Abstract
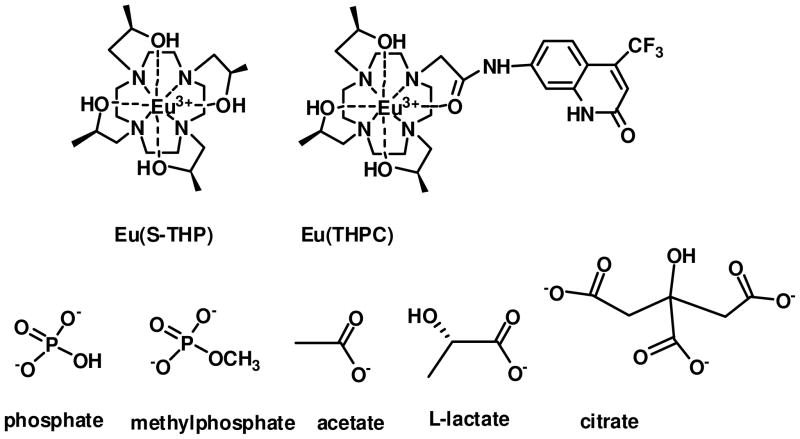
The Eu(III) complex of (1S,4S,7S,10S)-1,4,7,10-tetrakis(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane (S-THP) is studied as a sensor for biologically relevant anions. Anion interactions produce changes in the luminescence emission spectrum of the Eu(III) complex, in the 1H NMR spectrum, and correspondingly, in the PARACEST spectrum of the complex (PARACEST = paramagnetic chemical exchange saturation transfer). Direct excitation spectroscopy and luminescence lifetime studies of Eu(S-THP) give information about the speciation and nature of anion interactions including carbonate, acetate, lactate, citrate, phosphate and methylphosphate at pH 7.2. Data is consistent with the formation of both innersphere and outersphere complexes of Eu(S-THP) with acetate, lactate and carbonate. These anions have weak dissociation constants that range from 19–38 mM. Citrate binding to Eu(S-THP) is predominantly innersphere with a dissociation constant of 17 μM. Luminescence emission peak changes upon addition of anion to Eu(S-THP) show that there are two distinct binding events for phosphate and methylphosphate with dissociation constants of 0.3 mM and 3.0 mM for phosphate and 0.6 mM and 9.8 mM for methyl phosphate. Eu(THPC) contains an appended carbostyril derivative as an antenna to sensitize Eu(III) luminescence. Eu(THPC) binds phosphate and citrate with dissociation constants that are 10-fold less than that of the Eu(S-THP) parent, suggesting that functionalization through a pendent group disrupts the anion binding site. Eu(S-THP) functions as an anion responsive PARACEST agent through exchange of the alcohol protons with bulk water. The alcohol proton resonances of Eu(S-THP) shift downfield in the presence of acetate, lactate, citrate and methylphosphate, giving rise to distinct PARACEST peaks. In contrast, phosphate binds to Eu(S-THP) to suppress the PARACEST alcohol OH peak and carbonate does not markedly change the alcohol peak at 5 mM Eu(S-THP), 15 mM carbonate at pH 6.5 or 7.2. This work shows that the Eu(S-THP) complex has unique selectivity toward binding of biologically relevant anions and that anion binding results in changes in both the luminescence and PARACEST spectra of the complex.
Introduction
The design of compounds that facilitate multi-modal imaging is an active area of research.1 Such compounds that have multiple signaling components can be used to capitalize on the different types of information gained through each imaging modality. For example, one prevalent type of multimodal probe combines radiolabeled compounds for positron emission tomography (PET) with MRI contrast agents.2 Another well-developed dual modality probe combines agents that are used for optical and magnetic resonance imaging.3,4 Such probes capitalize on the high resolution and deep penetration of MRI and the high sensitivity but lower resolution of optical imaging. The popularity of this approach is demonstrated by the numerous examples of probes that contain a Gd(III) complex as a MRI contrast agent and an organic fluorophore as an optical probe. Another common approach for dual optical/MRI probes is to use two distinct Ln(III) ions, one with useful luminescence properties and the other with magnetic properties that are favorable for MRI contrast applications. For example, Gd(III) is used as a T1 MRI contrast agent in combination with luminescent lanthanide ions including Tb(III), Eu(III), or Nd(III).3,5 This approach necessitates linking two different lanthanide(III) complexes together to form a heterodinuclear complex or, alternately, employing two complexes containing different Ln(III) ions and assuming that the two complexes have similar tissue distribution for imaging.
An alternate strategy for dual probe design is to use a single lanthanide ion that has both favorable MRI contrast and luminescence properties. For example, Nd(III), Eu(III) and Yb(III) are being studied as MRI contrast agents6–10 and as optical probes that emit in the visible or infrared.11–13 These three Ln(III) ions function as PARACEST (paramagnetic chemical exchange saturation transfer) MRI contrast agents. PARACEST agents capitalize on the Ln(III) paramagnetic induced shift of ligand protons that are exchangeable with water. 14,15 Application of a long frequency-selective pulse at the frequency of the exchangeable proton results in partial saturation of those spins and, through exchange, decreases the intensity of the water proton resonance. Here we show that the favorable PARACEST and luminescence properties of a Eu(III) complex can be combined towards the development of a dual function probe.
An important goal for the design of both PARACEST agents and optical probes is to create responsive agents with signals that are modulated by environment. Important examples include lanthanide complexes that act as pH-responsive PARACEST agents.8,16 Enzyme responsive PARACEST agents are also of interest.17,18 Other examples include lanthanide(III) complexes that modulate contrast as a function of temperature to probe tissue temperature changes.19 Finally, binding of small molecule metabolites to lanthanide ion complexes has been shown to modulate both PARACEST20–23,24 and luminescence spectra.25–28
A major advantage for the development of responsive lanthanide MRI and optical probes is the incorporation of ratiometric imaging. Eu(III) complexes are used as ratiometric optical sensors by monitoring two different emission bands, most commonly the 5D0 → 7F1 (ΔJ = 1) and 5D0→7F2 (ΔJ = 2) bands.13,28 The magnetic dipole induced ΔJ = 1 transition is relatively insensitive to changes in coordination whereas the ΔJ = 2 and ΔJ = 4 transitions are hypersensitive and generally change substantially upon formation of a new complex. A ratiometric factor can be designed into PARACEST agents as well. This has been accomplished by comparison of either two different classes of exchangeable protons on the same complex or by using two complexes that contain different lanthanide ions with distinct chemical shifts for ligand protons.29
The Eu(III) complex of (1S,4S,7S,10S)-1,4,7,10-tetrakis(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane (S-THP, Chart 1) is one of the first lanthanide complexes to function as a PARACEST agent in water through exchange of alcohol protons.6,30,31 The PARACEST properties of this complex are modulated by pH and by binding to phosphate mono and diesters.20 Here we show that additional biologically important anions including carbonate, acetate, lactate, citrate and phosphate affect the PARACEST properties of the Eu(III) complex and also give rise to unique luminescence spectral signatures for these anions. Interaction of these anions with Eu(S-THP) are characterized by using direct excitation Eu(III) luminescence spectroscopy under physiologically relevant conditions to show unique outersphere and innersphere binding modes. Anion binding selectivity is distinct from previously studied Eu(III) complexes with amide pendent groups, a class of compounds under development as optical sensors.28 In addition, binding of these anions to the ligand sensitized luminescent complex, Eu(THPC) is compared. This work demonstrates that complexes of a single lanthanide ion, Eu(III), show promise for the development of PARACEST contrast agents and luminescent probes that are responsive to biologically relevant anions.
Chart 1.
Experimental Section
Materials
Monomethylphosphate (MMP),32 Eu(S-THP)(H2O)(CF3SO3)3, 33 1(4-nitrobenzyl)-1,4,7,10-tetraazacyclododecane (1)34, and 7-amino-trifluoromethyl-2-(1H)-quinolinone35 were prepared as previously reported.
