Abstract
Plant communities in the European Alps are assumed to be highly affected by climate change, as the temperature rise in this region is above the global average. It is predicted that higher temperatures will lead to advanced snowmelt dates and that the number of extreme weather events will increase. The aims of this study were to determine the impacts of extreme climatic events on flower phenology and to assess whether those impacts differed between lower and higher altitudes. In 2010, an experiment simulating advanced and delayed snowmelt as well as a drought event was conducted along an altitudinal transect approximately every 250 m (600–2000 m above sea level) in the Berchtesgaden National Park, Germany. The study showed that flower phenology was strongly affected by altitude; however, there were few effects of the manipulative treatments on flowering. The effects of advanced snowmelt were significantly greater at higher than at lower sites, but no significant difference was found between both altitudinal bands for the other treatments. The response of flower phenology to temperature declined through the season and the length of flowering duration was not significantly influenced by treatments. The stronger effect of advanced snowmelt at higher altitudes may be a response to differences in treatment intensity across the gradient. Consequently, shifts in the date of snowmelt due to global warming may affect species more at higher than at lower altitudes, as changes may be more pronounced at higher altitudes. These data indicate a rather low risk of drought events on flowering phenology in the Bavarian Alps.
Key words: advanced snowmelt, Alps, BBCH, climate change, delayed snowmelt, flowering
Introduction
Over the past 100 years, global annual mean temperatures have increased by about 0.7 °C (IPCC, 2007). However, some regions have been more affected by climate change than others. For the European Alps, a much stronger temperature increase of about 2 °C has been detected (Auer et al., 2007). In the future, it is predicted that temperatures will further rise, that rainfall distribution will change, and that extreme weather events, such as torrential rain and drought, will increase significantly in frequency (IPCC, 2007). Furthermore, a general reduction in the duration of snow cover will be caused by warmer temperatures, because the zero-degree isotherm will be displaced to higher altitudes (Beniston, 2003; Laghari et al., 2012). However, climate change scenarios for future snow conditions are rather vague. An increase in heavy snowfall events in winter may therefore also lead to a prolongation of snow cover duration.
As a result of the changing environment, alpine plant communities have already experienced and will suffer further negative impacts (e.g. Korner, 1992; Grabherr et al., 1994; Sala et al., 2000; Erschbamer et al., 2009). The effects of climate change on alpine vegetation will be especially pronounced at high altitudes, as abiotic factors such as climate prevail over biotic factors in these regions (Korner and Miglietta, 1994; Theurillat and Guisan, 2001).
Phenology, the study of the timing of recurring natural events, can be a tool for assessing climate change impacts on plant growth and development. Several studies have shown that the most important factors for plant development in alpine areas are temperature, date of snowmelt, and photoperiod (e.g. Price and Waser, 1998; Blionis et al., 2001; Keller and Korner, 2003). However, in the future, drought may also play an important role in the development of plants in the Alps due to an increasing probability of the occurrence of extreme weather events.
Shifts in plant phenology due to warmer temperatures have already been widely documented by analysing long-term datasets (e.g. Schwartz and Reiter, 2000; Sparks et al., 2000; Abu-Asab et al., 2001; Fitter and Fitter, 2002; Menzel et al., 2005, 2006) or have been confirmed by experimental studies (Marion et al., 1997; Hollister and Webber, 2000; Kudernatsch et al., 2008; De Frenne et al., 2010). Higher temperatures mainly advance plant phenology (e.g. Sparks et al., 2000; Menzel et al., 2006; Kudernatsch et al., 2008), which increases the risk of late frost damage in spring (Inouye, 2000, 2008; Wipf et al., 2009) and may cause shifts in plant community composition due to die off (Molau, 1997). Furthermore, changes in plant flowering patterns can cause an overlap of the flowering times of different species, which, in early summer, can lead to greater competitive pressure, because pollinator activity is very low at this time of year (Molau, 1997). Shifts in plant flowering times may also decrease population levels of pollinators (Inouye and McGuire, 1991), which may in turn also increase competitive pressure.
Timing, depth, and duration of snow cover determine the beginning of the growing season in alpine areas (Inouye and Wielgolaski, 2003). Thus, the development of many species in alpine or Arctic regions is highly dependent on the timing of snowmelt (Inouye et al., 2002; Stinson, 2004; Inouye, 2008). A prolongation in snow cover duration often delays plant phenology (Weaver and Collins, 1977; Inouye, 2008; Torp et al., 2010; Cooper et al., 2011), whereas a shortening of snow cover duration mostly advances the timing of plant development (e.g. Price and Waser, 1998; Dunne et al., 2003; Inouye et al., 2003; Wipf et al., 2009; Lambert et al., 2010; Wipf, 2010; Chen et al., 2011). However, phenological responses are highly species specific and differ among functional groups (Wipf and Rixen, 2010). An advanced snowmelt could potentially increase plant fitness by prolonging the growth period and hence resource allocation (Galen and Stanton, 1993; Stinson, 2004). However, an earlier start of flowering also increases the risk of late frost damage in spring. Thus, an earlier snowmelt may not necessarily lead to advanced flowering (Inouye, 2008). In the Australian Alps, for example, the timing of snowmelt only slightly affected the timing of flowering for the tested species (Venn and Morgan, 2007).
As with other plants, the responses of alpine plants to drought include wilting and reduced plant growth (Sangtarash et al., 2009) and seed set, or even extinction (Galen, 2000). Phenological responses to drought, however, are not consistent for alpine and other plants. Jentsch et al. (2009) reported an advance of the mid-flowering date by 4 d after a drought event, whereas Bloor et al. (2010) did not detect a significant effect of drought on grasses. In contrast, a delay in flowering phenology under dry conditions was reported for Mediterranean plants (Penuelas et al., 2004; Llorens and Penuelas, 2005; Prieto et al., 2008; Miranda et al., 2009).
In general, there have been several studies dealing with the impacts of a changing abiotic environment (shifts in the date of snowmelt or the occurrence of drought) on plant phenology. However, as far as we know, there have been only a few studies combining manipulative experiments with an altitudinal gradient (but see Dunne et al., 2003; Stinson, 2004) to assess whether impacts due to climate change differ between lower and higher altitudes. Altitudinal gradients naturally provide different temperature scenarios, because air temperature decreases by 0.54–0.58 °C per 100 m increase in altitude (Rolland, 2003). Thus, the current study focused not only on treatment effects but also on combined temperature changes, which are derived indirectly from altitudinal change. Consequently, the aims of this study were to test whether shifts in the date of snowmelt and drought events affected (i) the timing and (ii) the length of flowering phenology of different grassland species, and whether those impacts changed with (iii) elevation and (iv) season.
Materials and methods
Study site and experimental design
The study area was located in the northern part of the Berchtesgaden National Park, which is the only German national park in the Alps and is characterized by a large altitudinal range within a small area (StMUG, 2001).
Eleven observational sites were located along two valleys in the national park and ranged from ~800 m to ~2000 m above sea level (a.s.l.). To ensure a larger altitudinal gradient, three sites below 800 m beyond the borders of the park were added, starting at ~600 m. One other site outside the two valleys was also included to ensure a site at approximately every 250 m altitude difference. Thus, observations were conducted at a total of 15 different sites. The aspects of the sites were different: eight faced north, three faced west, three faced south, and one was on level ground.
