Abstract
Angiogenesis, a formation of neovessels, is regulated by the local balance between angiogenesis stimulators and inhibitors. A number of such endogenous regulators of angiogenesis have been found in the body. Recently, vasohibin-1 (VASH1) was isolated as a negative feedback regulator of angiogenesis produced by endothelial cells (ECs) and subsequently vasohibin-2 (VASH2) as a homologue of VASH1. It was then explored that VASH1 is expressed in ECs to terminate angiogenesis, whereas VASH2 is expressed in cells other than ECs to promote angiogenesis in the mouse model of angiogenesis. This review will focus on the vasohibin family members, which are novel regulators of angiogenesis.
Keywords: angiogenesis, endothelial cell, SVBP, vasohibin-1, vasohibin-2
The vasculature is primarily composed of luminal endothelial cells (ECs) and surrounding mural cells (smooth muscle cells or pericytes). ECs are multifunctional cells covering the entire luminal surface of all blood vessels. ECs remain G0 phase of the cell cycle and form an interface between the circulating blood between the lumen and the rest of the vessel wall, and maintain vascular homeostasis. Physiological function of ECs includes the transport of various molecules across the vascular wall, the regulation of the adhesion of leukocytes for extravasation, the manipulation of vascular tonus and the prevention of thrombotic events. However, when stimulated by angiogenic factors, ECs migrate, proliferate and form neovessels for angiogenesis. The initial step of angiogenesis is the extrication of mural cells from endothelial tubes for vascular destabilization. Subsequently, specialized ECs, the so-called tip cells, migrate by extending numerous filopodia, whereas following ECs, the so-called stalk cells, proliferate causing elongation of the sprouts to form immature tube-like structures. Finally, redistributed mural cells affix themselves to the newly formed vessels for vascular restabilization. By this process, ECs stop their proliferation, thus terminating angiogenesis (1).
The body contains a number of endogenous angiogenesis stimulators and inhibitors, and the local balance between them regulates this process of blood vessel formation. Angiogenesis stimulators are mostly growth factors and cytokines including vascular endothelial growth factor (VEGF), whereas angiogenesis inhibitors are variable and include hormones, chemokines, proteins accumulated in the extracellular matrix, proteolytic fragments of various proteins and so forth. In addition, the majority of angiogenesis inhibitors are extrinsic to the vasculature; some are constitutively expressed and act as barriers to prevent the invasion of sprouts, and the others are generated in response to stimuli and counteract this process (2).
Isolation of VASH1 and VASH2
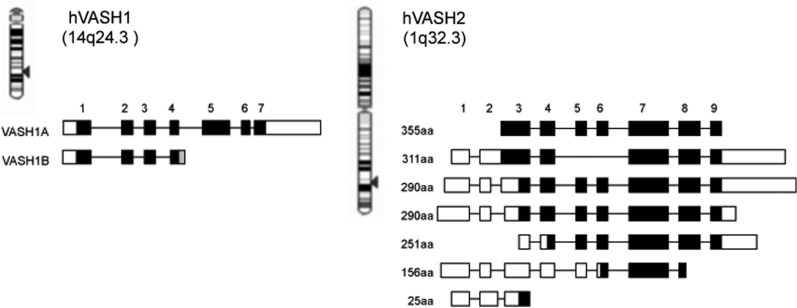
It could be hypothesized that ECs themselves might produce either angiogenesis stimulators or inhibitors as an autoregulatory or a feedback fashion. To test this hypothesis, cDNA microarray analysis was performed to detect VEGF-inducible genes in ECs (3). Among a variety of VEGF-inducible genes, an attention was focused on genes whose functions were undefined. In vitro functional assays for angiogenesis were performed, and one gene having anti-angiogenic activity was isolated. This gene was designated as vasohibin-1 (VASH1), and its in vivo anti-angiogenic activity was further confirmed (4). The gene for human VASH1 gene is located on chromosome 14q24.3 and consists of seven exons (Fig. 1). There are two isoforms of human VASH1: full-length VASH1A and the spliced variant VASH1B (Fig. 1). Human VASH1A protein is composed of 365 amino acid residues, whereas human VASH1B protein is composed of 204 amino acid residues, and this splicing variant maintains anti-angiogenic activity (5, 6).
Fig. 1.
VASH1 and VASH2 genes and their transcripts. Human VASH1 gene is encoded in 14q24.3, whereas human VASH2 gene is encoded in 1q32.3. There are multiple transcripts in both human VASH1 and VASH2. Black squares indicate encode proteins.