1-(4-nitrobenzyl)-(4S,7S, 10S)-4,7,10-tris(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane (2)
Compound 1 (0.3940 g, 0.001278 moles) was dissolved in methanol. A large excess of S-propylene oxide (0.5 ml) was added to the solution and the reaction was stirred under Ar(g) overnight. The reaction was then concentrated yielding 0.5893g (95% yield) of yellow solid. 1H NMR (400 MHz, (CD3)2S=O)): δ = 0.91 (d, 6H, CH3), 0.96 (d, 3H, CH3), 2.02–2.85 (cyclen CH2 and NCH2 pendent, 22H), 3.28 (s, 2H, N-CH2-Ar) 3.58 (3H, multiplet, CH(CH3) pendent), 4.99 (s,2H, OH), 5.07 (s, 1H, OH), 7.64 (d, J=8.4 Hz, 2H, Ar-NO2), 8.11 (d, J=8.4 Hz, 2H, Ar-NO2); 13C NMR (75.5 MHz, CD3)2S=O) δ = 21.0 (CH3) 51.4, 51.7, 52.4 (CH2, cyclen), 59.1 (CH2 pendent), 62.9 (CH2Ar), 63.3 (CH(CH3)), 123.6, 130.5, 146.8, 147.7 (Ar-NO2); ESI m/z: 482.4 M+1, 505.6 M+ Na+
(1S,4S,7S)-1,4,7-tris(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane (3)
10% Pd/C (0.476 g) in slight molar excess was added to a round bottom flask. MeOH was slowly added as Ar(g) was flushed through the system. 2 (0.50 g, 0.001037 moles) was added to the solution together with cyclohexene (8.3 ml). The mixture was heated to reflux for 2 hours. The reaction was filtered and washed with MeOH. The filtrate was evaporated to dryness in vacuo. Water (3 ml) was added to the residue and the pH adjusted to 12.5 by adding NH4OH. The reaction was extracted with hexanes (3 × 20 ml) to partially remove the 4-aminotoluene. The pH was readjusted to 12.5 and the aqueous layer was extracted with chloroform. The resulting organic layer was then concentrated. The resulting product was dissolved in chloroform and purified by silica gel chromatography and eluted with chloroform. The solvent was evaporated to yield 0.31g (88% yield) of solid. 1H NMR (500 MHz, CDCl3) 1.10 (d, J=6, 6H, CH3), 1.14 (d, J=6, 3H, CH3), 1.87–2.98 (CH2 cyclen and CH2 pendent), 3.94 (multiplet, CH(CH3 pendent), 5.23 (s, 2H, OH), 5.31 (s, 1H, OH); 13C NMR (500 MHz, CDCl3) 19.9, 20.6 (CH3) 49.3, 51.1, 52.5, (CH2 cyclen), 63.9 (CH2 pendent), 65.1 (CH(CH3)), ESI m/z: 347 M + H+, 370.2 M+ Na+.
Chloro-N-[(4-trifluromethyl-2-oxo-1,2-dihydro-7-quinolyl)ethanamide (4)
7-amino-4-trifluromethyl-2-(1H)-quinolinone (0.5g, 0.00218 moles) was dissolved in acetonitrile and a slight excess of chloroacetyl chloride (0.30 g, 0.20 ml, 0.00265 moles) was added. The reaction was stirred for 15 minutes until a precipitate formed. The reaction was filtered, the white solid was collected and dried under vacuum yielding 0.52g (78%). 1H NMR (400 MHz, (CD3)2S=O): δ = 4.27 (s, 2H, CH2), 6.81 (s, 1H, C=CH-CO), 7.36 (d, J=8.8 Hz, 1H, aryl), 7.6 (d, J=8.8 Hz, 1H, aryl), 7.9 (s, 1H, Aryl), 10.6 (s, ClCH2CONH), 12.2 (s, 1H, C=CH-CONH). 13C NMR (75.5 MHz, (CD3)2S=O)): 44, (CH2), 105.6 (Ar), 109.6 (Ar), 115.2 (Ar), 122.7 (q,1JC-F =275 Hz, CF3), 125.5 (Ar), 127.5 (Ar), 136.6 (q, 2JC-F= 30 Hz, C-CF3), 141.2 (Ar), 141.7 (Ar), 160.8 (C=O), 165.8 (C=O). ESI m/z: 305.7 M, 328.5 M + Na+.
1-[(4-trifluromethyl-2-oxo-1,2-dihydro-7-quinolyl)carbamoylmethyl]-(4S,7S,10S)-4,7,10-tris(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane (5, THPC)
Compound 3 (0.1 g, 0.000288 moles) was dissolved in DMF (5 ml). To this solution was added an equimolar amount of K2CO3 (0.039g, 0.000288 moles) and compound 4 (0.0879g, 0.000288 moles). The reaction was transferred to a capped 10 ml vial. The reaction was carried out in a conventional microwave (900 W). The microwave was adjusted to PW10 (approximately 90W). The reaction was carried out at this setting at 3 minute intervals and the reaction was monitored by using mass spectrometry. Water and chloroform were added to the reaction mixture. The organic layer was recovered and dried in vacuo yielding 0.095g (52% yield) of a yellow solid. The resulting solid was then precipitated from a mixture of ethyl acetate and hexane. The pure precipitate was isolated to give 0.021g (13 % yield). 1H NMR (400 MHz, CD3)2S=O)): 0.94 (d, 9H, CH3), 2.24–4 (19H, CH2 cyclen and CH(CH3) pendent), 4.1 (2H, s, CH2C(O)N), 6.79 (1H, s, C=CH-CO), 7.37 (J2=9.2 Hz, 1H, d, Ar), 7.62 (J2=8.8 Hz, 1H, d, Ar), 7.93 (1H, s, H3), 10.74 (1H, s, CH2CONH), 12.25 (1H, s, C=CH-CONH); 13C NMR (75.5 MHz, CDCl3) 20.8, 21.4 (CH3) 43.9(CH2 pendent), 50.3, 51.1, 52.2 (CH2, cyclen), 61.9, 62.8 (CH(CH3)), 105.5 (Ar), 109.6 (Ar), 115.2 (Ar), 120.1 (Ar), 122.6 (q,1JC-F =275 Hz, CF3), 125.5 (Ar), 136.6 (q, 2JC-F= 30Hz, C-CF3), 141.1 (Ar), 141.7 (Ar), 160.7 (C=O), 165.7 (C=O); ESI m/z: 615.2 M.
Eu(THPC)
THPC (5) (0.9 g, 0.000146 moles) was dissolved in acetonitrile and 0.9 equivalents of (Eu(CF3SO3)3) were added. The reaction was heated to 40 °C for 2 hours. The product was precipitated as a white solid upon addition of dichloromethane. ESI m/z: 765.2 M, 383.4 M/2. The complex was further characterized by using luminescence spectroscopy as described below.
CEST spectra
CEST experiments were acquired on an Inova-500 Spectrometer at room temperature, B1 = 800 Hz, with an irradiation time of 3 seconds and 1 ppm incremental steps. Measurements of the reduction of the percent water magnetization (Mz/Mo × 100) or saturation transfer (ST% = (1−Mz/Mo) × 100) had standard deviations of 1–2%. The concentration of Eu(III) complex for CEST experiments was 5.00 mM.
Luminescence Spectroscopy
Direct excitation, emission and luminescence lifetimes were obtained using a Spectra-Physics Quanta Ray PRO-270-10 Q-switched Nd:YAG pump laser (10 Hz, 60–70 mJ/pulse) and a MOPO SL as described previously. The 7F0 → 5D0 transition was obtained by directly exciting the Eu(S-THP) or Eu(THPC) complexes from 578 to 581 nm, while monitoring the emissions using a band pass filter (628 ± 27 nm). Peak fit analysis of the excitation spectra was carried out by using the program Peak Fit v4.12 (SeaSolve Software, Inc.). Emission spectra were obtained by direct excitation 7F0 → 5D0 of the Eu(III) complexes. The emission is dispersed by using 1/8-m f/3.3 monochromator (1 nm resolution) (CVI Laser Corporation CM110), and detected by a thermoelectrically-cooled photomultiplier tube (Hamamatsu, model R928). Time-resolved luminescence measurements were collected by using a digital Tektronix TDS 3034B oscilloscope. Three intensity decay measurements were collected, fit to a single exponential decay model, and averaged using the GraphPad Prism 4 v4.03 Software (GraphPad Software, Inc.). The single exponential decay was determined to be the best fit by the linearity of the log intensity versus time and the symmetric distribution of the residuals about zero. Luminescence lifetime measurements were reproducible within ± 7 %. Ligand sensitized luminescence for Eu(THPC) was carried out on a Cary Eclipse fluorimeter. Excitation spectra were recorded with 0.100 ms delay time and 5 ms gate time in the excitation range of 270–320 nm with λem= 615 nm. Emission spectra were recorded for the Eu(III) complex in the range of 565–720 nm with irradiation at 330 nm. Quantum yields were determined by comparison to rhodamine 101 as a standard.36
Luminescence titrations
Luminescence titrations were carried out in solutions containing 100 mM NaCl and 20 mM HEPES buffer, pH of 7.2. The concentration of Eu(S-THP) was varied from 1.00 to 0.100 mM. The anion stock solutions were adjusted to pH 7.2 prior to addition to the Eu(III) complex to avoid pH changes upon addition of large concentrations of anion. Binding constants for all anions except MMP were obtained by fitting either excitation intensity or emission band intensities as a function of anion concentration to Equation 1 in Sigma Plot 9.01. Here LS is the concentration of the Eu(III)-anion complex, Kd is the apparent dissociation constant, M is the total concentration of the Eu(III) complex, A is the anion concentration, ns is the number of binding sites (fixed at one), xM is the mole fraction of M, xLS is the mole fraction of LS, IM is the luminescence intensity of Eu(III) macrocycle complex and ILS is the intensity of LS. Single peak and ratiometric fits of MMP were fit to equation 2 for sequential binding of two anions per Eu(III) complex. Here Kd1 and Kd2 are dissociation constants, X = anion, Y = change in luminescence intensity and Bmax1 and Bmax2 are the maximum luminescence intensities for the first and second anion binding, respectively. Alternately, HypSpec (protonic software) was used to fit binding of two anions. Effective binding constants for phosphate in the presence of competing anions were calculated by using the program HYSS.
