Abstract
Why do humans menstruate while most mammals do not? Here, we present our answer to this long-debated question, arguing that (i) menstruation occurs as a mechanistic consequence of hormone-induced differentiation of the endometrium (referred to as spontaneous decidualization, or SD); (ii) SD evolved because of maternal-fetal conflict; and (iii) SD evolved by genetic assimilation of the decidualization reaction, which is induced by the fetus in non-menstruating species. The idea that menstruation occurs as a consequence of SD has been proposed in the past, but here we present a novel hypothesis on how SD evolved. We argue that decidualization became genetically stabilized in menstruating lineages, allowing females to prepare for pregnancy without any signal from the fetus. We present three models for the evolution of SD by genetic assimilation, based on recent advances in our understanding of the mechanisms of endometrial differentiation and implantation. Testing these models will ultimately shed light on the evolutionary significance of menstruation, as well as on the etiology of human reproductive disorders like endometriosis and recurrent pregnancy loss.
Keywords: genetic assimilation, maternal-fetal conflict, menstruation, molecular evolution
INTRODUCTION
Menstruation, the shedding of the superficial endometrium with associated bleeding, occurs in some species of placental mammals when progesterone levels fall at the end of an infertile reproductive cycle [1]. In non-menstruating mammals, tissue break-down and bleeding do not occur when progesterone levels fall. In the higher primates, some species of bats, and the elephant shrew, however, the regression of the corpus luteum and the consequent fall in progesterone triggers proteolysis of the extracellular matrix, cell death, and bleeding. Why do some mammals, in particular primates, menstruate while most do not? What is the adaptive value, if any, of menstruation? Many hypotheses have been put forth, including one arguing that menstruation evolved to protect against sperm-born pathogens and one claiming that menstruation is energetically less costly than maintaining the endometrium in an active state (Table 1; for a review see [2]). These ideas are flawed for a variety of reasons and thus fail to explain why menstruation evolved (Table 1; [2,3]). The most frequent problem is lack of consideration of the ancestral state and variation among placental mammals. In this paper we present our hypothesis on the mechanisms and evolutionary significance of menstruation.
Table 1.
Hypotheses on the significance of menstruation
| Hypothesis | Claim | Comments | References | |
|---|---|---|---|---|
| 1 | Sperm-born pathogen removal | Menstruation occurs to protect the uterus against colonization by pathogens transported by sperm. | Menstruation occurs weeks after copulation; the problem of sperm-born pathogens is not unique to menstruating species. | [2,3,56] |
| 2 | Energy conservation | Menstruation/endometrial resorption is energetically less costly than maintaining a differentiated endometrium | Maintaining a differentiated endometrium is no the alternative in other species. It also would not allow of ovulation, sperm transport or sperm capacitation. | [3,57] |
| 3 | Nonadaptive consequence of SD | Menstruation occurs as a nonadaptive consequence of SD of the endometrium. | This is consistent with known consequences of decidualization and the position adopted here. The remaining problem is to explain the evolution of SD. | [3] |
| 4 | Uterine preconditioning | Menstrual “preconditioning” protects uterine tissues from the hyperinflammation and oxidative stress associated with deep placentation in humans. | Claim ignores why menstruation may have evolved in ancestral primate species and menstruating non-primates; claim ignores benefits that SD itself might provide; there is no experimental evidence that endometrial shedding confers protection against oxidative stress during pregnancy. | [58] |
First, we will argue that it is not menstruation per se that is the adaptive trait, i.e. directly confers an adaptive advantage (first proposed by [3]). In fact, it can be argued that menstruation as such is costly to the female in terms of incapacitation and loss of blood. Rather, it is more plausible that menstruation occurs as an inevitable consequence of spontaneous decidualization (SD), the cyclical differentiation of the endometrial stroma in response to maternal hormones. Thus, to understand why menstruation evolved, it is necessary to understand the forces involved in SD. Here, we argue that SD evolved in some groups of placental mammal because of maternal-fetal conflict, and that it evolved by genetic assimilation of the decidualization reaction, which occurs in non-menstruating mammals in response to blastocyst implantation (rather than circulating progesterone). We propose three models of how SD may have evolved by genetic assimilation and how it can be tested experimentally.
THE MENSTRUATING SPECIES
In the primates, there is well-documented evidence that the catarrhines (humans, apes, and Old World monkeys) menstruate and that the strepsirrhines (e.g. lemurs and galagos) do not (see [2]; Fig. 1). The data on New World monkeys are ambiguous. There is clear evidence that at least one species in each of the genera Cebus, Ateles, Alouatta, and Lagothrix exhibits menstruation [4–7]. For other New World species, the data are conflicting and probably reflect the low expression of this character in the species studied and the different methods used to detect menstruation. There are also conflictin data for tarsiers, a group of primates more basal than the New World monkeys. Catchpole and Fulton [8] and Wright et al. [9] saw no external bleeding in regular vaginal smears of Tarsius syrichta and T. bancanus, respectively, while Hill et al. [10] saw external bleeding on a few occasions in one female of T. syrichta and Van Herwerden [11] saw extravasation (but no destruction of uterine tissue) in fixed uteri of T. bancanus. Thus, there is very weak if any evidence of menstruation in tarsiers. It is certain, however, that the rodents and lagomorphs, members of the sister taxon to the primates, do not menstruate (ss [12]; Fig. 1).
Figure 1.
Phylogeny showing the distribution of menstruation in placental mammals and the inferred states of ancestral lineages. Menstruating species/lineages are colored in pink, non-menstruating species/lineages in black. Species in which the character state is not known are not colored, and lineages of equivocal state are represented with black lines. Monodelphis represents the outgroup. Inference of ancestral states was performed in MacClade 4 by the parsimony method [55]. Note that there is strong evidence for three independent originations of menstruation among placental mammals.
