Abstract
Vascular endothelial growth factors (VEGFs) belong to the platelet-derived growth factor supergene family, and they play central roles in the regulation of angiogenesis and lymphangiogenesis. VEGF-A, the major factor for angiogenesis, binds to two tyrosine kinase (TK) receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1), and regulates endothelial cell proliferation, migration, vascular permeability, secretion and other endothelial functions. VEGFR-2 exhibits a strong TK activity towards pro-angiogenic signals, whereas the soluble VEGFR-1 (sFlt-1) functions as an endogenous VEGF inhibitor. sFlt-1 is abnormally overexpressed in the placenta of preeclampsia patients, resulting in the major symptoms of the disease due to abnormal trapping of VEGFs. The VEGF-VEGFR system is crucial for tumour angiogenesis, and anti-VEGF-VEGFR molecules are now widely used in the clinical field to treat cancer patients. The efficacy of these molecules in prolonging the overall survival of patients has been established; however, some cancers do not respond well and reduced tumour sensitivity to anti-VEGF signals may occur after long-term treatment. The molecular basis of tumour refractoriness should be determined to improve anti-angiogenic therapy.
Keywords: hypoxia, preeclampsia, tumor angiogenesis, VEGF, VEGFR
The closed circulatory system of vertebrates is essential for supplying oxygen and nutrients to the tissues in the body. In the recent decades, the molecular basis of angiogenesis, a new blood vessel formation from pre-existing blood vessels, has been extensively studied and a variety of signaling systems, such as vascular endothelial growth factor (VEGF)-VEGFR, Angiopoietin-Tie, Ephrin-Eph and Delta-Notch, were found to play important roles in angiogenesis. Among them, VEGF-VEGFR is crucial not only for physiological angiogenesis from early embryonic to adult stages but also for pathological angiogenesis, such as in cancer (1–5).
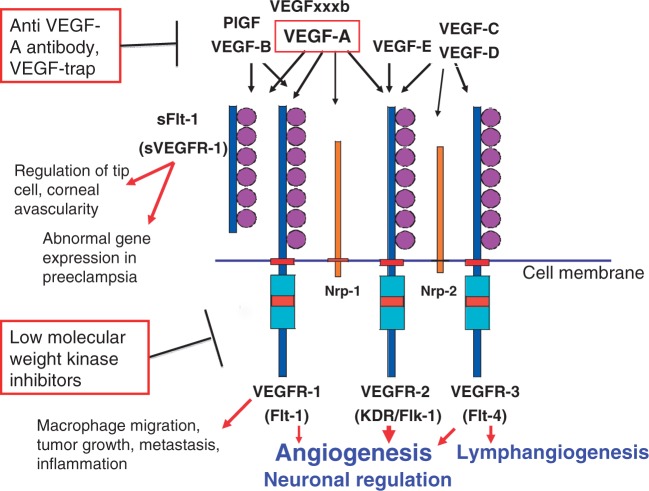
Phylogenetically, non-vertebrates such as Drosophila possess a single VEGFR-like receptor gene, which regulates cell migration and morphogenesis (6). However, this receptor in non-vertebrates does not have cell proliferation-stimulatory activity. In the fish, which are the earliest vertebrates, the VEGFR gene was duplicated to produce four genes and obtained cell proliferation-stimulatory activity to regulate angiogenesis and lymphangiogenesis (7). In parallel, the structurally related PDGFR gene family members were generated, and a strong cell proliferation signalling system was acquired to regulate smooth muscle cells and haematopoiesis. The number of VEGFR genes is conserved from amphibians to mammals, with three genes encoding three full-length receptors and one soluble molecule (sFlt-1/sVEGFR-1) (Fig. 1), which positively and negatively regulate the formation of blood vessels as well as lymph vessels.
Fig. 1.
