Abstract
Wheat yield depends on the number of grains per square metre, which in turn is related to the number of fertile florets at anthesis. The dynamics of floret generation/degeneration were studied in contrasting conditions of nitrogen (N) and water availability of modern, well-adapted, durum wheats in order to understand further the bases for grain number determination. Experiments were carried out during the 2008–2009 and 2009–2010 growing seasons at Lleida (NE Spain). The first experiment involved four cultivars (Claudio, Donduro, Simeto, and Vitron) and two contrasting N availabilities (50 kgN ha–1 and 250 kgN ha–1; N50 and N250) while experiment 2 included the two cultivars most contrasting in grain setting responsiveness to N in experiment 1, and two levels of N (N50 and N250), under irrigated (IR) and rainfed (RF) conditions. In addition, a detillering treatment was imposed on both cultivars under the IR+N250 condition. The number of fertile florets at anthesis was increased by ~30% in response to N fertilization (averaging across treatments and spikelet positions). The effect of N and water availability was evident on floret developmental rates from the third floret primordium onwards, as these florets in the central spikelets of all genotypes reached the stage of a fertile floret in N250 while in N50 they did not. In this study, clear differences were found between the cultivars in their responsiveness to N by producing more fertile florets at anthesis (through accelerating developmental rates of floret primordia), by increasing the likelihood of particular grains to be set, or by both traits.
Key words: Detillering, fertile floret, floret primordia, grain number, grain set, Triticum durum.
Introduction
A substantial increase in cereal yield is required to ensure food security (Foulkes et al., 2011). The better yield determination in cereals is understood, the more likely it is that such increases will be achieved. Due to its relevance, several papers have recently been published reviewing the physiological bases of yield determination (Slafer et al., 2005; Fischer, 2011; Foulkes et al., 2011). As yield is linearly related to grain number per unit of land area, understanding the mechanisms controlling grain number determination may be relevant to increase yield further (Fischer, 2011; Pedro et al., 2012). In turn, it has been established in a wide range of conditions that the number of fertile florets at anthesis is closely related to the number of grains at maturity; due to genotypic differences (Miralles et al., 1998) or environmental effects (Serrago et al., 2008; González et al., 2011). Therefore, studying floret generation/degeneration dynamics may be useful to understand further the bases for grain number and yield determination (Slafer et al., 2005).
Floret primordia develop in a rather narrow window of time, mostly coinciding with stem elongation (Kirby, 1988; Miralles and Slafer, 1999; González et al., 2011). That is why the stem elongation phase is considered a critical period for grain number and yield determination (e.g. Fischer, 2011).
In a previous study, it was found that nitrogen (N) availability affected the rate of floret development, and therefore floret primordia mortality, in one cultivar of durum wheat (Ferrante et al., 2010). It is known that there is genetic variation within well-adapted modern durum wheats in grain number determination due to differences either in spike growth during pre-anthesis or in fruiting efficiency (Pedro et al., 2011). However, no studies have been performed to determine whether this genetic variation is somehow associated with genotypic differences in floret developmental patterns. Regarding the effects of N fertilization on grain number in wheat, it seems clear that N effects mostly operate through affecting crop growth and therefore spike dry matter at anthesis (Fischer, 1993; Prystupa et al., 2004), though there were cases in the literature where more direct effects have been reported, through increasing fruiting efficiency (the number of grains set per unit spike dry matter at anthesis; Abbate et al., 1995). There have been no studies determining whether N effects on floret survival are simply a reflection of better crop growth due to increased availability of resources. Moreover, it was not determined whether differences between cultivars in dynamics of floret development and grain setting are related to their differences in growth. Recognizing whether the changes in floret primordia development are simply a response to availability of resources (N and water) or whether they are affected by N specifically is rather important to draw general physiological models of floret development (in turn critical to achieve a better understanding of the physiological determination of the number of fertile florets in wheat). Therefore, the aim of the present work was to study the dynamics of floret development and its consequence on grain number and yield in well-adapted durum wheats in response not only to N but also to water availability and to an increase in growth of developing main-shoot spikes through detillering.
Materials and methods
General conditions
Experiments were carried out outdoors during 2008–2009 (experment 1) and 2009–2010 (experiment 2) growing seasons at the premises of the School of Agronomy, University of Lleida, Spain (41°37’50’’ N, 0°35’27’’ E; altitude 180 m). The experimental system was in microcrops, within large cubic containers (1 m height and 1×1 m2 surface, as illustrated in Ferrante et al., 2010).
In order to ensure a low N availability in the control, the containers were filled with a sand:soil mixture (3:1 v/v). In each experimental unit, mineral N availability at sowing was very low, equivalent to 20 kgN ha–1.
Immediately before sowing, phosphorus fertilizer (triple superphosphate, 20 kgP ha–1) was uniformly mixed within the top 20cm of soil. Before tillering, micronutrients chelated with EDTA (400ml per 100 litres of water) were applied.
Microcrops were sown, within the optimal sowing period for cereals in the region, on 28 November 2008 and 26 November 2009. To guarantee maximum uniformity within each microcrop, seeds were placed manually on 1 m linear strips of masking tape and then covered with tissue paper, as illustrated in Ferrante et al. (2010). These strips were placed in the rows and covered with the soil mixture so that actual plant density was 300 or 250 plants m–2 uniformly distributed in 2008–2009 or 2009–2010, respectively. Diseases and insects were prevented by spraying recommended fungicides and insecticides at the doses suggested by their manufacturers. Weeds were removed by hand throughout the growing season.
Rainfall was 25% higher in the second than in the first growing season (269mm and 334mm fell during the 2008–2009 and 2009–2010 growing seasons, respectively). Temperatures during the stem elongation phase (jointing–anthesis) were relatively similar in both years. The average maximum/minimum daily temperatures during this period were 19.5/6.8 °C and 20.3/7.8 ºC in the 2008–2009 and 2009–2010 growing seasons, respectively. Temperatures were higher during grain filling in 2008–2009 (29.1/14.2 ºC) than in 2009–2010 (26.3/12.1 ºC).
Treatments and experimental design
Experiment 1
Treatments consisted of a factorial combination of four modern semi-dwarf durum wheat cultivars (Claudio, Donduro, Simeto, and Vitron) and two levels of N (low and high soil N availability). All microplots and treatments were well irrigated to avoid stress by water throughout the growing season.
The genotypes were carefully chosen to be well-adapted cultivars in the northern growing area of Spain (Ferrante et al., 2012), where this study took place. As cultivars were all well adapted, their developmental patterns are very similar, avoiding any confounding effects of time to anthesis on floret development and grain number determination. In this way, any variation does actually represent variation within elite germplasm, with flowering time and plant height already optimized.
N treatments consisted of a control with an initial soil N content equivalent to 50 kgN ha–1 (N50), achieved through a light fertilization (30 kgN ha–1), and a heavily fertilized treatment which received 230 kgN ha–1, resulting in an availability of 250 kgN ha–1 (N250). N fertilizer (urea) was applied together with irrigations. In N50, the light fertilization was applied at the onset of tillering (DC 2.1; Zadoks et al., 1974). In N250, fertilizer was applied by splitting the dose in three equal applications at the onset of tillering (DC 2.1), at mid-tillering (DC 2.3), and at the onset of stem elongation (DC 3.1).
Treatments were arranged in a completely randomized design with three replicates. All in all, there were 24 large containers, each one being a microcrop used as an experimental unit.
Experiment 2
Treatments consisted of a factorial combination of (i) two modern semi-dwarf durum wheat cultivars (Donduro and Vitron); (ii) two water treatments [rainfed (RF) and irrigated (IR)]; and (iii) two N treatments (low and high soil N availability).