| (1a) |
| (1b) |
| (2) |
Luminescence lifetime data was used to calculate the number of bound waters (q) by using the relationship shown in Eq. 3.37 This relationship reflects the quenching influence of O-H oscillators in water and alcohol ligands and amide N-H oscillators on luminescence lifetimes where A is a constant for Eu (A =1.20 ms−1) and α is for outersphere quenching (0.25 ms−1) and we assume that each hydroxyethyl group will contribute one O-H oscillator at 0.45 ms−1 (β) and each amide contributes 0.075 ms−1 (γ). In equation 3, A is the constant for Eu(III), and k=1/τ.
| (3a) |
| (3b) |
Results
Eu(III) macrocyclic complexes
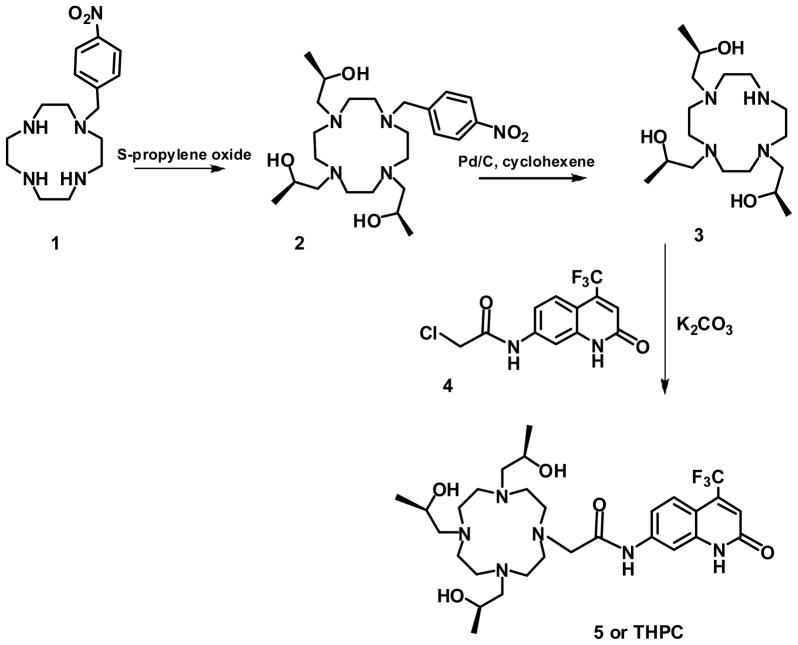
The luminescence properties of S-THP and THPC complexes of Eu(III) were studied in the presence of a series of biologically relevant anions in order to characterize their solution chemistry and to assess their potential as optical sensors (Chart 1). Eu(S-THP) was studied by using direct excitation luminescence spectroscopy because the S-THP ligand does not have an antenna for sensitizing luminescence. Eu(THPC), which contains a modified carbostyril as a luminescence sensitizing moiety for Eu(III), was studied both by direct excitation luminescence spectroscopy and by excitation of the THPC ligand. The THPC ligand was prepared as shown in scheme 1 by using a route with nitrobenzyl as a protecting group to prepare the trialkylated 1,4,7,10-tetraazacyclododecane for attachment of the carbostyril dye. Both complexes are strongly resistant to dissociation under physiologically relevant conditions.20
Scheme 1.
Luminescence spectroscopy of Eu(III) complexes
Anion binding to Eu(S-THP) and Eu(THPC) in buffered solutions at pH 7.2 was monitored through both excitation and emission luminescence spectroscopy. For excitation spectroscopy, the 7F0→ 5D0 transition of Eu(S-THP) and Eu(THPC) was monitored in order to characterize the number of species formed upon anion binding to the Eu(III) complexes. Monitoring the 7F0→ 5D0 transition is useful because both ground and excited states are non-degenerate, so that each peak corresponds to a distinct Eu(III) species,38 barring overlap of peaks. Emission spectra were recorded upon both direct 7F0→ 5D0 excitation and for, Eu(THPC) complexes, upon excitation of the carbostyril antenna. Luminescence lifetime data was collected for both complexes in order to calculate the number of bound waters (q) by using the relationship shown in Eq. 3.
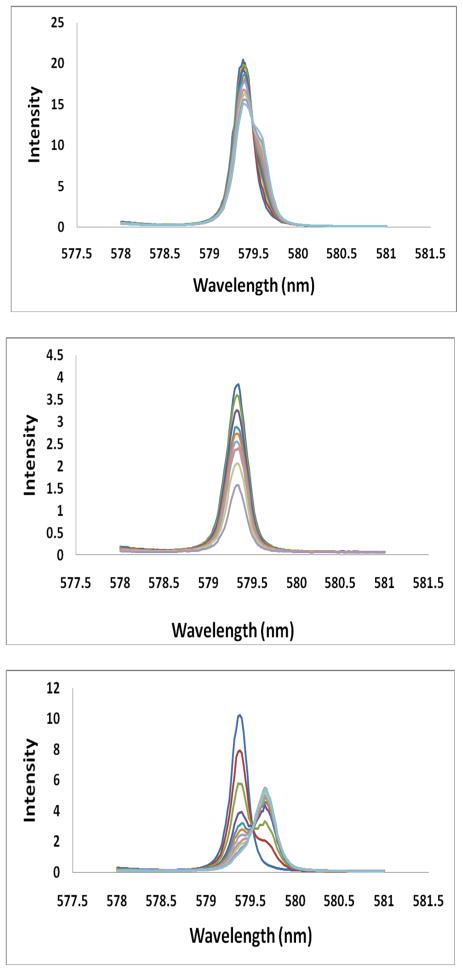
Direct excitation 7F0→ 5D0 spectra for Eu(S-THP) as a function of added anion are shown in Figure 1 and Figures S1, S2. At pH 7.2, Eu(S-THP) has a single excitation peak which has been assigned to the aquo complex [Eu(S-THP)(H2O)]3+ at 579.36 nm.34 Addition of phosphate or methylphosphate to Eu(S-THP) gives rise to new excitation peaks that are similar in frequency to that of the parent excitation peak. In contrast, addition of NaHCO3, lactate, carbonate or citrate to Eu(S-THP) results in a decrease in intensity at the 579.36 nm peak and the emergence of new red-shifted peaks at 579.57, 579.54, 579.63, 579.64 nm, respectively. For citrate, the parent peak at 579.36 nm largely disappears upon addition of excess citrate. Fitting of the excitation spectrum under conditions of fully formed Eu(S-THP)(citrate) complex shows one major peak at 579.64 nm and a minor one at 579.36 nm (Figure S2a). In contrast, acetate, lactate and carbonate give complexes with two excitation peaks, one red-shifted and one at close to the original excitation peak frequency (Figure 1, Figure S1).
Figure 1.
Eu(III) 7F0→5D0, excitation spectra (λem = 628 ± 27 nm). All solutions contain 20 mM HEPES, pH 7.2, 100 mM NaCl. Top: addition of lactate (0 – 40 mM) to 1.00 mM Eu(S-THP) gives decrease in 579.3nm peak, middle: phosphate (0 – 1.0 mM) added to 100 μM Eu(S-THP) gives decrease in 579.3 nm peak and bottom: citrate (0 – 1 mM) added to 25 μM Eu (S-THP) gives decrease in 579.3 nm peak and increase in 579.64 nm peak.
Luminescence lifetime data compiled in Table 1 are useful for making assignments of the complex composition and speciation in solution. The q values that represent the number of bound waters are calculated by using eq. 3. These data show that binding of all anions studied here decreases the apparent number of bound water molecules. The q number for the citrate complex of Eu(S-THP) is close to zero as expected for complete displacement of one bound water molecule to give an innersphere complex. The q numbers of the complexes formed upon addition of phosphate or methyl phosphate are higher than those of citrate with q numbers of 0.88 and 0.64 at a 10-fold excess of ligand or 0.41 and 0.34 at a 200-fold excess of ligand. These non-integral q numbers may be attributed, in part, to contributions from outersphere anion interactions as described below.
Table 1.
Luminescence lifetimes and number of bound waters (q) for Eu(III) complexes.
| complex | Excitation wavelength (nm) | Anion | [Anion] (mM) for lifetime | τH2O (ms) | τD2O (ms) | qe |
|---|---|---|---|---|---|---|
| Eu(S-THP) | 579.36 | Phosphatea | 1.0 | 0.271 | 0.893 | 0.64 |
| Eu(S-THP) | 579.36 | Phosphatea | 20 | 0.310 | 1.12 | 0.34 |
| Eu(S-THP) | 579.36 | MMPa | 1.5 | 0.249 | 0.808 | 0.88 |
| Eu(S-THP) | 579.36 | MMPa | 20 | 0.298 | 1.03 | 0.41 |
| Eu(S-THP) | 579.36 | Carbonateb | 40 | 0.267 | 0.856 | 0.64 |
| Eu(S-THP) | 579.36 | Acetateb | 40 | 0.280 | 0.911 | 0.50 |
| Eu(S-THP) | 579.36 | L-lactateb | 40 | 0.271 | 0.860 | 0.56 |
| Eu(S-THP) | 579.54 | Citratec | 1.0 | 0.333 | 1.10 | 0.070 |
| Eu(THPC) | 579.60 | Citrated | 0.70 | 0.323 | 1.45 | 0.76 |
| Eu(THPC) | 579.54 | Phosphated | 10 | 0.284 | 0.785 | 0.69 |
All samples contain 0.100 M NaCl, 20 mM HEPES at pH 7.2. Eu(S-THP) concentrations were: (a) 0.100 mM, (b)1.00 mM, (c) 25 μM, (d) Eu(THPC) was 25 μM. e. reported q values are ± 10% based on errors in luminescence lifetime measurements as reported in reference 21.