The annual mean temperature in the park ranges between –2 and 7 °C and annual mean precipitation is 1500–2600mm depending on altitude (StMUG, 2001). For sites below 1000 m a.s.l., maximum snow cover is reached in February at a mean depth of about 50cm. Sites over 1000 m have their maximum snow cover in March, ranging between 3 and 5 m at the highest altitudes (StMUG, 2001).
The lapse rate of air temperature (decrease in temperature with elevation) was about 0.45 °C per 100 m elevation (mean from March to August; Konnert, 2004). Growing season lengths (derived from days above a 10 °C threshold) varied from 5 months at 600 m to ~1 month at 2000 m (Konnert, 2004).
Experimental plots were established at each of the 15 study sites along the entire altitudinal gradient, consisting of three different treatments and a control, each plot sized 4×4 m. Plots were contained within a 10×10 m square, arranged in a 2×2 array. Treatments were the simulation of advanced and delayed snowmelt, as well as a drought event.
Advanced and delayed snowmelt
Advanced and delayed snowmelt was simulated by shovelling snow from advanced snowmelt plots onto delayed snowmelt plots until only a thin snow layer was left on the former; thus, the vegetation on the advanced snowmelt plots was not disturbed. Shovelling took place between the end of February and the beginning of April in 2010, depending on altitude. Snow depth along the gradient varied from 15 to 214cm on advanced snowmelt plots before shovelling. After shovelling, snow depth on delayed snowmelt plots ranged from 16 to 304cm depending on altitude. Snow melting date was defined as the day when near-surface air temperatures reached more than +5 °C on at least three consecutive days (for description of temperature measurements, see Environmental data, below).
Drought event
The drought event was simulated by rain-out shelters, which were installed, on average, 4 weeks after snowmelt in control plots depending on altitude (installed end of April to end of June; removed beginning of June to beginning of August). The drought period lasted 43±1 d, which is regarded as a 1000-year extreme event in this region (Jentsch and Beierkuhnlein, 2008). To allow air exchange, rain-out shelters were open at the front and rear. Rain-out shelters were 125cm high and constructed with aluminium tubes and cast-iron key clamps (B-One key clamps, Montfoort, The Netherlands). Shelter poles were covered with a transparent plastic sheet (0.2mm polyethylene; SPR 5, Hermann Meyer KG, Germany), which transfers nearly 90% of photosynthetically active radiation. The drought period ended when the shelters were removed. Over the altitudinal gradient, no significant difference in average or maximum near-surface air temperature (for description of temperature measurements, see Environmental data, below) between drought and control plots was detected with a paired t-test (P=0.6 and P=0.9, respectively) in the drought period. Minimum temperature, however, was significantly different between drought and control plots for the same period (paired t-test, P=0.012).
Species and phenological observations
Phenological observations of ten different species (eight herbs and two grasses) were conducted once a week from April to September 2010 on each plot following the Biologische Bundesanstalt, Bundessortenamt and Chemical Industry (BBCH) code. The BBCH code is a detailed growth stage key that includes intermediate stages as well as stages marking the end of phenophases. It allows the observation of the entire development cycle of all mono- and dicotyledonous plants using a decimal coding system (Meier, 2001). Using a detailed observation key like this, it is not necessary to be present at the exact start of the phenological stage, as the key allows recording of the frequency distribution of phenophases of a certain number of individual plants on each sampling date. Classical onset dates, as used in climate research studies, could be interpolated from these data using the ordinal logistic regression (OLR) method described by Cornelius et al. (2011). The OLR method provides information about the progression of stages (including the beginning, speed of passage, and end of secondary growth stages) and allows stages to be of unequal length (ordinal-scale approach). Furthermore, OLR is based on the frequency distribution over time, which includes the entire progression of plants in the model and not only the progression of a single stage.
In this study, the focus was on flower phenology, especially the beginning of flowering (forbs: first flowers open; grasses: first anthers visible), full flowering (forbs: 50% of flowers open; grasses: 50% of anthers mature), and end of flowering (forbs: petals dehydrated or fallen; grasses: all spikelets/panicles have completed flowering but some dehydrated anthers may remain). Campanula scheuchzeri Vill and Ranunculus montanus Willd. develop only one flower per individual, and thus definitions needed to be adjusted for the beginning of flowering (flower slightly open) and full flowering (flower expanded to full size). In each plot, 20 individuals per species were observed where possible. This number of individuals was considered large enough for further statistical analysis and small enough to make all observations achievable within a week. As plants of each species were not individually marked, partly different groups of individual plants were probably observed on consecutive sampling dates.