By searching in the database, one gene homologous to VASH1 was found and designated as vasohibin-2 (VASH2) (7). The gene for human VASH2 is located on chromosome 1q32.3. So far, nine exons for the VASH2 gene have been shown in the database to form multiple transcripts owing to alternative splicing (Fig. 1). The full-length human VASH2 was found to be expressed in cultured cells, which is composed of 355 amino acid residues (7). The overall homology between full-length human VASH1 and VASH2 is 52.5% at the amino acid level.
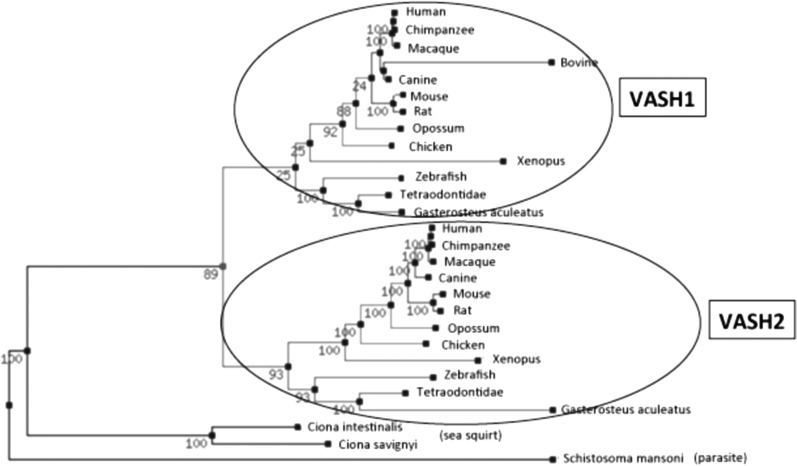
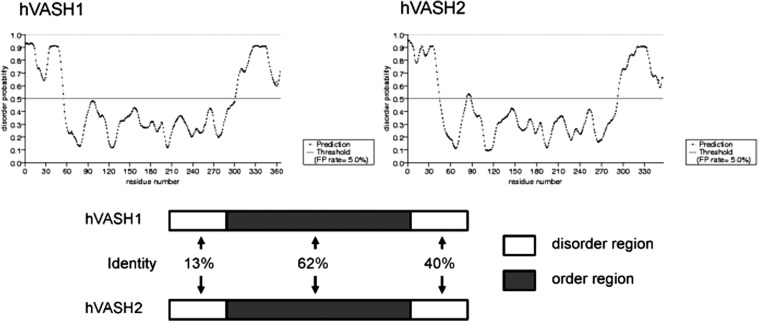
The phylogenic tree of vasohibin family proteins reveals that parasite or sea squirt possesses one common ancestry vasohibin gene, while vertebrates have VASH1 and VASH2 (Fig. 2). The homology between sea squirt vasohibin and human VASH1 or human VASH2 is about 40%. Moreover, amino acid sequences of vertebrate VASH1 and VASH2 are well conserved. Thus, a common ancestry gene seems to be divided into VASH1 and VASH2 during the evolution to vertebrate. No known functional motifs were found in the amino acid sequences in either VASH1 or VASH2. This makes extremely difficult to estimate the functions and compare three-dimensional structures of these two molecules. Instead, the order/disorder orientation of VASH1 and VASH2 proteins estimated by Protein Disorder Prediction System (http://prdos.hgc.jp/cgi-bin/top.cgi) would provide useful information. The order region defines stable in a three-dimensional composition, whereas the disorder region defines unstable in a three-dimensional composition. In addition, the disorder region is more important for determining the function of proteins. As shown in Fig. 3, VASH1 and VASH2 contain disorder regions in both N-terminus and C-terminus ends and order region in the centre. The overall order/disorder probability lines of VASH1 and VASH2 are considerably resemble, indicating the correspondence of these two molecules. However, when similarity of order and disorder area was compared, disorder areas are less resemble (Fig. 3). The differences in the disorder regions may indicate the distinctive function of VASH1 and VASH2.
Fig. 2.
The phylogenic tree of vasohibin family proteins. Parasite and sea squirt have one vasohibin ancestry gene, whereas vertebrates have VASH1 and VASH2. A common ancestry gene seems to be divided into VASH1 and VASH2 during the evolution to vertebrate.
Fig. 3.