Acetate, lactate and carbonate complexes have still larger q values of 0.5 to 0.6. However, the luminescence decays recovered upon excitation of either of the two Eu(III) excitation peaks for the acetate, lactate or carbonate complexes give lifetimes that are equivalent within experimental error. One explanation of this observation is that there are two distinct species with different q numbers, but that the equilibrium between these two species has a rate constant for interconversion that exceeds that for de-excitation of the two species.39 This would give a luminescence lifetime that is a weighted average of the two lifetimes for the distinct species. A q number of 0.5 may arise from nearly equal concentrations of a species with a q number close to zero and a species with a q number close to one. For the carbonate case, for example, this would correspond to the presence of both innersphere (q = 0, new peak at 579.57 nm) and outersphere (q = 1, unshifted peak, 579.34 nm) complexes. Based on the assignment of the unshifted excitation peak as an outersphere complex and the shifted peak as an innersphere complex and assuming that the intensity of the excitation peak of the outersphere complex is the same as that of the parent complex,40 the percentage of outersphere complex for Eu(S-THP) can be estimated from the change in intensity of the original excitation peak in the presence of excess anion. The percentages of outersphere anion complex of Eu(S-THP) are approximately 68%, 62%, 74% and 13% for carbonate, acetate, lactate and citrate, respectively. Alternately, the two different excitation peaks for carbonate, acetate, lactate and citrate may correspond to different isomers of the bound anion complex. However, this is a less likely alternative given that all four complexes have an excitation peak that matches that of Eu(S-THP)(H2O). An innersphere carboxylate group typically produces a red-shifted 7F0→ 5D0 Eu(III) excitation peak relative to the aquo complex.41
Binding isotherms were obtained from plots of the decrease in intensity of the Eu(S-THP) excitation peak at 579.3 nm as a function of anion concentration (Figure S3). Fitting of the data to a 1:1 binding isotherm (Eq. 1) gave the dissociation constants shown in Table 2. Binding constants were also obtained from the change in emission peaks as a function of added anion as described below.
Table 2.
Dissociation constants of Eu(S-THP) and Eu(THPC) anion complexes.
| Complex | Anions | Kd (mM) emission | Kd (mM) excitation |
|---|---|---|---|
| Phosphatea | 0.3, 3.0 | 0.3 | |
| Eu(S-THP) | MMPa | 0.7, 9.8 | 1.8 |
| Carbonateb | 33 | 38 | |
| Acetateb | 14 | 33 | |
| L-lactateb | 13 | 19 | |
| Citratec | 0.016 | 0.017 | |
| Eu(THPC) | Phosphatea | - | 4.2 |
| Citratea | - | 0.21 |
Solutions contained 100 mM NaCl, 20 mM HEPES, pH 7.2. Eu(S-THP) concentrations were: (a) 0.100 mM, (b) 1.00 mM, (c) 25 μM. Eu(THPC) was 100 μM.
The 7F0→ 5D0 excitation spectrum of the complex with appended sensitizing dye, Eu(THPC), shows one major excitation peak at 579.56 nm at pH 7.2 (Figure S4). The assignment of this peak to a species containing one water molecule (Eu(THPC)(OH2)) is supported by luminescence lifetime measurements that give a q number of 1.1 (250 ms (τH2O), 758 ms (τD2O)). Addition of phosphate produces a reduction in the major Eu(III) excitation peak, consistent with overlap of the excitation peak of the phosphate bound product with that of the parent complex. The new phosphate complex has an associated q value of 0.69, similar to that observed for the phosphate complex of Eu(S-THP) at low ratios of phosphate to Eu(III). Addition of citrate results in the appearance of a new peak at 579.88 nm and retention of the original peak at 579.56 nm. The q number associated with the 579.56 nm peak is 0.76, but the q number for the species that gives rise to the new peak at 579.88 nm cannot be accurately measured because it is too close to the larger peak at 579.56 nm. The observation of two excitation peaks with one at the same wavelength as the original peak and one red-shifted in conjunction with the high q value is reminiscent of the dual outersphere/innersphere binding proposed for acetate, lactate and carbonate with Eu(S-THP).
Emission spectroscopy
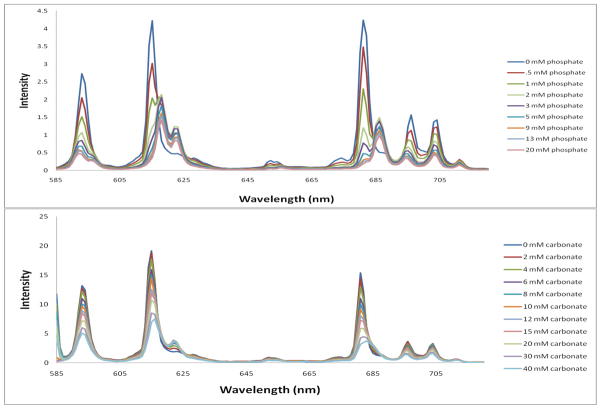
The emission spectrum of Eu(S-THP) was recorded upon binding of the biologically relevant anions shown in Scheme 1 upon direct excitation of the lanthanide ion (7F0 →5D0). Two trends were observed in the emission bands. The emission spectra of the carbonate, acetate or lactate adducts were distinctively different from those of the phosphate and phosphate ester emission spectra (Figure 2 and Figures S5–10). The ΔJ = 2 emission was split into two peaks instead of the three observed with phosphate. Also, as the intensity of the 615 nm peak decreased with increased carbonate, a new peak at 622 nm increased in intensity. The ΔJ = 4 emission peak at 681 nm decreases with an increase in carbonate concentration and a new peak arises at 685 nm. However, the new peak at 685 nm is not as pronounced as that observed upon binding with phosphate.
Figure 2.
Eu(III) emission spectra (λex at 579.36 nm) in 20 mM HEPES at pH 7.2, 100 mM NaCl upon addition of (a) phosphate with 100 μM Eu (S-THP) and (b) carbonate with 1.00 mM Eu(S-THP).
A plot of the ratio of the intensities of 622 nm/593 nm emission bands gives dissociation constants of 33, 14, 13 mM for carbonate, acetate and lactate respectively (Figure S11). The excitation peak of the citrate complex of Eu(S-THP) is well separated from that of the parent peak. Thus, excitation at the new red-shifted peak at 579.64 nm gave rise to an increase of all emission peaks as the new species increased in concentration. A plot of a single emission peak wavelength at 579.64 nm gave a binding constant of 16 μM, similar to that obtained by excitation spectroscopy.
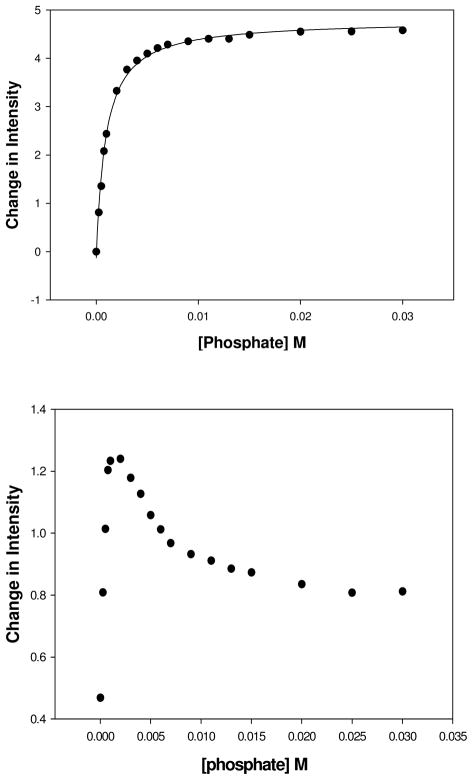
For phosphate and methylphosphate, the ΔJ = 1 emission peak decreased in intensity (Figure 2 and Figure S8–10). As the intensity of the major peak at 615 nm (ΔJ = 2) decreases with increasing concentrations of phosphate, two new peaks emerge at 618 and 622 nm. A plot of the ratio of the intensities of ΔJ=2/δJ=1 (622nm/593nm) gives data that are fit to a 1:1 binding isotherm for phosphate to give a binding constant of 0.30 mM. Examination of the two emission peaks individually suggested a more complex binding process. The ΔJ = 1 emission peak at 597 nm showed a monotonic decrease in intensity, but the ΔJ = 2 emission peak at 622 nm shows an increase in luminescence intensity up to 1 mM phosphate, followed by a decrease in intensity (Figure 3). Fitting of the data gives two binding constants of 0.30 mM and 3.0 mM respectively by fitting the binding isotherm to binding of two phosphate anions (Figure S12b). Similarly, emission binding isotherms for methylphosphate show evidence for two distinct binding events, presumably for two anions binding sequentially to the Eu(III) complex. In this case, the intensity of emission peaks in both the ΔJ = 1 and ΔJ = 2 transitions show a discontinuity as does the ratiometric plot, but to a lesser extent (Fig. S12). Fitting of the data to a 2:1 binding isotherm (eq. 2) or by using the HypSpec program gives two distinct dissociation constants of 0.70 and 9.8 mM as given in Table 2. The complexes formed in the binding processes for phosphate and methylphosphate are characterized by the number of Eu(III) bound waters at different concentrations of phosphate or phosphate ester (Table 1). For concentrations of phosphate or methylphosphate that correspond to the binding of the first anion, the q number (0.6 and 0.9, respectively) is relatively high, consistent with anion binding that does not fully displace a water molecule. At high concentrations of phosphate or methylphosphate the q number is lower (0.3 and 0.4, respectively), which is more consistent with replacement of the bound water for binding of the second anion. The interaction of phosphate and methyl phosphate with Eu(S-THP) is thus complicated. Despite the relatively high apparent hydration numbers, all four binding events must involve a change in the innersphere of the Eu(III) complex given that a change in the emission peaks reflects a perturbation in the coordination environment of the Eu(III) center.
