Abstract
Anthocyanin production is a characteristic response of flowering plants to unfavourable environmental conditions. The potential roles of flavonoids and anthocyanins in plant growth were investigated by growing Arabidopsis thaliana anthocyanin production mutants (transparent testa) under limiting nitrogen and high light conditions. Inability to produce kaempferol or subsequent intermediate compounds by some transparent testa lines was correlated with less biomass accumulation in mature plants compared with wild-type control plants under all growth conditions tested. However, under both limiting nitrogen and high light chronic stress conditions, mutant lines defective in later steps of the anthocyanin production pathway produced the same or more biomass than wild-type plants. No difference in senescence between transparent testa and wild-type plants was found using chlorophyll catabolism and SAG12 expression measurements, and no mutants were impaired in the ability to remobilize nutrients from the vegetative to reproductive tissues. Moreover, the absence of anthocyanin and/or upstream flavonoids does not affect the ability of plants to respond to limiting nitrogen by reducing photosynthetic capacity. These results support a role for kaempferol and quercetin accumulation in normal plant growth and development. Further, the absence of anthocyanins has no effect on plant growth under the chronic stress conditions tested.
Key words: Anthocyanin, Arabidopsis, flavonoid, growth, light, nitrogen, stress.
Introduction
Plants respond to environmental changes by implementing a number of physiological, metabolic, and developmental changes. Production of anthocyanin in Arabidopsis thaliana is a clear visible marker of plant response to unfavourable growth conditions (Chalker-Scott, 1999). Environmental stresses, in particular, are well known to stimulate production of anthocyanin. Low availability of nutrients such as nitrogen and/or phosphorus, wounding, pathogen infection, jasmonate treatment, drought, and ultraviolet, visible, and far-red radiation have all been associated with anthocyanin accumulation in various tissues (Kolesnikov and Zore, 1957; Bhatla and Pant, 1977; Hipskind et al., 1996; Diaz et al., 2006; Hughes et al., 2010). Previous studies have linked production of anthocyanin pigment to modulation of hormone responses, UV-B protection, photoperception, antimicrobial activity, and feeding deterrents against pathogens and herbivores (Dixon and Steele, 1999; Harborne and Williams, 2000; Winkel-Shirley, 2001). Anthocyanin is utilized by plants as a light attenuator in high light environments, especially during winter months in the leaves of the evergreen herb Galax urceolata (Hughes et al., 2005). The role of anthocyanin in the plant response and development to chronic unfavourable conditions of limiting nitrogen and high light has not been examined before in A. thaliana over the course of the whole plant life cycle.
Anthocyanins are naturally occurring secondary metabolites that belong to a group of chemicals called flavonoids, which are present in all orders of land plants (Rausher, 2006). The wide array of anthocyanin colours, including pink, red, orange, scarlet, purple, blue, or blue-black to yellow, stems from the structural diversity of these molecules due to various hydroxylation and glycosylation patterns of precursors (Bloor, 2001; Davies and Schwinn, 2003). Anthocyanins are present in many tissues such as leaves, stems, roots, tubers, fruits, and seeds (Williams and Grayer, 2004). However, they have primary importance in flowers, where their main function is to attract pollinators (Saito and Harborne, 1992). Some tissues only accumulate anthocyanins under specific environmental conditions (Schoeneweiss and Grunwald, 1979; Faragher, 1983; Leng et al., 2000).
Flavonoids are a large and diverse group of colourless polyphenolic secondary metabolites that are produced as intermediates in the anthocyanin biosynthesis pathway (Williams and Grayer, 2004). Although they have been linked to a variety of physiological and developmental processes, such as auxin transport, flavonoids are not essential for plant growth (Jacobs and Rubery, 1988). An important function of anthocyanin molecules appears to be protection from harmful effects of solar radiation. Arabidopsis mutants unable to produce epidermal flavonoids were found to be hypersensitive to UV-B radiation (Landry et al., 1995). The ability of flavonoids to absorb light at 280–320nm is thought to be utilized by plants to prevent DNA damage (Stapleton and Walbot, 1994). In Arabidopsis, flavonoids have been shown to prevent photooxidative and photoinhibitory damage (Havaux and Kloppstech, 2001). They are also known to induce virulence and nodulation genes (Mulligan and Long, 1989; Zerback et al., 1989). Relevant to this study, flavonoids are produced in response to various stresses as precursors for anthocyanin accumulation.