Order/disorder configuration of human VASH1 and VASH2 proteins. Order/disorder probability lines of human VASH1 and human VASH2 are shown on the top. Area above the line of disorder probability 0.5 is regarded as disorder region. Similarities of order and disorder regions are shown at the bottom.
Isolation of Small Vasohibin-Binding Protein
To understand the undefined characteristics of vasohibins, their possible binding partners were searched by using a yeast two-hybrid technique, and one candidate gene was discovered (8). This gene was registered in the database as hypothetical protein LOC374969 or coiled-coil domain containing 23. The binding of this protein to VASH1 and VASH2 was confirmed by using the BIAcore system. Because this protein is composed of 66 amino acids, this molecule was renamed as small vasohibin-binding protein (SVBP) (8). The database search revealed that SVBP is highly conserved between species. The analysis of the function of SVBP revealed that SVBP binds to vasohibins within the cells, makes a heterodimer with vasohibins and facilitates the secretion of vasohibins. The knockdown of SVBP impedes the secretion of vasohibins, and vasohibins remained in the cells are degraded via the proteasome–ubiquitin system (8). Because vasohibins lack classical signal sequence for secretion, it has been obscure whether vasohibins are secreted. The isolation of SVBP verifies vasohibins as secretory proteins, and SVBP acts as a secretory chaperone of vasohibins.
Expression and Function of VASH1 and VASH2
As one can see from its discovery, the expression of VASH1 in ECs is inducible. The VEGF receptor (VEGFR)-induced expression of VASH1 in ECs is mediated via VEGFR2 and its downstream PKCδ (5). VASH1 is induced not only by VEGF but also by fibroblast growth factor 2, another potent angiogenic factor (4, 5), and this induction is also mediated by PKCδ (5). Accordingly, the principal signalling pathways for the induction of VASH1 by these two representative angiogenic growth factors considerably overlap. Interestingly, this induction of VASH1 in ECs disappears under a hypoxic condition or in the presence of inflammatory cytokines, tumour necrosis factor-α and interleukin-1 (5).
In contrast, the expression of VASH2 seems to be not inducible but constitutive as it is not modulated by growth factors and cytokines. In connection with this expression pattern of VASH2, recently, VASH2 is found as the target of mir-200 (9). There is a cluster of miR-200bc/429 binding sites in the 3’ untranslated region of VASH2 mRNA, and mir-200b silences the expression of VASH2. Thus, the expression of VASH2 is augmented when the expression of mir-200b declines (9).
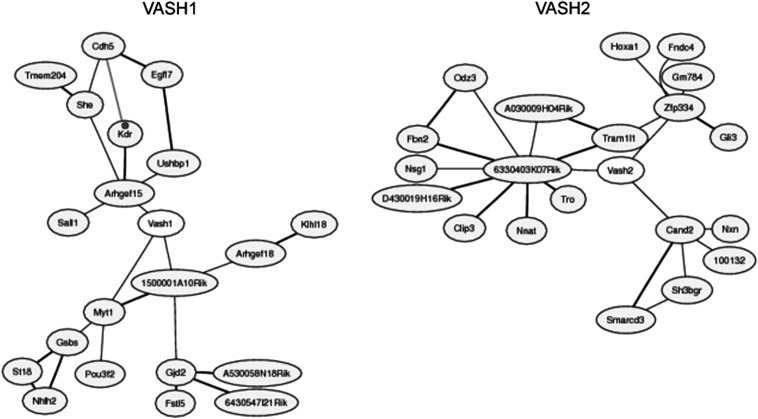
Large collections of microarray data contain information about concerted changes in transcript levels in the datasets beyond the original purpose of each dataset, and the co-expressed gene database (http://coxpresdb.jp/) using those datasets will determine the co-expressed genes with VASH1 and VASH2 are compared by the use of this database, and the results revealed that co-expressed genes with VASH1 and VASH2 are completely different (Fig. 4). This information may suggest that the cells expressing VASH1 and VASH2 are different.
Fig. 4.
Co-expressed genes with VASH1 and VASH2. Co-expressed genes with VASH1 are shown on the left and those with VASH2 are on the left.