The VEGF-VEGFR system and its involvement in various physiological and pathological processes. VEGF-A, particularly VEGF165, plays a central role in angiogenesis. VEGFxxxb has recently been reported to act as a natural antagonist to the VEGF-A. Three receptors and one soluble form of the VEGFR family members are highly conserved in most vertebrates, except for fish. Major pro-angiogenic signals are generated from VEGFR-2, whereas sFlt-1 maintains physiological corneal avascularity and induces several symptoms in PE. VEGFR-1 is expressed not only in endothelial cells but also in monocytes/macrophages and stimulates angiogenesis, inflammation and the malignant phenotypes of cancer. Nrp: co-receptors for VEGFs such as VEGF-A165, increasing the affinity between VEGF and its receptors, and the intracellular signalling. VEGFxxxb: an alternatively processed variant form of VEGF-A and suggested to be an endogenous competitor against VEGF-A.
This review briefly describes the structure and function of VEGF-VEGFR, the intimate relationship of VEGFR with preeclampsia (PE) and the development of anti-cancer drugs targeting VEGF-VEGFR signals as well as the questions that remain to be addressed.
Structure and Function of VEGFs
The mammalian genome encodes five VEGF family members, VEGF-A (also known as VEGF), placenta growth factor (PlGF), VEGF-B, VEGF-C and VEGF-D, which regulate vasculogenesis, angiogenesis and lymphangiogenesis (1–4). Particularly, VEGF-A is crucial for blood vessel formation during early embryogenesis. Not only the VEGF-A homozygous knockout mice but also its heterozygous mice (VEGF-A +/−) exhibit an embryonic lethal phenotype due to immature blood vessel formation, indicating that the local concentration of VEGF-A in the embryos has to be tightly regulated for proper angiogenesis (8, 9). Several VEGF-A subtypes are generated by alternative splicing. Among these, VEGF-A165 has the highest biological activity, with binding affinity for a co-receptor, neuropilin-1 (Nrp1). Recently, another alternatively spliced form of VEGF-A, VEGFxxxb, was reported. VEGFxxxb has a lower affinity for the receptor and competes with VEGF-A to negatively regulate angiogenesis (10).
VEGF-C and VEGF-D acquire a high affinity for VEGFR-3 after processing and stimulate lymphangiogenesis. VEGF-C, but not VEGF-D, is expressed during embryogenesis, and VEGF-C null mice (VEGF-C −/−) exhibit a lethal phenotype at the prenatal stage due to fluid accumulation in tissues because of poor lymphangiogenesis (11). PlGF and VEGF-B bind to and activate only VEGFR-1. Although the kinase activity of VEGFR-1 is relatively weak compared with that of VEGFR-2, PlGF stimulates survival signals in endothelial cells and promotes angiogenesis without a severe inflammatory response (12). VEGF-B was reported to be important for the stimulation and maintenance of the coronary artery system (13).
Dual Roles of VEGFRs in Angiogenesis
In 1990, we isolated a gene encoding a novel tyrosine kinase (TK) receptor from the human placenta. The TK receptor had seven immunoglobulin (Ig)-like domains in the extracellular region and a TK domain with a 60-amino acid-long kinase insert sequence (14). Based on the structural similarity, we named it Fms-like TK-1 (Flt-1) (Fig. 1). In 1992, Flt-1 was shown to bind VEGF/vascular permeability factor, now known as VEGFR-1 (15).
VEGFRs are structurally related to the platelet-derived growth factor (PDGF) receptor family, PDGFR/Fms/Kit/FLT3, with five Ig domains in the extracellular region. PDGFR family members also have a TK domain with a long kinase insert sequence. However, the intracellular signalling of VEGFRs is quite different from that of PDGFRs/Fms/Kit. It is well established that the PDGFR/Fms/Kit family has one or more tyr (Y)-x-x-met (M) motifs in the kinase insert and following autophosphorylation at this tyrosine, the motif binds to the SH2 domain in the p85 subunit of PI3K and significantly stimulates the PI3K-Akt and Ras pathways for cell proliferation and transformation (16). On the other hand, none of the kinase insert sequences of VEGFRs has the Y-x-x-M motif, and the PI3K pathway is usually not highly activated after stimulation with VEGF (3).
