Abstract
Heavy metal pollution often occurs together with organic contaminants. Brassinosteroids (BRs) induce plant tolerance to several abiotic stresses, including phenanthrene (PHE) and cadmium (Cd) stress. However, the role of BRs in PHE+Cd co-contamination-induced stress amelioration is unknown. Here, the interactive effects of PHE, Cd, and 24-epibrassinolide (EBR; a biologically active BR) were investigated in tomato plants. The application of Cd (100 µM) alone was more phytotoxic than PHE applied alone (100 µM); however, their combined application resulted in slightly improved photosynthetic activity and pigment content compared with Cd alone after a 40 d exposure. Accumulation of reactive oxygen species and membrane lipid peroxidation were induced by PHE and/or Cd; however, the differences in effect were insignificant between Cd and PHE+Cd. The foliar application of EBR (0.1 µM) to PHE- and/or Cd-stressed plants alleviated photosynthetic inhibition and oxidative stress by causing enhancement of the activity of the enzymes and related transcript levels of the antioxidant system, secondary metabolism, and the xenobiotic detoxification system. Additionally, PHE and/or Cd residues were significantly decreased in both the leaves and roots after application of EBR, more specifically in PHE+Cd-stressed plants when treated with EBR, indicating a possible improvement in detoxification of these pollutants. The findings thus suggest a potential interaction of EBR and PHE for Cd stress alleviation. These results advocate a positive role for EBR in reducing pollutant residues for food safety and also strengthening phytoremediation.
Key words: Brassinosteroids, food safety, heavy metal, photosynthesis, phytoremediation, polycyclic aromatic hydrocarbons (PAHs)
Introduction
Polycyclic aromatic hydrocarbons (PAHs) and cadmium (Cd) are two widespread environmental pollutants, well recognized for their toxic, mutagenic, and carcinogenic properties (Goldman et al., 2001; Sun et al., 2011; Adams et al., 2012). The severity of environmental contamination is further shown to be aggravated with mixed pollution of heavy metals and organic pollutants, prevalent among contaminated sites around the world (Sandrin and Maier, 2003; Pilon-Smits, 2005). Shortage of arable land in many developing countries results in growing of vegetables on and/or around polluted sites. Such plants grown in these polluted areas usually take up PAHs and heavy metals from the contaminated soil and/or air. Cd toxicity has emerged as one of the major agricultural problems in many soils around the world (Hasan et al., 2011). Evidence of PAH contamination of agricultural crops is also rapidly emerging (Mo et al., 2009). For instance, vegetables grown in the Pearl River Delta of southern China have been reported to contain toxic concentrations of PAHs as high as 5353 µg kg–1 (Mo et al., 2009). Contamination of food commodities including vegetables with Cd has also become a matter of grave concern in sustaining healthy agricultural practices (Falcó et al., 2005). Besides direct exposure to contaminants, dietary intake of Cd and PAHs has an adverse impact on potential health risk, such as childhood asthma, skin cancer, and lung cancer (Goldman et al., 2001; Falcó et al., 2005). Previous findings have shown that vegetables are one of the main sources of the total dietary intake of PAHs and Cd (Falcó et al., 2005; Mo et al., 2009). Therefore, research focusing on the reduction of xenobiotic residues in food commodities has received much attention in the past decade.
Cd is considered to be a non-essential trace element, highly phytotxoic at higher concentrations (López-Millán et al., 2009; Zhang et al., 2011; Masood et al., 2012). Cd has been shown to interfere with the uptake, transport, and utilization of essential nutrients and water, to decrease photosynthesis, change enzyme activities, and also to cause symptoms such as chlorosis, necrosis, and root browning in tomato (López-Millán et al., 2009; Hasan et al., 2011). Similarly, PAHs have also been shown to decrease biomass, photosynthetic pigments, and photosynthesis, alter antioxidant enzyme activities, and induce oxidative stress in Arabidopsis and tomato (Liu et al., 2009; Ahammed et al., 2012a , b ). Excessive production of reactive oxygen species (ROS) and subsequent oxidative damage may be the common mechanism of phytotoxicity induced by both Cd and PAHs in plants (Liu et al., 2009; Masood et al., 2012). ROS overload causes degradation of photosynthetic pigments and damage to photosynthetic machinery, which in turn decrease photosynthesis (Hasan et al., 2011; Ahammed et al., 2012b ; Masood et al., 2012). Interestingly, additive, independent, synergistic, as well as antagonistic toxicity to plants has been reported following mixed pollution of PAHs and heavy metals (Babu et al., 2001; Lin et al., 2008; Sun et al., 2011; Zhang et al., 2011). For instance, Babu et al. (2001) observed synergistic effects of photooxidized PAHs and Cu on photosynthesis and plant growth in Lemna gibba. In contrast, stress-ameliorative effects of fluoranthene (a type of PAH) against Pb and Cu on the growth of Triticum aestivum have also been reported by Wetzel et al. (1994). These authors observed antagonistic toxicity, where the negative effects of metal solutions were diminished by simultaneous addition of fluoranthene. Likewise, pyrene has been shown to alleviate Cu-induced biomass reduction in Zea mays L. (Lin et al., 2008). However, to date, the physiological and molecular mechanisms behind this synergistic or antagonistic toxicity of metal and PAHs in mixed pollution to plants is poorly understood.
Phytoremediation is a promising green technology using higher plants to restore contaminated soils and water following exposure to toxic organic and/or inorganic compounds (Pilon-Smits, 2005). In recent years, phytoremediation of co-contamination has become an appealing research avenue (Sun et al., 2011; Zhang et al., 2011). However, remediation of mixed pollution offers a complex problem, as different pollutants may influence the remediation processes through their interaction (Sandrin and Maier, 2003; Sun et al., 2011; Zhang et al., 2011). Biodegradation of organic pollutants has been shown to be affected by the presence of metals in both aerobic and anaerobic systems (Sandrin and Maier, 2003). Accordingly, interactions between metal and organic pollutants may influence stress responses and corresponding stress tolerance in plants.
Plants, being sessile, inherently possess the ability to detoxify xenobiotics for their survival (Coleman et al., 1997; Pilon-Smits, 2005). However, plants themselves suffer from phytotoxicity under high levels of toxin exposure because of rate-limiting antioxidant and detoxification capacity. Most often, the plant’s detoxification system is not very efficient in bringing about complete degradation of some recalcitrant foreign compounds (Dixit et al., 2011). Previous studies reveal that hormonal supplementation enhances plant tolerance and detoxification capacity under xenobiotic stress (Xia et al., 2009; Hasan et al., 2011; Ahammed et al., 2012a , b ).
Brassinosteroids (BRs) are a class of essential plant hormones with multiple roles in the induction of tolerance to various abiotic stresses such as high or low temperature, moisture stress, drought, salinity, pesticide injury, PAHs, and heavy metal stresses (Divi and Krishna, 2009; Xia et al., 2009; Hasan et al., 2011; Ahammed et al., 2012b ; Choudhary et al., 2012a , b ). BRs increase biomass, chlorophyll contents, photosynthesis, and the potential of antioxidants and detoxification to improve plant tolerance under stress. Previously, BRs have been reported to alleviate Cd as well as PAH stresses in different plants including tomato (Hasan et al., 2011; Ahammed et al., 2012b ). However, the role of BRs in mitigation of a co-contaminated system such as Cd+PAHs has not been studied yet. Keeping this in mind, it was hypothesized that BRs might have an anti-stress effect on the co-contamination by PAHs and Cd in tomato. Therefore, the present study was carried out to investigate the interactive effects of phenanthrene (PHE, a model PAH) and Cd on the growth, photosynthetic machinery, enzymes, and gene expression of the antioxidant and detoxification system, secondary metabolism, and pollutant residues in tomato plants. Foliar application of 24-epibrassinolide (EBR; an active BR) was used as a BR source to determine whether EBR could alleviate the PHE+Cd co-contamination-induced stress in tomato. This study will provide details of the underlying physiological and molecular mechanisms of the phytotoxicity of dual environmental pollution and the feasibility of EBR application to induce stress tolerance in tomato.
