig. 3C and D). Nitrogen was used for the sheath gas pressure (setting 50), ion sweep gas pressure (setting 10) and auxiliary gas pressure (setting 15), whereas argon was used as the collision gas for the collision-induced dissociation conditions (setting 1.5 mTorr). The ion spray voltage was set to a positive mode at 3,500 V or a negative mode at 2,500 V. The vaporizer gas temperature was set at 450°C, and the ion transfer capillary temperature was set at 270°C. The tube lens and collision energy for each SRM transition were set.
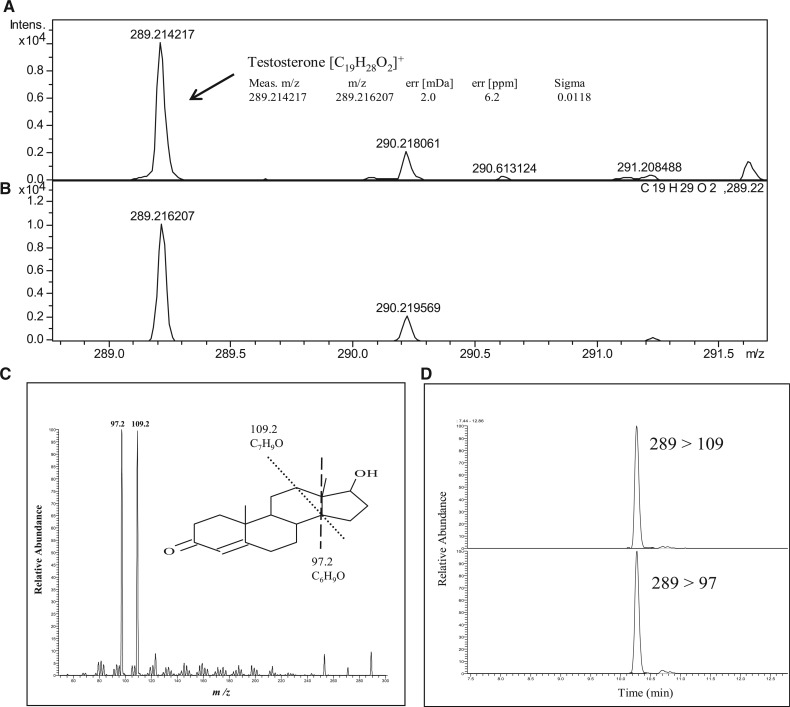
Fig. 3.
Identification and determination of steroids by the SigmaFit algorithm and LC-TOF MS analysis. The chromatograms of the preparations from the testis by TOF MS analysis developed in this study (A) and theoretical abundances of testosterone containing an isotope (B). A δ-value was calculated by the program ‘sigmaFit algorithm’ after comparing the two chromatograms A and B. The δ-value between 0 and 1.0 shows higher identification. In this case, small δ-value was observed at 0.0118 and the mass error was only 2.0 mDa showing that TS in the sample was identified. The identification of other steroids was performed by the same procedure using sigmaFit algorithm. Identically, TS precursors were identified from testis. Product-ion spectrums were obtained for TS by MS/MS analysis (C). SRM of TS was used for quantitative analysis as described in Materials and Methods section (D).
Calibration curves
Stock solutions of the steroid hormones and conjugated metabolites were used to prepare working standards of the steroid hormones and conjugated metabolites (at concentrations of 0.1, 0.5, 1.0, 5.0, 10.0, 50.0 and 100.0 ng/ml) by serial dilution in methanol; the isotopes of the internal standards TS-d3, E2-d2 were prepared at a concentration of 5.0 ng/ml. The calibration curves were plotted using the concentration ratios of the analytes to the internal standards (PGN, PGT, HPGT, ADS, TS, DCC, CCS/TS-d3, 17α and 17 β-E2/E2-d2) as the x axes and the peak area ratios of the analytes to the internal standards as the y axes. The calibration curves for the steroid hormones and conjugated metabolites showed excellent linearity over the concentration range used (R2 > 0.998), and the detection limits of these were shown in Table I (19). The steroid hormones and their conjugated metabolites were used as the standard in the addition method (20).
Table I.