We have previously shown that of the two major autophosphorylation sites in VEGFR-2, 1175-PY but not 1214-PY is essential for the VEGF-A-induced proliferation in endothelial cells (17). The 1175-phenylalanine (F) mutation of VEGFR-2 or the intracellular injection of an anti-1175-PY-specific blocking antibody significantly suppressed VEGF-dependent cell proliferation. Furthermore, 1175-PY was found to be the binding and activation site for phospholipase C (PLC)-γ, which activates the PKC-Ca++-c-Raf-MEK-MAPK pathway (18). Mice with the 1173-F but not the 1212-F single amino acid mutation in VEGFR-2 (flk-1 1173F/F mice; two amino acids shorter in mice compared with that in humans) exhibit an embryonic lethal phenotype at E8.5–9.0 due to a lack of blood vessel formation, very similar to VEGFR2 (flk-1) −/− mice (19, 20). This indicates that the 1175-PY-PLCγ pathway downstream from VEGFR-2 is essential for vasculogenesis during early embryogenesis.
Furthermore, Sase et al. (21) reported that in an in vitro embryonic stem cell–endothelial cell differentiation system, the VEGFR-2 1175-PY-PLCγ pathway is required for the formation of vascular endothelial cells from progenitor cells. In addition, Xiong et al. (22) have recently shown that this VEGFR-2 1175-PY-PLCγ-Ca++ pathway together with the protein kinase-A pathway is important for the secretion of von Willebrand factor from vascular endothelial cells, which then triggers the inflammatory response in blood vessels.
Unlike VEGFR-2, VEGFR-1 has a very high affinity for its ligand, VEGF, with the affinity being about one order of magnitude higher than that of VEGFR-2 (23). However, the kinase activity of VEGFR-1 is lower, about one-tenth that of VEGFR-2. The VEGFR-1 gene produces two major proteins: a full-length receptor and sFlt-1 (14, 24). These facts suggest that VEGFR-1 may negatively regulate angiogenesis under certain conditions. Indeed, VEGFR-2 (flk-1) knockout mice (flk-1 −/− mice) exhibit a lethal phenotype at E8.5–9.0 due to a lack of vasculogenesis, whereas VEGFR-1 (flt-1) knockout mice (flt-1 −/− mice) die at almost the same stage, E8.5–9.0, due to overgrowth of endothelial cells and disorganization of blood vessels in the embryo (25). This indicates that during early embryogenesis, the two VEGFRs have opposite roles in angiogenesis: VEGFR-2 is a positive signal transducer, whereas VEGFR-1 is a suppressor of VEGFR-2 signalling.
To distinguish between the two possibilities that the VEGFR-1, TK generates a negative signal against angiogenesis or the ligand-binding site of VEGFR-1 blocks VEGF activity by trapping VEGF, we generated flt-1 TK-deficient (TK−/−) mice. Surprisingly, angiogenesis was almost normal in the flt-1 TK−/− mice, and these mice were basically healthy (26). This demonstrates that VEGFR-1 negatively regulates angiogenesis during early embryogenesis by trapping VEGF and decreasing the pro-angiogenic signals from VEGFR-2. Using the flt-1 TK−/− mice, our study as well as recent studies by others have shown that VEGFR-1 signalling is important for tumour growth, metastasis, and chronic arthritis in mice (27–31). Furthermore, flt-1 TK−/− mice with an op/op genetic background showed a significant suppression of bone marrow reconstruction, indicating that VEGFR-1 signalling is partly involved in bone marrow formation (32).
In addition, recent reports suggest that VEGFR signalling plays a role in the neuronal system, in both sensory and motor neurons. Thus, the VEGF-VEGFR system could be an attractive target for the treatment of neuronal disorders such as amyotrophic lateral sclerosis (33, 34).
Importance of sFlt-1 (Soluble VEGFR-1) Upregulation in PE
sFlt-1 is encoded by a short mRNA of the VEGFR-1 gene, and its structure, i.e. six Ig domains with a 31-amino acid tail, is highly conserved in mammals, birds and amphibians (35, 36). sFlt-1 traps VEGF and may thus play an important role in the negative regulation of angiogenesis in animals. Indeed, Ambati et al. (37) reported that sFlt-1 is expressed in the eye to maintain avascularity in the cornea.