The cultivars were chosen because they differed in spike fertility response to N in experiment 1: Donduro responded mainly through increasing grain setting, while Vitron responded mainly through producing more fertile florets per spike (Claudio and Simeto had responses intermediate to those of Donduro and Vitron). Plants receiving the RF treatment were only irrigated at sowing (to guarantee germination and seedling emergence) and when N was applied (to incorporate the fertilizer into the soil and minimize N losses). Each of these irrigations in the RF treatment was of only 9mm. The plants receiving IR treatment, on the other hand, were periodically watered (depending on the conditions from once weekly in winter to daily during grain filling). At each opportunity, each microcrop was irrigated individually until water freely drained underneath the container. The two N treatments were the same as in experiment 1. In addition, a detillering treatment was imposed which consisted of removing all tillers immediately after they appeared, leaving only the main shoot in each plant. This additional treatment was only carried out in additional containers in each replicate, which were under N250 and IR conditions.
Treatments were arranged in a completely randomized design with three replicates, where again each microcrop was an experimental unit.
Measurements and analyses
The developmental stages beginning of stem elongation (DC 3.1), anthesis (DC 6.5), and physiological maturity (DC 9.5) were recorded when 50% of plants in a microcrop reached that stage. Thermal time was calculated as the summation of daily average temperature [(Tmax+Tmin)/2] (as a base temperature of 0 ºC was assumed).
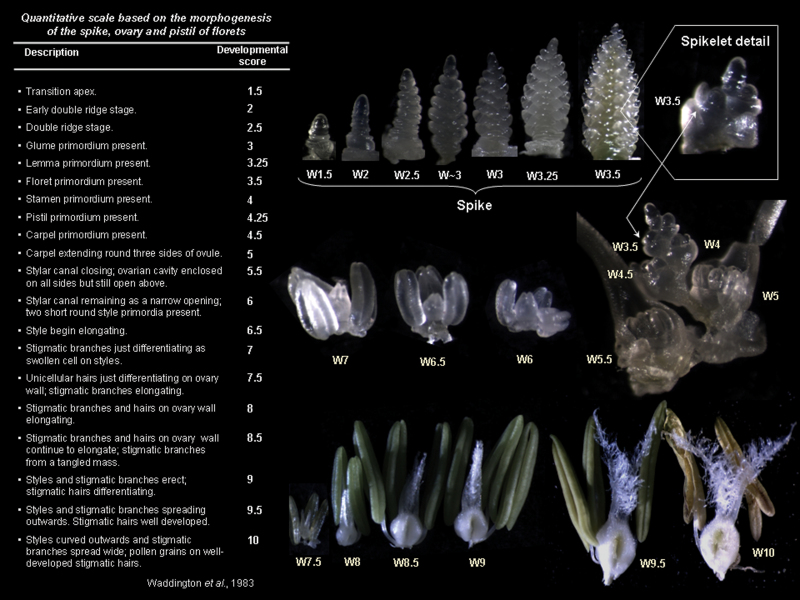
Once or twice a week, one plant per experimental unit was randomly harvested and its main shoot was dissected under a binocular microscope (Leica MZ 7.5, Leica Microscopy System Ltd, Heerbrugg, Switzerland) to determine the timing of double ridge formation firstly, and later that of terminal spikelet initiation (Kirby and Appleyard, 1984). From terminal spikelet initiation to flowering, one plant per experimental unit was randomly selected and harvested twice or three times a week. The spike of the main shoot was dissected again under the binocular microscope (i) to determine its length (through the image processing software Leica Application Suite version 3.3.0 or with a caliper, depending on the size); (ii) to count the total number of floret primordia; and (iii) to determine the developmental stage of each floret (floral score) within particular spikelets. For this determination, the scale proposed by Waddington et al. (1983) was followed (see Fig. 1), which is mostly based on pistil development from stage W3.5 (stamen primordia present) to stage W10 (styles curved and stigmatic branches spread wide, pollen grains on well-developed stigmatic hairs), as illustrated in Fig. 1. Floret primordia were considered as fertile florets when they were at W10 or immediately before that stage (when the stigmatic branches were curved with green anthers). The spikelets analysed were those in basal (fourth spikelet from the base), central (middle spikelet), and apical (fourth spikelet from the top) positions of the spike. Florets within the spikelets were numbered following the same procedure described by González et al. (2003), namely from F1 to Fn regarding their position with respect to the rachis, F1 being the floret primordium closest to the rachis, and Fn the most distal primordium whose development was scored in each particular case (ranging from F1 to F8, depending on the spikelet position and water×N treatments imposed on the different cultivars).
Fig. 1.
Floral developmental stages according to the Waddington scale (Waddington et al., 1983) with pictures taken from the experiments reported herein from early spikelet initiation at the transition of the apex to reproductive development to the stage of the fertile floret (W10), with details of selected developmental stages focused on the whole of spike development until the initiation of the terminal spikelet (W3.5) in each individual floret primordium from then onwards. (This figure is available in colour at JXB online.)
In both experiments, 10 spikes (main shoot) per experimental unit were randomly harvested at maturity. In each of these spikes, each spikelet was separated (following the same procedure described for mapping the number of fertile florets above) and the grains that were filled in each spikelet were determined, numbering them from the most proximal (G1) to the most distal position with respect to the rachis (G1–G4 depending on the particular spikelet position and treatments). Grain set (%) was calculated as the ratio of grain number to fertile floret number expressed as a percentage. The likelihood of particular grains (from G1 to G4) to be set at each spikelet was determined as the proportion of grains that was actually found set in the 10 spikes randomly selected at maturity for mapping the number of grains.
To determine the effect of treatments and their interactions on the different variables determined, the data were subjected to analysis of variance (SAS; SAS Institute, Cary, NC, USA), considering the factorial combination of two N availabilities (N50, N250) and four cultivars in experiment 1, and the factorial combination of two N availabilities (N50, N250), two water regimes (RF, IR), and two cultivars in experiment 2. To determine the dynamics of spike growth during pre-anthesis, the spike length values determined in successive samples during stem elongation were plotted against thermal time and the data were fitted with a bilinear model with a linear increase in spike length through thermal time until a maximum value when a plateau was reached.
Results
Floret development
All treatments in both experiments significantly affected the degree of development of most florets in all spikelet positions analysed, with the exception of several cases in which floret primordia developed normally to reach the stage of fertile florets at anthesis in all cultivars and any environmental condition, such as F1 in all cases, F2 in most cases, and F3 in exceptional cases (Supplementary Table S1 available at JXB online). Although the magnitude of the N effects was considerably larger than that of the other factors or their interactions, cultivar differences in both experiments and the effects of water and detillering in experiment 2 were also highly significant (Supplementary Table S1).