Figure 3.
Plot of the emission intensities of 0.100 mM Eu(S-THP) in 20 mM HEPES, pH 7.2, 100 mM NaCl, (λex = 579.36 nm) with the addition phosphate. Top: intensity at 593 nm, (bottom) Intensity at 622 nm.
The distinct emission bands of the phosphate complex in comparison to the carbonate complex allowed us to monitor phosphate binding in the presence of 20 mM carbonate (Figure S10). Data from a plot of the intensity ratio of emission peaks at 618/593 nm fit to a 1:1 binding curve gives a Kd of 14 mM for phosphate which, as expected, is higher than that for the experiment done in the absence of competing anions (Figure S11b, Table 2). This value is similar to that obtained by calculation of the effective binding constant (8.0 mM) when all equilibria are taken into consideration by using HYSS software.
Ligand sensitized luminescence
The Eu(THPC) complex contains a carbostyril derivative (cs124-CF3) to enable sensitization of Eu(III) luminescence.42–44 Characterization of the ligand and complex by UV-vis spectroscopy shows that the absorbance spectrum of the carbostyril moiety does not change appreciably when bound in the Eu(THPC) complex (Figure S13). Excitation at 340 nm, the peak maximum in the UV-visible absorbance spectrum of the dye gives rise to an emission peak characteristic of the carbostyril dye at 490 nm in addition to the characteristic emission bands of Eu(III) (Figure S14). The Eu(III) emission spectrum is observed more clearly upon time-gating the luminescence. The quantum yield for Eu(III) luminescence upon excitation at 340 nm was determined to be 0.19 by comparison to rhodamine 101 as a standard.36
Anion binding to Eu(THPC) was monitored by luminescence emission spectroscopy (Figures S15–S19). Excitation of the carbostyril antenna of Eu(THPC) leads to an increase of all of the emission peaks upon addition of citrate or phosphate. Plots of the luminescence emission intensity Eu(THPC) upon excitation at 340 nm as a function of anion are fit to a simple 1:1 binding isotherm to give dissociation constants for phosphate and citrate (Table 2). These dissociation constants are approximately 10-fold weaker than those of Eu(S-THP).
In comparison, direct 7F0 →5D0 excitation of Eu(THPC) gives the same general trend of decreasing emission peak intensity of the original complex emission peaks with an increase in new ΔJ = 2 and ΔJ = 4 emission peaks, similar to the titration of Eu(S-THP) with phosphate (Figures S18–S19). This shows how the relative emission peak intensities for the different species in the titrations depend on the mode of excitation. Additional differences observed in the emission spectra of the complexes of Eu(S-THP) taken on the MOPO system are attributed in part to the higher resolution of the monochromator of the MOPO system compared to that of the fluorimeter.
PARACEST
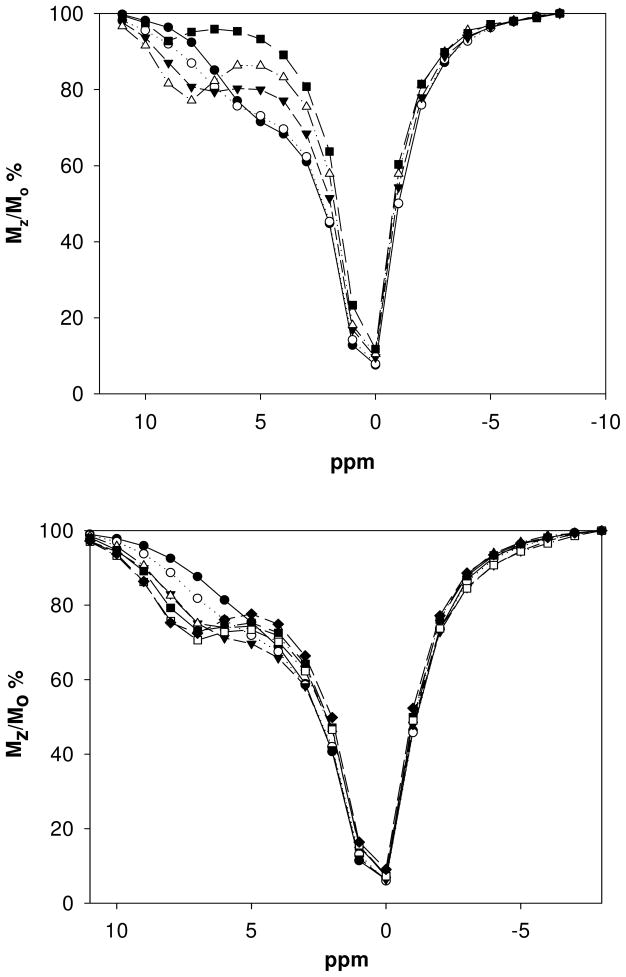
Eu(S-THP) is a pH-dependent PARACEST agent that functions best at a pH of approximately 5.0 in buffered solutions but shows a CEST effect over the pH range 3.5–6.5.6,40 As shown here, the CEST spectrum of the complex at pH 6.5 shows the percent change in water signal intensity (Mz/Mo) as a function of the frequency of the presaturation pulse (Figure 4). The spectrum has a shoulder at about 6 ppm (with bulk water set at zero ppm) that corresponds to exchange of the alcohol protons of Eu(S-THP) with bulk water. Addition of biologically significant anions including acetate, methylphosphate, lactate or citrate under these conditions gives rise to new Eu(S-THP) PARACEST peaks shifted downfield from the original shoulder at 6 ppm. The new PARACEST peak is similar for lactate and acetate and appears at 7 ppm and is slightly more shifted for citrate at 8 ppm. Methylphosphate shows more complicated behavior. A new CEST peak is observed at 4 ppm for up to 2 equivalents of methylphosphate. Addition of more methylphosphate gives rise to a new CEST peak at 8 ppm. This data supports two distinct binding events, similar to that observed by using luminescence spectroscopy. In contrast, addition of up to five equivalents of carbonate does not markedly change the PARACEST spectrum of Eu(S-THP) (Figure S20). Phosphate decreases the CEST shoulder at 6 ppm without the appearance of a new PARACEST peak.
Figure 4.
CEST spectra of 5.00 mM Eu(S-THP) in 20 mM Mes, 100 mM NaCl, pH 6.5, with addition of citrate (top) ● no citrate ○ 0.50 mM, ▼2.0 mM, ▲ 5.0 mM, ■ 7.0 citrate or with addition of acetate (bottom) ● no acetate ○ 5.0 mM, ▼10 mM, ▲ 15 mM, ■ 20 mM, □ 25 mM, ◆ 30 mM acetate.
Plots of Mz/Mzo as a function of anion concentration at pH 6.5 give dissociation constants of 34 mM, 20 mM for acetate and lactate at near neutral pH, respectively (Figure S21). These values are close to those calculated by using luminescence spectroscopy (Table 1). Similar plots for citrate were not amenable for binding analysis because CEST increases were complete at a 1:1 ratio, indicating strong binding under these conditions. In addition, the presence of more than one equivalent of citrate reduced the CEST peak. Plots of the CEST peak at 9 ppm upon addition of methylphosphate give a binding constant of 10 mM as reported previously.20 This corresponds closely to the weaker of the dissociation constants (9.8 mM) obtained by luminescence spectroscopy (Table 2).
More limited studies at pH 7.2 showed Eu(S-THP) PARACEST peaks for lactate (broad, 7 ppm), methylphosphate (8 ppm) and citrate (9 ppm). With the exception of citrate, the magnitude of the PARACEST alcohol peaks is diminished in comparison to those at pH 6.5 (Figure S22).