In 2003–2004, several researchers reported that an abnormal increase in the level of serum sFlt-1 in pregnant mothers is well correlated with the degree of PE (38–40) (Fig. 1). In the placenta, sFlt-1 is expressed in the trophoblast cells. The trophoblast layer is located between the umbilical capillaries on the foetal side and the maternal blood vessels, suggesting that sFlt-1 forms a molecular barrier against abnormal vascular permeability and abnormal angiogenesis, such as the fusion of fetal capillaries with maternal blood vessels, by trapping VEGF and PlGF. The two major symptoms of PE are hypertension and proteinuria. As discussed later, hypertension and proteinuria are also the most common adverse effects encountered in cancer patients treated with VEGF-neutralizing antibody (41). In addition, artificial expression of sFlt-1 using a vector in a pregnant rat model induced hypertension and proteinuria (38). These facts strongly suggest that sFlt-1 overexpression in PE is a crucial cause of PE symptoms.
Podocytes in the glomeruli of the kidney secrete VEGF at physiological levels and maintain the glomerular endothelial cells in a healthy state, producing primary urine without leakage of serum proteins. Suppression of VEGF secretion from podocytes results in severe proteinuria (42, 43). Thus, overexpression of the VEGF-trapping molecule, sFlt-1, may block podocyte-derived VEGF and induce glomerular endothelial damage, resulting in proteinuria in PE.
The molecular basis of hypertension under VEGF blockade remains to be clarified. VEGF signals stimulate eNOS and increase the production of NO, a vasodilator, in endothelial cells. Furthermore, VEGF is a strong vascular permeability factor (44). Thus, a decrease in both NO and permeability via VEGF trapping by sFlt-1 may cause hypertension.
Given that trophoblasts are the main source of sFlt-1 in PE, viral or bacterial infection or abnormal stress, such as hypoxia in the placenta, may induce these cells to overexpress sFlt-1 (45). In addition to sFlt-1, other factors such as sEndoglin, a TGFβ family member, promote the degree of PE (46). An sFlt-1-blocking agent that is safer to both foetus and mother can be a useful tool to control PE.
Anti-angiogenic Therapy in Cancer via Suppression of the VEGF-VEGFR System
In the 1970s, Folkman and coworkers (47) proposed that tumour angiogenesis is an attractive target for the treatment of cancer. From the 1980s to the present, the VEGF-VEGFR system has been demonstrated to be the major regulator of tumour angiogenesis (2–4) (Fig. 2). Solid tumours often become hypoxic due to a rapid growth of tumour cells. Hypoxic stress is a pivotal inducer of the VEGF gene via stabilization and activation of the HIF transcription factor: the 5′-upstream sequence of the VEGF gene has a HIF-response element motif, resulting in high gene expression. The VEGFRs, particularly VEGFR-2, are also upregulated to some extent under hypoxia. In 1993, Kim et al. (48) showed that an anti-human VEGF antibody efficiently suppressed the growth of human tumour xenografts transplanted in immune-deficient mice. This antibody can block only the human-type VEGF derived from tumour cells and not the mouse VEGF derived from the cells surrounding the tumours; however, tumour growth was significantly suppressed. These results suggest that tumour-derived VEGF plays a central role in tumour angiogenesis.
Fig. 2.