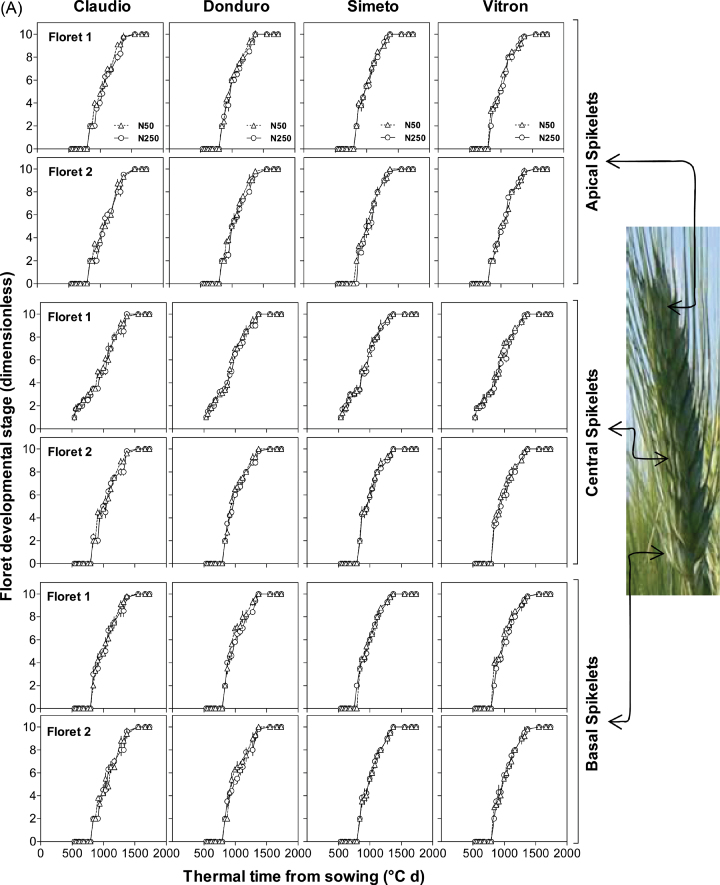
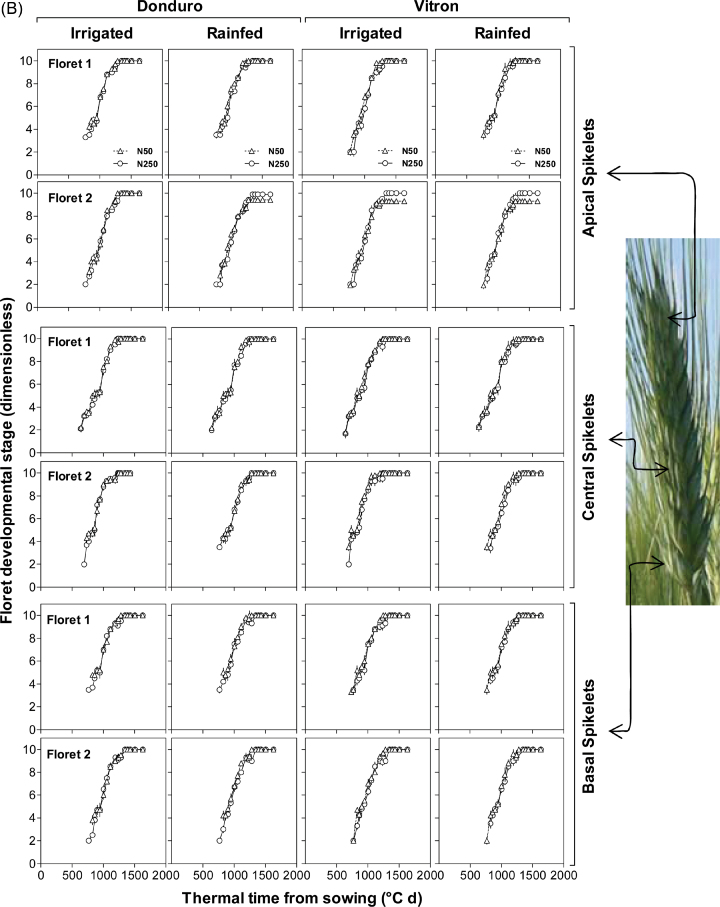
As evidenced in the analysis of variance, irrespective of the N fertilizer treatment, the two most proximal florets (F1 and F2) achieved the fertile floret stage in all cultivars for the three spikelet positions analysed in experiment 1 (Fig. 2A). In experiment 2, this was also the case, with the exception of three out of the 24 cases analysed: F2 did not reach the stage of fertile floret in the apical spikelets of Vitron in both water regimes and of Donduro in RF conditions (Fig. 2B). Overall, no clear effect of N availability was found in the developmental pattern of these two florets (Fig. 2).
Fig. 2.
Dynamics of floret development in four or two durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron (in experiment 1; A) or Donduro and Vitron (in experiment 2; B) under low (N50, open triangles) or high (N250, open circles) N availability (experiments 1 and 2; A and B, respectively), and irrigated or rainfed conditions (experiment 2; B) during the stem elongation phase for florets 1 and 2 proximal with respect to the rachis in each of the three spikelet categories considered (apical, central, and basal spikelets). Floret development was assessed through frequent determination of floret stages following the scores of the scale of Waddington et al. (1983). As scores lower than W3.5 correspond to spike rather than floret development, whenever a floret primordia at a stage of development earlier than that corresponding to W3.5 was observed, a score of W3 was assigned. (This figure is available in colour at JXB online.)
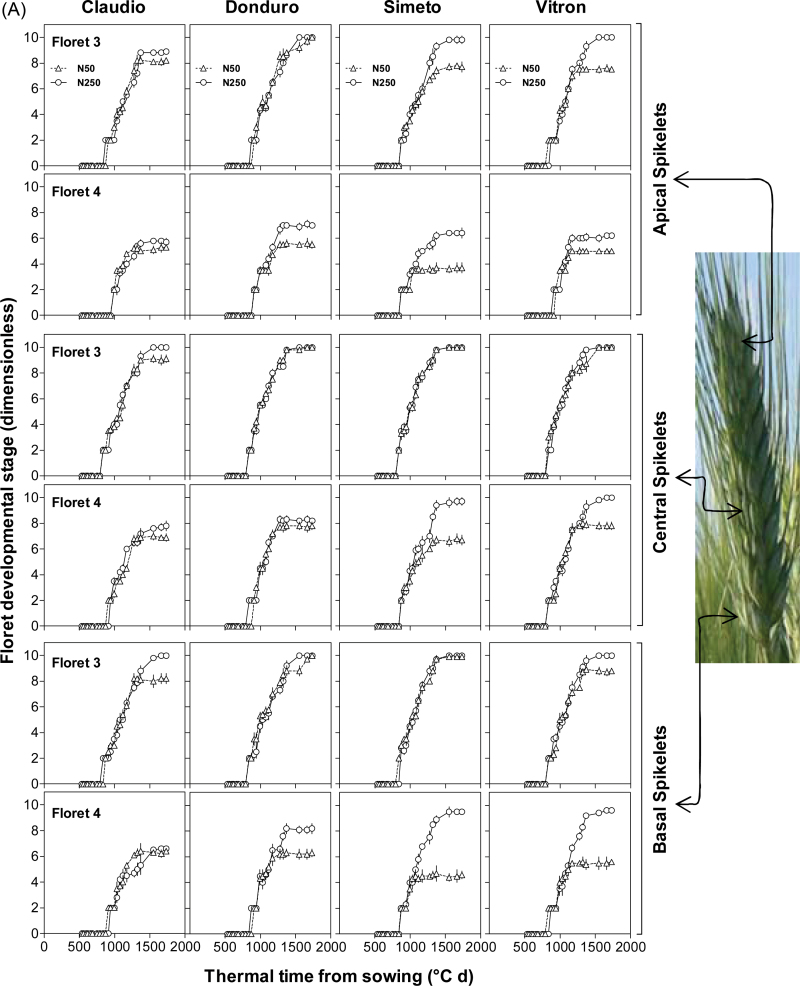
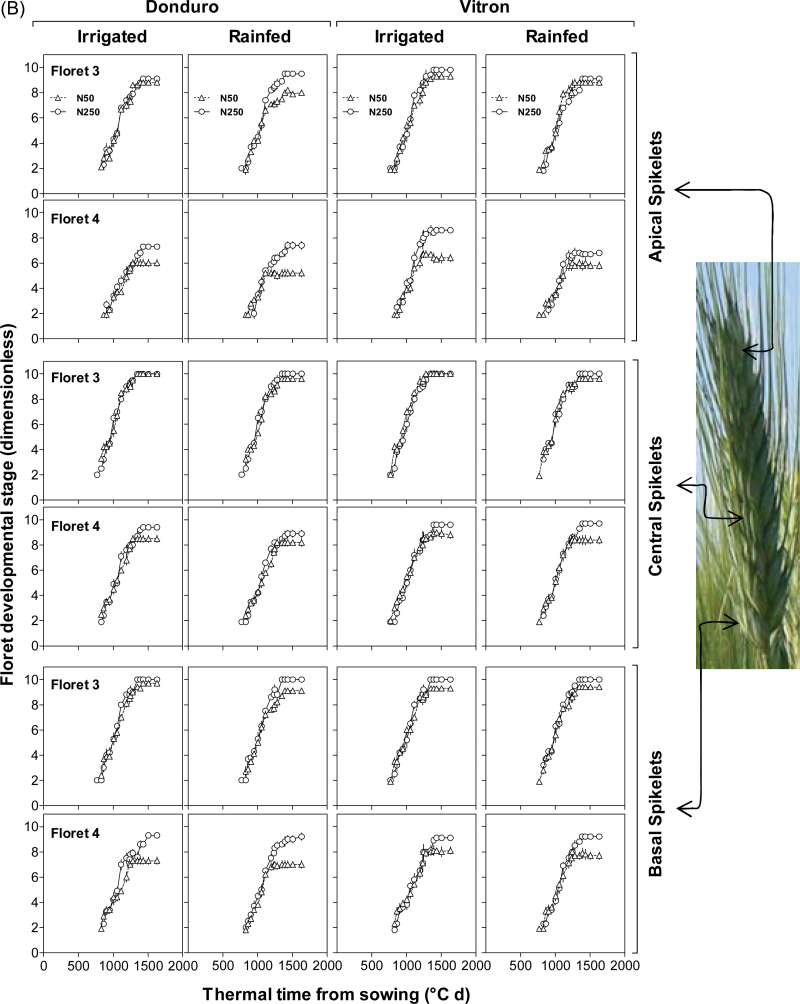
Differences between cultivars and effects of N availability became evident on the other, more distal florets (F3–F6, Fig. 3A, B; Supplementary Fig. S1 at JXB online). The third floret primordia responded clearly to N in those cultivars and spikelet positions in which the unfertilized control did not produce a fertile floret (Fig. 3). In all cases in which this N effect was clear, the reason why N-fertilized plants produced a third fertile floret was that its development continued normally while in the unfertilized plants it had stopped developing (Fig. 3A). Therefore, increasing N availability resulted in F3 being fertile in most of the cultivars and spikelet positions under IR conditions, with the only exception of the apical spikelet in Claudio (Fig. 3A). Under RF conditions of experiment 2, F3 did not reach the stage of fertile florets in the apical spikelets and did so in the central and basal spikelets of both cultivars studied only when plants were N fertilized (Fig. 3B).
Fig. 3.
Dynamics of floret development for florets 3 and 4 proximal with respect to the rachis in each of the three spikelet categories considered (apical, central, and basal spikelets) in experiments 1(A) and 2(B). Symbols are as in Fig. 2. Floret development was assessed as explained in Fig. 2. (This figure is available in colour at JXB online.)