The picture in bats (order Chiroptera) is less clear than in the primates. There is evidence that at least four bat species exhibit menstruation: Molossus ater, a molossid bat, and Glossophaga soricina, Carollia perspicillata, and Desmondus rotundus, all phyllostomid bats (see [13]; Fig. 1). Menstruation has not been detected or studied in the majority of bat species; thus, it is unclear if menstruation evolved twice within Chiroptera or is more widespread in the clade. Finally, there is well-documented evidence of menstruation in the elephant shrew (Elephantulus myurus), a species belonging to the clade Afrotheria [14].
There are cases of bleeding in other mammalian species, e.g. in dogs and tree shrews. In the case of the dog, bleeding occurs in proestrus (i.e. not at the time of progesterone withdrawal) and is derived from the vagina (i.e. not the uterus); thus it is clear that bleeding in this species does not represent menstruation (see [2]). In tree shrews bleeding is derived from the uterus but only occurs at the end of pseudopregnancy, which is induced by mating females in captivity to vasectomized males (pseudopregnancy is much like the luteal phase of the human menstrual cycle when progesterone levels are elvated for many days – see Fig. 2). Although bleeding in tree shrews is associated with progesterone withdrawal, it is dependent on a sterile copulation, which is not the case for any other menstruating species. It is also unclear if pseudopregnancy in tree shrews occurs at all in nature (see [2]). Thus, the consensus is that menstruation is restricted to the higher primates, some bats, and the elephant shrew, although it may be useful and important to study tree shrews to clarify the ultimate and proximate causes of menstruation (see later).
Figure 2.
Diagram showing development of the ovary and uterus, hormone levels (estradiol and progesterone), and key events (menstruation, ovulation, and decidualization) during the 28—day human menstrual cycle.
MENSTRUATION OCCURS AS A CONSEQUENCE OF SPONTANEOUS DECIDUALIZATION OF THE ENDOMETRIUM
Spontaneous decidualization is correlated with menstruation
In the majority of placental mammals with invasive placentation, differentiation of the endometrium (decidualization) occurs in the event of pregnancy, and specifically it initiates at the site where fetal tissues begin invasion into the endometrium. Decidualization is characterized by angiogenesis, an influx of natural killer cells to the uterus, and differentiation of endometrial stromal cells (ESCs) into decidual cells. These profound changes help establish a functional connection between maternal and fetal tissues, help restrain fetal invasion into the uterus, provide hemostasis and an immunotolerant environment (See Box 1; [15,16]). In contrast to the fetus-induced decidualization that occurs in non-menstruating species, decidualization in menstruating species occurs whether or not pregnancy takes place. Maternal hormones, specifically progesterone, appear to be the only signal require for decidualization to occur; this phenomenon is referred to as SD, i.e. without a signal from the fetus. In humans, SD initiates during the luteal phase of the menstrual cycle around spiral arterioles of the endometrium, about a week after ovulation and the beginning of progesterone production by the corpus luteum (Fig. 2). SD has also been documented to occur in Old World monkeys, apes, the elephant shrew, and in at least one of the menstruating bat species (Table 2; [14,17,18]). It has not been found to occur in any non-menstruating species.
BOX 1. Maternal-fetal conflict: Description and examples.
The conflict hypothesis posits that the relationship between the mother and fetus has been shaped by natural selection [21]. As the mother and fetus do not carry identical sets of genes, their evolutionary interests are not the same. Fetal genes will evolve to extract as much as possible from the mother to ensure their propagation (since these genes are not necessarily represented in future siblings), whereas maternal genes will evolve to promote the success of the current and future offspring to maximize their propagation. The conflict hypothesis predicts an evolutionary tug-of-war between maternal and fetal genomes, similar to virus-host interactions in which moves by one party are matched by countermoves of the other. Maternal-fetal conflict is manifest at the number of different organizational levels, from placental morphology and physiology to epigenetic marks on the genome. A few examples are described below:
Placental invasiveness and decidualization: The conflict hypothesis is invoked to explain why there is such a high degree of variation in invasiveness of the mammalian placenta, ranging from a superficial placenta that does not invade maternal tissues (epitheliochroial), to one that breaches the uterine epithelium (endotheliochorial), to one that breaches maternal blood vessels (hemochorial) (see [21]). While increased invasiveness likely evolved for the benefit of the fetus, the decidualization reaction, which only occurs in mammals with invasive placentation, is thought to have evolved as a protective mechanism for the mother (see [15,21]). A number of decidual features point to its role in fetal restraint: decidual morphology consists of a continuous block of enlarged cells joined by tight junctions [15]; the function of many decidual products is to inhibit the activity of invasive fetal proteins (e.g. see [16]); and uterine NK cells induce apoptosis in invading trophoblast cells [59]. Also, the occurrence of uncontrolled invasion during tubal and accrete pregnancies suggests that the maternal decidua is required for fetal restraint, as the decidual reaction in the fallopian tubes and at sites of accrete pregnancies is deficient and lacking NK cells [60,61].
Placental and maternal hormones involved in glucose metabolism: The presence during pregnancy of very high levels of placental and maternal hormones involved in glucose metabolism suggests that an evolutionary escalation between maternal and fetal genomes has taken place. During the third trimester of pregnancy blood glucose increases after a meal, but it does not return to baseline in response to insulin as quickly as it does in the non-pregnant state. In addition, the maternal insulin response during pregnancy is exaggerated relative to the non-pregnant state. It has been argued that insulin is less effective during pregnancy because fetal hormones manipulate the maternal response to insulin to make more glucose available to the fetus. Placental lactogen, one of the most abundant placental hormones, has been cited as a likely candidate [21]. In response to this fetal manipulation, the mother may have increased her production of insulin, evidenced by the peak levels of insulin during the third trimester (see [21] for more details).