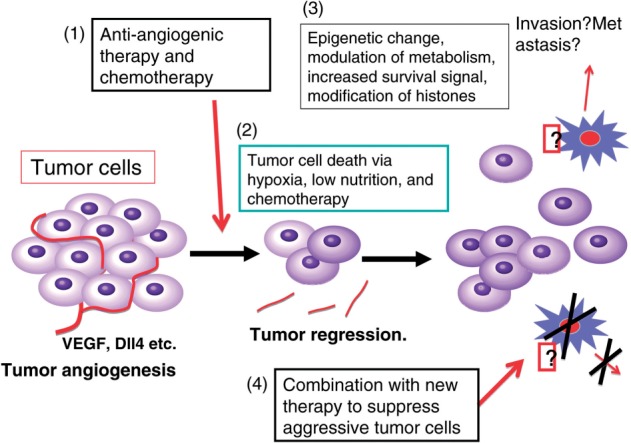
A hypothetical model of tumour responses to anti-angiogenic therapy. The VEGF-VEGFR system plays crucial roles in tumour angiogenesis. Therefore, anti-angiogenic therapy targeting VEGF signals efficiently suppresses cancer progression. However, during therapy, tumour cells may be subjected to hypoxia and/or low nutrient stresses for extended periods, and some of the cells may acquire epigenetic modifications and resistance to these stresses, resulting in a higher cell migration as well as an increased survival signal.
Till date, a VEGF-neutralizing antibody (bevacizumab) and small molecule kinase inhibitors (the multi-kinase inhibitors sunitinib and sorafenib) have been successfully developed for clinical use, although some adverse effects such as hypertension, proteinuria, thrombosis and bleeding have been observed. Bevacizumab was approved for the treatment of solid tumours, including colorectal, lung (non-epithelial, NSCLC) and breast cancers as well as glioblastomas. Kinase inhibitors are used for the treatment of renal and hepatocellular carcinomas as well as GIST. In the USA, the FDA approved bevacizumab in 2008 for the treatment of breast cancer because of a statistically significant efficacy in improving overall survival (OS); however, it was withdrawn in late 2011 due to a lack of clear efficacy data on OS in recent large-scale phase III studies, such as the E2100, AVADO and RIBBON1 clinical trials. On the other hand, other countries such as EU and Japan approved bevacizumab for breast cancer treatment based on a statistically significant improvement in progression-free survival (PFS).
These antiangiogenic drugs targeting the VEGF-VEGFR signal may induce tumour-suppressive effects through several pathways; (i) direct suppression of angiogenesis, (ii) promotion of the pre-existing endothelial cell apoptosis within tumour tissues, (iii) ‘vascular normalization’ which decreases abnormal vascular permeability and intratumoural pressure, resulting in an increase in the efficacy of chemotherapy and (iv) suppression of pro-angiogenic inflammatory reaction.
Other anti-VEGF-VEGFR drugs, such as VEGF-Trap (a VEGFR1-VEGFR2 fusion protein), VEGFR-neutralizing antibodies and VEGFR-derived peptides for immunotherapy, have been developed and are now under clinical trials.
Anti-angiogenic Therapy in the Treatment of Other (Non-cancer) Diseases
Age-related macular degeneration (AMD) is caused by abnormal angiogenesis with oedema in the retina and patients gradually lose visual activity. Abnormal activation of the VEGF-VEGFR system is intimately involved in the progression of this disease. Therefore, an aptamer against VEGF-A165, a VEGF-neutralizing antibody (Fab type) and VEGF-Trap are now approved for AMD treatment. Particularly, the neutralizing antibody not only slows down disease progression but also promotes partial recovery of visual activity and is thus widely used in the clinical field.
Unanswered Questions in the Current Anti-Angiogenic Therapy against Cancer
Essentially, all the current anti-angiogenic therapies in cancer treatment mainly target the VEGF-VEGFR system. Although their efficacies in improving OS and/or PFS were statistically significant or strongly suggested in glioblastoma (49), they were still insufficient. Until now, pancreatic cancer and gastric cancer have not shown a clear response to this therapy. Furthermore, breast cancer did not show a response with respect to OS in a large-scale phase III trial.
One important question is whether patients should continue anti-VEGF-VEGFR drugs, such as bevacizumab, when the cancer recurs. A recent phase III study in colorectal cancer, TML (ML18147), which was reported in the ASCO Annual Meeting 2012, indicates that bevacizumab should be continued because a combination of renewed chemotherapy with bevacizumab showed a better OS than that without bevacizumab. This suggests that the dependency of tumour angiogenesis on the VEGF-VEGFR system continues, at least partly, even after the recurrence of colorectal cancer.
