Abstract
Growth factor stimulations induce dynamic changes in the cytoskeleton beneath the plasma membrane. Among them is the formation of membrane ruffles organized in a circular array, called ‘circular dorsal ruffles’ (CDRs). Physiological functions of CDRs include downregulation of cell growth by desensitizing the signalling from growth factor receptors as well as rearrangement of adhesion sites at the onset of cell migration. For the formation of CDRs, not only the activators of actin polymerization, such as N-WASP and the Arp2/3-complex, but also membrane deforming proteins with BAR/F-BAR domains are necessary. Small GTPases are also involved in the formation of CDRs by controlling intracellular trafficking through endosomes. Moreover, recent analyses of another circular cytoskeletal structure, podosome rosettes, have revealed common molecular features shared with CDRs. Among them, the roles of PI3-kinase and phosphoinositide 5-phosphatase may hold the key to the induction of these circular structures.
Keywords: actin, circular dorsal ruffle, PI3-kinase, plasma membrane, podosome rosette
The cellular membrane is composed of a myriad number of lipid molecules. Building a robust limiting barrier that separates the cell’s outer and inner spaces, the lipid bi-layer is a highly flexible, fluid-like structure in which transmembrane as well as lipid-anchored proteins are embedded. A very close look at the surface of the plasma membrane reveals its ‘highly bumped’ structure. Analysis using a scanning electron microscope has shown that the cell extends membranous protrusions with various shapes: some are spiky, others are spread flatly and some others turn inward with wrinkled rims. Such local shapes of the plasma membrane are respectively called filopodia, lamellipodia and membrane ruffles (1). For the induction of these dynamic changes in membrane morphology, mechanical forces must be applied by the actin cytoskeleton. A wide range of extracellular stimuli is known to trigger polymerization and depolymerization cycles of actin filaments beneath the plasma membrane. The mechanism involves various signalling molecules, including N-WASP and WAVE, which activate the actin nucleation promoting factor Arp2/3 complex (2); Lin11, Isl-1 and Mec-3 kinase, which regulates actin polymerization through phosphorylation of cofilin (3) and Rho-mediated signalling, which promotes myosin-dependent contraction of the actin cytoskeleton (4).
Recent findings have shown that in certain groups of cells, stimulation by growth factors can induce a unique membrane structure driven by actin polymerization at the dorsal side of the plasma membrane. The most notable feature of this structure is its circular arrangement called the circular dorsal ruffles (CDRs) (5, 6). Clearly distinct from membrane ruffles that form at the cell periphery, CDRs appear as an ‘actin ring’ induced in the central area of the dorsal plasma membrane of fibroblasts (7, 8). Its movement seems highly organized, because the ruffled edges of the membrane are observed to constrict towards its centre in a coordinated manner, during which its circular shape is maintained (9, 10). Among many discussions concerning the physiological functions, bulk internalization of transmembrane proteins, together with their associated extracellular materials, is one of the most important roles of this dynamic membrane structure.
In this review, we will overview the molecular mechanism of CDR formation. The physiological function of CDRs has been enigmatic since long, but recent findings revealed its necessary roles in cell migration. The PI 3-kinase (PI3K) signalling pathway, including lipid phosphatases, has been recognized as a key regulator for the circular array of CDRs. We will also discuss another structure with circular membrane–cytoskeleton interfaces, called ‘podosome rosettes’. Although podosome rosettes are membrane protrusions formed at the ventral side of the plasma membrane (thus called invadopodia in cancer cells), they also share a number of factors in common with CDRs. By comparing the signalling pathways that regulate the formation of these two structures, we attempt to extract a common mechanism underpinning the induction and maintenance of the circular array of the actin-based membrane structures.
Physiological Functions of CDRs
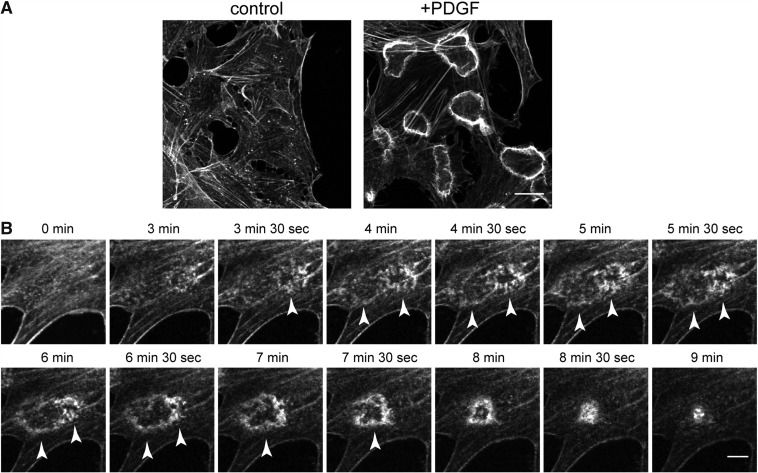
It has long been recognized that cells develop ‘membrane ruffles’ in response to stimulations with growth factors; membrane ruffles are small fin-shaped membrane regions with rims that curl up and then move centripetally. Membrane ruffles are divided into two categories according to their location within the cell: one is the ‘peripheral ruffle’, which forms at the cell edge, whereas the other is called the ‘dorsal ruffle’, which emerges at the dorsal side of the plasma membrane. The latter type of membrane ruffles are further divided into two groups, straight dorsal ruffles and CDRs. CDRs are typically ring-shaped, F-actin-rich structures found at the dorsal plasma membrane of cells treated with various growth factors (Fig. 1A). About 5–10 min after stimulation by growth factors, numerous short actin filaments, which are recognized as phase-dense dots under the light microscope, start to coalesce into a circular array with diameters of ∼10 ± 5 µm (Fig. 1B) (10, 11). When observed by scanning electron microscopy, each unit of the short actin filament can be recognized as small bumps emanating from the dorsal plasma membrane (5, 12). Each of these short actin dots then become bundled filaments that are subsequently assembled into a mature actin ring of the CDRs. At the later stages of CDR formation, the edges of the circular actin ring are gradually constricted to enclose its inner membrane area (Fig. 1B), wherein certain transmembrane receptors as well as their associated molecules are sequestered and then internalized into the cytosolic space.