The exchangeable proton that gives rise to the CEST peak was identified in 1H NMR studies (Figure S23–25). At pH 5 or greater, the alcohol proton resonance is broadened into the baseline.20,31 However, addition of acetate, citrate or methylphosphate at pH 6.2 gives rise to a new alcohol resonance at approximately 7–8 ppm downfield of bulk water that corresponds to the observed alcohol PARACEST peak under these conditions. Addition of phosphate to Eu(S-THP) also leads to the appearance of a new proton resonance in the region of the spectrum where the alcohol proton is normally observed (Figure S22). However, no new PARACEST peak is observed under these conditions. This observation suggests that the rate of alcohol proton exchange for the Eu(S-THP) complex of phosphate is too slow to produce a PARACEST peak. Carbonate does not give rise to a new alcohol resonance for Eu(S-THP), consistent with the lack of a new CEST peak. Citrate addition to Eu(S-THP) gives rise to two closely spaced new peaks at 8–9 ppm versus water protons, matching the frequency of the PARACEST peak for this complex.
Also noteworthy are the chemical shifts of the macrocyclic ligand proton resonances of Eu(S-THP) which shift upon addition of citrate, phosphate, acetate, or methylphosphate to give distinct 1H NMR spectra for each Eu(III) complex. The change in the macrocyclic complex 1H resonances upon anion binding is indicative of a new species formed by innersphere coordination to the Eu(III) center. Conversely, the fact that carbonate addition leaves the proton resonances of Eu(S-THP) unchanged is consistent with the predominance of an outersphere carbonate complex under these conditions. This corresponds to luminescence spectroscopy measurements that show carbonate has one of the highest percentages of outersphere complex formation.
Discussion
Luminescence characterization of Eu(III) complex interactions with anions
Luminescence spectroscopy is a useful tool to characterize the nature of anion binding to the Eu(III) complexes. In particular, monitoring the Eu(III) 7F0 →5D0 excitation peak in conjunction with time-resolved studies facilitates the identification of distinct species in solution. The excitation peak of Eu(S-THP) shifts to characteristic frequencies upon binding of anions containing carboxy groups (citrate, acetate, lactate and carbonate) or phosphate groups (phosphate, methylphosphate). Ligands containing carboxylate groups give red-shifted excitation peaks. Citrate primarily forms a Eu(S-THP) complex with a very red-shifted excitation peak as commonly observed for binding of carboxylate containing ligands41 and the bound water of Eu(S-THP) is replaced by citrate. In contrast, simple carboxylate ligands such as carbonate, acetate or lactate bind weakly and do not completely displace the bound water. A red-shifted excitation peak in addition to a peak at the same wavelength as the original complex is observed, consistent with the formation of both innersphere and outersphere complexes. This assignment is supported by the nearly identical luminescence lifetime obtained upon excitation at either peak and the q number which is consistent with an average of the outer and innersphere complexes. This is in line with our previous studies which report millimolar dissociation constants for outersphere complexation of anions such as phosphate diester monoanions.40
The excitation peaks of methyl phosphate and phosphate complexes of Eu(S-THP) or Eu(THPC) are not distinct from the parent aquo complex so that speciation information is not readily obtained from these experiments. Emission spectroscopy gives further information on binding of phosphate and phosphate esters. Data is consistent with sequential binding of two anions as suggested by changes in the intensity of the ΔJ = 2 emission peak for phosphate and by changes in both ΔJ = 2 and ΔJ = 1 for methylphosphate. Binding of the first anion only results in a small change in hydration, but clearly binding must also involve a change in the first coordination sphere in order to produce changes in the magnitude of the emission peak splitting and the intensity of the emission peaks. For comparison, purely outersphere complexes such as diethylphosphate do not perturb either the excitation or the emission spectrum of Eu(S-THP).40 However, if the phosphate or methylphosphate were to bind to the hydroxyl alcohols and water ligand and form strong hydrogen bonding interactions, this might increase the anionic charge at the oxygen donor groups sufficiently to perturb the inner coordination sphere without inducing a unit change in the hydration number. The lower q number for binding of the second anion may correspond to water ligand replacement. Interestingly, the more tightly bound complexes of methylphosphate and phosphate correspond to the more highly hydrated species. This suggests that there are strong anion interactions that do not involve replacement of the water ligand of Eu(S-THP)(H2O).
The Eu(S-THP) complex binds anions with a selectivity which is distinct from that of related complexes with pendent amide groups such as those studied by Parker26,45–47 or in our laboratory.7,34,48,49 One reason for the difference is that Eu(S-THP) forms both innersphere and outersphere complexes with anions whereas analogous amide complexes apparentlydo not.20,40,49 We attribute the prevalence of outersphere complexes in Eu(S-THP) to the fact that the Eu(III) center is nearly encapsulated by four hydroxyl groups adjacent to a single water ligand. Highly charged anionic ligands such as phosphate or citrate displace a water ligand from Eu(S-THP)(OH2), while weaker monoanionic ligands such as diethylphosphate or acetate form either exclusively outersphere complexes or a combination of outersphere and innersphere complexes. In the outersphere complexes, the hydroxyl groups of Eu(S-THP) form hydrogen bonds to the anion.40
Anion binding selectivity is dependent on the number of coordination sites available for innersphere binding. Eu(III) complexes with four pendent amide groups bind very weakly to phosphate ester anions or carbonate consistent with the difficulty of replacing the single bound water molecule.48 In contrast, Eu(III) macrocyclic complexes containing three pendent amide groups (septadentate ligands) and two bound waters bind tightly to phosphate esters and carbonate.7,26,46,47 Some of these Eu(III) septadentate macrocycles bind anions in a bidentate fashion. Thus lactate, which can form bidentate complexes, binds 100-fold more tightly than does acetate (10 uM versus 10 mM) to Eu(III) complexes with septadentate macrocycles.47 Eu(S-THP) has a distinct selectivity, in part due to its single replaceable water molecule, and shows similar affinities for lactate and acetate that are quite weak. Carbonate also binds relatively weakly to Eu(S-THP) with an affinity that is 100 to 1000-fold less than that of the amide complexes.7,50 Only citrate binding is fairly strong and, surprisingly, is comparable to that observed for Eu(III) complexes containing two available coordination sites.25 The larger negative charge on citrate would be expected to increase the binding to the cationic Eu(S-THP) complex. However, the 1000-fold tighter binding in comparison to acetate suggests that the citrate interacts with Eu(S-THP) through multiple acetate groups, perhaps through interactions with the hydroxyl groups of the macrocyclic complex. Displacement of an alcohol group in Eu(S-THP) is unlikely given that citrate binding is reversible and no Eu(III) is sequestered from the complex over a period of several days (data not shown).
Eu(S-THP)(OH2) and all its complexes with anions exhibit a single unsplit ΔJ = 1 emission peak consistent with small crystal field splitting due to weak alcohol donor groups. Direct excitation through the highly forbidden 7F0 →5D0 transition gives lower intensity emission peaks (ΔJ = 1, 2, 3, 4) for Eu(S-THP) anion complexes than for the parent aquo complex. A useful feature of the emission spectra of anion complexes of Eu(S-THP) is that the complexes have distinctly different intensities for components of the ΔJ = 2 or 4 emission bands based on the type of donor group. For phosphate versus carboxylate groups the emission peaks are sufficiently different to determine binding of one anion in the presence of the other. Plots of either the luminescence intensity of one component of the ΔJ = 2 emission peak or of the ratio of ΔJ = 2/ΔJ =1 emission bands give binding constants for binding of the various anions studied here.
Toward luminescent sensors
Eu(III) complexes are attractive for the development of optical sensors, in part due to their multiple sharp emission peaks, large Stokes’ shift and long luminescence lifetimes that facilitate time gating to improve signal over background fluorescence. In addition, there is generally a large change in intensity of the hypersensitive 5D0 →7F2 (ΔJ = 2) emission peak upon anion binding to the inner coordination sphere of Eu(III) relative to other emission peaks such as the 5D0 → 7F1 (ΔJ = 1). This feature enables the development of Eu(III) complexes as ratiometric sensors given that some emission peaks stay relatively constant and others change in intensity upon anion binding.28
Carbostyril dyes have been used to sensitize luminescence of both Tb(III) and Eu(III).42–44 In the present study, we used a fluorinated analog of carbostyril 124 to take advantage of its larger extinction coefficient compared to the unmodified analog.42–44 Excitation of the carbostyril dye of Eu(THPC) at 340 nm sensitized Eu(III) luminescence, albeit with a low quantum yield, about five-fold lower than that observed for Eu(III) bound to a carbostyril-124 appended macrocycle containing phosphinate pendent groups.42 Comparison of the emission peaks by direct excitation on the MOPO/laser system at 1 nm monochromator resolution shows that the ΔJ = 2 emission peak is slightly more dominant for Eu(THPC) than for Eu(S-THP) (Figure S18). In Eu(THPC) there is an amide pendent group that provides a stronger ligand field than the alcohol groups. In axially symmetric Eu(III) complexes, the separation of components in the 7F1 emission band is related to the crystal field coefficients.51 However, the ΔJ = 1 emission peak is not split despite the presence of the amide pendent.