Genomic imprinting: Conflict exists between genes expressed in the mother and fetus as described above, but it also occurs between maternally and paternally expressed genes in fetal tissues [21]. Genomic imprinting of genes expressed in fetal tissues is evidence of conflict at this level. Under this hypothesis, paternally expressed alleles will enhance fetal/placental growth, and maternally expressed alleles in the fetus will restrict fetal/placental growth. Interestingly, most known imprinted genes are involved in fetal/placental growth and expressed in the placenta [62]. The insulin and insulin-like-growth factor system provides good support for this hypothesis: IGF2, IGF2R, and INS, genes involved in placental and fetal growth and metabolism are imprinted and fit the pattern described above. For example, IGF2 is a gene expressed in the placenta that promotes trophoblast invasiveness, and is only expressed from the paternal allele [62].
Table 2.
Shared reproductive features of menstruating mammals
| Group | Spontaneous decidualization? | Type of placentation | Timing of mating | Ovulation (spontaneous or induced?) | Offspring |
|---|---|---|---|---|---|
| Higher primates (monkeys, apes, and humans) | Yes | Invasive (hemochorial), more so than in non-menstruating primates | Extended mating, not restricted to periovulatory period | Spontaneous | Usually 1 well-developed offspring/pregnancy |
| Bats (4 species) | Yes, at least 1 of 4 species | Invasive (hemochorial) | At least 1 species has extended mating, not restricted to periovulatory period | Spontaneous | Usually 1 well-developed offspring/pregnancy |
| Elephant shrew | Yes | Invasive (hemochorial) | Mating occurs over an extended period because ovulation lasts many days with over 100 eggs released | Spontaneous | Usually 2 well-developed offspring/pregnancy; only 3 pregnancies/lifetime |
Decidualization followed by progesterone withdrawal results in menstruation
Beyond the obvious correlation between menstruation and SD described above, there is evidence suggesting that there is a causal relationship between the two traits. In mice, menstruation can experimentally be induced if and only if decidualization has occurred prior to progesterone withdrawal [12]. Mice, which do not exhibit menstruation or SD under natural conditions, can be induced to undergo the decidualization reaction by scratching the surface of the endometrium during pseudopregnancy. If the source of progesterone is removed subsequent to decidualization, female mice shed their superficial endometrium and bleed into the uterine lumen. In control mice that are not given the uterine scratch, there is no bleeding or tissue breakdown after progesterone withdrawal.
On a more mechanistic level, it has been shown that differentiated human ESCs undergo apoptosis if their progesterone source is removed [19,20]. Labied et al. [20] studied expression of the pro-apoptotic FOXO1 transcription factor in differentiating human ESCs. They showed that the synthetic progestin medroxyprogesterone acetate (MPA) enhanced FOXO1 expression while simultaneously inducing its cytoplasmic retention and inactivation. Upon MPA withdrawal, there was rapid FOXO1 accumulation in the nucleus, increased BIM expression, a pro-apoptotic FOXO1 target gene, and a 2.5-fold increase in cell death. As differentiated ESC are a source of multiple factors implicated in tissue hemostasis, it is argued that progesterone withdrawal from differentiated stromal cells not only induces their apoptosis but also promotes focal bleeding, as the factors that promote hemostasis are no longer present.
In light of the evidence described above, it is clear that decidualization commits ESCs to an apoptotic pathway upon progesterone withdrawal. As menstruating individuals undergo the decidualization reaction every reproductive cycle, menstruation must occur if fertilization does not when progesterone levels drop. Thus, to elucidate how and why menstruation evolved, we must understand the selective forces and mechanisms involved in the evolution of SD.
WHY DID SPONTANEOUS DECIDUALIZATION EVOLVE?
When one analyzes the phylogenetic distribution of menstruation, and thus SD as argued above, it becomes evident that these traits evolved in concert multiple times in the various groups in which they are exhibited (Fig. 1). The elephant shrew is a member of the supraorder Afrotheria, the bats are part of the supraorder Laurasiatheria, and the primates are members of the clade Euarchontoglires (Fig. 1). Given the distribution of menstruation, it is highly unlikely that the ancestor of placental mammals exhibited menstruation. In fact, a parsimony analysis shows that the most parsimonious explanation for the occurrence of menstruation/SD is that it evolved at least three times: once in the primates, once in the elephant shrew, and at least once in the bats (Fig. 1). It is commonly understood that a trait that has evolved convergently has some adaptive value for species that possess the trait. Thus, we would expect that SD provides some benefit to the species in which it occurs.
Maternal-fetal conflict drives the evolution of uterine tissues
One must review the hypothesis of maternal-fetal conflict, described in detail by Haig ]21], to understand what the benefits of SD might be. According to the conflict hypothesis, the evolutionary interests of the mother and fetus are not the same (see Box 1). Natural selection acts on fetal genes to increase nutrient transfer to the fetus, and acts on maternal genes to limit excessive transfer. There are many examples that support the idea that there has been an evolutionary tug-of-war between maternal and fetal genomes, resulting in an evolutionary escalation (Box 1). With regards to the evolution of SD, two hypotheses have been put forward, both of which are based on the idea that conflict has driven the evolution of this maternal response.
Hypothesis 1: SD evolved for early protection against invasive fetal tissues
It has been argued that the decidualization reaction, which occurs in response to blastocyst implantation in most mammals, evolved in response to increased invasiveness of fetal tissues in some lineages ([3,21]; see Box 1). In line with this idea, SD may have evolved for the same reasons as the decidualization reaction itself, to protect the mother from an increasingly aggressive fetus. In the case of menstruating species, however, which exhibit some of the most invasive fetal tissues (e.g. in apes and humans; see Table 2), the decidualization reaction initiates in advance of the invasion, providing earlier and probably more effective protection in case of pregnancy (see [3]). In the primates, if one assumes that the evolution of menstruation/SD evolved in the stem lineage of anthropoid primates (i.e. the line leading to all monkeys and apes), this hypothesis provides a good explanation for how this might have occurred. Although tarsiers have invasive placentas, they develop differently from those of the anthropoid primates and are less invasive [22]. Thus, as the ancestral anthropoid fetus became more invasive, the mother may have responded with the differentiation of her endometrium in advance of the invasion, without any signal from the fetus. Moreover, as the primate fetus became even more aggressive over evolutionary time, with the evolution of interstitial implantation in the apes, the mother responded accordingly, with a more extensive and pronounced decidual reaction during the luteal phase of the menstrual cycle, as is evident in humans and chimpanzees [17].