Fig. 1.
CDR formation in response to growth factor stimulation. (A) CDR formation in NIH3T3 cells before (left panel) or after (right panel) PDGF stimulation. F-actin staining with rhodamine-phalloidin is shown. Bar, 10 µm. (B) Time-lapse images of actin cytoskeleton during CDR formation probed by Lifeact-mCherry transfected in NIH3T3 cells. Arrowheads indicate precursor and matured dorsal ruffles. Bar, 10 µm.
CDR formation has scarcely been observed in cancer cell lines (6) but has been reported in relatively naïve cell lines such as mouse embryonic fibroblast (MEF) (7, 13), Swiss3T3 (14), NIH3T3 (5, 10, 15), glia (12), human foreskin fibroblasts (8, 16), porcine aortic endothelial [when expressing exogenous platelet-derived growth factor receptor (PDGFR)] (17–19) and non-responder variant 6 (NR6) fibroblasts expressing wild-type human EGF (epidermal growth factor) receptor (EGFR) (20). Conditions that trigger CDR formation are stimulation by growth factors, including platelet-derived growth factor (PDGF) (8, 12), hepatocyte growth factor (21), EGF(20), as well as treatment with agents like 12-O-tetradecanoylphorbol-13-acetate that modify intracellular signalling pathways (22). Among these conditions, PDGF stimulation is the best characterized in the study of CDR formation. It has been shown that activation of the PDGF β-receptor (PDGFRβ), and not PDGFRα, efficiently induces CDRs (17, 19). The kinase insert domain of PDGFRβ has been observed to be important for this activation (18) but the exact mechanism for how this region is involved in the formation of CDRs remains unknown.
As briefly mentioned above, the main function of CDRs is thought to be the ‘bulk’ endocytosis of membrane-associated proteins. An excellent study by Orth et al. (20) first proposed that CDR is an important platform for sequestration and internalization of ligand-bound EGFR. Based on the comparison between NR6 cells (which form CDRs) and HeLa cells (which do not form CDRs), a clear discrepancy was observed: EGF internalization in NR6 cells is independent of clathrin but requires PI3K activity, whereas the opposite is true in the HeLa cells. Furthermore, it was also shown that CDR formation correlates well with the ability of EGF/EGFR endocytosis, and that the receptor–ligand complex was observed to be sequestered at the edges of the CDR ring in the NR6 cells (20). Welliver et al. (23) recently reported that the lateral diffusion of membrane-anchored proteins is limited within circular ruffles in macrophages stimulated by the macrophage colony stimulating factor. Although this study focuses on a sort of peripheral ruffle that curls up to form a relatively small circular macropinocytic cup (thus distinct from the CDR discussed here), the presence of a strong diffusion barrier at the edge of the circular ruffles could explain how the receptor molecules are trapped inside the CDR to be encapsulated within the endocytic vesicles.
Another important aspect of CDR is its involvement in cell migration. In resting cells, mature focal adhesions are interconnected with actin stress fibers for a strong attachment of the cell onto the substratum. Once stimulated by growth factors such as PDGF, cells need to disassemble these cytoskeletal structures to be transformed from the ‘static’ to the ‘motile’ state. It has been observed that stress fibres tend to decrease within the CDR rings formed in response to PDGF stimulation (5, 11). In addition, a lamellipodial plasma membrane extension was specifically induced in the area where the CDR was present. Although the exact mechanism for how the stress fibres are disassembled within the CDR remains unknown, a recent study on integrin trafficking could provide a clue to this observation. It was found that integrin β3-GFP accumulated at the edges of CDRs upon PDGF stimulation, and then internalized into endocytic compartments in a manner dependent on CDR formation, but not on clathrin-mediated endocytosis (9). The internalized integrin then passes through recycling endosomes, and is eventually transcytosed to the ventral side of the plasma membrane to create new adhesion sites. Such dynamic rearrangement of integrin molecules through their bulk endocytosis mediated by CDRs plays a pivotal role in the transition from the static to the motile state at the onset of cell migration. Interestingly, integrin β1 and its downstream kinase, integrin-linked-kinase, were shown to be required for PDGF-induced CDR formation (24, 25), indicating the existence of not only passive, but also active roles of integrin signalling in CDR formation.