On the other hand, breast cancer patients showed a better PFS but not OS in a large-scale phase III study. This suggests that at a later stage of anti-VEGF-VEGFR treatment, tumour cells might acquire a more aggressive phenotype, resulting in an acceleration of tumour growth/progression (Fig. 2). These accelerated tumour phenotypes may be acquired via several different mechanisms: (i) dependency of tumour angiogenesis on the VEGF-VEGFR system decreases, and other signalling systems such as FGF-FGFRs and HGF-c-Met stimulate tumour angiogenesis (50, 51); (ii) dependency of tumour growth on the blood vessels/angiogenesis decreases and tumour cells become more invasive (52) and (iii) tumour cells become more resistant to hypoxia and/or low nutrient conditions that could occur during anti-angiogenic therapy (53, 54). These secondary responses may occur in most tumours, including breast cancer. Thus, the molecular basis of these potential responses in tumours and the tumour microenvironment should be clarified to overcome the problems.
Conclusion
Recent studies on angiogenesis have demonstrated that several signalling systems, including VEGF-VEGFRs, play crucial roles under both physiological and pathological conditions and have heralded a new era in cancer treatment, introducing anti-angiogenic therapy. This new therapy has significantly improved the quality of life of patients with various solid tumours, but the efficacy is still insufficient. Signalling networks in angiogenesis as well as their modulation during anti-angiogenic therapy need to be extensively characterized.
Acknowledgements
Grant-in-aid Special Project Research on Cancer-Bioscience 17014020 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
None declared.
Glossary
Abbreviations
- AMD
age-related macular degeneration
- Flt-1
Fms-like TK-1
- Ig
immunoglobulin
- OS
overall survival
- Nrp1
neuropilin-1
- PIGF
placenta growth factor
- PDGF
platelet-derived growth factor
- PE
preeclampsia
- PFS
progression-free survival
- TK
tyrosine kinase
- VEGFs
vascular endothelial growth factors
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Shibuya M. Involvement of Flt-1 (VEGFR-1) in cancer and preeclampsia. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 2011;87:167–178. doi: 10.2183/pjab.87.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem. Soc. Trans. 2009;37:1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- 6.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 7.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 9.Carmellet P, Ferreira V, Breier G, Pollefeyt S, Kleckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawlling J, Moons L, Collen D, Resau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br. J. Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 12.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De, Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 13.Aase K, von, Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, Alitalo K, Betsholtz C, Eriksson U. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 14.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 15.De, Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 16.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Ueno H, Shibuya M. VEGF activates Protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 19.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X-F, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (vascular endothelial growth factor receptor-2) tyrosine residue-1173 in vasculogenesis in mice. Proc. Natl Acad. Sci. USA. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sase H, Watabe T, Kawasaki K, Miyazono K, Miyazawa K. VEGFR2-PLCgamma1 axis is essential for endothelial specification of VEGFR2+ vascular progenitor cells. J. Cell Sci. 2009;122:3303–3311. doi: 10.1242/jcs.049908. [DOI] [PubMed] [Google Scholar]
- 22.Xiong Y, Huo Y, Chen C, Zeng H, Lu X, Wei C, Ruan C, Zhang X, Hu Z, Shibuya M, Luo J. Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-gamma 1- and protein kinase A-dependent pathways. J. Biol. Chem. 2009;284:23217–23224. doi: 10.1074/jbc.M109.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawano A, Takahashi T, Yamaguchi S, Aonuma T, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for Placenta Growth Factor (PlGF), which is related to Vascular Endothelial Growth Factor (VEGF) Cell Growth Diff. 1996;7:213–221. [PubMed] [Google Scholar]