Development of F4 responded to N even more markedly than F3. However, F4 was fertile at anthesis only under high N in the central spikelets of Simeto and Vitron and in the basal spikelets of the latter in both experiments (Fig. 3), whilst in experiment 2 under RF conditions F4 never did reach the stage of fertile floret even under high N availability (Fig. 3B). Even when in most cases analysed F4 did not reach the stage of fertile floret, there was still a clear increase in developmental progress of F4 in response to N availability in virtually all cultivars and water regimes (Figs. 3A, B). In all cases, the effect of the treatment with high N was again that the development of F4 continued progressing normally after that in the unfertilized plants had stopped developing (Fig. 3A). This demonstrates a quantitative effect of N availability on development of florets, even though the effect may seem qualitative if analysed in terms of the final number of fertile florets (Supplementary Table S1 at JXB online).
Florets 5 and 6 did not reach the stage of fertile florets in any spikelet position of any treatment. However, again, in most cases, increases in N availability resulted in higher floral scores (Supplementary Fig. S1 at JXB online), evidencing again a quantitative effect of N availability on development of very distal florets which cannot be perceived if analysed in terms of number of fertile florets.
Detillering the plants under well-watered, high N conditions in experiment 2 did not affect the developmental patterns of F1 and F2 (which developed normally to reach the stage of fertile floret in all spikelets and both cultivars under N50+IR conditions; data not shown), but it did allow the development of distal florets in the main shoot to progress even more than when fertilized under irrigation (as illustrated for F3 and F4 in Supplementary Fig. S2 at JXB online). This indicates that the developmental progress of the floret primordia responded to the detillering similarly to how it responded to N fertilization.
Dynamics of living florets
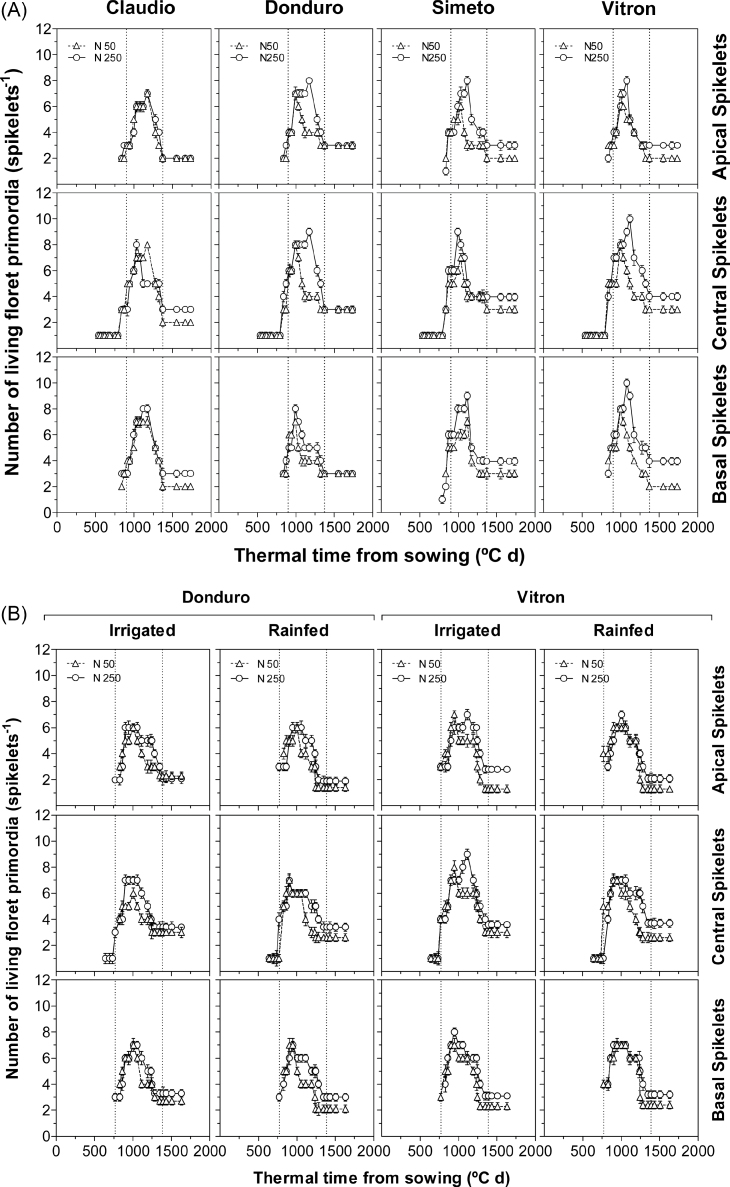
The pattern of generation and survival of living florets was characterized by an increase caused by the initiation of different florets in each of the spikelets until reaching a maximum number of floret primordia, many of which then degenerate and a certain number of florets survive, progressing normally in their development, determining the number of fertile florets for each specific spikelet (Fig. 4). The parameters of this pattern (the maximum number of florets initiated, the rate of floret degeneration, and the proportion of florets surviving to become fertile) were different for the analysed cultivars and spikelet positions, depended upon water availability, and almost invariably responded markedly to N by finally increasing the number of fertile florets at anthesis (Supplementary Table S2 at JXB online), the effect being less marked in Donduro than in the other cultivars (Fig. 4).