Molecular Mechanism of CDR Formation
Two major characteristics of CDRs, responsiveness to extracellular stimulation and highly organized morphology, require a variety of molecules for the regulation and construction of this actin-based structure. These include various kinds of signalling proteins such as protein kinases, small GTPases, adaptors and regulators of actin polymerization.
Regulators of actin polymerization
The treatment of cells with inhibitors of actin polymerization, such as cytochalasin D or latrunculin A, inhibits the formation of CDR structures (26, 27). Central to the actin polymerization at the CDR ring is the N-WASP-mediated activation of the Arp2/3 complex. N-WASP has been shown to localize at the rim of the CDR ring (11, 28), and N-WASP −/− as well as WIP −/− (an N-WASP-associated protein) MEFs exhibit almost no CDRs upon PDGF stimulation, unlike that observed in the wild-type MEFs that efficiently induce many CDRs (28, 29). Cortactin, another activator of the Arp2/3 complex, has also been reported to be necessary for CDR formation (11, 30). Involvement of the WASP family verprolin-homologous proteins (WAVEs), another major group of Arp2/3-activator proteins, in CDR formation has been controversial (13, 29). Not only actin polymerization, but also proteins associated with the actin cytoskeleton are important for CDR formation. For example, mammalian actin-binding protein 1 was demonstrated to localize at, as well as required for the formation of, CDRs (30). As for the adaptor proteins, Nck, in complex with a scaffolding protein Gab1, has been demonstrated to trigger the activation of N-WASP, resulting in local actin polymerization for CDR formation (31, 32). These studies support the involvement of activators and modulators of Arp2/3-dependent actin polymerization in the formation of CDRs.
Membrane-binding proteins
As described above, CDRs are morphologically composed of deep membrane invaginations as well as protrusions. Consistently, dynamin 2 (Dyn2), a large GTPase involved in membrane invagination and scission (33), is also observed to localize at CDRs (11). Overexpression of Dyn2 strongly induces the formation of CDRs (our unpublished observation), whereas a dominant-negative form of Dyn2 (K44A) inhibits PDGF-stimulated macropinocytosis (11, 34). Furthermore, the depletion of Dyn2 results in the inhibition of CDR formation (35). Despite these findings, it is still unclear how its GTPase activity is involved in the formation of CDRs. To understand this, numerous Dyn2-binding proteins, most of them containing SH3 domains that recognize the proline-rich domain of dynamin may be the key. It is of particular note that a group of such Dyn2-binding proteins have Bin-Amphiphysin-Rvs (BAR) and Fer-CIP4 homology-BAR (F-BAR) domains, functional modules that are directly involved in membrane deformation (36–38). These domains have abilities to bind membrane lipids and to deform the plasma membrane. Sorting nexin 9 (SNX9), which contains a BAR domain at its C-terminus and associates with N-WASP, has been shown to be critical for CDR formation (27). SNX9 stimulates N-WASP- and Arp2/3-mediated actin polymerization by binding to PI(4,5)P2 at the plasma membrane. In addition, expression of the BAR domain-containing protein Tuba stimulates CDR formation, whereas its knockdown inhibits CDR formation (39). Conversely, an Inverse BAR (I-BAR) domain-containing MIM/IRSp53, which induces membrane protrusion was also reported to localize at CDRs. These studies suggest that membrane-deforming proteins play important roles in CDR formation.
Recently, our group demonstrated that a new SH3 domain-containing protein, SH3YL1, is localized at, as well as is essential for the formation of, CDRs in PDGF-stimulated NIH3T3 cells (15). This protein has an evolutionarily conserved domain module in its N-terminal region that we named the ‘SYLF’ domain, which binds to several D5-phosphorylated phosphoinositides [most strongly to PI(3,4,5)P3]. Furthermore, the SH3 domain of SH3YL1 interacts with SHIP2, a PI(3,4,5)P3 5-phosphatase and depletion of these proteins suppresses CDR formation. Our model of roles for the phosphoinositide conversion mediated by the SH3YL1–SHIP2 complex in CDR formation is depicted in Fig. 2A.
Fig. 2.