Recovery tests of additive steroids and glucuronides into the blood and the testis in the determination method developed in this study.
| Blood (0.50 ml)a |
Testis (1.00 g)a |
Liver (1.00 g)a |
||||||
|---|---|---|---|---|---|---|---|---|
| Steroids | Added (ng/g) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | LOD (fmol/g) |
| PGN | 10.0 | 93.3 | 18.5 | 98.3 | 7.9 | 86.7 | 3.5 | |
| 5.0 | 91.0 | 18.4 | 104.3 | 14.8 | 85.0 | 15.3 | 14.2 | |
| 1.0 | 85.9 | 15.7 | 91.6 | 19.4 | 99.3 | 9.8 | ||
| PGT | 10.0 | 90.7 | 10.9 | |||||
| 5.0 | 94.6 | 8.2 | 92.0 | 10.5 | 86.6 | 16.0 | ||
| 1.0 | 90.1 | 6.4 | 97.7 | 16.5 | 107.1 | 15.3 | 3.2 | |
| 0.1 | 82.8 | 4.5 | 101.6 | 9.3 | ||||
| HPGT | 10.0 | 72.4 | 4.3 | |||||
| 5.0 | 110.3 | 2.3 | 105.2 | 7.0 | 75.8 | 19.8 | ||
| 1.0 | 86.0 | 9.4 | 89.4 | 10.4 | 89.7 | 6.7 | 2.8 | |
| 0.1 | 97.2 | 13.6 | 92.6 | 16.4 | ||||
| ADS | 10 | 105.2 | 7.0 | |||||
| 5.0 | 113.6 | 6.4 | 86.0 | 4.5 | 89.4 | 10.4 | ||
| 1.0 | 115.4 | 2.5 | 86.2 | 5.6 | 92.6 | 16.4 | 2.6 | |
| 0.1 | 100.7 | 7.6 | 102.0 | 5.3 | ||||
| TS | 10.0 | 98.6 | 4.5 | |||||
| 5.0 | 106.8 | 5.2 | 80.5 | 4.7 | 88.0 | 9.1 | ||
| 1.0 | 104.8 | 5.7 | 77.6 | 5.7 | 74.3 | 4.4 | 0.6 | |
| 0.1 | 110.6 | 7.8 | 96.4 | 15.1 | ||||
| 17α-Estradiol | 10.0 | 108.7 | 6.0 | |||||
| 5.0 | 91.9 | 14.1 | 108.0 | 1.0 | 89.7 | 20.5 | ||
| 1.0 | 73.3 | 2.1 | 109.5 | 4.0 | 89.0 | 7.9 | 25.3 | |
| 0.5 | 89.4 | 15.6 | 107.0 | 6.1 | ||||
| 17β-Estradiol | 10.0 | 99.3 | 3.1 | |||||
| 5.0 | 99.3 | 3.1 | 105.7 | 0.7 | 73.3 | 2.1 | ||
| 1.0 | 73.3 | 2.1 | 81.9 | 1.5 | 112.3 | 6.7 | 24.2 | |
| 0.5 | 112.3 | 6.7 | 96.9 | 18.4 | ||||
| CCS | 10.0 | 101.3 | 4.0 | |||||
| 5.0 | 82.7 | 9.4 | 84.8 | 16.4 | 88.9 | 9.6 | ||
| 1.0 | 80.7 | 4.3 | 100.6 | 13.9 | 104.3 | 2.9 | 0.4 | |
| 0.1 | 107.8 | 5.1 | 85.7 | 9.1 | ||||
| 11-DCC | 10.0 | 95.9 | 5.2 | |||||
| 5.0 | 78.6 | 7.4 | 116.9 | 1.7 | 97.6 | 2.0 | ||
| 1.0 | 90.0 | 11.3 | 78.1 | 4.3 | 100.7 | 21.5 | 9.2 | |
| 0.1 | 81.5 | 8.8 | 75.3 | 1.2 | ||||
| TS-17-glucuronide | 10.0 | 85.0 | 8.6 | |||||
| 5.0 | 91.8 | 18.8 | 85.0 | 2.6 | 95.4 | 16.4 | 34.6 | |
| 1.0 | 98.5 | 13.8 | 95.4 | 6.4 | 110.0 | 10.1 | ||
| 0.5 | 71.3 | 14.9 | 117.0 | 1.1 | ||||
| 17β-Estradiol-3-glucuronide | 10.0 | 103.5 | 4.3 | |||||
| 5.0 | 90.7 | 10.9 | 102.7 | 2.3 | 97.8 | 4.0 | 73.3 | |
| 1.0 | 86.6 | 16.0 | 109.2 | 7.7 | 119.1 | 0.5 | ||
| 0.5 | 107.1 | 15.3 | 79.1 | 12.3 | ||||
| 17β-Estradiol-3-sulfate | 10.0 | 90.3 | 17.1 | 106.4 | 1.4 | 70.2 | 6.5 | |
| 5.0 | 101.3 | 4.6 | 101.5 | 13.5 | 72.3 | 12.3 | 146.6 | |
| 1.0 | 82.3 | 13.2 | 91.3 | 12.5 | 71.2 | 15.3 | ||
Standard substrates ∼10-fold amount of the assay limits were added into the blood, liver and the testis. Standards substrates were extracted and determined by the assay method developed in this study. Recovery data obtained were shown with RSD. The accurate data having 70–120% recovery and RSD under 25% and LOD (signal/noise [S/N] > 3) were obtained, respectively, as previously indicated by FDA as a guideline (FDA 2001).