Fig. 4.
Dynamics of the number of living floret primordia from jointing to flowering in four or two durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron (in experiment 1; A) or Donduro and Vitron (in experiment 2; B) under low (N50, open triangles) and high (N250, open circles) N availabilities in each of the three spikelet categories considered (apical, central, and basal spikelets). Dotted lines indicate, for each panel, the timing of jointing (left line) and flowering (right line).
The maximum number of florets initiated tended to be increased in response to N availability in experiment 1 (Fig. 4A) but not in experiment 2 (Fig. 4B). In experiment 1 the magnitude of the increase varied among cultivars (averaged across spikelet positions the increase was 5, 14, 24, and 22% for Claudio, Donduro, Simeto, and Vitron, respectively; Fig. 4A). The timing to achieve the maximum number of floret primordia was either unaffected or slightly delayed in plants grown under high N (Fig. 4). This might actually be, again, an indirect reflection of N fertilization on maintaining normal development of floret primordia beyond the timing when these primordia stopped developing under low N conditions (see above).
As the final number of fertile florets per spikelet and per spike was consistently increased by N fertilization, responsiveness to N was related more to floret survival than to the magnitude of the maximum number of floret primordia (Fig. 4; Supplementary Table S2 at JXB online). This was also true for comparison among genotypes, beyond the responsiveness to N. In general, differences among cultivars seemed more related to their differences in floret survival than to the maximum number of floret primordia initiated (Fig. 4).
Increasing the allocation of dry matter to the main-shoot spikes due to detillering also increased the number of fertile florets consistently in all spikelet positions of both cultivars (on average the number of fertile florets was increased by 19.2±1.6%; Suppelementary Fig. S3 at JXB online) without a clear and consistent effect on the maximum number of floret primordia developed (on average the maximum number of floret primordia was not significantly increased by 7.5±3.4%; Suppelementary Fig. S3 at JXB online). Thus, the increased number of fertile florets per spikelet was almost exclusively due to an improved survival of the floret promordia developed.
Grain setting
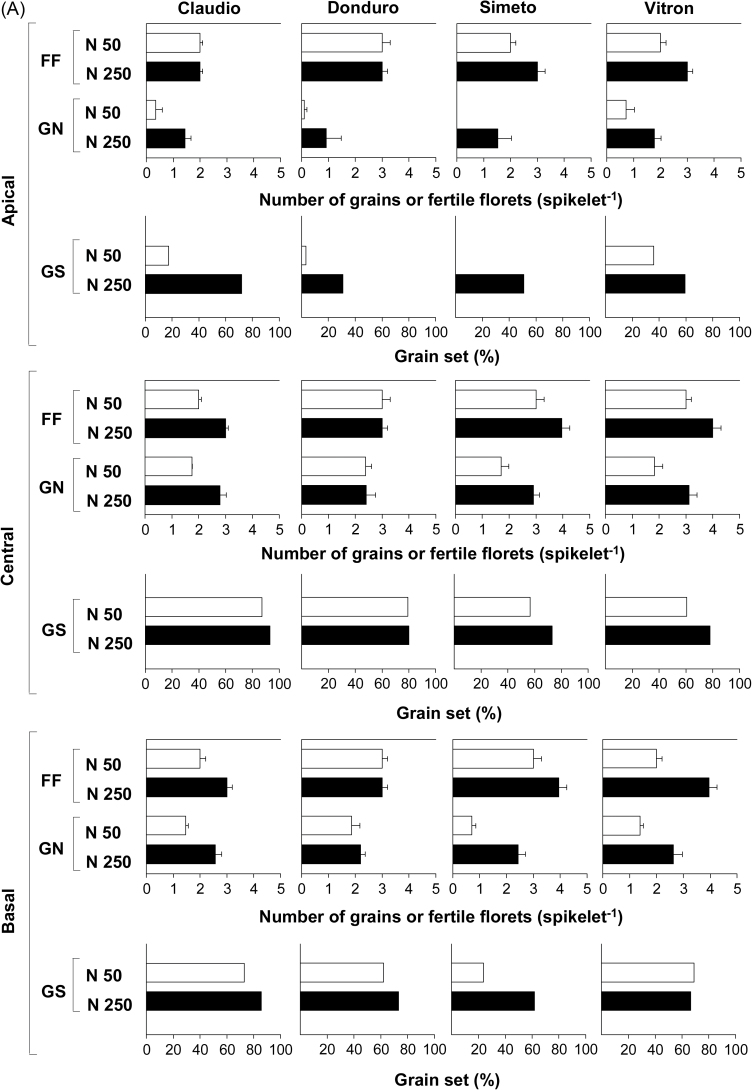
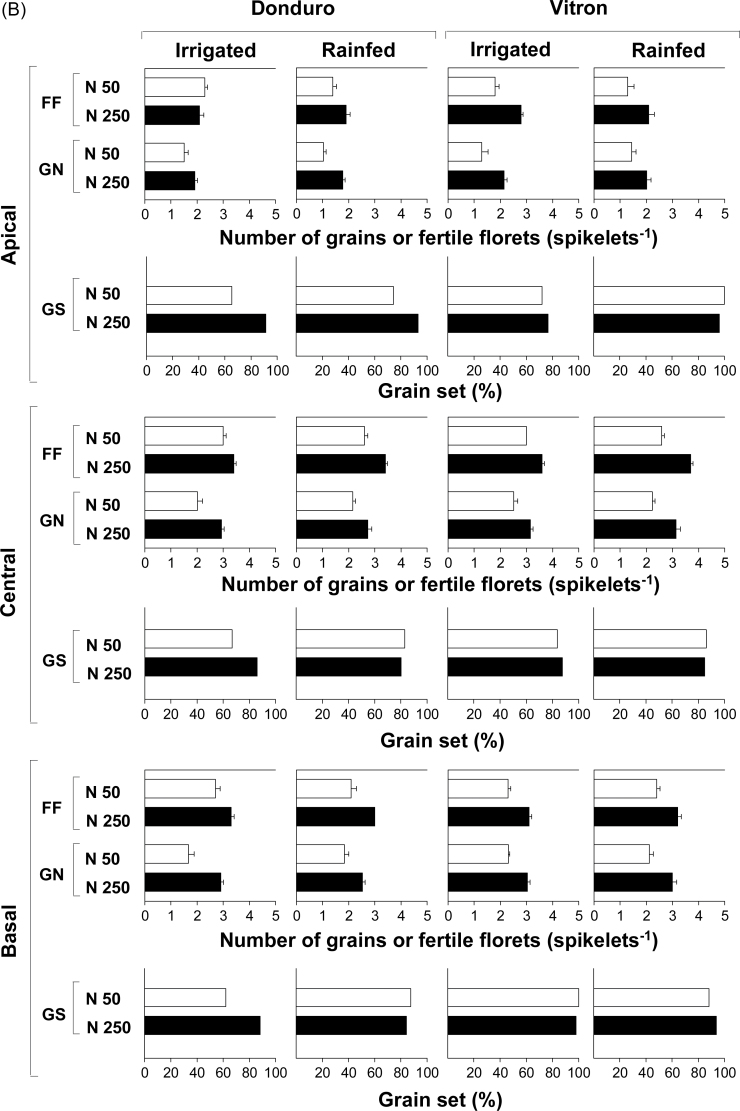
Grain number per spikelet at maturity was increased by N fertilization in all cultivars and spikelet positions (Fig. 5), with the exception of the central spikelets of Donduro in experiment 1 (Fig. 5A). In the case of the cultivar Vitron, the increase in grain number directly reflected the effect of N fertilization on the number of fertile florets (Fig. 5A) and under both water regimes in the second year (Fig. 5B). On the other hand, responsiveness of grain number to N in Donduro was mainly through increasing the percentage of fertile florets setting grains in apical and basal spikelets in experiment 1 (Fig. 5A) and in four out of the six combinations of spikelet positions and water regimes in the second experiment (Fig. 5B). In the two other cultivars, N fertilization increased both the number of fertile florets and the grain set percentage (Fig. 5A).