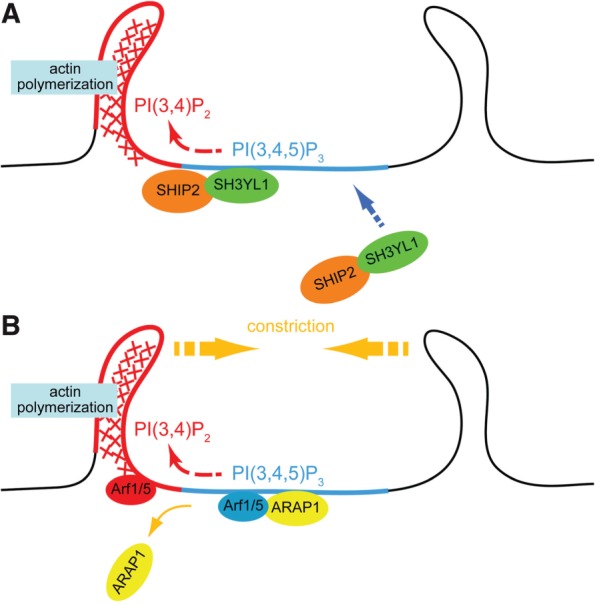
Models of CDR formation based on the phosphoinositide conversion from PI(3,4,5)P3 to PI(3,4)P2. (A) Upon stimulation with growth factors, PI3K is activated and produce PI(3,4,5)P3-rich area on the plasma membrane (blue). SH3YL1 is then recruited to the membrane via its SYLF domain that recognizes PI(3,4,5)P3. SH3YL1 simultaneously makes complex with a phosphoinositide 5-phosphatase SHIP2, resulting in a formation of PI(3,4)P2-enriched area (red) by dephosphorylation of PI(3,4,5)P3. Downstream target of PI(3,4)P2, such as Tapp1, is thought to be involved in actin polymerization. (B) ARAP1, which contains PH domains that collectively bind to PI(3,4,5)P3, is recruited to the plasma membrane and it then keeps Arf1/5 in a GDP-bound state (blue). When the phosphoinositide conversion from PI(3,4,5)P3 to PI(3,4)P2 takes place, ARAP1 detaches from the plasma membrane, allowing the activation of Arf1/5 into its GTP-bound form (red).
Protein kinases and small GTPases
Signalling pathways involved in the regulation of actin polymerization for the formation of CDRs also include various protein kinases and small GTPases. P21-activated kinase 1 (Pak1) has been demonstrated to localize at CDRs with F-actin after PDGF stimulation (14). In addition, the expression of the dominant active form of Pak1 stimulates the formation of CDRs and macropinocytosis (40). Furthermore, Pak1 interacts with the Arp2/3 complex (41) and PI3K (42), the activities of which are essential for CDR formation, suggesting that Pak1 is a key player that functions upstream of actin assembly.
On the other hand, expression of guanosine diphosphate (GDP)-bound dominant negative forms of Ras, Rac and Rab5 inhibit the induction of CDRs by growth factor stimulation (7, 43). Interestingly, it was also shown that Rab5-mediated endocytosis is required for the activation of Rac at early endosomes to induce CDR formation (44). Consistent with that report, we found that CDR formation is blocked by the overexpression of the Hrs FYVE domain, which masks PI(3)P on early endosomes (15). These studies indicate that the endocytic pathway through the early endosomes, mediated by small GTPases, is required for the induction of CDR formation.
Some GTPase activating protein (GAP) molecules for adenosine diphosphate (ADP)-ribosylaton factors (Arfs), such as ASAP1 [Arf GAP domain, SH3 domain, ankyrin repeat and pleckstrin homology (PH) domain] and ACAP1/2 (Arf GAP domain, coiled-coil, ankyrin repeat and PH domain 1/2) are localized at CDRs. It has also been shown that overexpression of those Arf GAPs strongly inhibits CDR formation (45, 46). Recently, we found that ARAP1 (Arf GAP with Rho GAP domain, ankyrin repeat and PH domain 1) regulates the ‘ring size’ of the CDRs in a manner dependent on its Arf GAP activity (10). Furthermore, expression of dominant negative mutants of Arf1 and Arf5, the substrates of ARAP1, induces the formation of larger CDRs. Together with the fact that the PH domains of ARAP1 collectively recognize PI(3,4,5)P3 at the plasma membrane (10), we propose a model of CDR size control by ARAP1-Arf1/5 under the control of phosphoinositide conversion from PI(3,4,5)P3 to PI(3,4)P2 (Fig. 2B).
Mathematical Models
The shape and the dynamics of CDRs as circular waves that propagate and then constrict have also been attracting researchers in the field of mathematical as well as theoretical biology. By the use of polydimethylsiloxane substrates with varying elasticity, it was found that the lifetimes, but not the sizes, of CDRs were extended as the substrate stiffness increased (47). This observation was explained by a mathematical model that conceives of an efficient replenishment of G-actin for CDR formation, which is accomplished by the destruction of well-developed stress fibres initially present in resting cells on the rigid substrate (47). The same study also predicts a key role for GAPs bound to WAVE, a Rac-dependent activator for Arp2/3. Such molecules, exemplified by the F-BAR domain-containing slit-robo GAPs (srGAPs) (48), could be recruited to the membrane in response to PDGF stimulation, and then form a belt of ‘suppressing zone’ for Rac activity, resulting in a spike of Rac-dependent actin polymerization that corresponds to the edge of the CDR (47).