aData presented mean values of n = 3–5.
Overall method recovery
Recovery tests were performed to search for the recovery rates and assess the accuracy of the method. Several concentration mixtures of the standards and internal TS-d3 and E2-d2 standards were added to 5.0 ng/ml. The samples were measured and evaluated for the recovery rate and relative standard deviation (RSD%) using LC-MS/MS. The accuracy and precision of the entire analytical procedure were evaluated by spiking the whole-organ samples (n = 3–5) with 0.1, 0.5, 1.0, 5.0 and 10.0 ng/ml or g at each concentration before using SPE with the working solution. The amount of endogenous steroid hormones and conjugated metabolites were subtracted from the spiked amounts of the analytes in the blood, testis and liver.
Results
Determination of steroids in organs
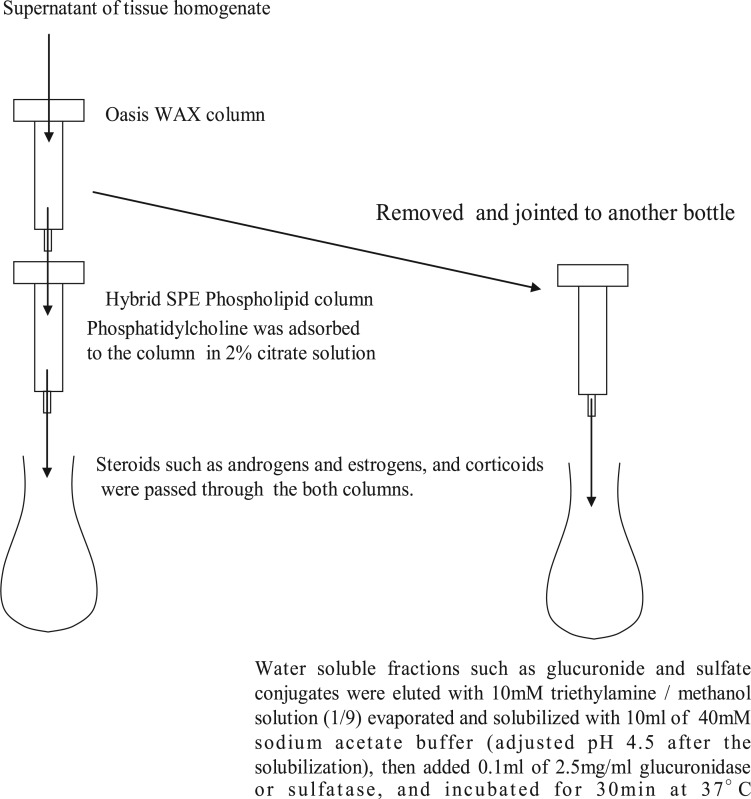
A simple method for preparing samples for LC-MS analysis with a high sensitivity and accuracy was improved to determine lipophilic compounds, such as TS in the blood and various tissues of rats. The total preparation scheme is shown in Fig. 1. The water-soluble compounds, such as the glucuronide and sulphate conjugates of the steroid hormones, were adsorbed onto an Oasis WAX column, and these compounds were eluted with 10 mM triethylamine methanol (1:9) solution. Because the androgens and estrogens passed through the HybridSPE Phospholipid column, only the corticoids and phosphatidylcholine, which inhibits the ESI in LC-MS analysis, were adsorbed to the column. Only the corticoids were eluted from the column with a 2% citrate solution as shown in Fig. 2. Using both columns allowed for the simultaneous analysis of the lipophilic steroid hormones and hydrophilic glucuronide and sulphate conjugates with a high degree of sensitivity and recovery as shown in Table I. The recovery tests for TS and the glucuronides were performed as described in the Materials and Methods section. High-accuracy results were obtained and showed significant trueness (70–120% recovery) with a high precision. All the substrate peaks in the LC-MS analysis indicated significant S/N values of <10. The quantitation limits of these substrates were determined and are shown in Table I (e.g. 1.0 ng of TS/g of liver and 0.1 ng of TS/ml of blood). This removal of phosphatidylcholine is a critical step for obtaining a highly sensitive and accurate LC-TOF MS analysis. Each steroid formula in the organs was detected using SigmaFit (Fig. 3). For example, the measured isotopic pattern of the positive ion of TS [C19H28O2]+ found in rat testis was identified using the developed method (Fig. 3A) and the theoretical isotopic abundance (Fig. 3B). The SigmaFit algorithm compares both the mass distances between the ions and their relative peak intensities with those predicted and expresses the degree of fit as a δ value between 0 and 1; a low δ value indicates a closer match (18, 21). In this case, the delta value was 0.0118, and the mass error was 2 mDa. Similarly, the identification of the steroid hormones was performed using the SigmaFit algorithm, and the identified steroids were then quantified by LC-MS/MS analysis (22). Other steroids were identified by same procedure as shown in Supplementary Fig. S3.