The general anthocyanin biosynthesis pathway is carried out in a series of biochemical reactions; mutants at each step of the anthocyanin production pathway are denoted transparent testa (tt), due to the absence of tannins in the seeds causing pale seed colour (Shirley et al., 1995). These mutants correspond to genes in structural (tt3, tt4, tt5, tt6, tt7, tt18) and regulatory (tt1, ttg, ttg1, PAP1) components of the pathway. Each tt mutant is unique with respect to which flavonoids are absent and/or accumulate in the plant (Peer et al., 2001). Therefore, these mutants can be used to identify the flavonoids that are of particular importance in plant stress response. Adverse environmental conditions are expected to highlight and/or magnify any differences between anthocyanin production mutants and wild-type plants.
Several parameters of plant development, with a particular focus on leaf senescence, were examined to assess the response of tt mutants to challenging environmental conditions. Leaf senescence is an active and regulated degenerative process leading to changes in gene expression, metabolism, and cell structure (Zhang et al., 2010; Ma et al., 2011). The earliest and most noticeable change in cell composition is chlorophyll breakdown, where carbon and nitrogen assimilation are replaced by catabolism of chlorophyll and associated macromolecules (Hortensteiner and Krautler, 2010). Chlorophyll breakdown can be used as an indicator of senescence and nutrient remobilization. Analysing leaf colour has been used previously to correlate leaf anthocyanin content and chlorophyll levels with senescence rates in sugar maple (Schaberg et al., 2008). Arabidopsis accumulates nutrients and biomass in rosette leaves until the floral transition; after the transition, nutrients are remobilized from rosette leaves to inflorescences (Diaz et al., 2005). Drought, nutrient limitation, extreme temperatures, and oxidative stress by UV-B irradiation and ozone can all lead to premature senescence (Lim et al., 2003). Production of anthocyanin and acceleration of senescence are well documented under limiting nitrogen and high light conditions (Hughes and Smith, 2007; Peng et al., 2008). It is important to note that anthocyanin production does not have a direct effect on leaf nitrogen content, as was demonstrated in evergreen angiosperms (Hughes et al., 2011).
The effects of both high light and growth under limiting nitrogen stress conditions can be monitored over the whole plant life cycle. Although the immediate metabolic, physiological, and gene expression changes associated with acute stresses are known, moderate stress conditions were used here to better understand the effect of the absence of anthocyanin and its precursors on plant growth and development over the whole life cycle, as opposed to a time point immediately following acute stress treatment (Davies, 2000; Martinez et al., 2005; Peng et al., 2007). Non-stress (optimal), limiting nitrogen, and high light conditions were used to examine growth and development of a set of anthocyanin biosynthesis mutants spanning the whole flavonoid pathway. Biomass accumulation in the upstream (tt4 and tt5) and the downstream mutant lines (tt6, tt7, tt3, and tt18) was studied under various growth conditions. The senescence rates of plants were compared by chlorophyll degradation measurements, as well as the expression of senescence-associated gene 12 (SAG12), Rubisco small subunit (RbcS), and chlorophyll a/b-binding protein (CAB1). The ability of the anthocyanin production mutants to degrade chlorophyll and subsequently accumulate inflorescence biomass was assessed under high light conditions to determine the photoprotective role of anthocyanin and flavonoids intermediates. The results support a role for flavonoids for normal plant growth and development. Further, under both limiting nitrogen and high light stress, the downstream mutants grew at least as well as the wild type, implying that anthocyanin production is not crucial under these conditions.