The average altitudinal range of species in this study was about 705 m but varied between 127 m for full flowering of Ranunculus acris L. and 1343 m for end of flowering of Ranunculus montanus (Table 1). All observed dates were converted to day of year (1 January=1, etc.; DOY).
Table 1.
Results of linear regression analysis of study site mean dates of three phenophases on altitude Numbers in bold are significant (P <0.05). Altitudinal ranges show the maximum difference in elevation and the elevation of the lowest and highest site for each species and phenophase. BF, beginning of flowering; FF, full flowering; EF, end of flowering; #, no variation recorded in this variable for this event.
| N | R 2 | P | Regression coefficients (±SE) (days/100 m) | Altitudinal ranges (low–high) (m) | ||
|---|---|---|---|---|---|---|
| Alchemilla vulgaris L. (Rosaceae) | BF | 4 | 0.119 | 0.655 | 0.8 (±1.6) | 534 (1045–1579) |
| FF | 7 | 0.827 | 0.005 | 4.8 (±1.0) | 865 (714–1579) | |
| EF | 7 | 0.550 | 0.050 | 2.4 (±1.0) | 619 (960–1579) | |
| Briza media L. (Poaceae) | BF | 7 | 0.844 | 0.003 | 3.4 (±0.7) | 689 (641–1330) |
| FF | 7 | 0.731 | 0.014 | 2.4 (±0.7) | 689 (641–1330) | |
| EF | 7 | 0.853 | 0.003 | 3.2 (±0.6) | 689 (641–1330) | |
| Campanula scheuchzeri Vill. (Campanulaceae) | BF | 3 | 0.776 | 0.314 | 5.2 (±2.8) | 273 (1552–1825) |
| FF | 5 | 0.535 | 0.160 | 1.4 (±0.8) | 865 (960–1825) | |
| EF | 8 | 0.642 | 0.017 | 1.9 (±0.6) | 865 (960–1825) | |
| Dactylis glomerata L. (Poaceae) | BF | 5 | 0.713 | 0.072 | 3.9 (±1.4) | 464 (641–1105) |
| FF | 5 | 0.526 | 0.166 | 2.5 (±1.4) | 464 (641–1105) | |
| EF | 5 | 0.331 | 0.311 | 1.3 (±1.1) | 464 (641–1105) | |
| Lotus corniculatus L. (Fabaceae) | BF | 6 | 0.794 | 0.017 | 3.6 (±0.8) | 1111 (714–1825) |
| FF | 6 | 0.878 | 0.006 | 3.1 (±0.6) | 1111 (714–1825) | |
| EF | 11 | 0.683 | 0.002 | 1.7 (±0.4) | 1270 (714–1984) | |
| Potentilla erecta (L.) Raeusch (Rosaceae) | BF | 4 | 0.917 | 0.043 | 3.6 (±0.8) | 762 (817–1579) |
| FF | 5 | 0.916 | 0.011 | 3.1 (±0.5) | 762 (817–1579) | |
| EF | 9 | 0.074 | 0.479 | 0.8 (±1.0) | 1167 (641–1808) | |
| Prunella vulgaris L. (Lamiaceae) | BF | 3 | 0.900 | 0.205 | 7.0 (±2.3) | 288 (817–1105) |
| FF | 5 | 0.816 | 0.036 | 2.7 (±0.7) | 865 (714–1579) | |
| EF | 8 | 0.769 | 0.004 | 2.5 (±0.6) | 938 (641–1579) | |
| Ranunculus acris L. (Ranunculaceae) | BF | # | # | # | # | # |
| FF | 3 | 0.894 | 0.211 | 7.9 (±2.7) | 127 (714–841) | |
| EF | 3 | 0.999 | 0.019 | 5.7 (±0.2) | 246 (714–960) | |
| Ranunculus montanus Willd (Ranunculaceae) | BF | # | # | # | # | # |
| FF | 3 | 0.953 | 0.139 | 5.1 (±1.1) | 780 (1045–1825) | |
| EF | 8 | 0.954 | <0.001 | 4.9 (±0.4) | 1343 (641–1984) | |
| Trifolium pratense L. (Fabaceae) | BF | 5 | 0.648 | 0.100 | 3.4 (±1.4) | 984 (841 –1825) |
| FF | 7 | 0.647 | 0.029 | 4.1 (±1.3) | 1111 (714–1825) | |
| EF | 10 | 0.684 | 0.003 | 4.1 (±1.0) | 1111 (714–1825) |
Environmental data
Temperature data was derived from iButton data loggers (Thermochron iButtons DS1921G#F5; Maxim Integrated Products, Sunnyvale, CA, USA), which were located in the middle of each treatment plot, recording the temperature at 2h intervals. For snowmelt treatments, iButton loggers were used to determine snow melting dates from subnivean temperatures, which were measured near the soil surface. Due to technical faults of the iButton data loggers, there was no information about snow melting dates for the sites at 817 and 1920 m. The amount of rain excluded from drought plots through rain-out shelters was estimated with the help of rain collectors next to the site. Averaged over all sites, mean precipitation was 379±71 l m–2 during the drought period. Linear regression analysis revealed no significant relationship between the amount of precipitation among sites along the altitudinal gradient (P=0.3). Soil moisture content was measured with a portable soil moisture meter (Delta-T Devices type HH2 + ThetaProbe ML2x sensors, Cambridge, UK) on average four times per plot during the drought period.
Statistical analyses
Linear regression models with data from control plots over the entire altitudinal gradient were conducted to test the effect of altitude on the timing of phenophases (beginning of flowering, full flowering, and end of flowering) for each species. To test whether the response to altitude changed with the timing of mean onset dates, weighted linear regression of significant altitudinal regression coefficients on mean dates was carried out. We weighted the dependent variable in dependence on its residuals. A mixed-effect analysis of covariance (ANCOVA) with Type I sums of squares was used for each species and phenophase (beginning of flowering, full flowering, and end of flowering) separately to test whether there were differences in phenology due to experimental treatments. In this model, site nested within altitude was considered as a random factor and treatment as a fixed factor. Altitude was included as a covariate to remove the effect of altitude from the treatment comparison. Tukey’s HSD for multiple comparisons was used when the model was significant. Mean onset dates were derived from the adjusted means from the mixed-effect model. ANCOVA was conducted separately for lower (600–1300 m) and higher (1300–2000 m) sites to detect changes in the response to experimental treatments over the altitudinal gradient.
Paired t-tests were used to test whether soil moisture content differed between control and treated plots during the drought period. Further t-tests were conducted to see whether there were differences in treatment effects between lower and higher sites.
All statistical analyses were performed with SPSS 19.0 (SPSS, Chicago, IL, USA).