There is an unexpected 10-fold decrease in binding affinity of Eu(THPC) toward both citrate and phosphate compared to Eu(S-THP). Phosphate esters do bind more weakly to analogous macrocyclic complexes of Eu(III) with four amide pendent groups compared to those with alcohol groups (Eu(S-THP)), but it is surprising that a single amide group would induce a ten-fold decrease in anion affinity. Previously we found that macrocycles with mixed amide and hydroxyethyl pendent groups have similar pKa values and hydration numbers in comparison to complexes containing all alcohol pendent groups consistent with no major decrease in Lewis acidity.52 We attribute this decrease in anion affinity to the bulky carbostyril group and to the loss of a pendent alcohol group that may act as a hydrogen donor to the anions upon binding. This work suggests that it may be necessary to attach the sensitizing dye to the macrocycle backbone in a region far from the anion binding site to maintain the unique anion binding selectivity of the Eu(S-THP) complex. Anion binding to Eu(THPC), similar to Eu(THP), gives complexes with a larger than expected q number (0.68 for phosphate and 0.76 for citrate). This suggests that the anion doesn’t completely displace the bound water or that the outersphere water and innersphere alcohol quenching constants give values that do not accurately reflect changes in the coordination sphere. The excitation spectrum of the citrate adduct of Eu(THPC) which shows two peaks, one at the wavelength of the unbound complex, is consistent with the presence of outer and innersphere complexes.
Binding of citrate or phosphate to Eu(THPC) resulted in an increase in the intensity of all emission peaks upon excitation at 340 nm so that ratiometric analysis was not feasible. Even the highly forbidden 7F0 →5D0 emission peak increased in intensity. This is in contrast to the ratiometric Eu(III) sensors of analogous macrocyclic complexes designed by Parker that have the ΔJ = 2 emission peak increases more substantially than the ΔJ = 1 emission peak upon replacement of water and anion binding. The increase in intensity of the emission peaks for Eu(THPC) is consistent with replacement of a water molecule of on the Eu(III) complex which quenches luminescence. However, the smaller relative increase in the hypersensitive ΔJ = 2 transition relative to other transitions is consistent with the weak interaction of the anions with Eu(THPC). In any case, we show here that Eu(THPC) is an optical probe for anions, but not a ratiometric optical probe.
PARACEST
Binding of an anion to Eu(S-THP) induces a marked change in the hyperfine shifted proton resonances of the complex. This includes the alcohol protons of Ln(S-THP) that give rise to the PARACEST effect. For complexes of citrate, phosphate, methylphosphate and acetate, all macrocyclic proton resonances shift upon binding of anion, consistent with the formation of innersphere complexes. This is reminiscent of the paramagnetic NMR properties observed for related Eu(III) complexes with pendent amide groups that demonstrate pronounced macrocycle proton shifts upon addition of axial ligands. The more polarizable anions such as carbonate shift the proton resonances the least and the least polarizable anions such as phosphate shift the proton resonances of the macrocycle the most.47,53 This trend is roughly what is observed here except that citrate shifts the CEST peak further than does acetate. This is consistent with additional interactions of the citrate with the complex.
Previous work has shown that Ln(III) PARACEST agents can be designed to function as responsive MRI contrast agents. For example, the PARACEST peak of a Eu(III) macrocyclic complex shifts due to exchange of the bound water molecule upon addition of cations such as Zn(II).54 A Eu(III) complex with three pendent amide groups and two available coordination sites shows a shift in the amide proton resonance upon binding of lactate. 24 The alcohol protons of Eu(S-THP) have the additional feature that they are in close proximity to the anion binding site of the Eu(III) complex. This potentially produces an anion induced change in the PARACEST spectrum both from hyperfine shifting of the resonance and by inducing a change in the rate constant of alcohol proton exchange from direct interaction of the anion with the alcohol protons. Indeed, the PARACEST effect of Eu(S-THP) is readily turned on and off by the addition of anions that bind outersphere as well as innersphere.31,40
A drawback to the use of alcohols as donor groups in PARACEST is the weak crystal field that is produced in Eu(S-THP) in comparison to that of analogous Eu(III) complexes with pendent amide groups.55 The smaller crystal field leads to less dispersion in the hyperfine shifted protons of the exchangeable alcohol groups and a small Δω, the chemical shift difference between the exchangeable proton and the bulk water resonance. As noted previously, the magnitude of splitting of the ΔJ = 1 transition is related to the second-order crystal field parameter which also predicts the magnitude of the dipolar induced shift.51 The ΔJ = 1 transition here is unsplit for both Eu(S-THP) and Eu(THPC), consistent with weak donor groups. Strategies to increase the Δω value include substitution of a Ln(III) with a larger hyperfine induced shift such as Yb(III)6 or the incorporation of stronger donor ligands in the pendent groups of the macrocycle to increase the ligand field factors.55
Conclusions
The Eu(S-THP) complex binds a range of biologically relevant mono, di and trianions with a 2000-fold difference in binding strength for the most tightly bound (citrate) and most weakly bound anion (carbonate). Anion binding produces changes in the luminescence spectrum and in the PARACEST spectrum of the complex. These changes are a first step for the development of anion responsive dual modality optical/MRI responsive agents based on a single lanthanide ion. Binding selectivity is partially determined by whether the anion is a sufficiently strong ligand to displace the single water on the Eu(III) center. Weak anions interact through outersphere hydrogen bonding to the alcohol groups while stronger ligands such as citrate bind innersphere but also may have additional interactions with the alcohol groups.
With the exception of citrate, the biologically relevant anions studied here bind relatively weakly to Eu(S-THP) and Eu(THPC) with millimolar binding constants. This is a useful magnitude of binding interaction because many of the anions studied here are present in milimolar concentrations in mammalian extracellular fluids56 including NaHCO3, phosphate, lactate or citrate.57 However, the selectivity of binding of Eu(S-THP) or Eu(THPC) to each of these anions will thus need to be increased in order to obtain an anion sensor in a biological milieu. This is despite the fact that distinct Eu(III) emission peaks arise for different types of anion donor groups. For example, methylphosphate binding to Eu(S-THP) can be distinguished from lactate by emission spectroscopy or by PARACEST spectroscopy. Rather, a higher anion binding selectivity is required so that the presence of competing anions will not markedly change the effective binding constant for the anion of interest.
Challenges for the implementation of dual modality PARACEST/optical probes based on Eu(THP) derivatives include the incorporation of ligands that more effectively sensitize Eu(III) luminescence. The carbostyril dye appended through an amide pendent group forms a Eu(III) complex with only a modest quantum yield. Previous studies showed that the low quantum yield in a related Eu(III) macrocyclic complex may be attributed to back energy transfer from the Eu(III) excited state to the carbostyril dye.42 The optimization of ligand antenna for energy transfer to Eu(III) and other lanthanide ions is an active area of current research.11,28 In addition, it would be useful to determine an alternate site of attachment of the sensitizing dye that does not interfere with anion binding and/or interaction with the alcohol groups. Another challenge is maintaining good water solubility for the PARACEST agent. While Eu(S-THP) is soluble in aqueous solution up to 10 mM, the addition of organic dyes for luminescence sensitization generally results in decreased solubility in water. For example, the lower aqueous solubility of Eu(THPC) made it prohibitive to carry out PARACEST experiments at the millimolar concentrations required to observe the CEST effect.
In summary, continued development of lanthanide ion complex dual modality probes is predicated based on their excellent luminescence and PARACEST MRI contrast properties. These complexes are of interest for the development of responsive probes because both the magnetic and optical properties of the lanthanide ion are modulated by anion binding. This promising feature may lead to the design of versatile magnetic resonance and optical probes that are highly sensitive to environment. Notably, both outersphere and innersphere ligand binding must be considered in the development of these responsive agents
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Science Foundation (CHE-0911375) and the National Institutes of Health (EB-04609) for support of this work and for a major instrumentation award (CHE-0321058) to build the MOPO laser system. We thank Peter Gans for suggestions on using Hyperspec software for binding analysis.