Materials and methods
Plant lines and growth conditions
All plants were grown in the Phytotron facility at the University of Guelph. Arabidopsis thaliana seeds were obtained from the ABRC stock center in Ohio. Structural mutants from different steps of the anthocyanin biosynthesis pathway were chosen to delineate whether anthocyanin or any upstream flavonoid precursors are correlated with any growth and/or developmental differences exhibited by affected lines under chronic unfavourable growth conditions. Loss-of-function lines of structural genes chalcone synthase (tt4), chalcone isomerase (tt5), flavanone 3-hydroxylase (tt6), flavonoid 3’-hydroxylase (tt7), dihydroflavonol 4-reductase (tt3), and anthocyanidin synthase (tt18) were tested here. Plants homozygous for mutant genes were easily identifiable by their pale seed coat (Shirley et al., 1995). Different mutants accumulate varying flavonoid compounds depending on the exact position of the mutation in the anthocyanin biosynthesis pathway. For example, tt4 has no detectable flavonoids, tt5 is able to produce and accumulate naringenin chalcone, tt6 is a leaky mutant that contains naringenin, naringenin chalcone, kaempferol, and quercetin, tt7 predominantly overaccumulates kaempferol and also contains some naringenin chalcone, and tt3 produces excess quercetin and kaempferol, and contains naringenin chalcone (Peer et al., 2001). The precise flavonoid content of the tt18 mutant line is currently unknown, but the position of the TT18 gene in the anthocyanin biosynthesis pathway suggests that tt18 mutants should be deficient in their ability to accumulate condensed tannins. All of the tested mutants are in the Landsberg background, except for anthocyanidin synthase (tt18), which is in the Columbia background. Due to difference in the background ecotypes, both Landsberg and Columbia wild-type plants were used as controls. None of the mutant lines produce visible anthocyanins under any of the growth conditions used in this study.
For limiting nitrogen condition experiments, the growth chamber was set at 150 µmol m–2 s–1, 16h day at 23 °C/8h night at 18 °C, and 75% relative humidity. Nutrient-free LB2 (Sun Gro Horticulture Canada Ltd) soil was used. The plants were supplied with nutrient solutions (10mM KH2PO4, 2mM MgSO4, 1mM CaCl2, 0.1mM Fe-EDTA, 50 µM H3BO4, 12 µM MnSO4, 1 µM CuSO4, 0.2 µM Na2MoO4) once a week. Limiting nitrogen conditions were achieved by supplying plants with 2.5mM KNO3 solution. Otherwise 10mM KNO3 was used to supply plants with a non-stress amount of nitrogen for other growth conditions. High light treatment experiments were performed by raising the amount of light to 400 µmol m–2 s–1. T5 Sylvania fp54 841 HO Eco light bulbs were used for these experiments.
Biomass measurements
Plants were harvested and frozen in liquid nitrogen prior to freeze-drying. After floral transition, the inflorescence was separated from the rosette. Samples were freeze-dried (~12h) using the Labconco 7934020 freeze-drying system. The dry samples were weighed using the Mettler AE163 analytical scale. The measurements were taken at four separate time points spanning the floral transition at ~21 d.
Chlorophyll and carotenoid measurements
Chlorophyll and carotenoid levels were measured by first extracting the pigments from freeze-dried samples. The samples were ground using chrome–steel beads until broken down into a fine powder. An 80% acetone:20% water solution was added to the samples and vortexed briefly to re-suspend plant tissue. The extraction was performed with 1.5ml of acetone solution 2–3 times or until the extraction solution was clear. A subsample of 250 µl of the extract was transferred to a PMMA cuvette along with 750 µl of fresh acetone solution, and absorbance at 480, 645, and 663nm was taken. The absorbance values were plugged into the following formulae in order to calculate the desired pigment concentrations in mg g–1 dry weight (Mackinney, 1941; Davies, 1976):
| Chlorophyll a=12.72×A663–2.58×A645 |
| Chlorophyll b=22.87×A645–4.67×A663 |
| Chlorophyll a+b=8.05×A663+20.29×A645 |
| Carotenoids=A480+0.114×A663–0.638×A645 |
RNA isolation and real-time PCR
Total RNA was isolated from frozen tissue with TRIzol reagent (Invitrogen). RNeasy (Qiagen) columns were used to purify the RNA samples. A superscript (Quanta) kit was used to synthesize cDNA. RbcS, CAB1, and SAG12 expression was determined using quantitative real-time PCR (qRT-PCR). Gene expression was measured using the 7300 Applied Biosystems Real Time PCR machine. The measurements of three technical and three biological replicates were carried out at two time points during plant development/growth for each gene of interest. For qRT-PCR, 25 µl reactions were performed using 12.5 µl of PerfeCTA SYBR Green SuperMix, ROX (Quanta), 5 µl of cDNA, 6.5 µl of H2O, and 1 µl of primers. The data were analysed using Sequence Detection Software Version 1.2.3 by Applied Biosystems. Relative quantification was achieved by comparing expression of genes of interest with the actin7 gene control, which was chosen based on eFP Browser results (bar.utoronto.ca).