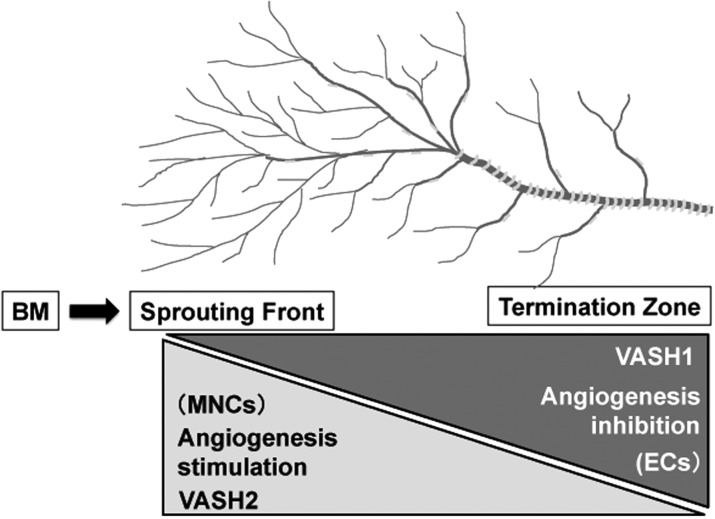
To disclose the precise relationship of VASH1 and VASH2, their spatiotemporal expression and function during angiogenesis were examined (10). The analysis using the mouse subcutaneous angiogenesis model revealed that VASH1 is expressed not in ECs at the sprouting front but in newly formed blood vessels behind the sprouting front where angiogenesis terminates. In contrast, VASH2 is expressed preferentially in mononuclear cells (MNCs) that are mobilized from the bone marrow and infiltrate the sprouting front. VASH1 and VASH2 knockout (KO) mice were generated and used in this analysis. VASH1 KO mice contain numerous immature microvessels in the area where angiogenesis should be terminated. This phenotype was gene dosage sensitive, as more enhanced angiogenesis was observed in VASH1 (−/−) mice (10). Importantly, newly formed immature microvessels in VASH1 (−/−) mice are functional, as indicated by blood flow (10). In contrast, VASH2 KO mice contain less neovessels in the sprouting front of angiogenesis. This phenotype was also gene dosage sensitive, as more impaired angiogenesis was observed in VASH2 (−/−) mice (10). Importantly, the infiltration of MNCs in VASH2 (−/−) mice is identical to that of wild-type mice (10). As described earlier, the expression of VASH1 is low in proliferating ECs at the sprouting front but high in non-proliferating ECs (10). In addition, angiogenesis in the VASH2 KO mice was deficient at the sprouting front (10). These results indicate that VASH1 is expressed in ECs in the termination zone of angiogenesis to terminate angiogenesis, whereas VASH2 is mainly expressed in MNCs in the sprouting front and promotes angiogenesis (Fig. 5).
Fig. 5.
Spacial expression and function of VASH1 and VASH2 in the regulation of angiogenesis. VASH1 is mainly expressed in ECs at the termination zone and halts angiogenesis. In contrast, VASH2 is mainly expressed in MNCs at the sprouting front mobilized from bone marrow (BM) and promotes angiogenesis.
VASH1 and VASH2 in Pathophysiology
Immunohistochemical analysis has revealed that VASH1 protein is present in ECs in the developing human or mouse embryo, but it is reduced in expression in the post-neonate (7). Nimmagadda et al. independently showed by in situ hybridization that VASH1 mRNA is expressed in a wide range of tissues and organs in the chicken embryo and suggested that the expression of VASH1 might not be limited to ECs (11). Indeed, VASH1 mRNA was detected in bone marrow hematopoietic stem cells (12) and striated muscles (13). Although the significance of these expression is not clear (14), immunohistochemical analysis preferentially detects VASH1 protein in ECs at the site of angiogenesis (4, 7). The expression of VASH1 was further investigated under various conditions accompanied by pathological angiogenesis and a related condition. The presence of VASH1 in ECs is evident in various cancers, atherosclerotic lesions, age-dependent macular degeneration, diabetic retinopathy, rheumatoid arthritis and arterial re-endothelialization after denudation (15–27). Even under pathological conditions, the extent of angiogenesis may vary. Tumours inoculated into VASH1 (−/−) mice contain numerous immature vessels, resulting in a growth advantage of the tumours (20). These observations suggest that VASH1 should regulate the course of angiogenesis under pathological conditions as well. Interestingly, Lu et al. recently reported that EZH2 silenced the VASH1 expression in ovarian cancers and that made the prognosis of ovarian cancers worse (28).