Materials and methods
Plant materials and growth conditions
Tomato (Solanum lycopersicum L. cv. Hezuo 903) seeds were purchased from the Zhejiang Academy of Agriculture, Hangzhou, China. Seeds were surface sterilized with 0.4% sodium hypochlorite for 15min followed by repeated washings with Milli-Q water. Sterilized seeds were sown in a mixture of peat and vermiculite (7:3, v:v). The experiment was carried out in a multispan greenhouse at the Institute of Vegetable Sciences, Zhejiang University. The approximate conditions inside the greenhouse during the experimental period were as follows: temperature 25/17 ºC (day/night), mean relative humidity 80%, photosynthetic photon flux density (PPFD) 800 µmol m–2 s–1, and a photoperiod of 14/10h (day/night). Upon the appearance of the first true fully expanded leaves, a group of eight seedlings was transplanted into a container (40 cm×25 cm×15cm) filled with Hoagland’s nutrient solution.
Treatments and biomass analysis
At the fourth leaf stage, the whole foliar region of the seedlings was sprayed with EBR and/or Milli-Q water (containing an equal ratio of ethanol used for the preparation of EBR solution). The working solution of 0.1 µM EBR (Sigma-Aldrich, St. Louis, MO, USA) was prepared by dissolving the solute in ethanol followed by dilution with Milli-Q water [ethanol:water (v/v)=1:10 000]. At 24h after EBR pre-treatment, seedlings were exposed to freshly prepared nutrient solution with or without PHE and/or Cd. PHE (purity 98%, Sigma-Aldrich, China) was initially dissolved in acetone and then diluted with Milli-Q water containing nutrient solution to a final concentration of 100 µM [acetone:water (v/v)=1:1000]. Controls as well as the rest of the treatments were exposed to the same ratio of acetone. Cd (100 µM) was supplied as CdCl2·2.5H2O (analytical grade) from a stock solution prepared in Milli-Q water. The concentrations of EBR, PHE, and Cd were selected on the basis of a preliminary experiment, previous studies, and others published reports (López-Millán et al., 2009; Xia et al., 2009; Ahammed et al., 2012a , b ). Thus, the experiment consisted of eight treatments with three replications (each replication means one container, containing eight seedlings). Nutrient solution was changed every 5 d with or without PHE and/or Cd to avoid metabolic effects of root exudates as well as to maintain the stable concentration. EBR was sprayed every 10 d. Forty days after imposition of stress, the experiment was terminated and plants were harvested after the determination of gas exchange and chlorophyll fluorescence parameters. For physiological, biochemical, and molecular analysis, leaves were immediately frozen in liquid nitrogen and stored at –80 °C until further analyses. Eight plants from each treatment were randomly selected and divided into shoots and roots. Selected plants were dried in an oven at 80 °C for 24h and then weighed to record their dry weights.
Determination of PHE and Cd contents
PHE was extracted from freeze-dried plant samples with dichloromethane and methanol (9:1, v:v) mixture by ultrasonication. The extracts were concentrated and cleaned up by passing them through an anhydrous Na2SO4/Florasil column, and eluted with dichloromethane. Elutes were concentrated and analysed by gas chromatography–mass spectrometry (Shimadzu GCMC-QP2010One Ultra System, Kyoto, Japan) following the method of Sun et al. (2011). To analyse Cd residue, dried leaf and root samples were ground to a fine powder and digested with HNO3/HClO4 (3:1, v:v). The Cd content was determined by atomic absorption spectrophotometry (Shimadzu AA-6300, Japan) as described in Masood et al. (2012).
Gas exchange measurements
Gas exchange parameters such as the CO2 assimilation rate (P n), stomatal conductance (g s), and the intercellular CO2 concentration (C i) were determined on the third fully expanded leaves using an infrared gas analyser (IRGA) portable photosynthesis system (LI-COR 6400, Lincoln, NE, USA). The measurement was performed within the time period 8.00–11.00h maintaining the air temperature, air relative humidity, CO2 concentration, and PPFD, at 25 °C, 80–90%, 400 µmol mol–1, and 1000 µmol m–2 s–1, respectively.
Chlorophyll a fluorescence quenching and chlorophyll content measurements
Chlorophyll fluorescence parameters were measured on the third fully expanded leaves after 30min of dark adaptation using an imaging pulse amplitude-modulated (PAM) fluorimeter (IMAG-MAXI; Heinz Walz, Effeltrich, Germany). Chlorophyll fluorescence parameters were measured and calculated as described by Xia et al. (2009) and Jiang et al. (2012). The maximum photochemical efficiency of photosystem II (PSII; F v/F m), the quantum efficiency of PSII (ΦPSII), and the photochemical quenching coefficient (qP) were determined for the same leaves as an area of interest. Photosynthetic pigments were quantified by pooling third leaves from at least three plants per treatment. Leaf chlorophyll (Chl a and Chl b) and carotenoids (Carts) were extracted in 80% acetone and their contents (µg/g FW) were analysed spectrophotometrically according to Lichtenthaler and Wellburn (1983).
Determination of H2O2 content and membrane damage (lipid peroxidation), and histochemical detection of H2O2 and O2∙– in leaves
H2O2 content in leaves was determined spectrophotometrically by a peroxidase assay according to Willekens et al. (1997). Accumulation of H2O2 in leaves was visually detected by staining with 3,3-diaminobenzidine (DAB) using the method of Thordal-Christensen et al. (1997). The O2∙─ accumulation was visualized using the nitroblue tetrazolium (NBT) staining procedure. NBT- and DAB-stained leaves were photographed using a digital camera (Canon DS126201, Japan). The level of lipid peroxidation in leaves was determined by quantifying the malondialdehyde (MDA) equivalents using 2-thiobarbituric acid (TBA) as described by Hodges et al. (1999).
Assay of antioxidant enzymes
The activities of antioxidant enzymes were assayed in leaves using spectrophotometric methods. Superoxide dismutase (SOD) activity was determined following the method of Giannopolitis and Ries (1977) based on the photochemical reduction of NBT. One unit of SOD activity was defined as the amount of enzyme required to inhibit 50% of the reduction rate of NBT as monitored at 560nm. The catalase (CAT) activity was assayed as described by Cakmak and Marschner (1992). Ascorbate peroxidase (APX) activity was measured according to the method described by Nakano and Asada (1981).
Determination of detoxification-related enzymes and glutathione content in the leaves
Glutathione S-transferase (GST) activity was assayed spectrophotometrically using a GST activity assay kit (Jiancheng Bio Co., Nanjing, China) following the manufacturer’s instructions (Xia et al., 2009). Peroxidase (POD) activity was assayed using guaiacol as substrate, as described by Cakmak and Marschner (1992). The method of Foyer and Halliwell (1976) was followed to determine glutathione reductase (GR) activity by monitoring the glutathione-dependent oxidation of NADPH at 340nm.
The glutathione content was determined according to Sgherri and Navari-Izzo (1995) by an enzymatic recycling method. Total glutathione was sequentially oxidized by 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) and reduced by NADPH in the presence of GR. Oxidized glutathione (GSSG) was assayed by derivatizing reduced glutathione (GSH) with 2-vinylpyridine. The GSH content was then calculated by subtracting GSSG from total glutathione.
Determination of secondary metabolism-related enzyme activity in leaves
The activity of phenylalanine ammonia-lyase (PAL) was assayed by a combination of the methods of Ruiz et al. (1999) and Nguyen et al. (2003) based on the yield of cinnamic acid. One unit of PAL activity was defined as the change in absorbance at A 290 ml–1 enzyme extract. Polyphenol peroxidase (PPO) activity was assayed by measuring the increase in absorbance at 370nm with caffeic acid as a substrate (Ruiz et al., 1999). The activity of shikimate dehydrogenase (SKDH) was determined as described by Diaz et al. (2001). Cinnamyl alcohol dehydrogenase (CAD) was assayed spectrophotometrically according to Mitchell et al. (1994) based on the oxidation of the appropriate hydroxycinnamyl alcohol at 30 °C. Protein contents were quantified according to Bradford (1976). All spectrophotometric analyses were conducted on a 96-well Enspire™ 2300 Multilabel Reader (PerklinElmer, Singapore).