Based on the fact that both convex and concave types of membrane curvature-sensing/generating proteins, MIM/IRSp53 and Tuba, have been found to be localized at CDRs (28, 39, 49), the roles of such curvature sensors/generators in CDR formation have also been discussed. A mathematical simulation, which conceives of involvement of activators of actin polymerization with convex or concave surfaces for their membrane binding, actually reproduced the formation of periodic membrane undulations (waves) (49). The model also predicted that a threshold value of the level of actin polymerization should exist to trigger this wave formation. Such properties seem consistent with the characteristics of real CDRs, the formation of which is induced only in response to extracellular stimuli. A problem unsolved by this study is that the simulation could have derived repeated wave formations, whereas in the real CDR the wave appears only once. This kind of feature could be at least partly reproduced by introducing a negative regulator of actin polymerization with curved membrane binding surfaces, exemplified by srGAP, as described above (47).
Common and Distinct Features of CDRs and Podosome Rosettes
Numerous characteristics of CDRs are similar to those of podosomes (Table I). A podosome is an actin-based adhesive structure formed at the ventral side of the plasma membrane in monocyte-derived cells such as macrophages and osteoclasts, and even fibroblasts transformed by oncogenes like src tyrosine kinase (5). It is a site of cell-substrate adhesion, where the enzymes involved in the degradation of the extracellular matrix (ECM) are secreted, such as matrix metalloproteinases. In cancer cells, a very similar structure called ‘invadopodium’ is known for its crucial role in invasion through the basement membrane during metastasis. Similar to CDRs, podosomes are also composed of numerous actin-based membrane invaginations and protrusions, the components of which include dynamin, WASP/N-WASP, Arp2/3, cortactin and FBP17 (50–55). Surrounded by such invaginated membranes, the highly bundled F-actin forms a ‘core’ of the membrane protrusion for the degradation of ECM during invasive cell migration.
Table I.
Comparison between signalling and cytoskeletal molecules involved in circular dorsal ruffles and podosome rosettes.
| Circular dorsal ruffles | Podosome rosettes | |
|---|---|---|
| PI signalling | ||
| PI3K | Yes (57) | Yes (58) |
| PIs 5-phosphatase | SHIP2 (15) | Synaptojanin 2 (58) |
| Accumulation of PI(3,4)P2 | Yes (15) | Yes (58) |
| PI(3,4)P2-binding proteins | TAPP1 (15, 68) | Tks4 (60), Tks5 (58, 61) |
| Small GTPase | ||
| Arf | Arf1, 5 (10) | Arf6 (56) |
| Rac | Yes (7, 43) | Yes (50, 58) |
| Rho | Yes (47) | Yes (62) |
| Actin machinery | ||
| Arp2/3 | Yes (11) | Yes (51) |
| N-WASP | Yes (29) | Yes (53) |
| WAVE | Yes (13) and No (29) | No (58) |
| Cytoskeletons other than actin | ||
| Microtubules | Yes (our unpublished results) | No (62) [Yes for podosome belts (51)] |
| Intermediate filaments | unknown | Yes (62) |
An interesting feature of podosomes is their assembling pattern dependent on the cell type. In osteoclasts, each podosome is first organized in a circular array with diameters ranging from ∼5 to 20 µm, which is called the ‘podosome rosette’ (or ring). These circular structures subsequently fused with each other, build up a ‘belt’ of an F-actin-rich area that embraces the whole periphery of the cell, thus referred to as the ‘podosome belt’. The podosome belt is thought to function as a ‘sealing zone’ by tightly packing the contact area between the basal membrane and the substrate for efficient degradation of the ECM (56). In v-Src-transformed fibroblasts, only the podosome rosette, and not the podosome belt, is present, indicating the existence of an additional mechanism for the fusion of podosome rosettes and the expansion of podosome belts.
Roles of microtubules and intermediate filaments
A role for microtubules in the formation of the podosome belt has been reported by several groups (63–65). It has also been observed by electron microscopy that microtubules are in very close proximity to the podosome core (59). As for the molecule linking between microtubules and actin during podosome belt formation, unconventional myosin X (Myo10), an actin motor protein that interacts with microtubules, is known to be involved in this process (66). Our unpublished data show that microtubules are also required for CDR formation by PDGF stimulation in NIH3T3 cells. This may indicate that the circular arrangement of actin-based membrane deformation is mediated, at least in part, by intracellular membrane traffic along the microtubules. Considering the size of their circular arrangements, CDRs are more similar to podosome ‘rosettes’, but not podosome ‘belts’, because CDR has never been observed to grow as large as the size of the entire cell. However, Pan et al. demonstrated that microtubules are not necessary for podosome ‘rosette’ formation in Src-transformed MEFs (62). Instead, the group revealed a previously unappreciated role of vimentin, a component of intermediate filaments, in the negative regulation of podosome rosette formation. Whether intermediate filaments are also involved in the regulation of CDRs awaits future studies.