Fig. 2.
Critical steps for separation of lipophilic steroids and hydrophilic conjugate forms of the steroids and removing various contaminants from the extracted preparations of organs.
Tissue distribution of steroids
The steroid concentrations in various organs were assayed using the MS procedure developed in this study, and the results are shown in Table II. The data showed that TS and CCS were distributed in all of the organs tested, and these two steroid hormones are known to play multiple roles in the respective organs and regulate the endocrine system in the entire body. Significant levels of two of the precursors for steroid hormone biosynthesis, PGT and 11-DCC, were also observed in the blood and all of the organs (Table II). This result suggests that the endo products TS and CCS and also the precursors were transported to the various target organs via the circulation from the endocrine organs. Interestingly, only a trace amount of CCS and PGN were detected in the liver before the removal of blood by perfusion with PBS (Supplementary Figs S1 and S2), but only PGN and no other steroids were detected in the liver after the perfusion (Table II). The levels of the two conjugate forms of various steroids in the organs are shown in Table III. The glucuronide conjugates of endo products, TS and CCS and all of the precursors, except PGN, were observed in the testis and adrenal gland. Higher levels of CCS and its precursors were observed in the adrenal gland (Table II), and the glucuronide forms were also detected at higher levels in the same organs (Table III). In contrast, the sulphate conjugates of the endo products and a portion of the precursors were observed only in the blood and hormone target organs, such as the brain, muscle and kidney (Table III). Both of these conjugation reactions may be performed in the respective organs, and/or the conjugates were transported via blood circulation from the endocrine organs. It is very interesting that steroid hormones and the glucuronide and sulphate conjugate forms were not detected in the liver (Table III).
Table II.
Steroid hormones and the precursors in the various organs of adult male rats.
| Brain |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Steroids | Blood | Testis | Adrenal glands | Liver | Kidney | Cerebrum | Cerebellum | Hippocampus | Muscle |
| TS | 5.4 ± 1.3 | 175.8 ± 41.8 | 0.105 ± 0.004 | – | 8.5 ± 1.5 | 9.1 ± 2.6 | 9.4 ± 2.5 | 1.17 ± 0.21 | 3.14 ± 0.90 |
| ADS | 0.57 ± 0.16 | 45.6 ± 8.8 | 0.15 ± 0.02 | – | 6.1 ± 2.7 | – | – | 0.052 ± 0.015 | 0.88 ± 0.09 |
| HPGT | – | 36.2 ± 9.5 | – | – | – | – | – | – | – |
| PGT | 2.21 ± 1.43 | 13.7 ± 5.3 | 28.5 ± 2.8 *a | – | 10.1 ± 0.4 | 15.67 ± 5.40 | 4.4 ± 0.36 | 0.083 ± 0.043 | 0.45 ± 0.15 |
| PGN | – | 25.4 ± 2.5 | 4.99 ± 0.86 *a | 304.2 ± 55.1 | 25.3 ± 4.4 | – | – | – | 216.2 ± 28.0 |
| 11-DCC | 20.4 ± 3.1 | 40.2 ± 17.8 | 17.2 ± 1.5 *a | – | 30.4 ± 1.6 | 11.2 ± 5.9 | 18.8 ± 1.0 | 0.368 ± 0.07 | 14.4 ± 3.4 |
| CCS | 140.3 ± 38.1 | 137.7 ± 24.1 | 38.2 ± 1.1 *a | – | 414.7 ± 127.8 | 101.0 ± 6.6 | 92.7 ± 6.5 | 2.55 ± 0.62 | 57.0 ± 19.3 |
Concentration of steroids and the precursors in rat organs were assayed by the method developed in this study as described in ‘Materials and Methods’ section. Data were shown as the means ± SD for 3–5 animals (nmol/ml or g of tissue weight). ‘*a’ and ‘–’ mean as ‘μmol/g’ and ‘LOD’, respectively.