Total RNA extraction, cDNA synthesis, and quantitative real-time PCR (qRT-PCR) analyses
Total RNA was extracted from ~100mg of leaf tissue using the Total RNA Miniprep Kit (Axygen Biosciences, CA, USA) according to the manufacturer’s protocol. Genomic DNA was removed with the RNeasy Mini Kit (Qiagen, Hilden, Germany). A 1 µg aliquot of total RNA was reverse-transcribed for the synthesis of cDNA using the ReverTra Ace qPCR RT Kit (Toyobo, Japan), following the manufacturer’s instructions. Gene-specific primers for qRT-PCR were designed based on the mRNA or expressed sequence tag (EST) for the corresponding genes as follows: Fe-SOD (F, 5’-GTATCACAGGGCGTATGTCG-3’; R, 5’-GGGCTTCATAGATTCCCAGA-3’), CAT1 (F, 5’-TGATCGC GAGAAGATACCTG-3’; R, 5’-CTTCCACGTTCATGGACAAC-3’), APX (F, 5’-TCTGAATTGGGATTTGCTGA-3’; R, 5’-CGTCTAAC GTAGCTGCCAAA-3’), GST1 (F, 5’-CTCTGGTTTGGAGCAATT CA-3’; R, 5’-AATTTCAGCTGGATGCCTTT-3’), POD (F, 5’- TGA TCGCGAGAAGATACCTG-3’; R, 5’-ATCACCATTGGCTTCTGA CA-3’), GR (F, 5’-TTGGTGGAACGTGTGTTCTT-3’; R, 5’-TCTC ATTCACTTCCCATCCA-3’), GSH1 (F, 5’-TTGCTTATGCATGTTG CTCA-3’; R, 5’-ACAACCTCGGCTACTTCGTT-3’), PAL (F, 5’-AG GAGATCGACAAGGTGT-3’; R, 5’-TAGCAGATTGGAAGAGGA-3’), PPO (F, 5’-CACCACCTCCTGATCTCTCA-3’; R, 5’-GGGACGAA TACGGAGCTTAG-3’), SKDH (F, 5’-ATATCTGGGTCACCTTTG GC-3’; R, 5’-AGATAAGGCCTCAGCTCCAA-3’), CAD (F, 5’-GC CGCTGACTCACTTGATTA-3’; R, 5’-TTCCATCAAGCTTCAACA GC-3’), and the actin gene (F, 5’-TGGTCGGAATGGGACAGAAG-3’; R, 5’-CTCAGTCAGGAGAACAGGGT-3’) as an internal control. For qRT-PCR, PCR products were amplified in triplicate using the SYBR Green PCR Master Mix (Applied Biosystems) in 25 µl qRT-PCRs in an iCycler iQ™ 96-well real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The PCR conditions consisted of denaturation at 95 °C for 3min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s. The software provided with the PCR system was used to calculate threshold cycle values, and quantification of mRNA levels was performed according to the method of Livak and Schmittgen (2001). The threshold cycle (Ct) value of actin was subtracted from that of the gene of interest to obtain a ΔCt value. The Ct value of the untreated control sample was subtracted from the ΔCt value to obtain a ΔΔCt value. The fold changes in expression level relative to the control were expressed as 2–ΔΔCt.
Statistical analyses
Data were statistically analysed and expressed as mean ±SD. Analysis of variance (ANOVA) was performed and treatment differences were compared using Tukey’s test (P < 0.05).
Results
Effects of EBR on biomass accumulation and PHE and Cd content
Exposure to PHE and/or Cd was observed to decrease the biomass accumulation in terms of dry weight significantly when compared with the untreated control (Fig. 1A). The inhibitory effects of Cd on biomass accumulation were much stronger than those of PHE (Fig. 1A). EBR application to PHE-, Cd-, and PHE+Cd-stressed plants was able to improve the dry weight by 49, 48, and 28%, respectively, in shoot, and 50, 32, and 21%, respectively, in root when compared with plants stressed only by PHE-, Cd-, and PHE+Cd (Fig. 1A). These EBR-induced increments were all statistically significant, with the exception of only PHE+Cd+EBR in root.
Fig. 1.
Interactive effects of PHE, Cd, and EBR on (A) plant biomass, (B) PHE residue, and (C) Cd content in tomato plants after 40 d of the respective treatments. The culture solution was co-contaminated with 100 µM PHE and 100 µM Cd. EBR (0.1 µM) was applied on the foliar portion. Bars of the same colour with different letters are significantly (P <0.05) different according to Tukey’s test. Data are the means, and the error bar indicates ±SD (n=8 for dry weight analysis and n=3 for pollutant residue analysis).
The leaves and roots showed no significant difference in terms of PHE accumulation when compared with plants exposed to PHE alone or the PHE+Cd combination (Fig. 1B). Application of EBR to plants stressed by only PHE was able to reduce PHE accumulation significantly in leaves by 21% over PHE alone treatment. Interestingly, EBR applied to PHE+Cd-stressed plants showed a 31% reduction in PHE accumulation in leaves in comparison with PHE+Cd-only treatments. It is worth mentioning that EBR when applied to PHE+Cd-treated plants could reduce PHE accumulation in leaves by a factor of 10% when compared with EBR applied to plants treated with PHE alone. EBR application was found to be more effective in decreasing the PHE accumulation in root by 32% and 50% compared with PHE- and PHE+Cd-stressed plants, respectively (Fig. 1B).
Analysis of Cd content revealed that addition of PHE with Cd in nutrient solution decreased Cd accumulation in leaves by 8.5%, but increased Cd content in roots by 18.3% when compared with Cd accumulation in Cd alone-treated plants (Fig. 1C). In contrast, EBR application to Cd-stressed plants remarkably decreased the Cd content in both the leaves and roots compared with Cd alone. EBR-induced reduction in Cd content was 10% and 27% in roots following Cd+EBR and PHE+Cd+EBR treatments, respectively, when compared with the respective pollutant-only treatments (Fig. 1C). These results provide evidence that EBR and PHE co-application to Cd only-stressed plants could significantly lower the Cd content when compared with PHE only treatments of Cd-stressed plants.
Effects of PHE–Cd co-contamination on leaf gas exchange of tomato plant and role of EBR
Leaf gas exchange parameters such as P n was significantly decreased by PHE and/or Cd stress (Fig. 2A). Compared with control, P n was decreased by 14, 62, and 56% following PHE, Cd, and PHE+Cd treatments, respectively. Application of EBR to PHE-, Cd-, and PHE+Cd-stressed plants was able to improve the P n significantly when compared with their respective treatments alone. It was interesting to find that co-application of EBR and PHE to Cd-stressed plants showed more significant improvement in the P n value (50%) when compared with PHE applied alone to Cd-stressed plants (Fig. 2A).
Fig. 2.
Interactive effects of PHE, Cd, and EBR on (A) leaf gas exchange and (B) chlorophyll fluorescence quenching parameters in tomato. All measurements were taken on the third fully expanded leaves after 40 d of the respective treatments. The culture solution was co-contaminated with 100 µM PHE and 100 µM Cd. EBR (0.1 µM) was applied on the foliar portion. Data are the means of six biological replicates (±SD). Means denoted by the same letter did not differ significantly at P < 0.05 according to Tukey’s test.
A significant reduction in g s was noted for Cd- and PHE+Cd-stressed plants when compared with untreated controls. Application of EBR alone to PHE-stressed plants produced no significant increase in g s value when compared with PHE alone-stressed plants. Feeding of EBR to Cd-stressed plants showed a significant improvement in the g s value, and co-application of EBR with PHE to Cd-stressed plants was able to improve g s values more significantly compared with either Cd-stressed or PHE+Cd-stressed plants (Fig. 2A).