Statistical analysis
Statistical analysis was carried out using the Statistix 9.0 software program. The mean values were analysed using one-way analysis of variance (ANOVA) least significant difference (LSD) test with P < 0.05. Columbia and Landsberg plants were analysed separately to account for developmental differences between the two ecotypes.
Results
Senescence-triggered yellowing of transparent testa rosette leaves is common among all mutant lines under all adverse growth conditions at 24 days after germination
Leaf yellowing is a convenient visible marker of leaf senescence and reflects chloroplast degradation in mesophyll cells, which is the first step in senescence-associated programmed cell death (Oh et al., 1997). The colour of senescent leaves is due to unmasking and retention of carotenoids rather than to the new biosynthesis of yellow pigments (Thomas et al., 2009). Chlorophyll levels are an easily measured parameter for determining the degree of senescence (Hortensteiner and Feller, 2002). Wild-type plants and anthocyanin production mutants do not display significant differences in the colour of rosette leaves prior to floral transition. However, shortly after the floral transition at 21 days after germination (DAG), the rosette leaves of all anthocyanin production mutants began losing chlorophyll. Approximately 24 DAG, wild-type plants accumulated visible amounts of anthocyanin and did not show the early yellowing observed in tt lines (Supplementary Fig. S1A available at JXB online). Limiting nitrogen and high light treatments of 400 µmol m–2 s–1 induced nearly identical early yellowing phenotypes in the tt lines (Supplementary Fig. S1B). The type of stress or the location of a mutation