Table III.
Glucuronide or sulfate-conjugated steroids in various organs adult male rats.
| Glucuronide conjugates | Blood | Testis | Adrenal glands | Liver | Kidney | Whole brain | Muscle |
|---|---|---|---|---|---|---|---|
| TS | 0.039 ± 0.009 | 0.023 ± 0.008 | – | – | 0.037 ± 0.020 | 0.024 ± 0.006 | 0.062 ± 0.005 |
| ADS | – | 0.021 ± 0.005 | – | – | – | – | – |
| HPGT | – | 0.052 ± 0.004 | – | – | – | – | – |
| PGT | – | 0.050 ± 0.012 | 4.39 ± 2.30 | – | – | 0.036 ± 0.012 | – |
| PGN | 4.39 ± 0.55 | 2.69 ± 1.42 | 5.71 ± 2.17 | 8.18 ± 3.81 | 9.83 ± 1.00 | 7.84 ± 0.46 | 6.99 ± 1.59 |
| 11-DCC | – | – | 4.55 ± 1.84 | – | – | 0.060 ± 0.028 | – |
| CCS | 0.077 ± 0.007 | 0.021 ± 0.016 | 6.07 ± 3.19 | – | 0.063 ± 0.010 | 0.070 ± 0.054 | 0.028 ± 0.015 |
| Sulfate conjugates | Blood | Testis | Adrenal glands | Liver | Kidney | Whole brain | Muscle |
|---|---|---|---|---|---|---|---|
| TS | 0.068 ± 0.006 | – | – | – | 0.041 ± 0.018 | 0.015 ± 0.011 | 0.032 ± 0.002 |
| ADS | – | – | – | – | – | – | – |
| 17α-HPGT | – | – | – | – | – | – | – |
| PGT | – | – | – | – | 0.042 ± 0.006 | 0.013 ± 0.005 | – |
| PGN | 6.12 ± 0.42 | – | – | 17.95 ± 3.50 | 15.99 ± 5.98 | 10.91 ± 1.43 | 8.66 ± 0.73 |
| 11-DCC | – | – | – | – | – | 0.012 ± 0.004 | – |
| CCS | 0.056 ± 0.002 | – | – | – | 0.067 ± 0.017 | 0.018 ± 0.008 | 0.036 ± 0.018 |
Tissue-conjugated steroids were assayed by the method developed in this study as shown in ‘Materials and Methods’ section. Data were shown as the means ± SD for 3–5 animals (nmol/ml or g of tissue weight). ‘–’ mean as ‘LOD’.
Discussion
Steroid assays play an important role in the clinical evaluation of a number of common endocrine disorders, and LC-MS analysis has several advantages over immunoassays for the measurement of steroids, such as an improved specificity and ability to quantify numerous steroids. To analyse both steroid hormones and water-soluble glucuronide and sulphate conjugates simultaneously in organs containing various matrices, an analysis should be conducted without the derivatization of the specimen (23) and with the removal of the matrix, which reduces the ionization efficiency of the ESI in LC-MS/MS analysis. An simultaneous LC-MS analysis was performed on the steroid hormones, glucuronide and sulphate conjugates contained in urine and water, with only trace amounts of matrix (6–9). We developed an accurate method to determine various steroids and also their conjugate forms in tissues using two different columns. The limitation of detection (LOD) in case of serum TS were reported previously as 920 fmol/ml by RIA (3), 600 fmol/ml by Gas-mass (5) and 50 fmol/ml by LC-mass (3), and the LOD value of our method developed in this study was 0.6 fmol/ml (Table I), corresponding ∼1,500-fold sensitivity of that using RIA. Neurosteroids and their precursors and metabolites in whole brain were identified and determined, however, some precursors and sulfate conjugates could not be detected by the method using Gas-mass analysis (24). In non-reproductive organs, such as the brain, the synthesis of steroid hormones has been shown through the detection of cytochrome P450scc and the mRNAs encoding the enzymes that synthesize steroid hormones. Because of the broad specificity of antibodies against small molecules, the accurate determination of steroid levels in organs is difficult using RIA and ELISA (25, 26). We have developed a method for the determination of steroids using an efficient preparation of organs with two columns, with the determination being performed using LC-TOF MS and LC-MS/MS analysis; highly accurate results were obtained (Table II). Serum TS in pregnant and immature rats could be detected by our method developed in this study, and they were very low levels (means ± SD; 0.11 ± 0.06 and 0.18 ± 0.08 nmol/ml, respectively), corresponding ∼2–4% of that in adult male rat (Table II). Previously, sex steroids were thought to originate exclusively from the gonads and adrenal glands; however, it is now accepted that local steroid synthesis occurs in a number of tissues, such as adipose tissue, the cardiovascular system and the brain (27). In adult male rat neurons, significant