Results
Abiotic treatment effects
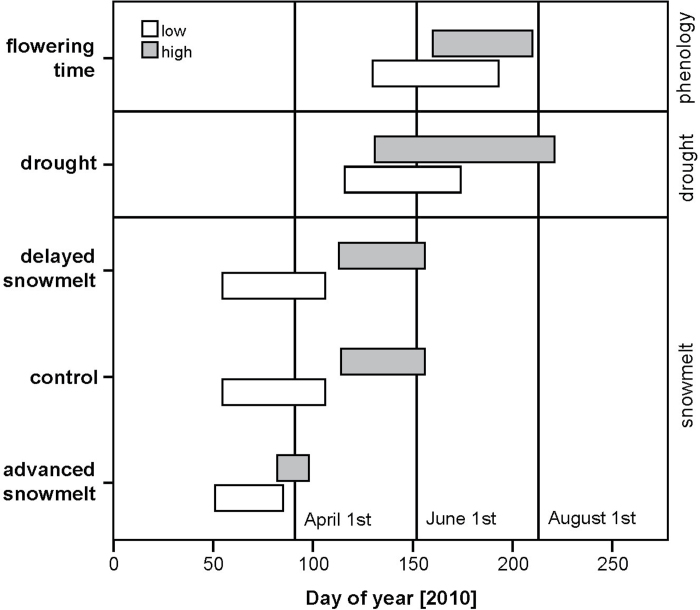
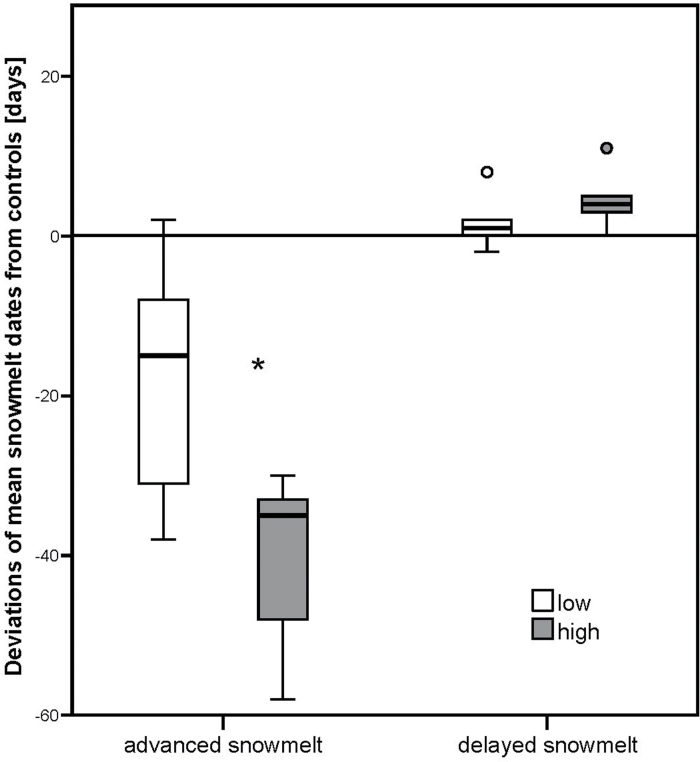
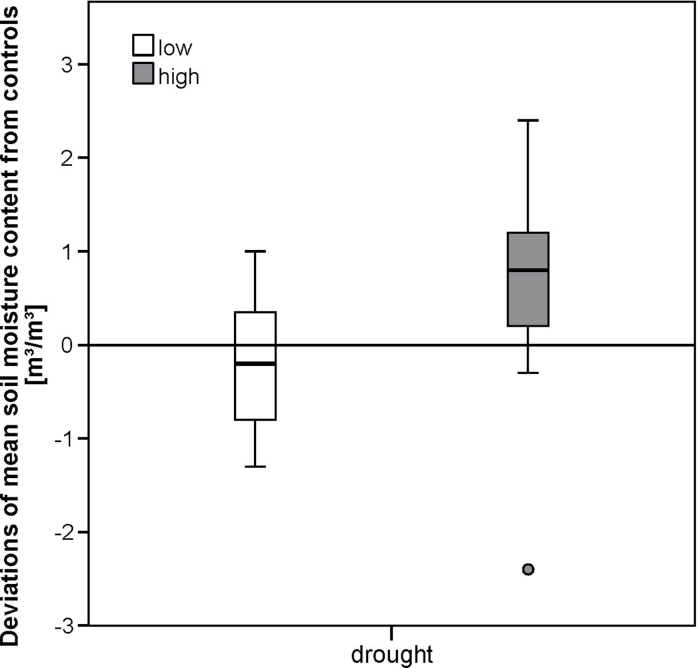
Averaged over the lower gradient from 600 to 1300 m, snow melted ~18 d earlier on advanced snowmelt plots than on control plots [between 20 February (DOY 51) and 23 March (DOY 82)] (Figs 1 and 2). At higher altitudes (1300–2000 m), the snow melting date on advanced snowmelt plots was between 23 March (DOY 82) and 8 April (DOY 98), which was about 40 d earlier in comparison with control plots (Figs 1 and 2). A t-test showed a significant difference in the advance of snow melting dates in comparison with control plots at lower and higher sites on advanced snowmelt plots (P=0.014). On delayed snowmelt plots, the mean snow melting date was about 2 d later at lower and about 5 d later at higher sites than on control plots (Fig. 2). The date of snowmelt on delayed plots was between 22 February (DOY 53) and 16 April (DOY 106) at lower sites and between 23 April (DOY 113) and 5 June (DOY 156) at higher sites (Fig. 1). A t-test showed no significant differences in the delay of snowmelt in comparison with controls between the lower and higher sites on delayed snowmelt plots (P=0.157, Fig. 2). During the drought period, soil moisture content was, averaged over all sites, significantly different between control and drought plots (paired t-test, P <0.001); however, the difference did not change with altitude, as a t-test was not significant different between the lower and higher sites (P=0.301, Fig. 3). No significant difference in soil moisture content was found between controls and either advanced or delayed snowmelt plots (paired t-test, P=0.786 or P=0.932, respectively).
Fig. 1.
Range in the date of snowmelt (advanced snowmelt, control, delayed snowmelt), duration of drought treatment, and flowering time of all species over the lower (600–1300 m) and the higher (1300–2000 m) altitudinal gradient in 2010.
Fig. 2.
Deviations from controls of mean snowmelt dates on advanced and delayed snowmelt plots, derived from each altitudinal site singly and then separated by low (600–1300 m) and high (1300–2000 m) altitudes. The asterisk indicates significant differences in snow melting date between lower and higher sites (P <0.05).
Fig. 3.
Deviations of soil moisture content on drought plots from controls, derived from each altitudinal site singly and then separated by low (600–1300 m) and high (1300–2000 m) sites.
Phenological shifts due to altitude
Linear regression models mostly showed significant responses of flowering phenology to altitude (Table 1). Averaged over all phenophases and species, there was a delay in onset dates of 3.4 d per 100 m increase in elevation. However, the altitudinal response differed strongly among species, being greatest for the end of flowering of R. acris (5.7 d per 100 m) and smallest for the end of flowering of Lotus corniculatus L. (1.7 d per 100 m).
Weighted linear regression analysis showed that phenophases occurring later in the year were significantly less responsive to altitudinal change than phenophases early in the year (P=0.043, R 2=0.593).
Phenological differences due to treatments
ANCOVA showed few significant differences in the timing of phenophases between treatments with differences found mainly at higher sites, except for Prunella vulgaris L., which showed a significant shift at the lower gradient (Tables 2 and 3).
Table 2.
Mixed-effect ANCOVA showing differences in phenological onset dates (beginning of flowering, full flowering, and end of flowering) between treatments [control (co), advanced snowmelt (ad), delayed snowmelt (de), and drought (dr)] for the lower part (600–1300 m) of the altitudinal gradient. Numbers in bold are significant (P <0.05). NA, data not available. Mean onset dates are derived as adjusted means from the model. Tukey’s HSD was conducted for multiple comparisons if the model was significant; letters indicate a significant difference between respective treatments.
| Beginning of flowering | Full flowering | End of flowering | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | co | ad | de | dr | P | co | ad | de | dr | P | co | ad | de | dr | |
| Alchemilla vulgaris L. | 140 | 137 | NA | 139 | 140 | 137 | 139 | 139 | 174 | 177 | NA | 177 | |||
| 0.622 | 0.153 | 0.369 | |||||||||||||
| Briza media L. | 173 | 172 | 174 | 172 | 175 | 177 | 179 | 173 | 181 | 181 | 182 | 180 | |||
| 0.485 | 0.236 | 0.103 | |||||||||||||
| Campanula scheuchzeri Vill. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Dactylis glomerata L. | 170 | 168 | 170 | 167 | 172 | 170 | 173 | 170 | 177 | 175 | 178 | 175 | |||
| 0.106 | 0.354 | 0.265 | |||||||||||||
| Lotus corniculatus L. | 166 | 156 | 170 | NA | 170 | 162 | 178 | NA | 189 | 188 | 200 | 198 | |||
| 0.253 | 0.223 | 0.160 | |||||||||||||
| Potentilla erecta (L.) Raeusch | 149 | 153 | 153 | 152 | 156 | 158 | 158 | 158 | 189 | 189 | 192 | 187 | |||
| 0.218 | 0.744 | 0.714 | |||||||||||||
| Prunella vulgaris L. | 183 | 183 | 181 | 185 | 185 | 183 | 187 | 185 | 193 | 191 | 196 | 191 | |||
| 0.800 | 0.290 | 0.047 | a | a, b | b | ||||||||||
| Ranunculus acris L. | 143 | 140 | 144 | 141 | 144 | 143 | 144 | 142 | 159 | 157 | 158 | 158 | |||
| 0.695 | 0.402 | 0.763 | |||||||||||||
| Ranunculus montanus Willd. | NA | NA | NA | NA | NA | 130 | 128 | 130 | 130 | 136 | 133 | 137 | 136 | ||
| 0.590 | 0.801 | ||||||||||||||
| Trifolium pratense L. | 167 | 167 | 162 | 169 | 173 | 172 | 170 | 171 | 184 | 181 | 181 | 181 | |||
| 0.391 | 0.147 | 0.634 | |||||||||||||
Table 3.