Roles of PI3K and lipid phosphatases
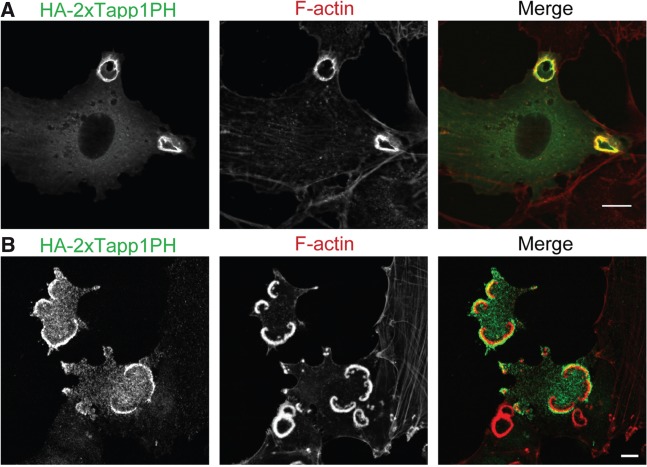
Results obtained by our recent studies have extracted a key role for phospholipid turnover mediated by PI3K and 5-phosphatases in the formation of a ‘ring’ structure, the common characteristic of CDRs and podosome rosettes (Table I). PI3K activity, as well as its product PI(3,4,5)P3, has been shown to be important for CDR formation(57). Consistenly, PI3K inhibitors such as wortmannin or LY294002 significantly inhibit CDR formation and macropinocytosis (13, 15, 67). In addition, our group has demonstrated that overexpression of the PH domain of Grp1, which binds specifically to PI(3,4,5)P3, blocked the formation of CDRs (15). We also found that the PI(3,4,5)P3 5-phosphatase SHIP2, which generates PI(3,4)P2, is localized at the CDRs and the knockdown of SHIP2 disrupts ‘circular’ dorsal ruffles, but not the peripheral ruffles (15). Moreover, the Tapp1 PH domain, which specifically binds to PI(3,4)P2, is also concentrated at CDRs (Fig. 3A) and overexpression of Tapp1 or its PH domain suppresses CDR formation (15, 68), suggesting that both SHIP2 and its product PI(3,4)P2 are essential for the ‘ring-shaped’ CDR. Essentially, podosome rosettes share a very similar property in their enrichment of, and requirement for, the PI3K products. In Src-transformed NIH3T3 cells, the PI(3,4)P2-specific probe Tapp1 PH domain was observed to localize at podosome rosettes (58) (Fig. 3B). In addition, treatment by LY294002 as well as overexpression of the Tapp1 PH domain also suppressed podosome rosette formation (58). The only discrepancy between these two circular structures is phosphoinositide 5-phosphatases involved in PI(3,4)P2 synthesis. Whereas CDR is dependent on SHIP2 as mentioned above, it is not required for podosome rosette formation. Instead, knockdown of synaptojanin 2, another phosphoinositide 5-phosphatase, was revealed to block podosome rosette formation (58).
Fig. 3.
Localizations of PI(3,4)P2 at CDRs and podosome rosettes. (A) NIH3T3 cells expressing HA-2 × Tapp1PH [a specific probe for PI(3,4)P2] were stimulated with PDGF for 5 min, and then stained with anti-HA antibodies as well as rhodamine-phalloidin. (B) NIH3T3 cells expressing an active form of Src (Y530F) were transfected with HA-2 × Tapp1PH, and then stained with anti-HA antibodies as well as rhodamine-phalloidin. Bars, 10 µm.
Next important question is the downstream targets of PI(3,4)P2 involved in the formation of each circular structure. In fact, many studies have shown that Tapp1, Tks4 and Tks5, which bind specifically to PI(3,4)P2, are required for CDR and podosome rosette formation, respectively (15, 58, 60, 61) (Table I). However, the detailed molecular mechanisms of how these PI(3,4)P2-binding proteins contribute to the organization of these ring-shaped actin structures still remains unclear.
Conclusions
This review has tried to summarize our current knowledge about two distinct circular structures mediated by actin–membrane interactions: CDRs and podosome rosettes. Despite many studies that have determined a large number of molecules involved in the formation of both structures, two fundamental questions remain to be answered: how do they become circular, and why are they circular? The roles of negative regulators in signal transduction, such as SHIP2, ArfGAPs and probably WAVE-associated srGAPs, may be the key to solving these questions. Considering the potential importance of these two circular actin-based membrane structures for the proper control of cell growth and cancer metastasis, unprecedented approaches based on the common principle underlying their morphology and dynamics will provide a new strategy for cancer research.
Funding
Grant-in-Aid for Creative Scientific Research from the Japan Society for the Promotion of Science (JSPS); Grant-in-Aid for Young Scientists from the JSPS; The Naito Foundation; Global COE (Centers of Excellence) Program from the JSPS.
Conflict of interest
None declared.
Acknowledgements
We are grateful to Dr Tsukasa Oikawa (Keio University) for providing data.