Fig. 5.
Number of fertile florets at anthesis (FF), number of grains at maturity (GN), and percentage of grain set (GS) in four or two durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron (in experiment 1; A) or Donduro and Vitron (in experiment 2; B) under low (N50, open bars) or high (N250, closed bars) N availability (experiments 1 and 2; A and B, respectively), and irrigated or rainfed conditions (experiment 2; B).
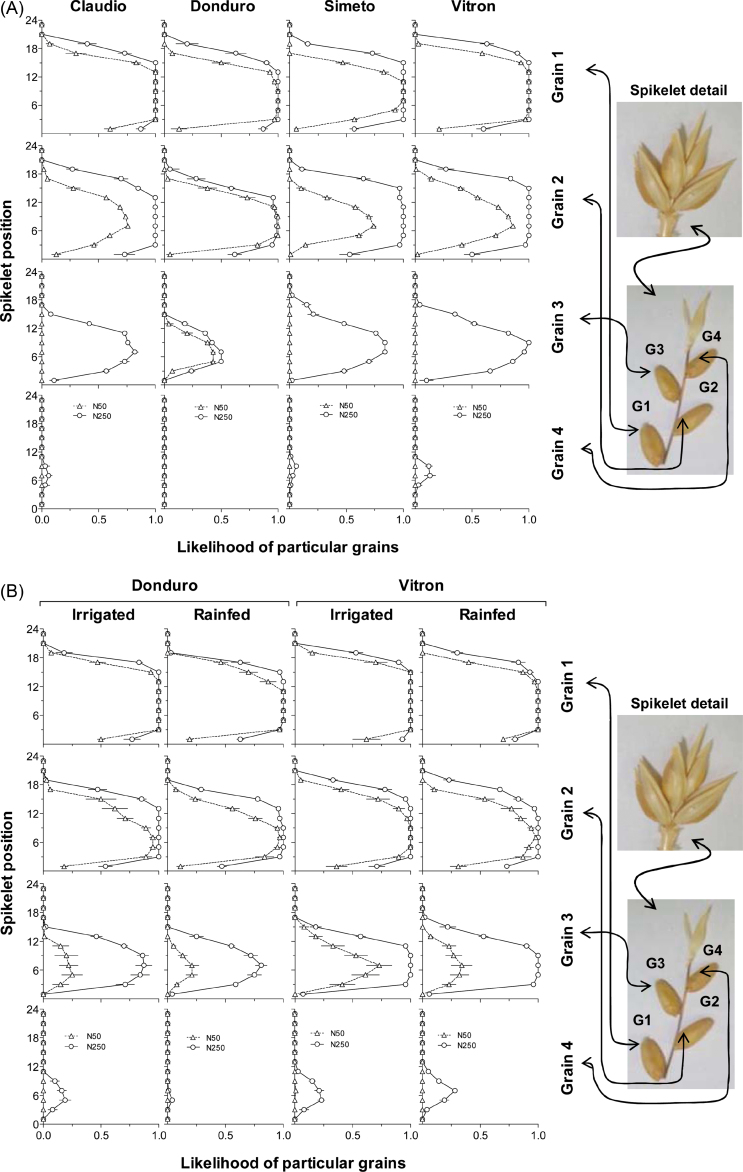
The differences in grain number described in detail for the three particular spikelets analysed systematically from the onset of stem elongation onwards represented well those of the whole spike, as revealed by the likelihood of particular grains (G1–G4) to be set throughout all spikelet positions, which varied depending on the cultivar, N availability, and grain position with respect to the rachis (Fig. 6). Regardless of N availability, the most proximal florets always set grains in most spikelets, and the effect of N fertilization on the likelihood of G1 to be set was restricted to extreme spikelet positions in both experiments and in both water regimes in experiment 2 (Figs. 6A, B; top row of panels). G2 showed a similar pattern to that described for G1, though with some improvement in likelihood of grain set under high N availability even in central spikelets in Simeto and Claudio in experiment 1 and under RF conditions of experiment 2 (Fig. 6B). For G3 and G4 (the most distal positions in which grains were occasionally set after anthesis and grown during the effective period of grain filling), there were clear increases in the likelihood of grain set in most spikelets in response to increased N availability.
Fig. 6.
Likelihood of particular grains (grains 1–4, from the top to the bottom rows of the panels) to be set at each spikelet position (from spikelet 1, at the base of the spike, to the uppermost spikelet) for cultivars: Claudio, Donduro, Simeto, and Vitron (in experiment 1; A) or Donduro and Vitron (in experiment 2; B) under low (N50, open triangles) or high (N250, open circles) N availability (experiments 1 and 2; A and B, respectively), and irrigated or rainfed conditions (experiment 2; B). (This figure is available in colour at JXB online.)
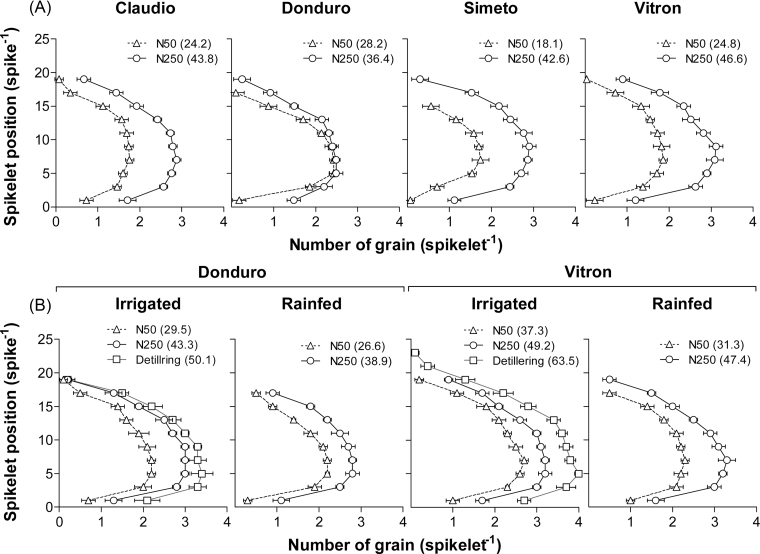
The integration of the cultivar×N effects on the development of floret primordia, their survival to produce fertile florets, and the likelihood of these florets setting grains determined the pattern of grains in the spikes and the final number of grains per spike. N treatments significantly affected the final number of grains along the spike in both experiments (Fig. 7; Supplementary Table S2 at JXB online). Water treatments produced on average smaller differences (Fig 7B), though still significant (Supplementary Table S2). The detillering treatment performed in experiment 2 resulted in an increased grain number over the values observed under IR and fertilized conditions, which was more noticeable in Vitron (29%) than in Donduro (16%; Fig. 7B).
Fig. 7.