Mixed-effect ANCOVA showing differences in phenological onset dates (beginning of flowering, full flowering, and end of flowering) between treatments [control (co), advanced snowmelt (ad), delayed snowmelt (de), and drought (dr)] for the higher part (1300–2000 m) of the altitudinal gradient. Numbers in bold are significant (P <0.05). NA data not available. Mean onset dates are derived as adjusted means from the model. Tukey’s HSD was conducted for multiple comparisons if the model was significant; letters indicate a significant difference between respective treatments.
| Beginning of flowering | Full flowering | End of flowering | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | co | ad | de | dr | P | co | ad | de | dr | P | co | ad | de | dr | |
| Alchemilla vulgaris L. | 160 | 154 | 166 | 161 | 170 | 161 | 172 | 169 | 187 | 187 | 189 | 185 | |||
| 0.005 | a | a b c | b | c | 0.028 | a | a b c | b | c | 0.255 | |||||
| Briza media L. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Campanula scheuchzeri Vill. | 209 | 211 | 213 | 207 | 210 | 211 | 214 | 207 | 216 | 219 | 225 | 217 | |||
| 0.122 | 0.123 | <0.001 | a | b | a b c | c | |||||||||
| Dactylis glomerata L. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lotus corniculatus L. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 202 | 200 | 200 | 202 | |
| 0.242 | |||||||||||||||
| Potentilla erecta (L.) Raeusch | 179 | 173 | 183 | 178 | 180 | 175 | 184 | 181 | NA | 195 | 200 | 192 | |||
| 0.318 | 0.192 | 0.036 | a | b | a b | ||||||||||
| Prunella vulgaris L. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ranunculus acris L. | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ranunculus montanus Willd. | 169 | 153 | 168 | 171 | 170 | 158 | 171 | 176 | 178 | 170 | 181 | 179 | |||
| 0.092 | 0.005 | a | a b c | b | c | 0.006 | a | a b c | b | c | |||||
| Trifolium pratense L. | NA | NA | NA | NA | NA | 199 | 196 | 201 | 198 | 210 | 207 | 211 | 210 | ||
| 0.610 | 0.410 | ||||||||||||||
Tukey’s HSD post-hoc tests showed a significant advance of 6–12 d for the beginning of flowering and full flowering of Alchemilla vulgaris L. on advanced snowmelt plots in comparison with control and other treatment plots (Table 3). Full flowering and end of flowering of R. montanus were also 8–18 d earlier on advanced snowmelt plots in comparison with the other treatments (Table 3). A significant delay of 5–9 d in the end of flowering of C. scheuchzeri and P. vulgaris was recorded on delayed snowmelt plots (Tables 2 and 3). For Potentilla erecta (L.) Raeusch, the end of flowering was significantly advanced on drought plots (by 3–8 d) in comparison with advanced and delayed snowmelt plots (no data available for control plots, Table 3).
The timing of phenophases, averaged over all species, was about 1–7 d earlier on advanced snowmelts plots in comparison with control plots (including non-significant results). The timing was 2–3 d later or about the same (0 to –2 d) on delayed snowmelt and drought plots, respectively.
On advanced snowmelt plots, effects were much greater at higher than at lower sites (mean response of –1 day on lower and –5 d on higher sites). On delayed snowmelt plots, the response was the same for lower and higher sites (mean delay of 3 d). The mean response to drought was –2 d for lower sites, but no significant difference in phenology was found at higher sites.
The effect of an advanced snowmelt appeared to be more pronounced earlier in the year, showing a response of –7 d on higher sites for the beginning of flowering and only –1 day for the end of flowering. The effect of delayed snowmelt and drought appeared to be consistent throughout the year.
Changes in duration of flower phenology
ANCOVA showed that, for all species, manipulative treatments had no significant effect on the duration of flower phenology except for A. vulgaris showing a prolongation of 7 d on advanced snowmelt plots in comparison with the control plots (Table 4). For P. erecta, flower duration was much longer at lower altitudes (33 d) than at higher altitudes (18 d) averaged over all treatments (Table 4).
Table 4.
Mixed-effect ANCOVA showing differences in the length of flowering period (days from beginning of flowering to end of flowering) between treatments [control (co), advanced snowmelt (ad), delayed snowmelt (de), and drought (dr)] over a lower (l) (600–1300 m) and a higher (h) (1300–2000 m) altitudinal gradient. Numbers in bold are significant (P <0.05). Tukey’s HSD was conducted for multiple comparisons if the model was significant; letters indicate a significant difference between respective treatments. NA, data not available.
| Duration (days) | ||||||
|---|---|---|---|---|---|---|
| P | co | ad | de | dr | ||
| Alchemilla vulgaris L. | Mean (l) | NA | NA | NA | NA | NA |
| Treatment (l) | ||||||
| Mean (h) | 0.010 | 30 | 37 | 25 | 27 | |
| Treatment (h) | a | a b c | b | c | ||
| Briza media L. | Mean (l) | 9 | 9 | 8 | 7 | |
| Treatment (l) | 0.552 | |||||
| Mean (h) | NA | NA | NA | NA | NA | |
| Treatment (h) | ||||||
| Campanula scheuchzeri Vill. | Mean (l) | NA | NA | NA | NA | NA |
| Treatment (l) | ||||||
| Mean (h) | 11 | 11 | 12 | 14 | ||
| Treatment (h) | 0.253 | |||||
| Dactylis glomerata L. | Mean (l) | 11 | 9 | 11 | 11 | |
| Treatment (l) | 0.396 | |||||
| Mean (h) | NA | NA | NA | NA | NA | |
| Treatment (h) | ||||||
| Lotus corniculatus L. | Mean (l) | 22 | 30 | 31 | ||
| Treatment (l) | 0.696 | |||||
| Mean (h) | NA | NA | NA | NA | NA | |
| Treatment (h) | ||||||
| Potentilla erecta (L.) Raeusch | Mean (l) | 33 | 31 | 35 | 31 | |
| Treatment (l) | 0.591 | |||||
| Mean (h) | 18 | 22 | 17 | 15 | ||
| Treatment (h) | 0.082 | |||||
| Prunella vulgaris L. | Mean (l) | 12 | 10 | 15 | 9 | |
| Treatment (l) | 0.935 | |||||
| Mean (h) | NA | NA | NA | NA | NA | |
| Treatment (h) | ||||||
| Ranunculus acris L. | Mean (l) | NA | NA | NA | NA | NA |
| Treatment (l) | ||||||
| Mean (h) | NA | NA | NA | NA | NA | |
| Treatment (h) | ||||||
| Ranunculus montanus Willd. | Mean (l) | NA | NA | NA | NA | NA |
| Treatment (l) | ||||||
| Mean (h) | 10 | 20 | 16 | 9 | ||
| Treatment (h) | 0.241 | |||||
| Trifolium pratense L. | Mean (l) | 12 | 12 | 15 | 10 | |
| Treatment (l) | 0.283 | |||||
| Mean (h) | NA | NA | NA | NA | NA | |
| Treatment (h) | ||||||
Discussion
The present study showed strong responses of the flower phenology of different grassland species to altitude. Furthermore, we demonstrated that advanced snowmelt had a greater influence on flower phenology at higher than at lower sites due to a stronger treatment effect at higher altitudes. However, altitude had no significant effect on responses to delayed snowmelt or drought, whereas treatment effects were rather small over the entire gradient. Flowering duration was mostly not influenced by manipulative treatments at both higher and lower sites.
Phenological response to altitudinal change
Averaged over all species and phenophases, there was a delay in flower phenology of 3.4 d per 100 m increase. This is in accordance with the results of Cornelius et al. (2012) who showed a delay in flower and leaf phenology of 3.8 d per 100 m increase for tree and herbaceous species in the same region. However, the response to altitude change is species specific, ranging between 1.7 and 5.7 d per 100 m, which is similar to the 1.7–6.9 d per 100 m shown by Cornelius et al. (2012). Most altitudinal studies refer to tree species (e.g. Roetzer and Chmielewski, 2001; Dittmar and Elling, 2006; Migliavacca et al., 2008; Vitasse et al., 2009; Moser et al., 2010); however, Ziello et al. (2009) showed, based on COST725 data for the Alpine region, a delay in the beginning of flowering of Dactylis glomerata L. of 2.8 d per 100 m. This is slightly less sensitive to altitude than the non-significant response of D. glomerata in our study (3.9 d per 100 m). We assume that if we had had a greater number of observations in the present study these values would have been more similar.