The menstruating bats and the elephant shrew also have invasive placentation ([14,18,23]; Table 2). Thus, it is plausible that SD also evolved in these groups in response to increasingly aggressive fetal tissues, but a detailed phylogenetic analysis of placental invasiveness in sister species would be required to test this idea.
Hypothesis 2: SD evolved for embryo selection
A more recent idea, with experimental support, argues that SD evolved to allow the mother to sense embryo quality upon implantation. Teklenburg et al. [24] used a human co-culture model to study the interaction between decidualizing ESCs and blastocysts and found that ESCs trigger a strong response against impaired embryos but only upon differentiation into decidual cells. These authors argue that SD evolved to compensate for the high rate of chromosomal abnormalities in human embryos, allowing the mother to limit her investment in bad embryos. In support of this hypothesis, the same group showed that women with impaired decidualization responses are not able to sense embryo quality, evidenced by increased fecundity but also recurrent pregnancy failure [25].
To further test this idea, it would be necessary to study the prevalence of chromosomal abnormalities in other species with SD/menstruation, as SD is not unique to humans as discussed above. There is some evidence that rhesus macaques have similar rates to humans of chromosomal instability in preimplantation embryos [26]. It is also known that fertilization involving ageing gametes, which results in developmentally impaired embryos, is a crucial problem in humans and any species with extended copulation during the reproductive cycle (see [27]) Most mammals only copulate in a short window around the time of ovulation of are induced to ovulate after copulation. Interestingly, the menstruating primates, at least one of the menstruating bats, and the elephant shrew are somewhat unique in having extended copulation (Table 2). Thus, although it is still unclear why extended copulation evolved (see [27]), it is possible that SD allows the mother to limit maternal investment in impaired embryos, which may occur at higher frequency in these species because of extended copulation.
Although both of explanation for the evolutionary significance of SD – protection from fetal invasiveness and embryo selection – require further testing, it is fairly clear that some sort of conflict at the maternal-fetal interface has driven the evolution of SD in menstruating species. What is not yet clear is how SD evolved. Below we present a novel hypothesis on the molecular mechanisms involved in the evolution of SD.
HOW DID SPONTANEOUS DECIDUALIZATION EVOVLE?
Cyclic adenosine monophosphate (cAMP) signaling pathway is activated during spontaneous and fetus induced decidualization
In non-menstruating species with invasive placentation, signals from both the mother (progesterone) and the implanting fetus (mechanical stimulation and cytokines) are required for decidualization. A scratch to the uterus is enough to initiate decidualization in the progesterone-primed rodent uterus, but fetus-derived cytokines are also suspected to be involved in decidualization (see [28,29]). While the mechanisms are not fully understood, there are multiple signaling pathways turned on in the uterus during decidualization induced by the fetus, including the BMP2, WNT, JAK/STAT, and cAMP dependent pathways [30–35]. While the mechanisms of SD are even less understood, we do know that only signals from the mother, i.e. progesterone, is required: human ESCs will begin differentiation in vitro after a week of progesterone stimulation [19]. As progesterone does not cause an immediate decidualization reaction, in vivo or in vitro, there must be intermediate or other signals involves. It is known, e.g., that the second messenger cAMP is essential for inducing and maintaining differentiation of human and rodent ESCs in vitro [36,37]. cAMP, which is generated upon ligand binding to a G-protein coupled receptor (GPCR), is elevated in human endometrial tissue from the secretory relative to proliferative phase of the menstrual cycle [38]. In vitro experiments show that cAMP is a much more potent differentiation agent of ESCs than progesterone, and when a protein kinase A (PKA) inhibitor that blocks the effects of cAMP is applied to human ESCs exposed to progesterone in culture, their differentiation is dramatically inhibited [39].
Environmentally induced phenotypes like decidualization can be internally stabilized by genetic assimilation
One appealing hypothesis on how SD evolved is that ancestral species evolved mechanisms that could substitute for the fetal signals/stimulation required for decidualization in species that lack SD. Under this scenario, the ancestral anthropoid primate, e.g., evolved the ability to activate some or all of the same signaling pathways turned on by the fetus, without any fetus required. This hypothesis, where an environmentally induced phenotype (i.e. decidualization induced by the implanting fetus) becomes genetically stabilized (i.e. SD, where no fetus is required), is a well-documented phenomenon termed “genetic assimilation”, of which there are multiple examples in nature and in the laboratory (see Box 2). These examples are used to argue that genetic assimilation is important in the evolution of novel traits (see [40,41]); in fact, experiments in the laboratory and computer modeling have shown that genetic assimilation can result in rapid changes in phenotype [42].
BOX 2. Genetic assimilation and the evolution of novel traits.
The concept of genetic assimilation describes how a novel trait can evolve by genetic stabilization of a phenotype that was previously induced by an environmental stimulus. If having the internally induced trait confers greater fitness to individuals than the trait requiring the outside stimulus, the internally induced trait is selected for in the population (see [40,63] for more detail). Below are some examples of genetic assimilation:
Bithorax phenotype in Drosophila: A classic example of genetic assimilation involves the ether-induced bithorax mutation in fruit flies. Waddington [64] exposed Drosophila embryos to ether in the laboratory, which induced the bithorax phenotype in adult flies (four wings instead of two). After many generations of ether exposure and artificial selection for the bithorax phenotype, the selected flies had the mutant phenotype even when they were not exposed to ether. Hence, the environmentally induced phenotype (bithorax by ether) was genetically stabilized (bithorax with no ether required). Later experiments showed that the Ultrabithorax gene was involved in genetic assimilation of the bithorax phenotype [65].