In contrast, the C i value was increased by 16, 17, and 20% for PHE-, Cd-, and PHE+Cd-stressed plants, respectively, when compared with untreated control. Application of EBR to PHE-stressed plants produced a significant decrease in C i, however, no significant change in C i was recorded for Cd-stressed plants. The co-application of EBR and PHE was able to decrease C i significantly when compared with plants stressed with PHE+Cd alone (Fig. 2A).
EBR influences PHE–Cd co-contamination effects on chlorophyll fluorescence and pigments in tomato leaves
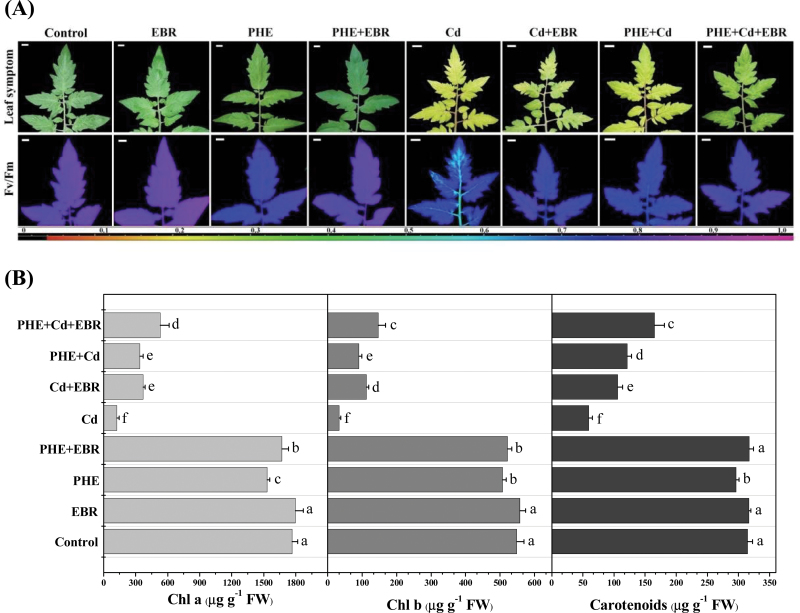
Changes in chlorophyll fluorescence parameters in response to PHE and/or Cd are shown in Figs 2B and 3A. A significant decrease in F v/F m and ΦPSII in PHE-, Cd-, and PHE+Cd-stressed plants was observed when compared with untreated control. The maximum decrease in F v/F m, ΦPSII, and qP was recorded under Cd alone, followed by PHE+Cd and PHE. The respective pseudocoloured images for F v/F m (Fig. 3A) also confirm the significant impact of the pollutants under study on F v/F m values. Application of EBR to Cd and PHE+Cd-stressed seedlings was able to increase F v/F m and ΦPSII significantly over Cd and PHE+Cd alone treatments. EBR application to PHE+Cd-stressed plants produced a significant increase in F v/F m compared with PHE+Cd- and Cd+EBR-stressed plants. However, ΦPSII values showed no significant difference for PHE+Cd+EBR versus plants stressed with Cd+EBR alone.
Fig. 3.
Effects of PHE, Cd, and EBR alone or in combination on the maximum photochemical efficiency of PSII (F v/F m) and chlorophyll contents in tomato leaves after 40 d of the respective treatments. (A) Original image of the third leaves (upper panels) and pseudocolour image of F v/F m (lower panels); (B) photosynthetic pigment contents under different treatments. Data are the average of three replicates and are presented as the mean ±SD. Means denoted by the same letters did not differ significantly at P < 0.05 according to Tukey’s test. Horizontal bars=1cm.
Application of PHE and Cd alone or in combination significantly reduced the photosynthetic pigments Chl a, Chl b, and Carts (Fig. 3B). When compared with untreated control, the maximum decrease in Chl a was recorded under Cd alone (93%), followed by PHE+Cd (81%) and PHE alone (13%). Chl b and Carts showed a similar trend following exposure to Cd and PHE pollutants. Observations made with the naked eye (leaf symptoms) clearly indicated severe chlorosis under Cd and PHE+Cd treatment, which was consistent with the respective pigment data (Fig. 3A, B). In contrast, EBR application together with Cd and PHE+Cd was able to increase the photosynthetic pigments significantly compared with Cd- and PHE+Cd only treatments. Similar to the chlorophyll fluorescence results, the most significant improvement contributed by EBR was observed under Cd+EBR. In this treatment Chl a, Chl b, and Cart contents were increased by 197, 237, and 79%, respectively, compared with Cd alone.
Effects of PHE–Cd co-contamination on O2∙– and H2O2 accumulation and lipid peroxidation in tomato leaves, and role of EBR
ROS accumulation and MDA contents were remarkably increased in leaves following exposure to PHE and/or Cd when compared with untreated control (Fig. 4). About 87% H2O2 accumulation was recorded under Cd treatment, followed by 83% under PHE+Cd and 40% under PHE alone over untreated control (Fig. 4B). EBR applications to PHE-, Cd-, and PHE+Cd-stressed plants showed a significant reduction in H2O2 when compared with their respective treatments without EBR. The H2O2 content was decreased by 21, 25, and 24% following PHE+EBR, Cd+EBR, and PHE+Cd+EBR (Fig. 4B), respectively. It was interesting to observe that co-application of EBR and PHE was more effective in reducing the H2O2 content when compared with plants stressed with PHE+Cd alone (Fig. 4B). Histochemical observations for H2O2 detection in leaves with DAB staining are also fairly in agreement with the biochemical data; however, slightly increased H2O2 was visualized under Cd-only treatment compared with PHE+Cd (Fig. 4A lower panel). A similar trend for the O2∙─ accumulation in leaves was also observed (Fig. 4A upper panel). Likewise, MDA contents were increased by 24, 22, and 8% compared with untreated control following PHE+Cd, Cd, and PHE, respectively. However, EBR application on stressed plants significantly decreased MDA contents by 9, 9, and 5% compared with their respective treatments without EBR (Fig. 4C). Corresponding NBT- and DAB-stained leaves also showed less ROS accumulation following EBR application in Cd-, PHE-, and PHE+Cd-stressed plants (Fig. 4A).
Fig. 4.
Interactive effects of PHE, Cd, and EBR on (A) O2∙– (upper panel) and H2O2 accumulation (lower panel), (B) H2O2 contents, and (C) MDA contents in tomato leaves after 40 d of the respective treatments. Accumulation of O2∙– and H2O2 in leaves was detected by NBT and DAB staining, respectively. Each value in the graph shows the mean with the standard deviation of three replicates. Means denoted by the same letters did not differ significantly at P < 0.05 according to Tukey’s test. Horizontal bars=2cm.
EBR induces changes in the effects of PHE–Cd co-contamination on antioxidant enzymes and gene expression in tomato leaves
Tomato plants stressed with Cd and PHE showed significant changes in antioxidant enzyme activities. The activity of SOD and APX was significantly increased by PHE and/or Cd compared with untreated control. Compared with control, SOD was increased by 10, 21, and 55% following PHE, Cd, and PHE+Cd, respectively; while the respective increases for APX were 59, 61, and 174% when compared with untreated control (Fig. 5A). CAT was induced by PHE and PHE+Cd (49% and 59%, respectively), but was decreased by Cd alone (by 15%) compared with untreated control (Fig. 5A). Application of EBR alone or in combination with Cd, PHE, and PHE+Cd produced increased SOD, CAT, and APX compared with untreated control or their respective Cd, PHE, and PHE+Cd alone treatments. The EBR supplemented increases were highest under Cd+EBR treatment compared with Cd alone, which were 23, 25, and 43% higher for SOD, CAT, and APX, respectively (Fig. 5A).
Fig. 5.