Glossary
Abbreviations
- Arf
adenosine diphosphate (ADP)-ribosylaton factor
- CDRs
circular dorsal ruffles
- Dyn2
dynamin 2
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- F-BAR
Fer-CIP4 homology-BAR
- GAP
GTPase activating protein
- MEF
mouse embryonic fibroblast
- NR6
non-responder variant 6
- Pak1
P21-activated kinase 1
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PI3K
PI 3-kinase
- SNX9
sorting nexin 9
- srGAP
slit-robo GAP
References
- 1.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 3.Bernard O. Lim kinases, regulators of actin dynamics. Int. J. Biochem. Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 5.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell. Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 6.Orth JD, McNiven MA. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 2006;66:11094–11096. doi: 10.1158/0008-5472.CAN-06-3397. [DOI] [PubMed] [Google Scholar]
- 7.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 8.Mellstrom K, Heldin CH, Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp. Cell Res. 1988;177:347–359. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- 9.Gu Z, Noss EH, Hsu VW, Brenner MB. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J. Cell Biol. 2011;193:61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa J, Tsujita K, Takenawa T, Itoh T. ARAP1 regulates the ring size of circular dorsal ruffles through Arf1 and Arf5. Mol. Biol. Cell. 2012;23:2481–2489. doi: 10.1091/mbc.E12-01-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell. 2003;14:1085–1096. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellstrom K, Hoglund AS, Nister M, Heldin CH, Westermark B, Lindberg U. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J. Muscle Res. Cell Motil. 1983;4:589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- 13.Suetsugu S, Yamazaki D, Kurisu S, Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 14.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J. Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa J, Tokuda E, Tenno T, Tsujita K, Sawai H, Hiroaki H, Takenawa T, Itoh T. SH3YL1 regulates dorsal ruffle formation by a novel phosphoinositide-binding domain. J. Cell Biol. 2011;193:901–916. doi: 10.1083/jcb.201012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nister M, Hammacher A, Mellstrom K, Siegbahn A, Ronnstrand L, Westermark B, Heldin CH. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988;52:791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- 17.Westermark B, Siegbahn A, Heldin CH, Claesson-Welsh L. B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proc. Natl Acad. Sci. USA. 1990;87:128–132. doi: 10.1073/pnas.87.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvidsson AK, Heldin CH, Claesson-Welsh L. Transduction of circular membrane ruffling by the platelet-derived growth factor beta-receptor is dependent on its kinase insert. Cell Growth Differ. 1992;3:881–887. [PubMed] [Google Scholar]
- 19.Eriksson A, Siegbahn A, Westermark B, Heldin CH, Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992;11:543–550. doi: 10.1002/j.1460-2075.1992.tb05085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66:3603–3610. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- 21.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur. J. Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- 22.Schliwa M, Nakamura T, Porter KR, Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J. Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welliver TP, Chang SL, Linderman JJ, Swanson JA. Ruffles limit diffusion in the plasma membrane during macropinosome formation. J. Cell Sci. 2011;124:4106–4114. doi: 10.1242/jcs.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King SJ, Worth DC, Scales TM, Monypenny J, Jones GE, Parsons M. Beta1 integrins regulate fibroblast chemotaxis through control of N-WASP stability. EMBO J. 2011;30:1705–1718. doi: 10.1038/emboj.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azimifar SB, Bottcher RT, Zanivan S, Grashoff C, Kruger M, Legate KR, Mann M, Fassler R. Induction of membrane circular dorsal ruffles requires co-signalling of integrin-ILK-complex and EGF receptor. J. Cell Sci. 2012;125:435–448. doi: 10.1242/jcs.091652. [DOI] [PubMed] [Google Scholar]
- 26.Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J. 2000;19:4589–4600. doi: 10.1093/emboj/19.17.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarar D, Waterman-Storer CM, Schmid SL. SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev. Cell. 2007;13:43–56. doi: 10.1016/j.devcel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Anton IM, Saville SP, Byrne MJ, Curcio C, Ramesh N, Hartwig JH, Geha RS. WIP participates in actin reorganization and ruffle formation induced by PDGF. J. Cell Sci. 2003;116:2443–2451. doi: 10.1242/jcs.00433. [DOI] [PubMed] [Google Scholar]
- 29.Legg JA, Bompard G, Dawson J, Morris HL, Andrew N, Cooper L, Johnston SA, Tramountanis G, Machesky LM. N-WASP involvement in dorsal ruffle formation in mouse embryonic fibroblasts. Mol. Biol. Cell. 2007;18:678–687. doi: 10.1091/mbc.E06-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortesio CL, Perrin BJ, Bennin DA, Huttenlocher A. Actin-binding protein-1 interacts with WASp-interacting protein to regulate growth factor-induced dorsal ruffle formation. Mol. Biol. Cell. 2010;21:186–197. doi: 10.1091/mbc.E09-02-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruusala A, Pawson T, Heldin CH, Aspenstrom P. Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor beta receptor. J. Biol. Chem. 2008;283:30034–30044. doi: 10.1074/jbc.M800913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abella JV, Vaillancourt R, Frigault MM, Ponzo MG, Zuo D, Sangwan V, Larose L, Park M. The Gab1 scaffold regulates RTK-dependent dorsal ruffle formation through the adaptor Nck. J. Cell Sci. 2010;123:1306–1319. doi: 10.1242/jcs.062570. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlunck G, Damke H, Kiosses WB, Rusk N, Symons MH, Waterman-Storer CM, Schmid SL, Schwartz MA. Modulation of Rac localization and function by dynamin. Mol. Biol. Cell. 2004;15:256–267. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu YW, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL. Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells. Mol. Biol. Cell. 2008;19:5347–5359. doi: 10.1091/mbc.E08-08-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim. Biophys. Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Heath RJ, Insall RH. F-BAR domains: multifunctional regulators of membrane curvature. J. Cell Sci. 2008;121:1951–1954. doi: 10.1242/jcs.023895. [DOI] [PubMed] [Google Scholar]