The initial analysis on VASH2 revealed that VASH2 protein was also present in ECs in the developing human or mouse embryo and faded in the post-neonate (7). However, when the expression of VASH2 was examined in postnatal angiogenesis, it was shown not in ECs but in infiltrating MNCs (10). The analysis was extended to pathological conditions including cancers thereafter and showed the expression of VASH2 in cancer cells of gastric cancer (29), hepatocellular carcinoma (30) and ovarian serous adenocarcinoma (9). This increased expression of VASH2 in cancer cells can be mediated by modulating the methylation of its promoter region (27) or the decrease of mir-200b (9). Importantly, the knockdown of VASH2 expression in cancer cells prominently inhibited both tumour growth and tumour angiogenesis (9, 27). These results indicate that VASH2 is expressed in cancer cells and promotes tumour growth by stimulating angiogenesis.
Therapeutic Application of Vasohibin
Because of the anti-angiogenic potential, VASH1 can be applied to treat diseases associated with angiogenesis. Its efficacy in anti-angiogenic treatment has been reported in animal models of cancers, ocular (retinal or choroidal) angiogenesis, arterial intimal stenosis, diabetic nephropathy and pulmonary diseases such as pulmonary fibrosis and bronchiolitis obliterans (4, 14, 19, 31–41).
Anti-angiogenic drugs that are now available in the clinics target VEGF-mediated signals. Those drugs are used for the treatment of cancers and age-related macular degeneration, but have some problems. Because VEGF acts as survival factor of ECs, such drugs cause cardiovascular toxicities including hypertension and proteinuria due to the impairment of normal ECs (42). However, VASH1 does not cause hypertension and proteinuria (43). Another problem is drug resistance due to the switch from VEGF to other angiogenic stimulators (44). As VASH1 inhibits not only VEGF-induced angiogenesis but also angiogenesis induced by various angiogenesis stimulators (34), VASH1 can be an alternative option for the treatment of pathological angiogenesis.
Because of the broad-spectrum anti-angiogenic activity of VASH1, the possible effect of VASH1 on lymphangiogenesis was examined (34). Peripheral lymphatic vessels are composed of a single layer of lymphatic ECs without mural cell coverage, and their function is to collect fluid lost from blood vessels and to maintain immune responses, lipid uptake and tissue homeostasis. Recently, attention has focused on lymphangiogenesis, because it has been shown to be related to lymph node (LN) metastasis of cancers. Angiogenesis is counter balanced by various endogenous inhibitors. However, little is known about endogenous inhibitors of lymphangiogenesis. The effect of VASH1 in the corneal micropocket assay was carefully examined, and it revealed broad-spectrum anti-angiogenic and anti-lymphangiogenic activities of VASH1 (34). In addition, VASH1 inhibited tumour lymphangiogenesis and tumour LN metastasis (34). Accordingly, VASH1 is the first molecule intrinsic to the endothelium that exhibits anti-lymphangiogenic activities.
Concluding Remarks
This study has focused on the VASH family members, VASH1 and VASH2. VASH1 and VASH2 are highly conserved between species, and their roles in the regulation of angiogenesis are distinct and perhaps contradictory. VASH1 is mainly expressed in ECs for the termination of angiogenesis, whereas VASH2 is expressed in infiltrating MNCs or cancer cells to stimulate angiogenesis. It has been recently described that VASH1 induces prolyl hydroxylase–mediated degradation of hypoxia-inducible factor-1α in human umbilical vein ECs (45). However, its significance on the effect of VASH1 is unclear. Apparently, further studies including their receptor and its downstream signalling are required to disclose the entire function of these unique family proteins.
Funding
This author is supported by a grant from the program Grant-in-Aid for Scientific Research on Priority Areas from the Japanese Ministry of Education, Science, Sports and Culture; Health and Labour Sciences research grants (22112006); Third Term Comprehensive Control Research for Cancer (H22-3-008); Japanese Ministry of Health, Labour, and Welfare (22300323).
Conflict of interest
None declared.