Interactive effects of PHE, Cd, and EBR on (A) antioxidant enzyme activities and (B) related gene expression. Each value in the graph shows the mean with the standard deviation of three replicates. Means denoted by the same letters did not differ significantly at P < 0.05, according to Tukey’s test.
qRT-PCR analysis also supported the changes in the activities of SOD, CAT, and APX induced under pollutant stress and EBR applications (Fig. 5B). The Fe-SOD, CAT1, and APX transcript levels were shown to be induced by PHE and/or Cd, while EBR application was able to enhance expression further over the respective pollutant alone treatments (Fig. 5B). Fe-SOD and APX transcript levels were maximum under EBR alone (2-fold) followed by PHE+EBR and PHE, while the highest CAT1 transcript level was observed under PHE+Cd+EBR (6-fold) followed by Cd+EBR and PHE+Cd (Fig. 5B).
Effects of PHE–Cd co-contamination and EBR on detoxification-related enzyme activity and gene expression
The activities of detoxification-related enzymes such as GST, POD, and GR were increased by PHE and/or Cd compared with untreated control (Fig. 6A). Similarly, PHE and/or Cd treatments induced the expression of GST1, POD, and GR1 genes compared with untreated control (Fig. 6B). EBR application alone or with pollutants further increased GST, GR, and POD activity as well as the related gene expression compared with untreated control and pollutant-only treatments, respectively. The highest induction by EBR was observed in Cd+EBR for GR, which was 55% over Cd alone. For POD and GST, the maximum increments by EBR was recorded in PHE+EBR, which were 42% and 27%, respectively, compared with PHE alone.
Fig. 6.
Effects of EBR on PHE and Cd detoxification-related enzyme activities, glutathione content, and related gene expression. Each value in the graph shows the mean with the standard deviation of three replicates. Means denoted by the same letters did not differ significantly at P < 0.05 according to Tukey’s test.
The GSH content was increased by 39, 220, and 237% following PHE, Cd, and PHE+Cd treatments (Fig. 6A), whereas the GSH1 transcript level was induced by 24, 36, and 24%, respectively, over that of untreated control (Fig. 6B). EBR application to PHE- and Cd-stressed plants further increased GSH content by 39, 17, and 22% following PHE+EBR, Cd+EBR, and PHE+Cd+EBR treatments, respectively, whereas the GSH1 transcript level was induced by 44, 52, and 21%, respectively, compared with their respective pollutant alone treatments.
Interactive effects of PHE, Cd, and EBR on plant secondary metabolism-related enzyme activity and gene expression in tomato leaves
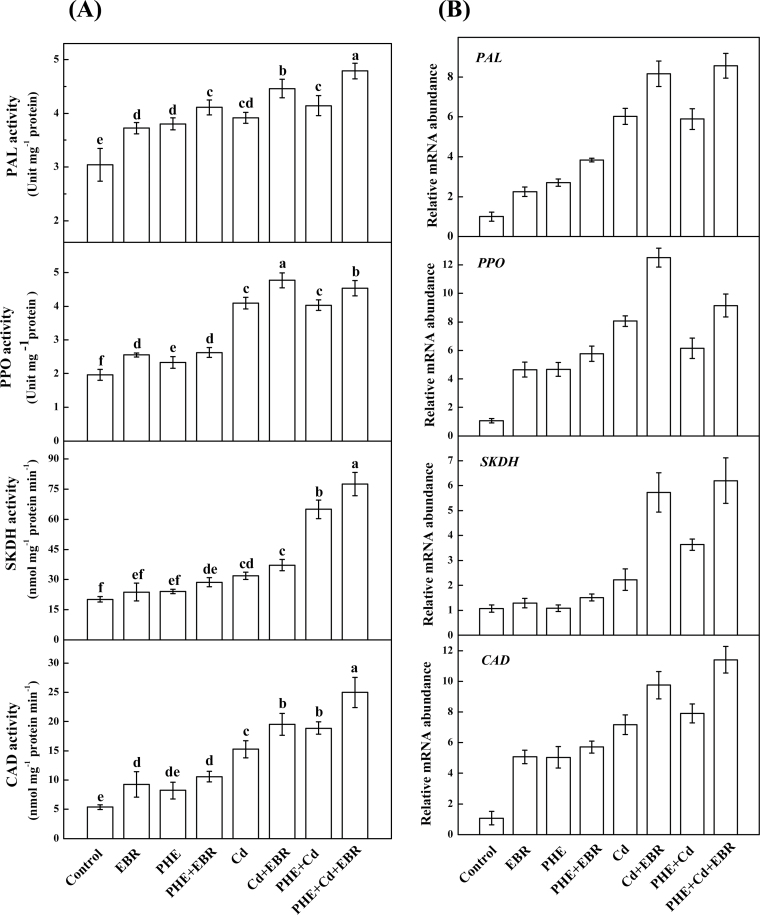
As shown in Fig. 7A, PAL and PPO activities were induced by PHE and/or Cd. Compared with pollutant-only treatments, the PAL activity increased by 8.1, 13.8, and 15.6% following PHE+EBR, Cd+EBR, and PHE+Cd+EBR, respectively, whereas PPO activity was induced consecutively by 12.8, 16.6, and 12.6%. These results show that EBR when applied to PHE+Cd-stressed plants could improve PAL activity more significantly than in PHE+Cd- and Cd+EBR-stressed plants. A synergistic induction of SKDH and CAD activities was also observed by co-application of PHE and Cd. When compared with pollutant-only treatments, EBR-induced increases in SKDH and CAD activity were 19.4% and 32.3%, respectively, following PHE+Cd+EBR (Fig. 7A). EBR applied with PHE to Cd-stressed plants produced a significant increase in the transcript level of CAD by 44% (compared with PHE+Cd) and by 17.5% (compared with Cd+EBR), thereby showing the positive interplay of EBR and PHE in the induction of CAD expression under Cd and PHE stress (Fig. 7B). The SKDH transcript level was also found to be enhanced with EBR applied to PHE+Cd-stressed plants by 71% with regard to PHE+Cd and 8.3% with regard to Cd+EBR. In line with the enzyme activity, the transcript levels of PAL and PPO were induced by PHE and/or Cd, and application of EBR further increased their expression levels. EBR-induced up-regulation of SKDH and CAD transcripts was remarkable when EBR was applied with Cd and PHE+Cd (Fig. 7B).
Fig. 7.
Interactive effects of PHE, Cd, and EBR on secondary metabolism-related (A) enzyme activities and (B) gene expression. Each value in the graph shows the mean with the standard deviation of three replicates. Means denoted by the same letters did not differ significantly at P < 0.05 according to Tukey’s test.
Discussion
Plants possess an inherent capacity to degrade or sequester a large number of toxic compounds (Coleman et al., 1997). To date it is not clear to what extent PAH exposure can trigger the stress signalling pathway that is common to other abiotic or biotic stressors in plants. There is no concrete evidence for the existence of stress signalling components specific to PAH stress in plants (Alkio et al., 2005). Importantly, plant tolerance to heavy metals such as Cd is conferred by a specific physiological mechanism that jointly enables the plant to function normally even in the presence of high levels of potentially toxic elements (Pilon-Smits, 2005). In recent years, an extensive body of work has been published on BR-induced plant tolerance to various abiotic stresses including temperature, drought, salinity, organic pollutants, and heavy metals (Xia et al., 2009; Cui et al., 2011; Hasan et al., 2011; Ahammed et al., 2012a , b ; Choudhary et al., 2012a , b ). So far the implication of BRs in the mitigation of mixed environmental pollution has not been studied. Here, an enhanced tolerance to and detoxification of PHE and Cd in combination by foliar application of EBR in tomato has been demonstrated.