Phenophases later in the year were significantly less sensitive to altitude than phenophases early in the year, which was also confirmed by Cornelius et al. (2012). A weaker response to temperature of species flowering in May and June in comparison with earlier spring flowering species was also shown by Fitter and Fitter (2002) and Menzel et al. (2006), who found that, because of high temperature variability in spring, the earlier the species, the stronger the sensitivity to temperature. However, in the present study as well as that of Cornelius et al. (2012), early (March to May) and late (September to October) species and phases are missing. Thus, it appears that response to temperature is declining not only in spring but consistently throughout the year.
Phenological response to treatments
The experiment showed only a few significant differences in the timing of flowering due to the manipulative treatments. Earlier snowmelt advanced flower phenology in most cases, although the effect was only significant for four species and phenophases. An earlier snowmelt is in accordance with the results of other studies (Price and Waser, 1998; Dunne et al., 2003; Lambert et al., 2010; Wipf, 2010). For example, Wipf et al. (2009) showed an advance of flower phenology of up to 10 d (present study 1–7 d). The response of species to later snowmelt was rather small; only the end of flowering phenophase was sometimes significantly delayed. Delayed timing due to later snowmelt was also shown in other studies (Weaver and Collins, 1977; Torp et al., 2010; Chen et al., 2011; Cooper et al., 2011) that demonstrated a delay in the beginning of flowering and peak flowering of about 6–8 d for alpine species. In our study, the response was smaller with a non-significant delay of 2–3 d between delayed snowmelt and control plots. Hoye et al. (2007), however, showed that the result of delayed snowmelt was not necessarily later flowering but could also be unchanged. Thus, the response to delayed snowmelt also appears to be species specific. Wipf and Rixen (2010) suggested that, in general, the least responsive and least consistent responses to shifts in the date of snowmelt were in the grasses, whilst forbs were a little more responsive. Furthermore, the advanced snowmelt treatment was very early in the year, thus early flowering species such as A. vulgaris or R. montanus were affected, whereas an effect on late flowering species such as C. scheuchzeri or P. vulgaris was rather unlikely. Late-flowering species as well as phenophases later in the season were less responsive to snowmelt than early-flowering species or phenophases early in the season because these are controlled by temperature (Price and Waser, 1998; Dunne et al., 2003; Wipf, 2010).
Across species and phenophases, drought treatment did not influence flowering significantly except for P. vulgaris. No effect of drought on plant phenology was also found for different grass species in an alpine meadow in France (Bloor et al., 2010) and for the onset of growth of Globularia alypum L. in a Mediterranean shrubland (Bernal et al., 2011). However, flowering of G. alypum was delayed by drought (Prieto et al., 2008). A delay in flowering time after a drought period was also demonstrated for other Mediterranean plants (Llorens and Penuelas, 2005). In contrast, Jentsch et al. (2009) showed an advance of the mid-flowering date by 4 d after a drought period of 32 d. Thus, plant response to drought appears to be highly species specific (Bernal et al., 2011) and ecosystem dependent. In the present study, soil moisture content on drought plots was 42% on average, which was probably not low enough to simulate a drought event that affects plant phenology (Fig. 3).
The flowering durations of species were mostly not significantly affected by the manipulative treatments. As far as we know, studies dealing with the impacts of snowmelt date on flower duration are rare and contradictory. Price and Waser (1998) showed that early snowmelt was associated with a longer flowering duration, which agrees with our prolongation of the flowering duration of A. vulgaris on advanced snowmelt plots. However, Wipf (2010) demonstrated that flowering duration was not affected by snowmelt timing, which agrees with the results of all other species in our study. Studies dealing with the impacts of drought on flowering duration are also rare and contradictory. Jentsch et al. (2009) reported a lengthening of the flowering period after a drought event, whereas Llorens and Penuelas (2005) reported both a shortening and a lengthening of the flowering duration of two different Mediterranean dwarf shrubs. In our study, the intensity of drought was probably not sufficient to cause shifts in flowering duration. However, general conclusions from ambiguous results as shown in these studies should be drawn with care due to slight differences in flowering duration definitions among studies.
Shifts in the phenological response due to changes in altitude
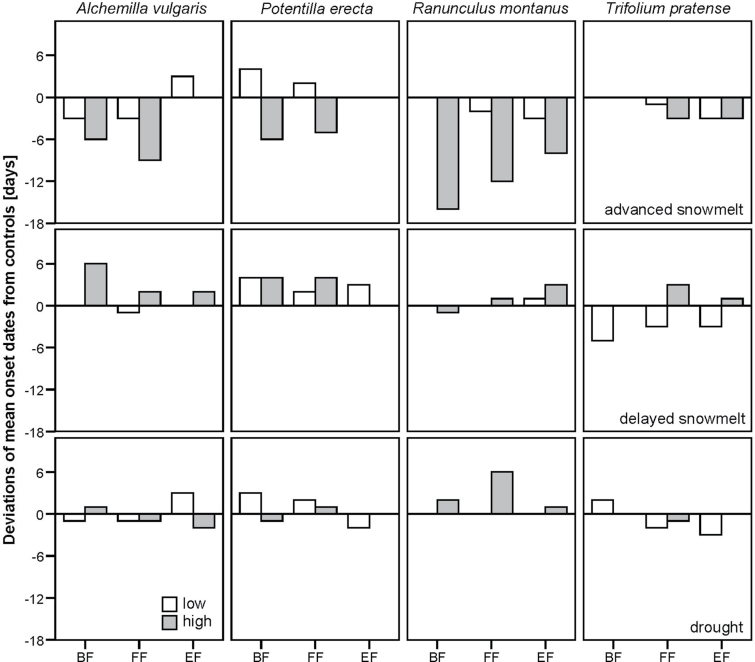
We had data for both the lower and the higher altitudes for only a few species. Ideally, species would have been distributed over the entire altitudinal gradient and also located in each treatment plot. However, due to restrictions in their natural distribution, species are often not spread over the entire gradient. Thus, only four species were monitored on both lower and higher sites. For these species, the impacts of treatments on flower phenology are additionally illustrated in Fig. 4. For advanced snowmelt plots, treatment effects were more pronounced at higher sites; however, other treatments showed no significant difference between altitude bands. Thus, on advanced snowmelt plots, the phenological response was most likely stronger at higher altitudes due to a larger treatment effect and not because species reacted more sensitively at higher than at lower altitudes. For delayed snowmelt and drought plots, no significant differences in treatment effects between lower and higher altitudes were detected. Thus, phenological differences were not significant, which indicates that species responded similarly at different altitudes. Defila and Clot (2005) showed from a 50-year time series in Switzerland that the total proportion of significant trends was higher in alpine regions (higher than 1000 m a.s.l.; 42%) and lower in lowland regions (lower than 600 m a.s.l.; 33%). However, considering the intensity of trends, the results showed an advance of full flowering of 32 d in the lowland and 20 d in the alpine region.
Fig. 4.