in the anthocyanin biosynthesis pathway did not affect the timing or severity of yellowing of the rosette leaves of tt lines. The anthocyanin mutant lines used in this study have been shown to not produce any anthocyanins (see, for example, Rausher, 2006). It was confirmed that these did not produce any anthocyanins by spectrophotometric analysis even under high light at the onset of senescence.
Establishing the baseline by growing plants under non-stress/optimal growth conditions
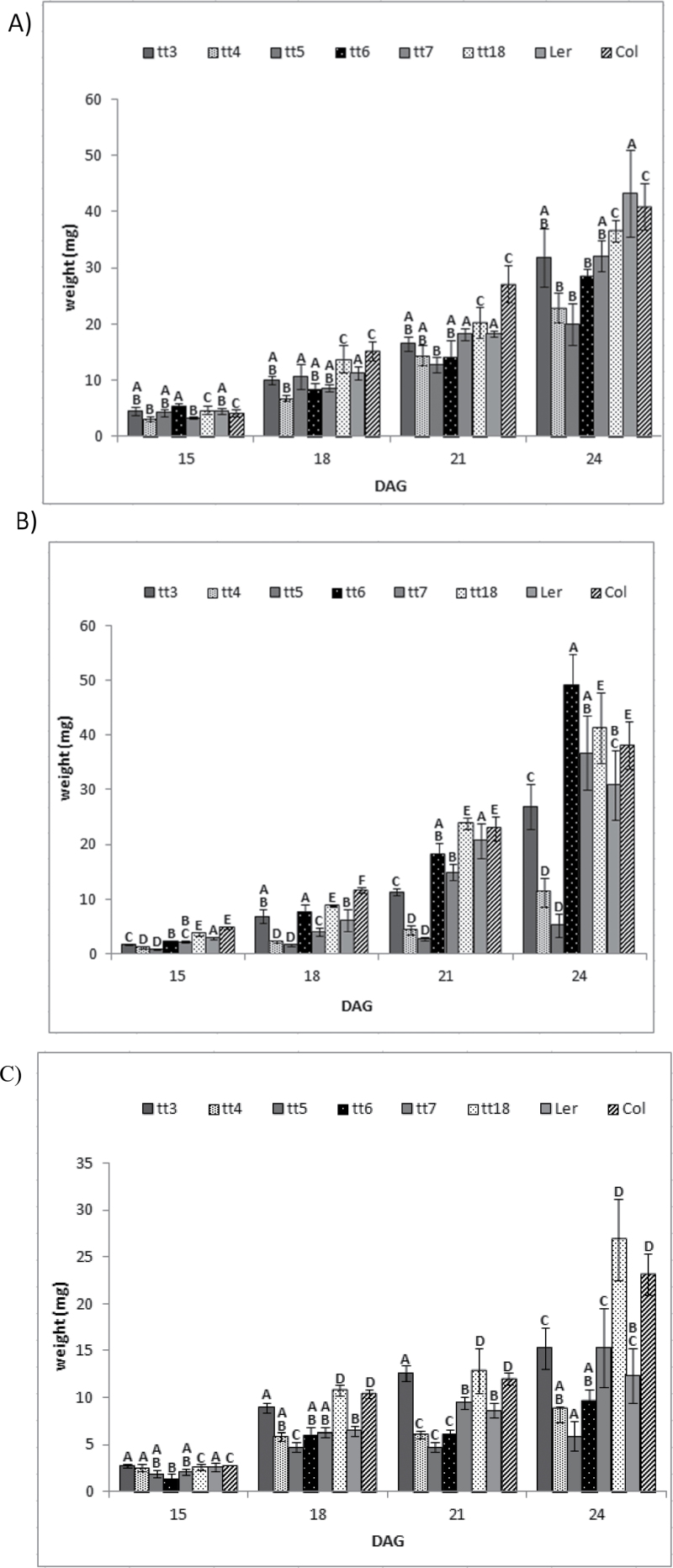
Adverse environmental conditions, such as insufficient nutrient levels, result in a significant decrease in shoot biomass accumulation (Hermans et al., 2006). Therefore, the negative impact of a treatment on plant growth and development can be assessed by measuring dry weight accumulation during the life cycle. The total dry weights of anthocyanin production mutants were determined and compared with those of wild-type plants. Biomass accumulation during early stages of development was nearly identical regardless of growth conditions. Differences in total plant biomass were observed between various anthocyanin production mutants as well as the two different ecotypes of Arabidopsis, particularly towards later stages of growth (Fig. 1). The need to use two different ecotypes as wild-type controls was reinforced by this result.
Fig. 1.
Biomass accumulation of anthocyanin production mutants and wild-type controls pre- and post-floral transition at 20 DAG. Plants were grown under optimal conditions (A), high light (B), and limiting nitrogen (C). All data are the mean ±SD. Bars with the same letters are not statistically different according to the one-way ANOVA LSD test (P < 0.05, n=5).