In the current study, Cd showed a stronger effect on biomass compared with PHE in terms of dry weight reduction (Fig. 1). The findings can be supported by those of Zhang et al. (2011), who also observed similar phenomena in Juncus subsecundus. The antagonistic interactions of PAHs and heavy metals can be explained by the report that a pyrene concentration of 50, 100, and 500mg kg–1 in the soil tends to alleviate Cu- (200mg kg–1 and 400mg kg–1) induced growth inhibition in Z. mays (Lin et al., 2008). Zhang et al. (2011) also inferred that PAHs might be used as a carbon source to stimulate microbial blooming or microbial composition modification in the rhizosphere, which might be the reason behind such stress ameliorative behaviour of PAHs against Cd recorded in the present investigation. Thus they concluded that a positive effect of PAH metabolites and/or phytohormones produced by microorganisms on plant growth might be the underlying reason for PAH-mediated Cd toxicity alleviation. Previous studies showed that BRs could alleviate the phytotoxicity of PHE or Cd alone in tomato (Hasan et al., 2011; Ahammed et al., 2012a , b ). Similar results were recorded in the current study (Fig. 1). Moreover, it was noticed that application of EBR and PHE to Cd-stressed plants could alleviate Cd-induced biomass reduction more significantly than Cd+EBR- or PHE+Cd-only treatments. The positive impact of BRs on biomass production in the current study can be supported by the observations of Xie et al. (2011) who showed that BRs could induce cellulose biosynthesis by controlling the expression of CELLULOSE SYNTHASE genes which significantly contribute to the total biomass accumulation in Arabidopsis.
The leaf gas exchange study showed that both stomatal and non-stomatal factors were involved in the photosynthetic inhibition of PHE-stressed plants (Ahammed et al., 2012a , b ). PHE as well as Cd has been reported to inhibit P n and g s in tomato, which supports the current observation (López-Millán et al., 2009; Hasan et al., 2011; Ahammed et al., 2012b ). Thus, biomass reduction by PHE or Cd stress may be the consequence of reduction in chlorophyll content and consequent inhibition of photosynthesis (Ci et al., 2010; Ahammed et al., 2012b ). It was also observed that PHE+Cd co-contamination could inhibit P n, but the value of P n was slightly higher than with Cd alone and lower than with PHE alone. Decreased P n could be a consequence of the inhibition of selected reaction steps of the Benson–Calvin cycle and RuBisCO activity by these contaminants. Moreover, the reduction in the CO2 assimilation rate might be attributed to altered stomatal opening and subsequent decline in g s (Fig. 2A). It has previously been reported that application of exogenous EBR increases photosynthetic CO2 assimilation in cucumber plants, which may provide an important mechanism for increased growth and yield in EBR-treated plants (Yu et al., 2004). In agreement with earlier studies, EBR follow-up treatment significantly increased CO2 assimilation over pollutants alone in the current study (Xia et al., 2009; Ahammed et al., 2012a , b ). EBR induces transcripts for photosynthetic genes and the activity of enzymes involved in the Benson–Calvin cycle, and thus improves photosynthetic capacity (Jiang et al., 2012). One interesting observation might be that Cd treatment reduced P n and g s of PHE-treated plants; however, when EBR is applied, this Cd-mediated reduction of PHE-stressed plants is slightly less for P n or was not observable for g s. It may suggest another piece of evidence that EBR application may protect stressed plants through physiological interaction with Cd and/or PHE.
PHE or Cd have been reported to decrease F v/F m, ΦPSII, and qP in different plant species including tomato (López-Millán et al., 2009; Ci et al., 2010; Liu et al., 2011; Ahammed et al., 2012a , b ). A decrease in F v/F m indicates the negative effects of these pollutants on the photochemical reactions which may affect the efficiency of PSII photochemistry by blocking electron transport. Interestingly, a significant effect of PHE on increasing F v/F m of Cd-treated plants was observed. This PHE-mediated increase was found to be slightly higher when EBR was co-applied with PHE. It might suggest a positive interaction of EBR and PHE to protect photosynthesis of plants against Cd stress. Leaf damage symptoms of these treatments are in line with this assumption (Fig. 3A). In addition, EBR seemed to protect against oxidative stress induced by Cd, but PHE did not exert such an effect. Moreover, the potential interaction of EBR and PHE could not be via a reduction of oxidative stress (measured as H2O2 and MDA contents). The value of ΦPSII corresponds to the electron transport in the thylakoid membrane and thus a decrease in ΦPSII is related to the electron transport chain in thylakoid membrane (Váňová et al., 2009). These phenomena revealed that inhibition of photosynthesis is the result of damage to the PSII reaction centre of the leaf. However, the current study showed that EBR application alone or with PHE and/or Cd could increase F v/F m (Fig. 2B). The promoting effects of BRs on F v/F m have been reported under different stresses such as PHE, pesticide, and chilling, in agreement with the present results (Xia et al., 2009; Cui et al., 2011; Ahammed et al., 2012a , b ).
Leaf chlorosis is one of the common signs of Cd toxicity (Liu et al., 2011). Cd inhibits chlorophyll formation by interfering with protochlorophyllide production (Ci et al., 2010; Hasan et al., 2011). Heavy metals can also replace the central Mg from chlorophyll molecules in in vivo conditions. Moreover, enzymatic degradation of chlorophyll by chlorophyllase is also triggered by Cd (Hasan et al., 2011). PHE-induced leaf pigment loss may be a result of degradation and/or reduction in formation of photosynthetic pigments (Ahammed et al., 2012a , b ). A decrease in chlorophyll content undoubtedly affects the photosynthetic efficiency. Importantly, EBR application on stressed plants significantly increased the chlorophyll content, possibly by stimulating transcription of genes implicated in the biosynthesis of pigments (Bajguz, 2011).
Overproduction of ROS and subsequent oxidative stress is the common mechanism of phytotoxicity for both PHE and Cd (Liu et al., 2009; Masood et al., 2012). Enhanced levels of ROS in cells resulted in pigment loss, reduction in photosynthetic CO2 assimilation, and decreased protein and RNA levels (Mittler, 2002; Masood et al., 2012). Moreover, the critical enzyme of the Benson–Calvin cycle, RuBisCO, is directly fragmented by ROS (Ishida et al., 1999). As evidence of ROS generation, significantly increased O2∙─, H2O2, and MDA levels were observed under Cd and PHE+Cd treatments, followed by PHE alone (Fig. 4). MDA is an indicator of lipid peroxidation, signifying the damage due to harmful ROS. The current observations confirmed that the tomato plants were under oxidative stress following PHE and/or Cd treatments. However, O2∙─, H2O2, and MDA contents were not synergistically stimulated by PHE and Cd. EBR application was able to decrease the toxic O2∙─, H2O2, and MDA contents in the stressed plants, which is consistent with previous reports on BR-induced alleviation of stress due to heavy metals and PAHs (Hasan et al., 2011; Ahammed et al., 2012a , b ). Oxidative stress alleviation is generally attributed to enhanced antioxidant activity and subsequent ROS scavenging by BRs under stressful conditions (Cui et al., 2011). Indeed, the plant antioxidant system is activated to avoid oxidative damage by PHE- and/or Cd-induced ROS (Liu et al., 2009; Masood et al., 2012). Here in the present study, SOD and APX were induced by PHE and/or Cd, while CAT activities were only inhibited by Cd alone. Meng et al. (2009) observed similar phenomena under 100 µM Cd stress in Brassica napus L. In general, decreased SOD activity suggests that O2∙─ generation has exceeded the elimination ability of SOD, or the ROS might have inactivated enzymes. In the present study, co-application of PHE and Cd produced a positive effect on antioxidant enzyme activities, as much higher activity was observed following PHE+Cd than Cd alone. The transcript levels of Fe-SOD, CAT1, and APX were induced by PHE and/or Cd (Fig. 5B). However, co-application of PHE and Cd did not show an additive up-regulation of the expression level of Fe-SOD and APX, which suggested a post-transcriptional impact on the induction of the respective enzyme activity for ROS scavenging. Enhanced antioxidant enzyme activity has a positive role in minimizing the ROS level in order to maintain the normal plant physiological activity under stressful regimes (Mittler, 2002). Similar to the present observation, a synergistic up-regulation in SOD activity was observed under PHE plus arsenic co-contamination in Pteris vitatta (Sun et al., 2011). The present investigation showed that EBR application could further increase the activity of antioxidant enzymes and the transcript level of the related genes under PHE and/or Cd stress, which is in line with earlier reports (Hasan et al., 2011; Ahammed et al., 2012a , b ). Recently, it has been shown that EBR-induced oxidative stress tolerance is associated with increased expression of genes encoding antioxidant enzymes in cucumber (Cui et al., 2011). Thus the increased transcript level of these enzymes might be the result of de novo synthesis and/or activation of the enzymes in response to BR-mediated transcription and/or translation of specific genes (Bajguz, 2000).