Deviations from controls of mean onset dates from the ANCOVA model for each studied phenophase (BF, beginning of flowering; FF, full flowering; EF, end of flowering) separately by low (600–1300 m) and high (1300–2000 m) sites for the species Alchemilla vulgaris L., Potentilla erecta (L.) Raeusch, Ranunculus montanus Willd, and Trifolium pratense L., observed at low and high altitudes.
The length of flowering of P. erecta was greater at lower altitudes for all treatments, which led to the conclusion that duration was probably influenced by higher temperatures at lower sites. Tyler (2001) showed that the length of the flowering period was only slightly influenced by temperature because the timing of the onset of both start and end of phenophases advances in response to temperature increase; thus, the total length is not affected. However, Cornelius et al. (2012) showed that, although for most species flowering duration was not influenced by temperature, some species prolonged their flowering duration mainly due to a weaker response of end of flowering, which is related to the declining sensitivity to temperature change over the season, as also shown in the present study.
Conclusion
Our results suggest that changes in the abiotic environment such as shifts in the date of snowmelt only influence the timing of flowering if the effect is rather distinctive. As this will probably be the case at higher altitudes, species there may be more affected by global climate change. Furthermore, this study showed that a 1000-year extreme drought event in the Bavarian Alps did not substantially influence the phenology of grassland species. Thus, the risk of severe impacts of droughts on flowering phenology will be rather low here. Consequently, shifts in temperature and in the date of snowmelt will constitute the main factors that alter plant phenology under future climate change; however, the magnitude of change will depend strongly on the species.
Acknowledgements
The study was pursued within the framework of the joint research centre FORKAST and was funded by the Bavarian Climate Programme 2020. We thank Tim Sparks for his valuable comments on this paper. We also thank the Berchtesgaden National Park for providing infrastructure and Inga Eschenlohr for her field work.
Glossary
Abbreviations:
- ANCOVA
analysis of covariance
- a.s.l.
above sea level
- BBCH
Biologische Bundesanstalt, Bundessortenamt and Chemical Industry
- DOY
day of year
- OLR
ordinal logistic regression.
References
- Abu-Asab MS, Peterson PM, Shetler SG, Orli SS. 2001. Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodiversity and Conservation. 10, 597–612 [Google Scholar]
- Auer I, Böhm R, Jurkovic A, et al. 2007. HISTALP—historical instrumental climatological surface time series of the Greater Alpine Region. International Journal of Climatology. 27, 17–46 [Google Scholar]
- Beniston M. 2003. Climatic change in mountain regions: a review of possible impacts. Climatic Change. 59, 5–31 [Google Scholar]
- Bernal M, Estiarte M, Penuelas J. 2011. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora . Plant Biology. 13, 252–257 [DOI] [PubMed] [Google Scholar]
- Blionis GJ, Halley JM, Vokou D. 2001. Flowering phenology of Campanula on Mt Olympos, Greece. Ecography. 24, 696–706 [Google Scholar]
- Bloor JM, Pichon P, Falcimagne R, Leadley P, Soussana JF. 2010. Effects of warming, summer drought, and CO2 enrichment on aboveground biomass production, flowering phenology, and community structure in an upland grassland ecosystem. Ecosystems. 13, 888–900 [Google Scholar]
- Chen W, Wu Y, Wu Ning, Luo P. 2011. Variation in phenology and population distribution pattern of three alpine species along the snowmelt gradient. Bulletin of Botanical Research. 31, 206–212 [Google Scholar]
- Cooper EJ, Dullinger S, Semenchuk P. 2011. Late snowmelt delays plant development and results in lower reproductive success in the High Arctic. Plant Science. 180, 157–167 [DOI] [PubMed] [Google Scholar]
- Cornelius C, Estrella N, Franz H, Menzel A. 2012. Linking altitudinal gradients and temperature responses of plant phenology in the Bavarian Alps. Plant Biologydoi: 10.1111/j.1438–8677.2012.00577.x [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cornelius C, Petermeier H, Estrella N, Menzel A. 2011. A comparison of methods to estimate seasonal phenological development from BBCH scale recording. International Journal of Biometeorology. 55, 867––877 [DOI] [PubMed] [Google Scholar]
- De Frenne P, De Schrijver A, Graae BJ, Gruwez R, Tack W, Vandelook F, Hermy M, Verheyen K. 2010. The use of open-top chambers in forests for evaluating warming effects on herbaceous understorey plants. Ecological Research. 25, 163–171 [Google Scholar]
- Defila C, Clot B. 2005. Phytophenological trends in the Swiss Alps, 1952–2002. Meteorologische Zeitschrift. 15, 191–196 [Google Scholar]
- Dittmar C, Elling W. 2006. Phenological phases of common beech (Fagus sylvatica L.) and their dependence on region and altitude in Southern Germany. European Journal of Forest Research. 125, 181–188 [Google Scholar]
- Dunne JA, Harte J, Taylor KJ. 2003. Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecological Monographs. 73, 69–86 [Google Scholar]
- Erschbamer B, Kiebacher T, Mallaun M, Unterluggauer P. 2009. Short-term signals of climate change along an altitudinal gradient in the South Alps. Plant Ecology. 202, 79–89 [Google Scholar]
- Fitter AH, Fitter RSR. 2002. Rapid changes in flowering time in British plants. Science. 296, 1689–1691 [DOI] [PubMed] [Google Scholar]
- Galen C. 2000. High and dry: drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). American Naturalist. 156, 72–83 [DOI] [PubMed] [Google Scholar]
- Galen C, Stanton ML. 1993. Short-term responses of Alpine buttercups to experimental manipulations of growing-season length. Ecology. 74, 1052–1058 [Google Scholar]
- Grabherr G, Gottfried M, Pauli H. 1994. Climate effects on mountain plants. Nature. 369, 448 [DOI] [PubMed] [Google Scholar]
- Hollister RD, Webber PJ. 2000. Biotic validation of small open-top chambers in a tundra ecosystem. Global Change Biology. 6, 835–842 [Google Scholar]
- Hoye TT, Ellebjerg SM, Philipp M. 2007. The impact of climate on flowering in the High Arctic—the case of Dryas in a hybrid zone. Arctic Antarctic and Alpine Research. 39, 412–421 [Google Scholar]
- Inouye DW. 2000. The ecological and evolutionary significance of frost in the context of climate change. Ecology Letters. 3, 457–463 [Google Scholar]
- Inouye DW. 2008. Effects of climate change on phenoloy, frost damage, and floral abundance of montane wildflowers. Ecology. 89, 353–362 [DOI] [PubMed] [Google Scholar]
- Inouye DW, McGuire AD. 1991. Effects of snowpack on timing and abundance of flowering in Delphinium nelsonii (Ranunculaceae): implications for climate change. American Journal of Botany. 78, 997–1001 [Google Scholar]
- Inouye DW, Morales MA, Dodge GJ. 2002. Variation in timing and abundance of flowering by Delphinium barbeyi Huth (Ranunculaceae): the roles of snowpack, frost, and La Nina, in the context of climate change. Oecologia. 130, 543–550 [DOI] [PubMed] [Google Scholar]
- Inouye DW, Saavedra F, Lee-Yang W. 2003. Environmental influences on the phenology and abundance of flowering by Androsace septentrionalis (Primulaceae). American Journal of Botany. 90, 905–910 [DOI] [PubMed] [Google Scholar]