- 24.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong GH, Rossant J, Gertsentein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 26.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl Acad. Sci. USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by Vascular Endothelial Growth Factor Receptor-1 is involved in lung specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, Shibuya M. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocyte/macrophages. Blood. 2006;108:1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu M, Yamamoto S, Osawa T, Shibuya M. VEGF-1 signaling promotes mobilization of macrophage-lineage cells from bone marrow and stimulateolid tumor growth. Cancer Res. 2010;70:8211–8221. doi: 10.1158/0008-5472.CAN-10-0202. [DOI] [PubMed] [Google Scholar]
- 31.Kerber M, Reiss Y, Wickersheim A, Jugold M, Kiessling F, Heil M, Tchaikovski V, Waltenberger J, Shibuya M, Plate KH, Machein MR. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342–7351. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 32.Niida S, Kondo T, Hiratsuka S, Hayashi S-I, Amizuka N, Noda T, Ikeda K, Shibuya M. Vascular endothelial growth factor receptor-1 signaling is essential for osteoclast development and bone-marrow formation in CSF-1-deficient mice. Proc. Natl Acad. Sci. USA. 2005;102:14016–14021. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van, Damme P, Rutten B, Man WY, De, Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat. Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 34.Dhondt J, Peeraer E, Verheyen A, Nuydens R, Buysschaert I, Poesen K, Van Geyte K, Beerens M, Shibuya M, Haigh JJ, Meert T, Carmeliet P, Lambrechts D. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. 2011;25:1461–1473. doi: 10.1096/fj.10-170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo K, Hiratsuka S, Subbalakshmi E, Matsushime H, Shibuya M. Genomic organization of the flt-1 gene encoding for Vascular Endothelial Growth Factor (VEGF) Receptor-1 suggests an intimate evolutionary relationship between the 7-Ig and the 5-Ig tyrosine kinase receptors. Gene. 1998;208:297–305. doi: 10.1016/s0378-1119(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi S, Iwata K, Shibuya M. Soluble Flt-1 (soluble VEGFR-1), a potent natural anti-angiogenic molecule in mammals, is phylogenetically conserved in avians. Biochem. Biophys. Res. Commun. 2002;291:554–559. doi: 10.1006/bbrc.2002.6478. [DOI] [PubMed] [Google Scholar]
- 37.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St, Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De, Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J. Clin. Endocrinol. Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 40.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N. Engl J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 41.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Ji Y, Liu X, Lu X, Su W, Zhang D, Hao F, Yi F, Guo L, Li X, Zheng Y. Podocyte-specific VEGF down-regulation and pathophysiological development. IUBMB Life. 2010;62:677–683. doi: 10.1002/iub.368. [DOI] [PubMed] [Google Scholar]
- 43.Veron D, Villegas G, Aggarwal PK, Bertuccio C, Jimenez J, Velazquez H, Reidy K, Abrahamson DR, Moeckel G, Kashgarian M, Tufro A. Acute Podocyte Vascular Endothelial Growth Factor (VEGF-A) knockdown disrupts alpha(V)beta(3) integrin signaling in the glomerulus. PLoS One. 2012;7:e40589. doi: 10.1371/journal.pone.0040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 46.Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF and sEndoglin) in preeclampsia—a step forward but not the definitive answer. J. Reprod. Immunol. 2009;82:106–111. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 48.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 49.Peak SJ, Levin VA. Role of bevacizumab therapy in the management of glioblastoma. Cancer Manag. Res. 2010;2:97–104. [PMC free article] [PubMed] [Google Scholar]
- 50.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 51.You WK, Sennino B, Williamson CW, Falcón B, Hashizume H, Yao LC, Aftab DT, McDonald DM. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71:4758–4768. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Ellika S, Schultz L, Mikkelsen T. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J. Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 53.Osawa T, Muramatsu M, Wang F, Tsuchida R, Kodama T, Minami T, Shibuya M. Increased expression of histone demethylase JHDM1D under nutrient starvation suppresses tumor growth via downregulating angiogenesis. Proc. Natl Acad. Sci. USA. 2011;108:20725–20729. doi: 10.1073/pnas.1108462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Awale S, Lu J, Kalauni SK, Kurashima Y, Tezuka Y, Kadota S, Esumi H. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 2006;66:1751–1757. doi: 10.1158/0008-5472.CAN-05-3143. [DOI] [PubMed] [Google Scholar]