Plant GSTs are the key enzymes that catalyse the conjugation reaction of xenobiotics with GSH in phase II (Coleman et al., 1997). In the current experiments, GST activity and the GST1 transcript level were induced by PHE and/or Cd, which were further increased by follow-up treatment with EBR (Fig. 6). GST activity was reported to be increased in Arabidopsis exposed to 100 µM Cd (Skorzynska-Polit et al., 2010). Enhanced GST and GST1 suggested enforcement of the detoxification reaction. Moreover, enhanced POD activity due to EBR application might play a crucial role in the xenobiotic conversion, a part of the detoxification process (Xia et al., 2009). Likewise, an up-regulation in GR activity may reduce GSSG to GSH and supply more GSH to strengthen the detoxification process. Previously, GSH has been reported to be induced by both PHE and Cd (Ahammed et al., 2012a , b ; Masood et al., 2012), which is in agreement with the current observation. It is well known that GSH and phytochelatins play a vital role in Cd detoxification and subsequent stress tolerance (Masood et al., 2012). The GSH content has been shown to increase positively under PHE plus arsenic co-contamination compared with PHE or arsenic alone in P. vitatta (Sun et al., 2011). The present study also observed a similar phenomenon for GSH content in PHE+Cd-treated tomato plants. EBR was reported to improve xenobiotic metabolism by enhancing the activities of GST, POD, and GR and related gene expression (Xia et al., 2009; Ahammed et al., 2012a ). Consistent with previous reports, here it was shown that EBR-induced alleviation of PHE+Cd stress might be associated with enhanced activity of detoxification enzymes and related gene expression.
An enhancement of the activity of PAL may stimulate subsequent reactions in the phenylpropanoid pathway to produce specific phenylpropanoid derivatives such as phenols and flavonoids (Dixon and Paiva, 1995). Phenolic compounds play a vital role in the alleviation of oxidative stress as they are involved in the detoxification of ROS (Wang et al., 2011). The current observation of up-regulation of CAD activity and the CAD1 transcript level signifies an enhancement in the synthesis of cinnamyl alcohols and is considered as a specific marker for lignification (Mitchell et al., 1994). Similarly, Wang et al. (2011) reported enhanced catalytic activities of PAL, PPO, and SKDH under Pb treatment, which supports the current data (Fig. 7). Secondary metabolism-related enzymes such as PAL, PPO, SKDH, and CAD as well as their transcripts were induced by EBR, PHE, and/or Cd (Fig. 7). The highest increments were recorded when pollutants were in combination. Importantly, EBR follow-up treatment on stressed plants further increased those activities which might influence the respective secondary metabolite synthesis required for active antioxidative protection (Fig. 7).
Earlier studies have shown that the combination of certain concentrations of metals (e.g. Cd) and PAHs could stimulate both metal and PAH uptake compared with metal alone, while growth and biomass were also increased (Lin et al., 2008; Zhang et al., 2011). Although some possible interpretations have been suggested, the underlying mechanism for this improved growth with high concentration of pollutants was not clear. Slightly increased PHE and Cd content accompanied by increased biomass was also observed following PHE+Cd compared with Cd alone (Fig. 1). As the content of PHE and Cd was not decreased, the improved growth could be contributed by the enhanced antioxidant potential and subsequent stress alleviation in the co-contaminated system. López-Millán et al. (2009) reported very high accumulation of Cd in leaves (1075 µg g–1 DW) and roots (4731 µg g–1 DW) following application of 100 µM Cd in tomato, which supports the current findings (Fig. 1C). On the other hand, EBR-induced biomass improvement may also be associated with enhanced degradation and detoxification of pollutant as PHE and Cd contents were significantly decreased in leaves and roots by EBR supplementation (Fig. 1B, C) (Ahammed et al., 2012a ). It is to be noted that either PHE or EBR could reduce Cd content; however, the combined treatment (PHE+EBR) provoked a similar Cd content reduction. This indicates that the EBR and PHE effects on Cd accumulation were not additive. It is speculated that the reduction of leaf Cd content by EBR might be through PHE. In contrast, EBR reduced root PHE content, while this pollutant enhanced the amount of Cd in the root. However, EBR greatly decreased Cd content in the roots, which suggests that such a reduction in Cd level could be independent of PHE in roots.
In summary, it was observed that PHE and/or Cd treatment resulted in a decreased growth and photosynthetic capacity. PHE- and/or Cd-induced inhibition of photosynthesis can be attributed to: (i) stomatal and non-stomatal limitation; (ii) reduction in photosynthetic pigments; and (iii) destruction of the photosynthetic apparatus. Single or mixed pollution induces oxidative stress. However, mixed pollution by PHE and Cd was less phytotoxic than Cd alone. EBR application alleviated the negative effects of Cd and PHE on growth and photosynthesis by improving antioxidant enzyme activity, xenobiotic detoxification capacity, and secondary metabolism. The findings also show positive interactions of EBR and PHE when applied together for Cd stress alleviation. All the studied parameters clearly indicate an anti-stress property of EBR against Cd and PHE co-contamination. These findings suggest the potential feasibility of BR application to reduce pollutant residues for food safety. Further studies using advanced molecular techniques and mutant analyses are required to better understand the detailed mechanism of BR-induced PHE+Cd stress tolerance in plants.
Acknowledgements
This work was supported by the National Basic Research Program of China (2009CB119000), the National Natural Science Foundation of China (31071790), and the National Key Technology R&D Program of China (2011BAD12B04).