Sex determination in turtles: Gonadal sex determination in turtles is environmentally controlled: in general, male turtles are produced at low temperatures and females at higher temperatures. In some species of turtles, however, sex has evolved from being environmentally to genetically determined. While it is unclear what the selective pressures were that caused these shifts to genetically determined sex, shorter lifespans and warmer climates have been suggested. Whatever the ultimate cause, it is clear that some species of turtles have genetically assimilated a process that was previously controlled by the environment ([66]; see also discussion in [63]).
Embryonic stem length and Hsp90 in Arabidopsis: Hsp90, a protein found in a variety of multicellular eukaryotes, chaperones other proteins and keeps them in their proper structure. Hence, Hsp90 buffers the effects of mutations in these other proteins. Experiments in many model systems have shown that during periods of environmental stress, or when the amount of Hsp90 is manipulated experimentally, cryptic variation becomes phenotypically exposed. For example, inhibition of Hsp90 in Arabidopsis can lead to short embryonic stems [67,68]. Shorter stems can then be selected for and genetically assimilated, becoming independent of the amount of Hsp90.
It is not difficult to envisage how SD evolved with the phenomenon of genetic assimilation in mind. Individuals who were able to genetically assimilate the decidualization reaction, i.e. those that evolved SD, experienced greater fitness benefits than those requiring the signal from the fetus as their uterus was prepared before the arrival of an invasive conceptus. These benefits much have been greater than the fitness costs to the female due to menstruation, which may include resource loss and temporary incapacitation. Thus, menstruation is likely a deleterious pleiotropic effect of the beneficial trait, SD. The benefits of SD probably only exceed the costs of menstruation for mothers under certain selective pressures, such as those in conflict with a very aggressive fetus or those with a high incidence of impaired embryos (see above). This cost/benefit balance likely explains why it evolved only in some groups and not others.
SD evolved by genetic assimilation of the cAMP signaling pathway originally activated by the fetus
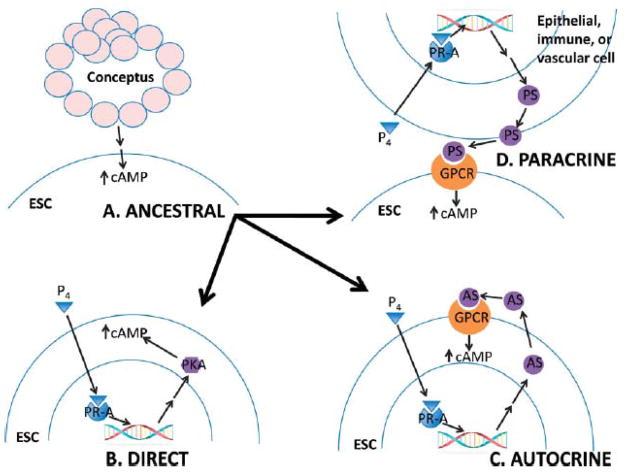
With the various signaling pathways already established in species that undergo decidualization in response to the fetus, we can infer three principal ways how SD may have evolved at the mechanistic level. From the evidence reviewed above, it is clear that SD in humans is the result of two converging signaling events: one mediated through progesterone and the nuclear receptor progesterone receptor A isoform (PR-A), and the other through cAMP-dependent PKA signaling. The progesterone signal is and always was under the control of the maternal organism. On the other hand it is tempting to speculate that the second, cAMP dependent, pathway is signaling pathway activated by the fetus in non-menstruating species, and was taken over by maternal signals in species with SD. It is clear that PKA and cAMP-stimulating agents are unpregulated at the implantation site and necessary for decidualization in the number of placental mammals, including rodents, nonhuman primates, and hoofed animals [35,43–47]. Hence, the evolution of SD might have proceeded via maternal activation of the cAMP signaling pathway that ancestrally was activated by the fetus. Explaining the evolution of SD then boils down to the question of how maternal signal(s) gained access to signaling pathways(s) that were ancestrally activated by the fetus. We propose three not mutually exclusive mechanisms that explain how this could have evolved: a direct, and autocrine, and a paracrine mechanism (Fig. 3).
Figure 3.
Diagram of proposed mechanisms (B, direct; C, autocrine; D, paracrine) to explain how maternal signal(s) gained access to signaling pathway(s) that were ancestrally activated by the fetus (A). ESC, endometrial stromal cell; cAMP, cyclic adenosine monophosphate; P4, progesterone; PKA, protein pathway; GPCR, G-protein coupled receptor; AS, autocrine signal; PS, paracrine signal.
Genetic assimilation of the cAMP pathway: Direct mechanism
A direct mechanism would involve a gene upstream in the PKA signaling pathway evolving progesterone responsiveness in ESSCs of species with SD (Fig. 3B). As mentioned above, progesterone exposure of human ESCs leads to an accumulation of cAMP [39]. cAMP levels in cells are controlled not only by the activity of GPCRs, but also by phosphodiesterases that break down cAMP in the cell. Thus, one possibility, e.g., is that genes coding for phosphodiesterases or genes whose proteins repress phosphodiesterases evolved responsiveness to liganded progesterone/PR-A. It is also known that G-proteins can be activated independently from GPCR-activation, by proteins that have been termed receptor-independent activators of G-protein signaling (AGS proteins; [48]). Thus, it is possible that a gene coding for an AGS protein was brought under transcriptional control of PR-A in ESCs, leading to G-protein activation and increased intracellular cAMP concentrations in response to progesterone stimulation.