Glossary
Abbreviations
- EC
endothelial cell
- FGF-2
fibroblast growth factor 2
- KO
knockout
- MNC
mononuclear cell
- SVBP
small vasohibin-binding protein
- VASH1
vasohibin-1
- VASH2
vasohibin-2
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato Y. Update on endogenous inhibitors of angiogenesis. Endothelium. 2006;13:147–155. doi: 10.1080/1062332060069110. [DOI] [PubMed] [Google Scholar]
- 3.Abe M, Sato Y. cDNA microarray analysis of the gene expression profile of VEGF-induced human umbilical vein endothelial cells. Angiogenesis. 2001;4:289–298. doi: 10.1023/a:1016018617152. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda H, Sato Y. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J. Clin. Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu K, Watanabe K, Yamashita H, Abe M, Yoshimatsu H, Ohta H, Sonoda H, Sato Y. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem. Biophys. Res. Commun. 2005;327:700–706. doi: 10.1016/j.bbrc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 6.Kern J, Bauer M, Rychli K, Wojta J, Ritsch A, Gastl G, Gunsilius E, Untergasser G. Alternative splicing of vasohibin-1 generates an inhibitor of endothelial cell proliferation, migration, and capillary tube formation. Arterioscler. Thromb. Vasc. Biol. 2008;28:478–484. doi: 10.1161/ATVBAHA.107.160432. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya T, Watanabe K, Yamashita H, Shimizu K, Miyashita H, Abe M, Moriya T, Ohta H, Sonoda H, Shimosegawa T, Tabayashi K, Sato Y. Isolation and characterization of vasohibin-2 as a homologue of VEGF-inducible endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler. Thromb. Vasc. Biol. 2006;26:1051–1057. doi: 10.1161/01.ATV.0000216747.66660.26. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Kobayashi M, Miyashita H, Ohta H, Sonoda H, Sato Y. Isolation of a small vasohibin-binding protein (SVBP) and its role in vasohibin secretion. J. Cell Sci. 2010;123:3094–4101. doi: 10.1242/jcs.067538. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Koyanagi T, Suzuki Y, Saga Y, Kanomata N, Moriya T, Suzuki M, Sato Y. Vasohibin-2 expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol. Cancer Res. 2012;10:1135–1146. doi: 10.1158/1541-7786.MCR-12-0098-T. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T, Sato Y. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood. 2010;113:4810–4818. doi: 10.1182/blood-2008-07-170316. [DOI] [PubMed] [Google Scholar]
- 11.Nimmagadda S, Geetha-Loganathan P, Pröls F, Scaal M, Christ B, Huang R. Expression pattern of vasohibin during chick development. Dev. Dyn. 2007;236:1358–1362. doi: 10.1002/dvdy.21134. [DOI] [PubMed] [Google Scholar]
- 12.Naito H, Kidoya H, Sato Y, Takakura N. Induction and expression of anti-angiogenic vasohibins in the hematopoietic stem/progenitor cell population. J. Biochem. 2009;145:653–659. doi: 10.1093/jb/mvp021. [DOI] [PubMed] [Google Scholar]
- 13.Kishlyansky M, Vojnovic J, Roudier E, Gineste C, Decary S, Forn P, Bergeron R, Desplanches D, Birot O. Striated muscle angio-adaptation requires changes in vasohibin-1 expression pattern. Biochem. Biophys. Res. Commun. 2010;399:359–364. doi: 10.1016/j.bbrc.2010.07.076. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y. Is vasohibin-1 for more than angiogenesis inhibition? J. Biochem. 2011;149:229–230. doi: 10.1093/jb/mvq152. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita H, Abe M, Watanabe K, Shimizu K, Moriya T, Sato A, Satomi S, Ohta H, Sonoda H, Sato Y. Vasohibin prevents arterial neointimal formation through angiogenesis inhibition. Biochem. Biophys. Res. Commun. 2006;345:919–925. doi: 10.1016/j.bbrc.2006.04.176. [DOI] [PubMed] [Google Scholar]