localization and protein expression were demonstrated for both cytochromes P45017α and P450 aromatase by means of immunohistochemical staining, western blot analysis and RT-PCR (28). The presence of a conversion activity from PGN to estradiol through TS was also observed. Estradiol synthesis has been demonstrated in cultured rat hippocampus slices and dispersed cells (29). In this study, we could additively confirm the presence of a biosynthesis system for neurosteroids in rat hippocampus by the new detection of steroid precursors, such as ADS and PGT (Table II). Rat Leydig cells expressing 11β-hydroxylase have been demonstrated using RT-PCR, western blot analysis and immunohistochemistry (30), and it was suggested that the enzyme may be involved in the regulation of glucocorticoid metabolism within the testis through the local biosynthesis of endogenous inhibitors, 11β-hydroxylated steroids, of 11β-HSD1 CCS metabolism (31, 32). However, 11β-hydroxylase catalyses the conversion of 11-DCC to CCS, and we found a significant level of 11-DCC in the testis (Table II), indicating the possibility of the biosynthesis of CCS via the 11β-hydroxylation of the 11-DCC detected in rat testis. We also observed significant levels of 11-DCC in the brain, suggesting the possibility of CCS biosynthesis in the brain, as previously reported in the hippocampus (33). Recently, neurosteroid glucuronides have been found in mouse brain by LC-MS analysis, and the authors showed the formation of those glucuronides in vitro using a brain homogenate S-9 fraction (34). It is interesting that the adrenal steroids, CCS, 11-hydroxycorticosterone and PGT, were observed at high concentrations, and the conjugate forms were also found in higher levels in the adrenal gland (Tables II and III), suggesting that each steroid was glucuronidated after the synthesis in the adrenal gland, as demonstrated in the brain (34). The glucuronide forms of the adrenal steroids CCS and 11-DCC were detected in the blood and whole organs at high concentrations (Table III), suggesting that these glucuronides were delivered to the target cells that express a glucuronide transporter and that the steroid can perform its respective function after deconjugation. Neurosteroid sulphates have not been previously found in the rodent brain (35, 36), however, sulphate conjugates of PGT, 11-DCC, CCS and TS were detected in the brain at very low levels (10–20 pmol/g) (Table III) in this and earlier studies (37, 38). Because the glucuronides and sulphates of major steroids were detected in the blood and kidney at similar levels, these conjugates were excreted into the urine via the kidneys. Our results suggested that the glucuronide and sulphate conjugates are the excreted forms of steroid hormones and also the transported forms, as previously reported with regard to estradiol sulphate circulation (39). These steroid hormones, their precursors and conjugate forms were not detected in the liver perfused with PBS except PGN (Tables II, III; Supplementary Data). A further comprehensive computer search of the steroid metabolites of phases I and II in the MS spectra was performed using the software ‘metabolitetools’ (Version 2.0, Bruker Daltonics Co.), but no corresponding peaks were observed in the liver.
We conclude the following: (i) steroid hormones were delivered from the endocrine organs to the target organs, together with the many precursors, which may then be converted to steroid hormones in each organ; (ii) those steroids were glucuronidated after their biosynthesis in the hormone-producing organs as delivery forms; and (iii) interestingly, these metabolisms were performed without any significant hepatic participation.
Supplementary Data
Supplementary Data are available at JB Online.
Acknowledgements
This work was supported by Grant-in-aid and Support Project to Assist Private Universities in Developing Bases for Research from the Ministry of Education, Science, Sports and Culture and Technology in Japan. The authors are grateful to Mr Michio Sasaki of the Association of Meat Science and Technology Institute for his helpful suggestions.
Conflict of Interest
None declared.