Genetic assimilation of the cAMP pathway: Autocrine mechanism
An autocrine mechanism would be one in which the production of a signaling molecule that is able to activate the cAMP-dependent PKA pathway is brought under control of progesterone/PR-A in ESCs (Fig. 3C). For example, a signaling molecule known to be a potent differentiation agent of mammalian ESC is relaxin (see [36,49]). When the membrane-bound relaxin receptor is activated by ligand, it inhibits a phosphodiesterase; thus, relaxin binding leads to an accumulation of cAMP. It has been shown that relaxin is present in the non-pregnant human endometrium in vivo [50], that it is produced by human ESCs cultured with MPA [51], and that progesterone regulates relaxin by a response element in the untranslated region (5′UTR) in a human placental cell line [52]. Thus, on scenario is that relaxin evolved progesterone-responsiveness in ESCs of ancestral lineages of species with SD, and acts as an autocrine signal activating the cAMP pathway in ESCs.
Genetic assimilation of the cAMP pathway: Paracrine mechanism
Decidualization is a reaction that is not limited to ESCs but includes immune cells such as natural killer cells and macrophages, luminal and glandular epithelial cells, and cells comprising the vasculature. Thus, a third mechanism by which maternal signal(s) might have gained access to fetally-induced signaling pathways would be the production of a signaling molecule in response to progesterone in one of these other cell types, that in turn activates the cAMP pathway in ESCs (Fig. 3D). For example, prostaglandins are signaling molecules that are ligands for GPCRs and known to be strong differentiation stimuli for human and rodent ESCs [34]. The enzymes involved in the synthesis of prostaglandins are produced in primate endometrial epithelial cells and in human perivascular cells during the luteal phase of the menstrual cycle, which is where SD initiates in primates. [53,54].
CONCLUSIONS AND PROSPECTS
As others have suggested, we argue here that menstruation occurs as a mechanistic consequence of SD, which is evidenced by the correlation between SD and menstruation across species and by experiments showing that differentiated ESCs are committed to apoptosis upon progesterone withdrawal. We think SD evolved in the higher primates, some bats, and the elephant shrew because of conflict between mother and fetus, and we discuss two possible selective forces that may have been involved: invasive embryos, and a high incidence of impaired embryos. We argue that SD evolved by genetic assimilation of the decidualization signal originally sent by the fetus. We suggest three classes of mechanisms by which this could have occurred: direct, autocrine, and paracrine mechanisms. These mechanistic models make obvious predicitons and are testable in the lab. For example, the direct and autocrine models can be tested by comparison of gene function in ESCs from species with spontaneous and fetally-induced decidualization. If either of these models were correct, we would expect an upregulation of cAMP-stimulating agents in response to progesterone in menstruating species like humans, but not in non-menstruating species such as the mouse.
Results from experiments like those described above will elucidate the evolutionary pathway from induced to spontaneous decidualization, allowing us to answer long-unanswered questions about the evolutionary significance of menstruation. In addition, they will provide mechanistic insights that might be useful in the treatment of common reproductive disorders such as endometriosis, endometrial cancer, preeclampsia, and recurrent pregnancy loss. These disorders involved dysfunctional endometrial responses during the menstrual cycle and pregnancy. Thus, clarifying mechanisms of the normal endometrial response to maternal hormones, i.e. SD, will facilitate identification of genes with abnormal function in women with these disorders. An analysis of how SD came about in evolution can aid in identifying these critical molecular mechanisms.
Acknowledgments
Research in the Wagner lab on this topic was done in part under the NIH subcontract N01-HD-2-3342 and the John Templeton Foundation grant # 12793. This research was supported in part by the Intramural Research Division of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Financial support by the Yale University Science Development Fund is gratefully acknowledged.
ABBREVIATIONS
- cAMP
cyclic adenosine monophosphate
- ESC
endometrial stromal cell
- GPCR
G-protein coupled receptor
- MPA
medroxyprogesterone acetate
- PKA
protein kinase A
- PR-A
progesterone receptor A isoform
- SD
spontaneous decidualization
- 5′UTR
five prime untranslated region
References
- 1.Maybin JA, Critchley HOD. Progesterone: a pivotal hormone at menstruation. In: Guller S, Bulletti C, DeZiegler D, editors. Reproductive Science. Oxford: Blackwell Science Publ; 2011. pp. 88–97. [DOI] [PubMed] [Google Scholar]
- 2.Martin RD. The evolution of human reproduction: a primatological perspective. Yearb Phys Anthropol. 2007;50:59–84. doi: 10.1002/ajpa.20734. [DOI] [PubMed] [Google Scholar]
- 3.Finn CA. Menstruation: a nonadaptive consequence of uterine evolution. Q Rev Biol. 1998;73:163–73. doi: 10.1086/420183. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey EW. The reproductive cycle of New World monkeys. Am J Anat. 1939;64:381–405. [Google Scholar]
- 5.Castella H, McCombs HL. Reproductive cycle of new world monkey -gynecologic problems in a breeding colony. Fertil Steril. 1968;19:213. doi: 10.1016/s0015-0282(16)36607-9. [DOI] [PubMed] [Google Scholar]
- 6.Campbell CJ, Shideler SE, Todd HE, Lasley BL. Fecal analysis of ovarian cycles in female black-handed spider monkeys (Ateles geoffroyi) Am J Primatol. 2001;54:79–89. doi: 10.1002/ajp.1014. [DOI] [PubMed] [Google Scholar]