- 16.Yoshinaga K, Ito K, Moriya T, Nagase S, Takano T, Niikura H, Yaegashi N, Sato Y. Expression of vasohibin as a novel endothelium-derived angiogenesis inhibitor in endometrial cancer. Cancer Sci. 2008;99:914–919. doi: 10.1111/j.1349-7006.2008.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakusawa R, Abe T, Sato H, Yoshida M, Kunikata H, Sato Y, Nishida K. Expression of vasohibin, an antiangiogenic factor, in human choroidal neovascular membranes. Am. J. Ophthalmol. 2008;146:235–243. doi: 10.1016/j.ajo.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Tamaki K, Moriya T, Sato Y, Ishida T, Maruo Y, Yoshinaga K, Ohuchi N, Sasano H. Vasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci. 2008;100:88–94. doi: 10.1111/j.1349-7006.2008.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato H, Abe T, Wakusawa R, Asai N, Kunikata H, Ohta H, Sonoda H, Sato Y, Nishida K. Vitreous levels of vasohibin-1 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetologia. 2009;52:359–361. doi: 10.1007/s00125-008-1229-z. [DOI] [PubMed] [Google Scholar]
- 20.Hosaka T, Kimura H, Heishi T, Suzuki Y, Miyashita H, Ohta H, Sonoda H, Moriya T, Suzuki S, Kondo T, Sato Y. Vasohibin-1 expressed in endothelium of tumor vessels regulates angiogenesis. Am. J. Pathol. 2009;175:430–439. doi: 10.2353/ajpath.2009.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake K, Nishida K, Kadota Y, Yamasaki H, Nasu T, Saitou D, Tanabe K, Sonoda H, Sato Y, Maeshima Y, Makino H. Inflammatory cytokine-induced expression of vasohibin-1 by rheumatoid synovial fibroblasts. Acta Med. Okayama. 2009;63:349–358. doi: 10.18926/AMO/31820. [DOI] [PubMed] [Google Scholar]
- 22.Tamaki K, Sasano H, Maruo Y, Takahashi Y, Miyashita M, Moriya T, Sato Y, Hirakawa H, Tamaki N, Watanabe M, Ishida T, Ohuchi N. Vasohibin-1 as a potential predictor of aggressive behavior of ductal carcinoma in situ of the breast. Cancer Sci. 2010;101:1051–1058. doi: 10.1111/j.1349-7006.2009.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshinaga K, Ito K, Moriya T, Nagase S, Takano T, Niikura H, Sasano H, Yaegashi N, Sato Y. Roles of intrinsic angiogenesis inhibitor, vasohibin, in cervical carcinomas. Cancer Sci. 2011;102:446–451. doi: 10.1111/j.1349-7006.2010.01812.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki Y, Kosaka T, Mikami S, Kikuchi E, Tanaka N, Maeda T, Ishida M, Miyajima A, Nakagawa K, Okada Y, Sato Y, Oya M. The prognostic significance of vasohibin-1 expression in patients with upper urinary tract urothelial carcinoma. Clin. Cancer Res. 2012;18:4145–4153. doi: 10.1158/1078-0432.CCR-12-0073. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Tian X, Zhang C, Wang Q. Upregulation of vasohibin-1 expression with angiogenesis and poor prognosis of hepatocellular carcinoma after curative surgery. Med. Oncol. 2012;29:2727–2736. doi: 10.1007/s12032-011-0106-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhao G, Yang Y, Tang Y, Han R, Sun Y. Reduced expression of vasohibin-1 is associated with clinicopathological features in renal cell carcinoma. Med. Oncol. 2012 doi: 10.1007/s12032-012-0313-x. PMID: 22865127 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Bai X, Margariti A, Hu Y, Sato Y, Zeng L, Ivetic A, Habi O, Mason JC, Wang X, Xu Q. PKCδ-deficiency accelerates neointimal lesion of mouse injured artery involving delayed reendothelialization and vasohibin-1 accumulation. Arterioscler. Thromb. Vasc. Biol. 2010;30:2467–2474. doi: 10.1161/ATVBAHA.110.215723. [DOI] [PubMed] [Google Scholar]
- 28.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, Ravoori MK, Shahzad MM, Lee JW, Mora E, Langley RR, Carroll AR, Matsuo K, Spannuth WA, Schmandt R, Jennings NB, Goodman BW, Jaffe RB, Nick AM, Kim HS, Guven EO, Chen YH, Li LY, Hsu MC, Coleman RL, Calin GA, Denkbas EB, Lim JY, Lee JS, Kundra V, Birrer MJ, Hung MC, Lopez-Berestein G, Sood AK. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Z, Kauttu T, Seppänen H, Vainionpää S, Ye Y, Wang S, Mustonen H, Puolakkainen P. Vasohibin-1 and vasohibin-2 expression in gastric cancer cells and TAMs. Med. Oncol. 2012;29:2718–2726. doi: 10.1007/s12032-012-0212-1. [DOI] [PubMed] [Google Scholar]