References
- 1.Jennings LE, Long NJ. Chem Commun. 2009:3511–3524. doi: 10.1039/b821903f. [DOI] [PubMed] [Google Scholar]
- 2.Frullano L, Ciprian C, Benner T, Sherry AD, Caravan P. Angew Chem Int Ed. 2010;49:2382–2384. doi: 10.1002/anie.201000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent S, Elst LV, Wautier M, Galaup C, Muller RN, Picard C. Bioorg & Med Chem Lett. 2007;17:6230–6233. doi: 10.1016/j.bmcl.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Pellegatti L, Zhang J, Drahos B, Villette S, Suzenet F, Guillaumet G, Petoud S, Toth E. Chem Commun. 2008:6591–6593. doi: 10.1039/b817343e. [DOI] [PubMed] [Google Scholar]
- 5.Chengelis DAY, Adrienne M, Badger Paul D, Shade Chad M, Petoud Stephane. J Am Chem Soc. 2005:16752–16753. doi: 10.1021/ja0511725. [DOI] [PubMed] [Google Scholar]
- 6.Huang C-HM, Janet R. Inorg Chem. 2009:7237–7243. doi: 10.1021/ic900696f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nwe K, Andolina CM, Huang C-H, Morrow JR. Bioconjug Chem. 2009;20:1375–1382. doi: 10.1021/bc900146z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aime S, Castelli DD, Terreno E. Angew Chem Int Ed. 2002;41:4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. Acc Chem Res. 2003;36:783–790. doi: 10.1021/ar020228m. [DOI] [PubMed] [Google Scholar]
- 10.Wojciechowski F, Suchy M, Li AX, Hassan AA, Bartha R, Hudson RHE. Bioconjugate Chem. 2007;18:1624–1635. doi: 10.1021/bc0701287. [DOI] [PubMed] [Google Scholar]
- 11.Moore EG, Samuel APS, Raymond KN. Acc Chem Res. 2009;42:542–552. doi: 10.1021/ar800211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunzli J-C. Chem Rev. 2010;110:2729–2755. doi: 10.1021/cr900362e. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery CPM, Benjamin S, New, Elizabeth J, Pal Robert, Parker David. Acc Chem Res. 2009:925–937. doi: 10.1021/ar800174z. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Chem Rev. 2010;110:2960–3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aime S, Castelli DD, Crich SG, Gianolio E, Terreno E. Acc Chem Res. 2009;42:822–831. doi: 10.1021/ar800192p. [DOI] [PubMed] [Google Scholar]
- 16.De Leon-Rodriguez LM, Lubag AJM, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Acc Chem Res. 2009;42:948–957. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo B, Raam MS, Rosenblum RM, Pagel MD. Contrast Media & Molecular Imaging. 2007;2:189–198. doi: 10.1002/cmmi.145. [DOI] [PubMed] [Google Scholar]
- 18.Chauvin T, Durand P, Bernier M, Meudal H, Doan B-T, Noury F, Badet B, Beloeil J-C, Toth E. Angew Chem Int Ed. 2008;47:4370–4372. doi: 10.1002/anie.200800809. [DOI] [PubMed] [Google Scholar]
- 19.Li AX, Wojciechowski F, Suchy M, Jones CK, Hudson RHE, Menon RS, Bartha R. Magn Reson Med. 2008;59:374–381. doi: 10.1002/mrm.21482. [DOI] [PubMed] [Google Scholar]
- 20.Huang C-H, Morrow JR. J Am Chem Soc. 2009;131:4206–4207. doi: 10.1021/ja900290z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andolina CM, Holthoff WG, Page PM, Mathews RA, Morrow JR, Bright FV. Applied Spectroscopy. 2009;63:483–493. doi: 10.1366/000370209788346959. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Trokowski R, Zhang S, Malloy CR, Sherry AD. Magn Reson Med. 2008;60:1047–1055. doi: 10.1002/mrm.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Li Y, Pagel MD. Magn Reson Med. 2007;58:1249–1256. doi: 10.1002/mrm.21428. [DOI] [PubMed] [Google Scholar]
- 24.Aime S, Delli Castelli D, Fedeli F, Terreno E. J Am Chem Soc. 2002;124:9364–9365. doi: 10.1021/ja0264044. [DOI] [PubMed] [Google Scholar]
- 25.Pal R, Parker D, Costello LC. Org Biomol Chem. 2009;7:1525–1528. doi: 10.1039/b901251f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson P, Bretonniere Y, Parker D. Chem Commun. 2004:438–439. doi: 10.1039/b313496m. [DOI] [PubMed] [Google Scholar]
- 27.Bretonniere Y, Cann MJ, Parker D, Slater R. Org Biomol Chem. 2004;2:1624–1632. doi: 10.1039/b400734b. [DOI] [PubMed] [Google Scholar]
- 28.New EJ, Parker D, Smith DG, Walton JW. Curr Opin Chem Biol. 2010;14:238–246. doi: 10.1016/j.cbpa.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Ali MM, Lui G, Shah T, Flask CA, Pagel MD. Acc Chem Res. 2009;42:915–924. doi: 10.1021/ar8002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C-HH Jacob, Ratnakar S James, Sherry A Dean, Morrow Janet. R Inorg Chem. 2010:5963–5970. doi: 10.1021/ic1004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C-H. PhD thesis. University at Buffalo, State University of New York; 2009. [Google Scholar]
- 32.Grzyska Piotr K, Czyryca Przemyslaw G, Golightly J, Small K, Larsen P, Hoff Richard H, Hengge Alvan C. J Org Chem. 2002;67:1214–20. doi: 10.1021/jo016104p. [DOI] [PubMed] [Google Scholar]
- 33.Chin KOA, Morrow JR, Lake CH, Churchill MR. Inorg Chem. 1994;33:656–64. [Google Scholar]
- 34.Chappell LL, Voss DA, Horrocks WD, Morrow JR. Inorg Chem. 1998;37:3989–3998. doi: 10.1021/ic980191v. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Selvin PRJ. Photochem Photobiol. 2000;135:27–32. [Google Scholar]
- 36.Eaton DF. Pure & Appl Chem. 1988;60:1107–1114. [Google Scholar]
- 37.Beeby AC, Ian M, Dickins Rachel S, Faulkner Stephen, Parker David, Royle Louise, de Sousa Alvaro S, Williams JA Gareth, Woods Mark. J Chem Soc, Perkin Trans 2. 1999:493–504. [Google Scholar]
- 38.Horrocks WD. Jr Adv Inorg Biochem. 1982:201–61. [Google Scholar]
- 39.Horrocks WD, Jr, Arkle VK, Liotta FJ, Sudnick DR. J Am Chem Soc. 1983;105:3455–9. [Google Scholar]
- 40.Huang C-H, Hammell J, Ratnakar SJ, Sherry AD, Morrow JR. Inorg Chem. 2010;49:5963–5970. doi: 10.1021/ic1004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey STH, William DeW., Jr Inorg Chim Acta. 1995:383–90. [Google Scholar]
- 42.Parker D, Williams JAGJ. Chem Soc Perkin Trans 2. 1996:1581–1586. [Google Scholar]
- 43.Ge P, Selvin PR. Bioconj Chem. 2004;15:1088–1094. doi: 10.1021/bc049915j. [DOI] [PubMed] [Google Scholar]
- 44.Xiao M, Selvin PR. J Am Chem Soc. 2001;123:7067–7073. doi: 10.1021/ja0031669. [DOI] [PubMed] [Google Scholar]
- 45.Bretonniere YC, Martin J, Parker David, Slater Rachel Chem Commun. 2002:1930–1931. doi: 10.1039/b206286k. [DOI] [PubMed] [Google Scholar]
- 46.Bruce JID, Rachel S, Govenlock Linda J, Gunnlaugsson Thorfinnur, Lopinski Stefan, Lowe Mark P, Parker David, Peacock Robert D, Perry Justin JB, Aime Silvio, et al. J Am Chem Soc. 2000:9674–9684. [Google Scholar]
- 47.Dickins RSA Silvio, Batsanov Andrei S, Beeby Andrew, Botta Mauro, Bruce James I, Howard Judith AK, Love Christine S, Parker David, Peacock Robert D, et al. J Am Chem Soc. 2002:12697–12705. doi: 10.1021/ja020836x. [DOI] [PubMed] [Google Scholar]
- 48.Amin SV, David A, Jr, Horrocks William DeW, Lake Charles H, Churchill Melvyn Rowen, Morrow Janet. R Inorg Chem. 1995:3294–300. [Google Scholar]
- 49.Nwe K, Andolina CM, Morrow JR. J Am Chem Soc. 2008;130:14861–14871. doi: 10.1021/ja8037799. [DOI] [PubMed] [Google Scholar]
- 50.Atkinson PB Yann, Parker David. Chem Commun. 2004:438–439. doi: 10.1039/b313496m. [DOI] [PubMed] [Google Scholar]
- 51.Bryden CC, Reilley CN. Anal Chem. 1982;54:610–15. [Google Scholar]
- 52.Chappell LLV, David A, Jr, Horrocks William DeW, Jr, Morrow Janet R. Inorg Chem. 1998:3989–3998. doi: 10.1021/ic980191v. [DOI] [PubMed] [Google Scholar]
- 53.Atkinson PB Yann, Parker David, Muller Gilles. Helv Chim Acta. 2005:391–405. [Google Scholar]
- 54.Trokowski R, Ren J, Kalman FK, Sherry AD. Angew Chem Int Ed. 2005;44:6920–6923. doi: 10.1002/anie.200502173. [DOI] [PubMed] [Google Scholar]
- 55.Woods M, Woessner DE, Zhao P, Pasha A, Yang MY, Huang CH, Vasalitiy O, Morrow JR, Sherry AD. J Am Chem Soc. 2006;128:10155–10162. doi: 10.1021/ja061498t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bretonniere YC, MJ, Parker D, Slater R. Chem Commun. 2002:1930–1931. doi: 10.1039/b206286k. [DOI] [PubMed] [Google Scholar]
- 57.Forsythe IJ, Wishart DS. Curr Prot Bioinformatics. 2009:14.8.1–14.8.45. doi: 10.1002/0471250953.bi1408s25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.