- 7.Wright EM, Bush DE. Reproductive cycle of capuchin (Cebus apella) Lab Anim Sci. 1977;27:651–4. [PubMed] [Google Scholar]
- 8.Catchpole HR, Fulton JF. The oestrus cycle in Tarsius: observations on a captive pair. J Mammal. 1943;24:90–3. [Google Scholar]
- 9.Wright PC, Izard MK, Simons EL. Reproductive-cycles in Tarsius bancanus. Am J Primatol. 1986;11:207–15. doi: 10.1002/ajp.1350110302. [DOI] [PubMed] [Google Scholar]
- 10.Hill WCO, Porter A, Southwick MD. The natural history, endoparasites and pseudo-parasites of the tarsiers (Tarsius carbonarius) recently living in the societys menagerie. Proc Zool Soc Lond. 1952;122:79. [Google Scholar]
- 11.Van Herwerden MA. Some remarks on the polyoestrus of Primates. Anat Rec. 1925;30:221–3. [Google Scholar]
- 12.Xu XB, He B, Wang JD. Menstrual-like changes in mice are provoked through the pharmacologic withdrawal of progesterone using mifepristone following induction of decidualization. Hum Reprod. 2007;22:3184–91. doi: 10.1093/humrep/dem312. [DOI] [PubMed] [Google Scholar]
- 13.Rasweiler J, Badwaik N. Anatomy and physiology of the female reproductive tract. In: Crichton EG, Krutzsch PH, editors. Reproductive Biology of Bats. London: Academic Press; 2000. [Google Scholar]
- 14.van der Horst C, Gillman J. The menstrual cycle in Elephantulus. S Afr J Med Sci. 1941;6:27–42. [Google Scholar]
- 15.Wooding P, Burton G. Comparative Placentation: Structures, Functions and Evolution. Berlin: Springer-Verlag; 2008. p. 301. [Google Scholar]
- 16.Esadeg S, He H, Pijnenborg R, Van Leuven F, et al. Alpha-2 macroglobulin controls trophoblast positioning in mouse implantation sites. Placenta. 2003;24:912–21. doi: 10.1016/s0143-4004(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 17.Dollar JR, Graham CE, Reyes FI. Postovulatory predecidual development in the baboon, chimpanzee, and human. Am J Primatol. 1982;3:307–13. doi: 10.1002/ajp.1350030130. [DOI] [PubMed] [Google Scholar]
- 18.Rasweiler JJ. Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater. Am J Anat. 1991;191:1–22. doi: 10.1002/aja.1001910102. [DOI] [PubMed] [Google Scholar]
- 19.Brosens JJ, Gellersen B. Death or survival – progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–98. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- 20.Labied S, Kajihara T, Madureira PA, Fusi L, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. doi: 10.1210/me.2005-0275. [DOI] [PubMed] [Google Scholar]
- 21.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol. 1993;68:495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- 22.Carter AM. Animal models of human placentation – A review. Placenta. 2007;28:S41–7. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Carter AM, Mess A. Evolution of the placenta and associated reproductive characters in bats. J Exp Zool B. 2008;310B:428–49. doi: 10.1002/jez.b.21216. [DOI] [PubMed] [Google Scholar]
- 24.Teklenburg G, Salker M, Molokhia M, Lavery S, et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5:e10258. doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salker M, Teklenburg G, Molokhia M, Lavery S, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupont C, Froenicke L, Lyons LA, Bavister BD, et al. Chromosomal instability in rhesus macaque preimplantation embryos. Fertil Steril. 2009;91:1230–7. doi: 10.1016/j.fertnstert.2008.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin RD. Human reproduction: a comparative background for medical hypotheses. J Reprod Immunol. 2003;59:111–35. doi: 10.1016/s0165-0378(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 28.Herington JL, Bany BM. Do molecular signals from the conceptus influence endometrium decidualization in rodents? J Exp Zool B. 2009;312B:797–816. doi: 10.1002/jez.b.21308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. IJDB. 2010;54:295–302. doi: 10.1387/ijdb.082829pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franco HL, Dai D, Lee KY, Rubel CA, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–87. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee P, Sapru K, Strakova Z, Fazleabas AT. Chorionic gonadotropin regulates prostaglandin E synthase via a phosphatidylinositol 3-kinase-extracellular regulatory kinase pathway in a human endometrial epithelial cell line: implications for endometrial responses for embryo implantation. Endocrinology. 2009;150:4326–37. doi: 10.1210/en.2009-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menkhorst E, Salamonsen L, Robb L, Dimitriadis E. IL11 antagonist inhibits uterine stromal differentiation, causing pregnancy failure in mice. Biol Reprod. 2009;80:920–7. doi: 10.1095/biolreprod.108.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55:795–810. doi: 10.1507/endocrj.k08e-067. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134:635–43. doi: 10.1530/REP-07-0328. [DOI] [PubMed] [Google Scholar]
- 35.Rosario GX, Katkam RR, Nimbkar-Joshi S, Modi DN, et al. Expression of endometrial protein kinase A during early pregnancy in bonnet monkeys (Macaca radiata) Biol Reprod. 2009;81:1172–81. doi: 10.1095/biolreprod.109.077339. [DOI] [PubMed] [Google Scholar]
- 36.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–72. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K, Yamauchi N, Watanabe R, Oozono S, et al. In vitro decidualization of rat endometrial stromal cells. Cell Tissue Res. 2009;335:575–83. doi: 10.1007/s00441-008-0734-1. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka N, Miyazaki K, Tashiro H, Mizutani H, et al. Changes in adenylyl cyclase activity in human endometrium during the menstrual-cycle and in human decidua during pregnancy. J Reprod Fertil. 1993;98:33–9. doi: 10.1530/jrf.0.0980033. [DOI] [PubMed] [Google Scholar]
- 39.Brar AK, Frank GR, Kessler CA, Cedars MI, et al. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6:301–7. doi: 10.1007/BF02820507. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert SF, Epel D. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sunderland, MA: Sinauer; 2009. [Google Scholar]