This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details)
© 2012 The Author(s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- Adams SV, Passarelli MN, Newcomb PA. 2012. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occupational and Environmental Medicine. 69, 153–156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahammed GJ, Gao CJ, Ogweno JO, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ. 2012. a Brassinosteroids induce plant tolerance against phenanthrene by enhancing degradation and detoxification in Solanum lycopersicum L. Ecotoxicology and Environmental Safety. 80, 28–36 . [DOI] [PubMed] [Google Scholar]
- Ahammed GJ, Yuan HL, Ogweno JO, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ. 2012. b Brassinosteroid alleviates phenanthrene and pyrene phytotoxicity by increasing detoxification activity and photosynthesis in tomato. Chemosphere. 86, 546–555 . [DOI] [PubMed] [Google Scholar]
- Alkio M, Tabuchi TM, Wang XC, Colón-Carmona A. 2005. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. Journal of Experimental Botany. 56, 2983–2994 . [DOI] [PubMed] [Google Scholar]
- Babu TS, Marder JB, Tripuranthakam S, Dixon DG, Greenberg BM. 2001. Synergistic effects of a photooxidized polycyclic aromatic hydrocarbon and copper on photosynthesis and plant growth: evidence that in vivo formation of reactive oxygen species is a mechanism of copper toxicity. Environmental Toxicology and Chemistry. 20, 1351–1358 . [DOI] [PubMed] [Google Scholar]
- Bajguz A. 2000. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris . Plant Physiology and Biochemistry. 38, 209–215 . [Google Scholar]
- Bajguz A. 2011. Suppresion of Chlorella vulgaris growth by cadmium, lead and copper stress and its restoration by endogenous brassinolide. Archives of Environmental Contamination and Toxicology. 60, 406–416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 72, 248–254 . [DOI] [PubMed] [Google Scholar]
- Cakmak I, Marschner H. 1992. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiology. 98, 1222–1227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary SP, Oral V, Bhardwaj R, Yu J-Q, Tran LS. 2012. a Interactions of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus . Journal of Experimental Botany. 63, 5659–5675 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary SP, Yu J-Q, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. 2012. b Benefits of brassinosteroid crosstalk. Trends in Plant Science. 17, 594–605 . [DOI] [PubMed] [Google Scholar]
- Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W. 2010. Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiologiae Plantarum. 32, 365–373 . [Google Scholar]
- Coleman J, Blake-Kalff M, Davies E. 1997. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends in Plant Science. 2, 144–151 . [Google Scholar]
- Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen ZX, Yu JQ. 2011. Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant, Cell and Environment. 34, 347–358 . [DOI] [PubMed] [Google Scholar]
- Diaz J, Bernal A, Pomar F, Merino F. 2001. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Science. 161, 179–188 . [Google Scholar]
- Divi UK, Krishna P. 2009. Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnology. 26, 131–136 . [DOI] [PubMed] [Google Scholar]
- Dixit P, Mukherjee PK, Sherkhane PD, Kale SP, Eapen S. 2011. Enhanced tolerance and remediation of anthracene by transgenic tobacco plants expressing a fungal glutathione transferase gene. Journal of Hazardous Material. 192, 270–276 . [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. The Plant Cell. 7, 1085–1097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcó G, Bocio A, Llobet JM, Domingo JL. 2005. Health risks of dietary intake of environmental pollutants by elite sportsmen and sportswomen. Food and Chemical Toxicology. 43, 1713–1721 . [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 133, 21–25 . [DOI] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. 1977. Superoxide dismutases: I. Occurrence in hgher plants. Plant Physiology. 59, 309–314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R, Enewold L, Pellizzari E, Beach JB, Bowman ED, Krishnan SS, Shields PG. 2001. Smoking increases carcinogenic polycyclic aromatic hydrocarbons in human lung tissue. Cancer Research. 61, 6367–6371 . [PubMed] [Google Scholar]
- Hasan SA, Hayat S, Ahmad A. 2011. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere. 84, 1446–1451 . [DOI] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 207, 604–611 . [DOI] [PubMed] [Google Scholar]
- Ishida H, Makino A, Mae T. 1999. Fragmentation of the large subunit of ribulose-1,5-bisphosphate carboxylase by reactive oxygen species occurs near Gly-329. Journal of Biological Chemistry. 274, 5222–5226 . [DOI] [PubMed] [Google Scholar]
- Jiang YP, Cheng F, Zhou YH, Xia XJ, Shi K, Yu JQ. 2012. Interactive effects of CO2 enrichment and brassinosteroid on CO2 assimilation and photosynthetic electron transport in Cucumis sativus . Environmental and Experimental Botany. 75, 98–106 . [Google Scholar]
- Lichtenthaler HK, Wellburn AR. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions. 11, 591–592 . [Google Scholar]
- Lin Q, Shen KL, Zhao HM, Li WH. 2008. Growth response of Zea mays L. in pyrene–copper co-contaminated soil and the fate of pollutants. Journal of Hazardous Materials. 150, 515–521 . [DOI] [PubMed] [Google Scholar]
- Liu C, Guo J, Cui Y, Lü T, Zhang X, Shi G. 2011. Effects of cadmium and salicylic acid on growth, spectral reflectance and photosynthesis of castor bean seedlings. Plant and Soil. 344, 131–141 . [Google Scholar]
- Liu H, Weisman D, Ye YB, Cui B, Huang YH, Colon-Carmona A, Wang ZH. 2009. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana . Plant Science. 176, 375–382 . [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25, 402–408 . [DOI] [PubMed] [Google Scholar]
- López-Millán AF, Sagardoy R, Solanas M, Abadía A, Abadía J. 2009. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environmental and Experimental Botany. 65, 376–385 . [Google Scholar]
- Masood A, Iqbal N, Khan NA. 2012. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant, Cell and Environment. 35, 524–533 . [DOI] [PubMed] [Google Scholar]
- Meng H, Hua S, Shamsi I, Jilani G, Li Y, Jiang L. 2009. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regulation. 58, 47–59 . [Google Scholar]
- Mitchell HJ, Hall JL, Barber MS. 1994. Elicitor-induced cinnamyl alcohol-dehydrogenase activity in lignifying wheat (Triticum aestivum L) leaves. Plant Physiology. 104, 551–556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science7, 405–410 . [DOI] [PubMed] [Google Scholar]
- Mo CH, Cai QY, Tang SR, Zeng QY, Wu QT. 2009. Polycyclic aromatic hydrocarbons and phthalic acid esters in vegetables from nine farms of the Pearl River Delta, South China. Archives of Environmental Contamination and Toxicology. 56, 181–189 . [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 22, 867–880 . [Google Scholar]
- Nguyen TBT, Ketsa S, van Doorn WG. 2003. Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biology and Technology. 30, 187–193 . [Google Scholar]
- Pilon-Smits E. 2005. Phytoremediation. Annual Review of Plant Biology. 56, 15–39 . [DOI] [PubMed] [Google Scholar]
- Ruiz JM, Garcia PC, Rivero RM, Romero L. 1999. Response of phenolic metabolism to the application of carbendazim plus boron in tobacco. Physiologia Plantarum. 106, 151–157 . [Google Scholar]
- Sandrin TR, Maier RM. 2003. Impact of metals on the biodegradation of organic pollutants. Environmental Health Perspectives. 111, 1093–1101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgherri CLM, Navari-Izzo F. 1995. Sunflower seedlings subjected to increasing water deficit stress: oxidative stress and defence mechanisms. Physiologia Plantarum. 93, 25–30 . [Google Scholar]
- Skorzynska-Polit E, Drazkiewicz M, Krupa Z. 2010. Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiologiae Plantarum. 32, 169–175 . [Google Scholar]
- Sun L, Yan X, Liao X, Wen Y, Chong Z, Liang T. 2011. Interactions of arsenic and phenanthrene on their uptake and antioxidative response in Pteris vittata L. Environmental Pollution. 159, 3398–3405 . [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. The Plant Journal. 11, 1187–1194 . [Google Scholar]
- Váňová L, Kummerová M, Klemš M, Zezulka Š. 2009. Fluoranthene influences endogenous abscisic acid level and primary photosynthetic processes in pea (Pisum sativum L.) plants in vitro . Plant Growth Regulation. 57, 39–47 . [Google Scholar]
- Wang C, Lu J, Zhang SH, Wang PF, Hou J, Qian J. 2011. Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natans . Ecotoxicology and Environmental Safety. 74, 1297–1303 . [DOI] [PubMed] [Google Scholar]
- Wetzel A, Alexander T, Brandt S, Haas R, Werner D. 1994. Reduction by fluoranthene of copper and lead accumulation in Triticum aestivum L. Bulletin of Environmental Contamination and Toxicology. 53, 856–862 . [DOI] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inze D, Van Camp W. 1997. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO Journal. 16, 4806–4816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Zhang Y, Wu JX, Wang JT, Zhou YH, Shi K, Yu YL, Yu JQ. 2009. Brassinosteroids promote metabolism of pesticides in cucumber. Journal of Agricultural and Food Chemistry. 57, 8406–8413 . [DOI] [PubMed] [Google Scholar]
- Xie L, Yang C, Wang X. 2011. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis . Journal of Experimental Botany. 62, 4495–4506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JQ, Huang LF, Hu WH, Zhou YH, Mao WH, Ye SF, Nogues S. 2004. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus . Journal of Experimental Botany. 55, 1135–1143 . [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Rengel Z, Meney K, Pantelic L, Tomanovic R. 2011. Polynuclear aromatic hydrocarbons (PAHs) mediate cadmium toxicity to an emergent wetland species. Journal of Hazardous Materials. 189, 119–126 . [DOI] [PubMed] [Google Scholar]