Glossary
Abbreviations
- LC-MS
liquid chromatograph-mass spectrometry
- SRM
selected reaction monitoring
References
- 1.Holst JP, Soldin OP, Guo T, Soldin SJ. Steroid hormones: relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004;24:105–118. doi: 10.1016/j.cll.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funderburgh LJ, Zipf WB, Sotos JF. Direct measurement of testosterone in a pediatric center, with use of a radioimmunoassay kit and unextracted serum. Clin. Chem. 1983;29:1796–1798. [PubMed] [Google Scholar]
- 3.Turpeinen U, Linko S, Itkonen O, Hämäläinen E. Determination of testosterone in serum by liquid chromatography–tandem mass spectrometry. Scand. J. Clin. Lab. Invest. 2008;68:50–57. doi: 10.1080/00365510701496496. [DOI] [PubMed] [Google Scholar]
- 4.Cawood ML, Field HP, Ford CG, Gillingwater S, Kicman A, Cowan D, Barth JH. Testosterone measurement by isotope dilution–liquid chromatography–tandem mass spectrometry: validation of a method for routine clinical practice. Clin. Chem. 2005;51:1472–1479. doi: 10.1373/clinchem.2004.044503. [DOI] [PubMed] [Google Scholar]
- 5.Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope dilution-gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin. Chem. 2003;49:1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Nakada N, Yasojima M, Yamashita N, Johnson AC, Tanaka H. Rapid determination of free and conjugated estrogen in different water matrices by liquid chromatography-tandem mass spectrometry. Chemosphere. 2009;77:1440–1446. doi: 10.1016/j.chemosphere.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 7.Weng Y, Xie F, Xu L, Zagorevski D, Spink DC, Ding X. Analysis of testosterone and dihydrotestosterone in mouse tissues by liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal. Biochem. 2010;402:121–128. doi: 10.1016/j.ab.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozo OJ, Deventer K, Eenoo PV, Delbeke FT. Efficient approach for the comprehensive detection of unknown anabolic steroids and metabolites in human urine by liquid chromatography-electrospray-tandem mass spectrometry. Anal. Chem. 2008;80:1709–1720. doi: 10.1021/ac7020757. [DOI] [PubMed] [Google Scholar]
- 9.Faupel-badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Comparison of liquid chromatography-tandem mass spectrometry, RIA,and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol. Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buiarelli F, Coccioli F, Merolle M, Neri B, Terracciano A. Development of a liquid chromatography–tandem mass spectrometry method for the identification of natural androgen steroids and their conjugates in urine samples. Anal. Chim. Acta. 2004;526:113–120. [Google Scholar]
- 11.Kumar V, Nakada N, Yasojima M, Yamashita N, Johnson AC, Tanaka H. Rapid determination of free and conjugated estrogen in different water matrices by liquid chromatography–tandem mass spectrometry. Chemosphere. 2009;77:1440–1446. doi: 10.1016/j.chemosphere.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Hernandéz F, Sancho JV, Pozo OJ. Critical review of the application of liquid chro matography/mass spectrometry to the determination of pesticide residues in biological samples. Anal. Bioanal. Chem. 2005;382:934–946. doi: 10.1007/s00216-005-3185-5. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PJ. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray-tandem mass spectrometry. Clin. Biochem. 2005;38:328–334. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Honour JW. Steroid assays in paediatric endocrinology. J. Clin. Res. Pediatr. Endocrinol. 2010;2:1–16. doi: 10.4274/jcrpe.v2i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita K, Miyashiro Y, Maekubo H, Okuyama M, Honma S, Takahashi M, Numazawa M. Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chromatography electrospray ionization tandem mass spectrometry. Steroids. 2009;74:920–926. doi: 10.1016/j.steroids.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Pelander A, Ojanpera I, Laks S, Rasanen I, Vuori E. Toxicological screening with formulabased metabolite identification by liquid chromatography/time-of-flight mass spectrometry. Anal. Chem. 2003;75:5710–5718. doi: 10.1021/ac030162o. [DOI] [PubMed] [Google Scholar]
- 17.Gergov M, Boucher B, Ojanpera I, Vuori E. Toxicological screening of urine for drugs by liquid chromatography/time-of-flight mass spectrometry with automated target library search based on elemental formulas. Rapid Commun. Mass Spectrom. 2001;15:521–526. doi: 10.1002/rcm.260. [DOI] [PubMed] [Google Scholar]