- 30.Xue X, Gao W, Sun B, Xu Y, Han B, Wang F, Zhang Y, Sun J, Wei J, Lu Z, Zhu Y, Sato Y, Sekido Y, Miao Y, Kondo Y. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene. 2012 doi: 10.1038/onc.2012.177. 2012 [Epub ahead of print; doi:10.1038/onc.2012.177] [DOI] [PubMed] [Google Scholar]
- 31.Shen J, Yang X, Xiao WH, Hackett SF, Sato Y, Campochiaro PA. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006;20:723–725. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
- 32.Nasu T, Maeshima Y, Kinomura M, Hirokoshi-Kawahara K, Tanabe K, Sugiyama H, Sonoda H, Sato Y, Makino H. Vasohibin-1, a negative feedback regulator of angiogenesis, ameliorates renal alterations in a mouse model of diabetic nephropathy. Diabetes. 2009;58:2365–2375. doi: 10.2337/db08-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Zhou K, Wang S, Shi Z, Yang Z. Recombinant adenovirus encoding vasohibin prevents tumor angiogenesis and inhibits tumor growth. Cancer Sci. 2010;101:448–452. doi: 10.1111/j.1349-7006.2009.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, Hojo K, Matsumoto M, Yamauchi C, Ohta H, Sonoda H, Sato Y. Endogenous angiogenesis inhibitor vasohibin1 exhibits a broad-spectrum anti-lymphangiogenic activity and suppresses lymph node metastasis. Am. J. Pathol. 2010;176:1950–1958. doi: 10.2353/ajpath.2010.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Zhu H, Yang X, Bi Y, Cui S. Vasohibin attenuates bleomycin induced pulmonary fibrosis via inhibition of angiogenesis in mice. Pathology. 2010;42:457–462. doi: 10.3109/00313025.2010.493864. [DOI] [PubMed] [Google Scholar]
- 36.Zhou SY, Xie ZL, Xiao O, Yang XR, Heng BC, Sato Y. Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol. Vis. 2010;16:1389–1398. [PMC free article] [PubMed] [Google Scholar]
- 37.Saito D, Maeshima Y, Nasu T, Yamasaki H, Tanabe K, Sugiyama H, Sonoda H, Sato Y, Makino H. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am. J. Physiol. Renal Physiol. 2011;300:F873–F886. doi: 10.1152/ajprenal.00503.2010. [DOI] [PubMed] [Google Scholar]
- 38.Wakusawa R, Abe T, Sato H, Sonoda H, Sato M, Mitsuda Y, Takakura T, Fukushima T, Onami H, Nagai N, Ishikawa Y, Nishida K, Sato Y. Suppression of choroidal neovascularization by vasohibin-1, a vascular endothelium-derived angiogenic inhibitor. Invest. Ophthalmol. Vis. Sci. 2011;52:3272–3280. doi: 10.1167/iovs.10-6295. [DOI] [PubMed] [Google Scholar]
- 39.Onami H, Nagai N, Machida S, Kumasaka N, Wakusawa R, Ishikawa Y, Sonoda H, Sato Y, Abe T. Reduction of laser-induced choroidal neovascularization by intravitreal vasohibin-1 in monkey eyes. Retina. 2012;32:1204–1213. doi: 10.1097/IAE.0b013e318233ad0b. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Fan K, Wang S, Liu Z, Zheng Z. Dual targeting of glioma U251 cells with nanoparticles prevents tumor angiogenesis and inhibits tumor growth. Curr. Neurovasc. Res. 2012;9:133–138. doi: 10.2174/156720212800410902. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe T, Okada Y, Hoshikawa Y, Eba S, Notsuda H, Watanabe Y, Ohishi H, Sato Y, Kondo T. A potent anti-angiogenic factor, vasohibin-1, ameliorates experimental bronchiolitis obliterans. Transplant. Proc. 2012;44:1155–1157. doi: 10.1016/j.transproceed.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 42.des Guetz G, Uzzan B, Chouahnia K, Morère JF. Cardiovascular toxicity of anti-angiogenic drugs. Target Oncol. 2011;6:197–202. doi: 10.1007/s11523-011-0204-7. [DOI] [PubMed] [Google Scholar]
- 43.Miyashita H, Suzuki H, Ohkuchi A, Sato Y. Mutual balance between vasohibin-1 and soluble VEGFR-1 in endothelial cells. Pharmaceuticals. 2011;4:1551–1577. [Google Scholar]
- 44.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozako T, Matsumoto N, Kuramoto Y, Sakata A, Motonagare R, Aikawa A, Imoto M, Toda A, Honda S, Shimeno H, Soeda S. Vasohibin induces prolyl hydroxylase-mediated degradation of hypoxia-inducible factor-1α in human umbilical vein endothelial cells. FEBS Lett. 2012;586:1067–1072. doi: 10.1016/j.febslet.2012.03.007. [DOI] [PubMed] [Google Scholar]