- Inouye DW, Wielgolaski FE. 2003. High altitude climates.. In: Schwartz MD, ed. Phenology: an integrative environmental science. Dordrecht: Kluwer Academic Publisher; 195–214 [Google Scholar]
- IPCC 2007. Climate change 2007: synthesis report. Fourth assessment report of the Intergovernmental Panel on Climate Change Core Writing Team, Pachauri RK, Reisinger A, eds. Geneva, Switzerland: Intergovernmental Panel on Climate Change; [Google Scholar]
- Jentsch A, Beierkuhnlein C. 2008. Research frontiers in climate change: effects of extreme meteorological events on ecosystems. Comptes Rendus Geoscience. 340, 621–628 [Google Scholar]
- Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. 2009. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology. 15, 837–849 [Google Scholar]
- Keller F, Korner C. 2003. The role of photoperiodism in alpine plant development. Arctic Antarctic and Alpine Research. 35, 361–368 [Google Scholar]
- Konnert V. 2004. Standortkarte Nationalpark Berchtesgaden. Forschungsbericht des Nationalparks Berchtesgaden. 49, 1–151 [Google Scholar]
- Korner C, Miglietta F. 1994. Long-term effects of naturally elevated CO2 on Mediterranean grassland and forest trees. Oecologia. 99, 343–351 [DOI] [PubMed] [Google Scholar]
- Korner C. 1992. Response of alpine vegetation to global climate change. Catena Supplement. 22, 85–96 [Google Scholar]
- Kudernatsch T, Fischer A, Bernhardt-Romermann M, Abs C. 2008. Short-term effects of temperature enhancement on growth and reproduction of alpine grassland species. Basic and Applied Ecology. 9, 263–274 [Google Scholar]
- Laghari AN, Vanham D, Rauch W. 2012. To what extent does climate change result in a shift in Alpine hydrology? A case study in the Austrian Alps. Hydrological Sciences Journal. 57, 103–117 [Google Scholar]
- Lambert AM, Miller-Rushing AJ, Inouye DW. 2010. Changes in snowmelt date and summer precipitation affect the flowering phenology of Erythronium grandiflorum (Glacier Lily; Liliaceae). American Journal of Botany. 97, 1431–1437 [DOI] [PubMed] [Google Scholar]
- Llorens L, Penuelas J. 2005. Experimental evidence of future drier and warmer conditions affecting flowering of two co-occurring Mediterranean shrubs. International Journal of Plant Sciences. 166, 235–245 [Google Scholar]
- Marion GM, Henry GHR, Freckman DW, et al. 1997. Open-top designs for manipulating field temperature in high-latitude ecosystems. Global Change Biology. 3, 20–32 [Google Scholar]
- Meier U. 2001. Growth stages of plants. BBCH Monograph. Berlin: Blackwell Wissenschafts-Verlag Berlin; [Google Scholar]
- Menzel A, Estrella N, Testka A. 2005. Temperature response rates from long-term phenological records. Climate Research. 30, 21–28 [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology. 12, 1969–1976 [Google Scholar]
- Migliavacca M, Cremonese E, Colombo R, et al. 2008. European larch phenology in the Alps: can we grasp the role of ecological factors by combining field observations and inverse modelling?. International Journal of Biometeorology. 52, 587–605 [DOI] [PubMed] [Google Scholar]
- Miranda JD, Padilla FM, Pugnaire FI. 2009. Response of a Mediterranean semiarid community to changing patterns of water supply. Perspectives in Plant Ecology Evolution and Systematics. 11, 255–266 [Google Scholar]
- Molau U. 1997. Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: Cassiope tetragona and Ranunculus nivalis . Global Change Biology. 3, 97–107 [Google Scholar]
- Moser L, Fonti P, Buentgen U, Esper J, Luterbacher J, Franzen J, Frank D. 2010. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiology. 30, 225–233 [DOI] [PubMed] [Google Scholar]
- Penuelas J, Filella I, Zhang XY, Llorens L, Ogaya R, Lloret F, Comas P, Estiarte M, Terradas J. 2004. Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytologist. 161, 837–846 [DOI] [PubMed] [Google Scholar]
- Price MV, Waser NM. 1998. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology. 79, 1261–1271 [Google Scholar]
- Prieto P, Penuelas J, Ogaya R, Estiarte M. 2008. Precipitation-dependent flowering of Globularia alypum and Erica multiflora in Mediterranean shrubland under experimental drought and warming, and its inter-annual variability. Annals of Botany. 102, 275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetzer T, Chmielewski FM. 2001. Phenological maps of Europe. Climate Research. 18, 249–257 [Google Scholar]
- Rolland C. 2003. Spatial and seasonal variations of air temperature lapse rates in Alpine regions. Journal of Climate. 16, 1032–1046 [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, et al. 2000. Biodiversity—global biodiversity scenarios for the year 2100. Science. 287, 1770–1774 [DOI] [PubMed] [Google Scholar]
- Sangtarash M, Qaderi M, Chinnappa C, Reid MD. 2009. Differential responses of two Stellaria longipes ecotypes to ultraviolet-B radiation and drought stress. Flora. 204, 593–603 [Google Scholar]
- Schwartz MD, Reiter BE. 2000. Changes in North American spring. International Journal of Climatology. 20, 929–932 [Google Scholar]
- Sparks TH, Jeffree EP, Jeffree CE. 2000. An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. International Journal of Biometeorology. 44, 82–87 [DOI] [PubMed] [Google Scholar]
- Stinson KA. 2004. Natural selection favors rapid reproductive phenology in Potentilla pulcherrima (Rosaceae) at opposite ends of a subalpine snowmelt gradient. American Journal of Botany. 91, 531–539 [DOI] [PubMed] [Google Scholar]
- StMUG (Staatsministerium für Umwelt, Gesundheit und Verbraucherschutz). 2001. Nationalparkplan. Bayerisches Staatsministerium für Landesentwicklung und Umweltfragen ed. Munich; Germany: [Google Scholar]
- Theurillat JP, Guisan A. 2001. Potential impact of climate change on vegetation in the European Alps: a review. Climatic Change. 50, 77––109 [Google Scholar]
- Torp M, Witzell J, Baxter R, Olofsson J. 2010. The effect of snow on plant chemistry and invertebrate herbivory: experimental manipulations along a natural snow gradient. Ecosystems. 13, 741–751 [Google Scholar]
- Tyler G. 2001. Relationships between climate and flowering of eight herbs in a Swedish deciduous forest. Annals of Botany. 87, 623–630 [Google Scholar]
- Venn SE, Morgan JW. 2007. Phytomass and phenology of three alpine snowpatch species across a natural snowmelt gradient. Australian Journal of Botany. 55, 450–456 [Google Scholar]
- Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. 2009. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research. 39, 1259–1269 [Google Scholar]
- Weaver T, Collins D. 1977. Possible effects of weather-modification (increased snowpack) on Festuca idahoensis meadows. Journal of Range Management. 30, 451–456 [Google Scholar]
- Wipf S, Rixen C. 2010. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Research. 29, 95–109 [Google Scholar]
- Wipf S, Stoeckli V, Bebi P. 2009. Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Climatic Change. 94, 105–121 [Google Scholar]
- Wipf S. 2010. Phenology, growth, and fecundity of eight subarctic tundra species in response to snowmelt manipulations. Plant Ecology. 207, 53–66 [Google Scholar]
- Ziello C, Estrella N, Kostova M, Koch E, Menzel A. 2009. Influence of altitude on phenology of selected plant species in the Alpine region (1971–2000). Climate Research. 39, 227–234 [Google Scholar]