- 18.Ojanperä S, Pelander A, Pelzing M, Krebs I, Vuori E, Ojanperä I. Isotopic pattern and accurate mass determination in urine drug screening by liquid chromatography/ time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:1161–1167. doi: 10.1002/rcm.2429. [DOI] [PubMed] [Google Scholar]
- 19.FDA Draft Guidance (2001) Bioanalytical Methods Validation for Human Studies. 2001 [Google Scholar]
- 20.Stüber M, Reemtsma T. Evaluation of three calibration methods to compensate matrix effects in environmental analysis with LC-ESI-MS. Anal. Bioanal. Chem. 2004;378:910–916. doi: 10.1007/s00216-003-2442-8. [DOI] [PubMed] [Google Scholar]
- 21.Polettini A, Gottardo R, Pascali JP, Tagliaro F. Implementation and performance evaluation of a database of chemical formulas for the screening of pharmaco/ toxicologically relevant compounds in biological samples using electrospray ionization-time-of-flight mass spectrometry. Anal. Chem. 2008;80:3050–3057. doi: 10.1021/ac800071n. [DOI] [PubMed] [Google Scholar]
- 22.Peters RJ, Bolck YJ, Rutgers P, Stolker AA, Nielen MW. Multi-residue screening of veterinary drugs in egg, fish and meat using high-resolution liquid chromatography accurate mass time-of-flight mass spectrometry. J. Chromatogr. A. 2009;1216:8206–8216. doi: 10.1016/j.chroma.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Weng Y, Xie F, Xu L, Zagorevski D, Spink DC, Ding X. Analysis of testosterone and dihydrotestosterone in mouse tissues by liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal. Biochem. 2010;402:121–128. doi: 10.1016/j.ab.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebner MJ, Corol DI, Havlíková H, Honour JW, Fry JP. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology. 2006;147:179–190. doi: 10.1210/en.2005-1065. [DOI] [PubMed] [Google Scholar]
- 25.Faupel-badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol. Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M, Baker SD, Yan X, Zhao Y, Wright WW, Zirkin BR, Jarow JP. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids. 2004;69:721–726. doi: 10.1016/j.steroids.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr. Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 28.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl Acad. Sci. USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neuro. Sci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang GM, Ge RS, Latif SA, Morris DJ, Hardy MP. Expression of 11beta-hydroxylase in rat Leydig cells. Endocrinology. 2002;143:621–626. doi: 10.1210/endo.143.2.8638. [DOI] [PubMed] [Google Scholar]
- 31.Monder C, Miroff Y, Marandici A, Hardy MP. 11beta-Hydroxysteroid dehydrogenase alleviates glucocorticoid-mediated inhibition of steroidogenesis in rat Leydig cells. Endocrinology. 1994;134:1199–1204. doi: 10.1210/endo.134.3.8119160. [DOI] [PubMed] [Google Scholar]
- 32.Monder C, Hardy MP, Blanchard RJ, Blanchard DC. Comparative aspects of 11beta-hydroxysteroid dehydrogenase—Testicular 11beta-hydroxysteroid dehydrogenase: development of a model for the mediation of Leydig cell function by corticosteroids. Steroids. 1994;59:69–73. doi: 10.1016/0039-128x(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 33.Higo S, Hojo Y, Ishii H, Komatsuzaki Y, Ooishi Y, Murakami G, Mukai H, Yamazaki T, Nakahara D, Barron A, Kimoto T, Kawato S. Endogenous synthesis of corticosteroids in the hippocampus. PLoS One. 2011;6:e21631. doi: 10.1371/journal.pone.0021631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallonen SE, Tammimäki A, Piepponen P, Raattamaa H, Ketola RA, Kostiainen R. Discovery of neurosteroid glucuronides in mouse brain. Anal. Chim. Acta. 2009;651:69–74. doi: 10.1016/j.aca.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Sjövall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal. Chem. 2003;75:5835–5846. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- 36.Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjövall J, Baulieu EE. Novel lipoidal derivatives of pregnenolone and dehydro-epiandrosterone and absence of their sulfated counterparts in rodent brain. J. Lipid Res. 2004;45:2287. doi: 10.1194/jlr.M400244-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Corpéchot C, Synguelakis M, Talha S, Axelson M, Sjövall J, Vihko R, Baulieu EE, Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 38.Corpéchot C, Robel P, Axelson M, Sjövall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl Acad. Sci. USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood CE. Fetal hypothalamus-pituitary-adrenal responses to estradiol sulfate. Endocrinology. 2011;152:4966–4973. doi: 10.1210/en.2011-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.