Grain number in each spikelet position on the main-shoot spike for the durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron (in experiment 1; A) or Donduro and Vitron (in experiment 2; B) under low (N50, open triangles) and high (N250, open circles) N availabilities; and irrigated or rainfed conditions (experiment 2; B). Open squares symbols correspond to detillering treatments. Values in parentheses correspond to the number of grains per spike.
Pre-anthesis spike growth
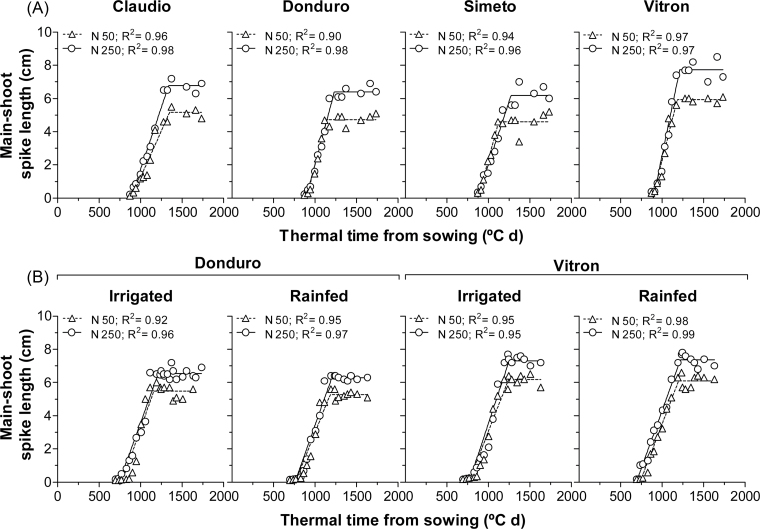
There was a generalized effect of resource availability on spike growth: spikes at flowering were consistently longer under high than under low N in all cultivars in experiment 1 (Fig. 8A) and for Vitron and Donduro under both RF and IR conditions in experiment 2 (Fig. 8B). In general, the differences due to N were evident towards the end of the spike growth period (Fig. 8). The growth of the spikes of Vitron responded to N more than those of Donduro across the range of conditions given by the two experiments and the water regimes (Fig. 8); and the number of grains per spike also tended to respond more sharply to N in Vitron than in Donduro (Fig. 7).
Fig. 8.
Dynamics of the main-shoot spike length from the onset of stem elongation to the beginning of grain growth for the durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron (in experiment 1; A) or Donduro and Vitron (in experiment 2; B) under low (N50, open triangles) and high (N250, open circles) N availabilities; and irrigated or rainfed conditions (experiment 2; B). For each spike length, a bilinear model [Y=A+B×X×(X≤C)+B×C×(X>C)] was used where Y=spike length (cm) and X=thermal time from sowing (ºC d).
Discussion
Fertile florets and grain setting
In the present work, it was confirmed that increasing N availability during early phases of wheat growth resulted in an increased number of grains (Fischer, 1993; Abbate et al., 1995; Prystupa et al., 2004). What is new is that such an effect operated through increasing both the number of fertile florets and the percentage of them setting grains, with genotypic variation—within modern well-adapted cultivars—on which of this two traits was dominantly responsive to N. Thus, responsiveness to N was almost exclusively due to an increased number of fertile florets (Vitron; experiments 1 and 2), to an increased proportion of fertile florets setting grains (Donduro; experiments 1 and 2), or to both processes (Claudio and Simeto; experiment 1).
The finding that increasing N availability allows a larger number of florets to become fertile is in line with what was reported in a previous explorative study, which comprised only one cultivar of wheat (Ferrante et al., 2010). Here it was confirmed that differences in yield determination between contrasting modern cultivars (with similar phenology and height), as well as yield responsiveness to improved resource availability (this improvement being through a specific management practice, such as N fertilization, or through an unspecific boost in overall resource availability from removal of competing spikes) were a reflection of responsiveness of floret primordia at different spikelets. Slightly increasing the rate of floret development, through an improved supply of resources, determined an increased likelihood of more distal florets achieving the stage of fertile floret: this difference took place irrespective of the spikelet position and was then reflected throughout the whole spike. This showed that understanding the dynamics of floret initiation and survival may be instrumental in achieving a more comprehensive perception of wheat yield determination as affected by genotypic and environmental factors (González et al., 2011, and references therein).
Thus, responsiveness to N seemed to have operated somewhat differently among cultivars. As spike growth was increased by increased N availability in all the cultivars, it is speculated that the increased spike growth may result in an increased number of fertile florets (e.g. Vitron) or in having larger florets which would have increased the likelihood of setting a grain (e.g. Donduro). Although they was not measured in the present study, eventual differences among cultivars in water-soluble carbohydrates (WSCs) in the stems around anthesis may also explain part of the differences in number of fertile florets: when WSCs increase, grain number tends to decrease (Rebetzke et al., 2008).
Floret development and degeneration
The number of grains per spike is mainly the consequence of the dynamics of floret initiation and degeneration to produce fertile florets at anthesis (Kirby, 1988). It has been established that the introgression of dwarfing genes increased grain number per spike due to an improved survival of floret primordia, with little or no effect on the maximum number of initiated floret primordia (e.g. Miralles et al., 1998; González et al., 2011). Differences were found among cultivars of similar height in terms of number of fertile florets, also mainly related to their rates of floret survival rather than to their differences in maximum number of floret primordia initiated. This showed that the mechanism identified for the introgression of dwarfing genes was not specific but generic, and that genotypic differences are mostly related to floret survival. This supports the notion that the process would be mediated by source–sink balances. As the metabolic cost required to initiate floret primordia would be negligible compared with that required to maintain floret growth to the stage of fertile floret, plants would initiate a large number of primordia and then, depending on the source strength (spike growth), a certain proportion of those primordia would end in being fertile florets, making floret survival far more relevant than floret initiation in the determination of the final number of fertile florets. In other words, the plasticity of a given component could be expected to be inversely related to the cost/benefit associated with that component (Sadras and Slafer, 2012). Therefore, the crop would accommodate environmental variation by preferential production of components which involve low cost or net fitness benefits (Bloom et al., 1985). Similarly, and in line with this notion, N fertilization consistently increased the number of fertile florets per spike through improving the survival of floret primordia rather than through stimulating a larger number of initiated floret primordia. This was in line with what González et al. (2005) found when they subjected wheat to different photoperiods during stem elongation: the relatively short photoperiods extended the duration of floret development, not by greatly affecting the maximum number of primordia initiated but through increasing the rate of floret survival to produce fertile florets at anthesis.
Possible causes of floret degeneration
According to González et al. (2005), three categories of floret primordia can be distinguished: (i) the two most proximal florets (F1 and F2) which frequently do develop normally through all the stages, reaching the stage of fertile florets at anthesis in most spikelets and under most conditions; (ii) the following floret primordia positioned in more distal positions of the spikelet (F3–F5), which may either progress normally and produce fertile florets or degenerate during spike growth, depending on the conditions and the spikelet position in the spike; and (iii) floret primordia in the most distal positions of the spikelet (F6 to F7–F10; the maximum number of floret primordia initiated per spikelet depends on the spikelet position in the spike) which invariably degenerate and virtually never reach the stage of fertile florets. Bancal (2008, 2009) suggested that the onset of floret death may be a purely developmental process, triggered by the stage of development of the most proximal florets, and then the likelihood of florets in intermediate positions in the spikelets to become fertile florets would be related to their own stage of development in relation to the developmental rate of F1, and irrespective of assimilate availability. In a recent paper, analysing together a rather large body of experimental data of floret development in response to photoperiod and shading, González et al. (2011) concluded that the fate of floret primordia in the intermediate positions of the spikelet is actually related to the increase in assimilate supply during spike growth. Here further support is provided for the evidence that the fate of floret primordia in intermediate positions of the spikelet reflects the availability of assimilates, in this case as affected by a common agronomic practice such as N fertilization, and not only through physiological manipulations of the environment (altering photoperiods or shading the crops).