- 38.Itoh T, Takenawa T. Mechanisms of membrane deformation by lipid-binding domains. Prog. Lipid Res. 2009;48:298–305. doi: 10.1016/j.plipres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs EM, Makar RS, Gertler FB. Tuba stimulates intracellular N-WASP-dependent actin assembly. J. Cell Sci. 2006;119:2715–2726. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- 40.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell. 2000;11:3341–3352. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004;5:154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papakonstanti EA, Stournaras C. Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol. Biol. Cell. 2002;13:2946–2962. doi: 10.1091/mbc.02-01-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooshmand-Rad R, Claesson-Welsh L, Wennstrom S, Yokote K, Siegbahn A, Heldin CH. Involvement of phosphatidylinositide 3'-kinase and Rac in platelet-derived growth factor-induced actin reorganization and chemotaxis. Exp. Cell Res. 1997;234:434–441. doi: 10.1006/excr.1997.3636. [DOI] [PubMed] [Google Scholar]
- 44.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 45.Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, Cooper JA. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl Acad. Sci. USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA. ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol. 2000;151:627–638. doi: 10.1083/jcb.151.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Y, Lai T, Koh CG, LeDuc PR, Chiam KH. Investigating circular dorsal ruffles through varying substrate stiffness and mathematical modeling. Biophys. J. 2011;101:2122–2130. doi: 10.1016/j.bpj.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong WC, Rao Y. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- 49.Peleg B, Disanza A, Scita G, Gov N. Propagating cell-membrane waves driven by curved activators of actin polymerization. PLoS One. 2011;6:e18635. doi: 10.1371/journal.pone.0018635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–1149. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- 51.Linder S, Higgs H, Hufner K, Schwarz K, Pannicke U, Aepfelbacher M. The polarization defect of Wiskott-Aldrich syndrome macrophages is linked to dislocalization of the Arp2/3 complex. J. Immunol. 2000;165:221–225. doi: 10.4049/jimmunol.165.1.221. [DOI] [PubMed] [Google Scholar]
- 52.Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, De Camilli P. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 54.Tsuboi S, Takada H, Hara T, Mochizuki N, Funyu T, Saitoh H, Terayama Y, Yamaya K, Ohyama C, Nonoyama S, Ochs HD. FBP17 mediates a common molecular step in the formation of podosomes and phagocytic cups in macrophages. J. Biol. Chem. 2009;284:8548–8556. doi: 10.1074/jbc.M805638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaff M, Jurdic P. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J. Cell Sci. 2001;114:2775–2786. doi: 10.1242/jcs.114.15.2775. [DOI] [PubMed] [Google Scholar]
- 56.Heckel T, Czupalla C, Expirto Santo AI, Anitei M, Arantzazu Sanchez-Fernandez M, Mosch K, Krause E, Hoflack B. Src-dependent repression of ARF6 is required to maintain podosome-rich sealing zones in bone-digesting osteoclasts. Proc. Natl Acad. Sci. USA. 2009;106:1451–1456. doi: 10.1073/pnas.0804464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wymann M, Arcaro A. Platelet-derived growth factor-induced phosphatidylinositol 3-kinase activation mediates actin rearrangements in fibroblasts. Biochem. J. 1994;298(Pt 3):517–520. doi: 10.1042/bj2980517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans JG, Correia I, Krasavina O, Watson N, Matsudaira P. Macrophage podosomes assemble at the leading lamella by growth and fragmentation. J. Cell Biol. 2003;161:697–705. doi: 10.1083/jcb.200212037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol. Biol. Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seals DF, Azucena EF, Jr., Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Pan YR, Chen CL, Chen HC. FAK is required for the assembly of podosome rosettes. J. Cell Biol. 2011;195:113–129. doi: 10.1083/jcb.201103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babb SG, Matsudaira P, Sato M, Correia I, Lim SS. Fimbrin in podosomes of monocyte-derived osteoclasts. Cell Motil. Cytoskeleton. 1997;37:308–325. doi: 10.1002/(SICI)1097-0169(1997)37:4<308::AID-CM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 64.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. J. Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMichael BK, Cheney RE, Lee BS. Myosin X regulates sealing zone patterning in osteoclasts through linkage of podosomes and microtubules. J. Biol. Chem. 2010;285:9506–9515. doi: 10.1074/jbc.M109.017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hogan A, Yakubchyk Y, Chabot J, Obagi C, Daher E, Maekawa K, Gee SH. The phosphoinositol 3,4-bisphosphate-binding protein TAPP1 interacts with syntrophins and regulates actin cytoskeletal organization. J. Biol. Chem. 2004;279:53717–53724. doi: 10.1074/jbc.M410654200. [DOI] [PubMed] [Google Scholar]