- 41.Lande R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J Evol Biol. 2009;22:1435–46. doi: 10.1111/j.1420-9101.2009.01754.x. [DOI] [PubMed] [Google Scholar]
- 42.Behera N, Nanjundiah V. Phenotypic plasticity can potentiate rapid evolutionary change. J Theor Biol. 2004;226:177–84. doi: 10.1016/j.jtbi.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Kim JJ, Wang J, Bambra C, Das SK, et al. Expression of cyclooxygenase-1 and -2 in the Baboon endometrium during the menstrual cycle and pregnancy. Endocrinology. 1999;140:2672–8. doi: 10.1210/endo.140.6.6716. [DOI] [PubMed] [Google Scholar]
- 44.St-Louis I, Singh M, Brasseur K, Leblanc V, et al. Expression of COX-1 and COX-2 in the endometrium of cyclic, pregnant and in a model of pseudopregnant rats and their regulation by sex steroids. Reprod Biol Endocrinol. 2010;8:12. doi: 10.1186/1477-7827-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pakrasi PL, Jain AK. Cyclooxygenase-2 derived PGE2 and PG12 play an important role via EP2 and PPAR delta receptors in early steps of oil induced decidualization in mice. Placenta. 2008;29:523–30. doi: 10.1016/j.placenta.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Cheng JG, Stewart CL. Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol Reprod. 2003;68:401–4. doi: 10.1095/biolreprod.102.009589. [DOI] [PubMed] [Google Scholar]
- 47.Charpigny G, Reinaud P, Tamby JP, Creminon C, et al. Cyclooxygenase-2 unlike cyclooxygenase-1 is highly expressed in ovine embryos during the implantation period. Biol Reprod. 1997;57:1032–40. doi: 10.1095/biolreprod57.5.1032. [DOI] [PubMed] [Google Scholar]
- 48.Blumer JB, Smrcka AV, Lanier SM. Mechanistic pathways and biological roles for receptor-independent activators of Gprotein signaling. Pharmacol Ther. 2007;113:488–506. doi: 10.1016/j.pharmthera.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parry LJ, Vodstrcil LA. Relaxin and Related Peptides. Berlin: Springer-Verlag Berlin; 2007. Relaxin Physiology in the Female Reproductive Tract during Pregnancy; pp. 34–48. [DOI] [PubMed] [Google Scholar]
- 50.Ykijarvinen H, Wahlstrom T, Seppala M. Human-endometrium contains relaxin that is progesterone-dependent. Acta Obstet Gynecol Scand. 1985;64:663–5. doi: 10.3109/00016348509158210. [DOI] [PubMed] [Google Scholar]
- 51.Palejwala S, Tseng L, Wojtczuk A, Weiss G, et al. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod. 2002;66:1743–8. doi: 10.1095/biolreprod66.6.1743. [DOI] [PubMed] [Google Scholar]
- 52.Garibay-Tupas JL, Okazaki KJ, Tashima LS, Yamamoto S, et al. Regulation of the human relaxin genes H1 and H2 by steroid hormones. Mol Cell Endocrinol. 2004;219:115–25. doi: 10.1016/j.mce.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Marions L, Danielsson KG. Expression of cyclo-oxygenase in human endometrium during the implantation period. Mol Hum Reprod. 1999;5:961–5. doi: 10.1093/molehr/5.10.961. [DOI] [PubMed] [Google Scholar]
- 54.Stavreus-Evers A, Koraen L, Scott JE, Zhang P, et al. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A(2) in the luteal phase human endometrium and ovary. Fertil Steril. 2005;83:156–62. doi: 10.1016/j.fertnstert.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 55.Maddison W. Phylogenetic interpretations of character evolution using the computer- program MACCLADE. J Gen Physiol. 1993;102:A9–10. [Google Scholar]
- 56.Profet M. Menstruation as a defense against pathogens transported by sperm. Q Rev Biol. 1993;68:335–86. doi: 10.1086/418170. [DOI] [PubMed] [Google Scholar]
- 57.Strassmann BI. The evolution of endometrial cycles and menstruation. Q Rev Biol. 1996;71:181–220. doi: 10.1086/419369. [DOI] [PubMed] [Google Scholar]
- 58.Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, et al. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstetrics Gynecol. 2009;200:615.e1–6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 59.von Rango U, Krusche CA, Kertschanska S, Alfer J, et al. Apoptosis of extravillous trophoblast cells limits the trophoblast invasion in uterine but not in tubal pregnancy during first trimester. Placenta. 2003;24:929–40. doi: 10.1016/s0143-4004(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 60.von Rango U, Classen-Linke I, Kertschanska S, Kemp B, et al. Effects of trophoblast invasion on the distribution of leukocytes in uterine and tubal implantation sites. Fertil Steril. 2001;76:116–24. doi: 10.1016/s0015-0282(01)01859-3. [DOI] [PubMed] [Google Scholar]
- 61.Floridon C, Nielsen O, Holund B, Sweep F, et al. Does plasminogen activator inhibitor-1 (PAI-1) control trophoblast invasion? A study of fetal and maternal tissue in intrauterine, tubal and molar pregnancies. Placenta. 2000;21:754–62. doi: 10.1053/plac.2000.0573. [DOI] [PubMed] [Google Scholar]
- 62.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 63.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]
- 64.Waddington CH. Genetic assimilation of the bithorax phenotype. Evolution. 1956;10:1–13. [Google Scholar]
- 65.Gibson G, Hogness DS. Effect of polymorphism in the Drosophila regulatory gene Ultrabithorax on homeotic stability. Science. 1996;271:200–3. doi: 10.1126/science.271.5246.200. [DOI] [PubMed] [Google Scholar]
- 66.Janzen FJ, Paukstis GL. Environmental sex determination in reptiles – ecology, evolution, and experimental-design. Q Rev Biol. 1991;66:149–79. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- 67.Sangster TA, Salathia N, Lee HN, Watanabe E, et al. HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105:2969–74. doi: 10.1073/pnas.0712210105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sangster TA, Salathia N, Undurraga S, Milo R, et al. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci. 2008;105:2963–8. doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]