In the present study, spike weight was not measured throughout stem elongation, but spike length was measured. Using the latter as a proxy for spike growth, it was found that the effect of N on spike length was mostly restricted to the second half of the spike growth period, which is also the period of floret death in which the responsiveness of the number of fertile florets to N availability has been largely determined.
Furthermore, the parallelism of the responses to N fertilization and to detillering treatment indicates that both treatments operated in the same way. Therefore, yield responsiveness to N was in general related to grain number increases as an indirect response to N through its effect on increasing growth, and the same response may be achieved by any environmental or nutritional factor affecting crop growth during the period of floret death. This interpretation is based not only on the fact that the relationship of grain number to spike dry matter was not improved if grain number is regressed against spike N (Ferrante et al., 2012, and references therein), but also on the fact that detillering did increase grain number through the same relationships to spike dry matter (Ferrante et al., 2012). Therefore, it seems reasonable to assume that any environmental factor modifying the availability of resources or the efficiency in using these resources would affect floret development in a similar way to that shown in the present study.
In conclusion, in this study, clear differences were found, depending on the cultivars, in the responsiveness of grain number and yield to N (experiments 1 and 2), water availability or the removal of competing shoots (experiment 2). Improved spike fertility was due both to producing more fertile florets at anthesis and to reducing the percentage of failure of fertile florets in becoming grains.
Responsiveness of the number of fertile florets was determined by a developmental response of floret primordia, which under higher N×water conditions continued developing normally in some distal florets of the spikelets at any position of the spike.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Dynamics of floret development in four or two durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron, or Donduro and Vitron under low or high N availability, and irrigated or rainfed conditions during the stem elongation phase for florets 5 and 6 proximal with respect to the rachis in each of the three spikelet categories considered (apical, central, and basal spikelets).
Figure S2. Dynamics of floret primordia development during the stem elongation phase for florets 3 and 4 in apical (top panels), central (middle panels), and basal (bottom panels) spikelet positions for two durum wheat cultivars, Donduro and Vitron, grown under high N and irrigated conditions with plants tillering freely or subjected to a detillering treatment.
Figure S3. Dynamics of the number of living floret primordia from jointing to flowering for two durum wheat cultivars, Donduro and Vitron, grown under high N and irrigated conditions with plants tillering freely or subjected to a detillering treatment.
Table S1. Mean squares of the stage of development (Waddington et al., 1983) reached at anthesis of the crop by floret primordia (F1–F6) for the apical, central and basal spikelets analysed in both growing seasons (2008/09 and 2009/10).
Table S2. Mean squares of grain number per spike, maximum number of living florets (MNLF), number of fertile florets (FF) and survival ratio (SR) for the apical, central, and basal spikelets analysed in both growing seasons (2008/09 and 2009/10).
Acknowledgements
We thank the team of the crop physiology laboratory of the UdL for technical assistance. Funding was provided by the Spanish Ministry of Science and Innovation through project AGL2009-11964. AF held a FPU, AP2006-03719, scholarship from the Spanish Ministry of Education.
References
- Abbate PE, Andrade FH, Culot JP. 1995. The effects of radiation and nitrogen on number of grains in wheat. Journal of Agricultural Science. 124, 351–360 [Google Scholar]
- Bancal P. 2008. Positive contribution of stem growth to grain number per spike in wheat. Field Crops Research. 105, 27–39 [Google Scholar]
- Bancal P. 2009. Early development and enlargement of wheat floret primordia suggest a role of partitioning within spike to grain set. Field Crops Research. 110, 44–53 [Google Scholar]
- Bloom AJ, Chapin FSI, Mooney HA. 1985. Resource limitation in plants—an economic analogy. Annual Review of Ecology and Systematics. 16, 363–392 [Google Scholar]
- Ferrante A, Savin R, Slafer GA. 2010. Floret development of durum wheat in response to nitrogen availability. Journal of Experimental Botany. 61, 4351–4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Savin R, Slafer GA. 2012. Differences in yield physiology between modern, well adapted durum wheat cultivars grown under contrasting conditions. Field Crops Research. 136, 52–64 [Google Scholar]
- Fischer RA. 1993. Irrigated spring wheat and timing and amount of nitrogen fertilizer. II. Physiology of grain yield response. Field Crops Research. 33, 57–80 [Google Scholar]
- Fischer RA. 2011. Wheat physiology: a review of recent developments. Crop and Pasture Science. 62, 95–114 [Google Scholar]
- Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP. 2011. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. Journal of Experimental Botany. 62, 469–486 [DOI] [PubMed] [Google Scholar]
- González FG, Miralles DJ, Slafer GA. 2011. Wheat floret survival as related to pre-anthesis spike growth. Journal of Experimental Botany. 62, 4889–4901 [DOI] [PubMed] [Google Scholar]
- González FG, Slafer GA, Miralles DJ. 2003. Floret development and spike growth as affected by photoperiod during stem elongation in wheat. Field Crops Research. 81, 29–38 [Google Scholar]
- González FG, Slafer GA, Miralles DJ. 2005. Floret development and survival in wheat plants exposed to contrasting photoperiod and radiation environments during stem elongation. Functional Plant Biology. 32, 189–197 [DOI] [PubMed] [Google Scholar]
- Kirby EJM. 1988. Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops Research. 18, 127–140 [Google Scholar]
- Kirby EJM, Appleyard M. 1984. Cereal development guide. 2ndedn. Coventry: Arable Unit, National Agricultural Centre; [Google Scholar]
- Miralles DJ, Katz SD, Colloca A, Slafer GA. 1998. Floret development in near isogenic wheat lines differing in plant height. Field Crops Research. 59, 21–30 [Google Scholar]
- Miralles DJ, Slafer GA. 1999. Wheat development. In: Satorre EH, Slafer GA, eds. Wheat: ecology and physiology of yield determination. New York: Food Product Press; 13–43 [Google Scholar]
- Pedro A, Savin R, Habash DZ, Slafer GA. 2011. Physiological attributes associated with yield and stability in selected lines of a durum wheat population. Euphytica. 180, 195–208 [Google Scholar]
- Pedro A, Savin R, Parry MAJ, Slafer GA. 2012. Selection for high grain number per unit stem length through four generations from mutants in a durum wheat population to increase yields of individual plants and crops. Field Crops Research. 12, 59–70 [Google Scholar]
- Prystupa P, Slafer GA, Savin R. 2004. Grain number and its relationship with dry matter, N and P in the spikes at heading in response to N×P fertilization in barley. Field Crops Research. 90, 245–254 [Google Scholar]
- Rebetzke JG, van Herwaarden AF, Jenkins C, Weiss M, Lewis D, Ruuska S, Tabe L, Fettell NA, Richards RA. 2008. Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Australian Journal of Agricultural Research. 59, 891–905 [Google Scholar]
- Sadras VO, Slafer GA. 2012. Environmental modulation of yield components in cereals: heritabilities reveal a hierarchy of phenotypic plasticities. Field Crops Research. 127, 215–224 [Google Scholar]
- Serrago RA, Miralles DJ, Slafer GA. 2008. Floret fertility in wheat as affected by photoperiod during stem elongation and removal of spikelets at booting. European Journal of Agronomy. 28, 301–308 [Google Scholar]
- Slafer GA, Araus JL, Royo C, García Del Moral LF. 2005. Promising eco-physiological traits for genetic improvement of cereal yields in Mediterranean environments. Annals of Applied Biology. 146, 61–70 [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. 1983. A quantitative scale of spike initial and pistil development in barley and wheat. Annual of Botany. 51, 119–130 [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research. 14, 415–421 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.