Two different growth conditions, limiting nitrogen and high light, were compared with non-stress growth conditions (10mM NO3 and 150 µmol m–2 s–1 light level) to test the effect of anthocyanin production on plant fitness and to establish a baseline for chronic stress treatments (Fig. 1A). Plants grown under non-stress conditions did not accumulate visible amounts of anthocyanin at 24 DAG (Supplementary Fig. S1C at JXB online). Anthocyanin production mutants did not display early leaf yellowing observed under nitrogen limitation and high light growth conditions at 24 DAG. Under non-stress growth conditions, tt4 and tt5 lines accumulated the least biomass compared with the rest of the plants at 24 DAG (Fig. 1A). The tt6 mutants grew somewhat better in comparison with tt4 and tt5, but did not grow as well as other mutants or the wild-type control. The more downstream mutants tt3, tt7, and tt18 accumulated the most biomass out of all tt lines used in this experiment in comparison with wild-type plants (Fig. 1). There was a clear difference between anthocyanin production mutants upstream and downstream of the flavonoid 3’-hydroxylase step in the anthocyanin biosynthesis pathway. The mutant lines in the first three steps of the anthocyanin biosynthesis pathway (tt4, tt5, and tt6) accumulated between 34% and 54% less biomass compared with wild-type controls, while the mutant lines in the subsequent steps (tt7, tt3, and tt18) showed a reduction of 11–26% as calculated from Fig. 1. Overall, anthocyanin production mutants did not grow as well as wild-type controls with respect to total biomass accumulation at 24 DAG. Therefore, in order to assess the effect that a given treatment has on anthocyanin production mutants, one has to account for the difference observed under optimal/non-stress growth conditions. The anthocyanin production mutants were compared with their respective wild-type control lines under any given treatment.
Growth pattern of anthocyanin production mutants did not change when grown under limiting nitrogen
Landsberg and Columbia wild-type plants responded differently to the limiting nitrogen condition. The decrease in biomass of Landsberg wild-type plants was more prominent than that of Columbia wild-type plants, suggesting that the Landsberg ecotype is more sensitive to the limiting nitrogen treatments used here. A similar pattern in growth variation was observed between different mutants under nitrogen limitation compared with non-stress growth conditions. Mutants in the later steps (tt3, tt7, and tt18) of the anthocyanin biosynthesis pathway were able to outperform those in the early steps (tt4 and tt5). The tt4 and tt5 plants once again accumulated less biomass—8.88mg and 5.92g, respectively—relative to the wild-type control (12.32g) under the limiting nitrogen condition at 24 DAG (Fig. 1C). On the other hand, tt3, tt7, and tt18 mutants were able to accumulate slightly more biomass, compared with their respective wild-type controls grown under the limiting nitrogen condition. Early step anthocyanin biosynthesis mutants once again performed more poorly compared with the more downstream mutants, which is consistent with the finding under non-stress growth conditions (Fig. 1A, C).
Chronic exposure to moderately high light throughout the plant life cycle has a significant effect on the tt4 and tt5 mutant lines
Previous research linked anthocyanin production to protection from excess solar radiation (Takahashi et al., 1991; Ahmad et al., 1995). In contrast to previous studies on protective properties of anthocyanin that focused on short-term effects of high light treatments, the performance of anthocyanin production mutants was examined relative to that of wild-type plants under chronic, moderately challenging conditions over the entire plant life cycle. High light treatment was defined as 400 µmol m–2 s–1 because the wild-type plants produced visible amounts of anthocyanin, suggesting that irradiance was perceived as a stressor, and Arabidopsis plants were able to grow and set seed under these conditions. This treatment allowed better monitoring of the effects of high light on biomass accumulation and chlorophyll levels over the entire life cycle of Arabidopsis.
Significantly larger differences in biomass accumulation between mutant lines in the first two steps of the anthocyanin biosynthesis pathway (tt4 and tt5) and the mutant lines in the subsequent steps (tt6, tt7, tt3, and tt18) under high light compared with the other growth conditions tested were observed (Fig. 1B). Mutants defective in the first two steps of the anthocyanin production pathway (tt4 and tt5) accumulated 11.9mg and 5.2mg, respectively, compared with 30.9mg for the wild-type control plants. On the other hand, the remaining lines were equal to or performed slightly better than the wild-type control. Two downstream mutant lines, tt6 and tt7, showed a significant increase in biomass accumulation compared with the wild-type control under high light conditions. The difference in biomass accumulation in tt18 plants relative to the wild-type control under high light treatment was not significant. Due to growth differences between Landsberg and Columbia ecotypes under certain conditions, the anthocyanin production mutants were only compared with their respective wild-type controls (Fig. 1B, C).
Chlorophyll levels are not correlated with presence/absence of anthocyanin and the upstream flavonoid precursors
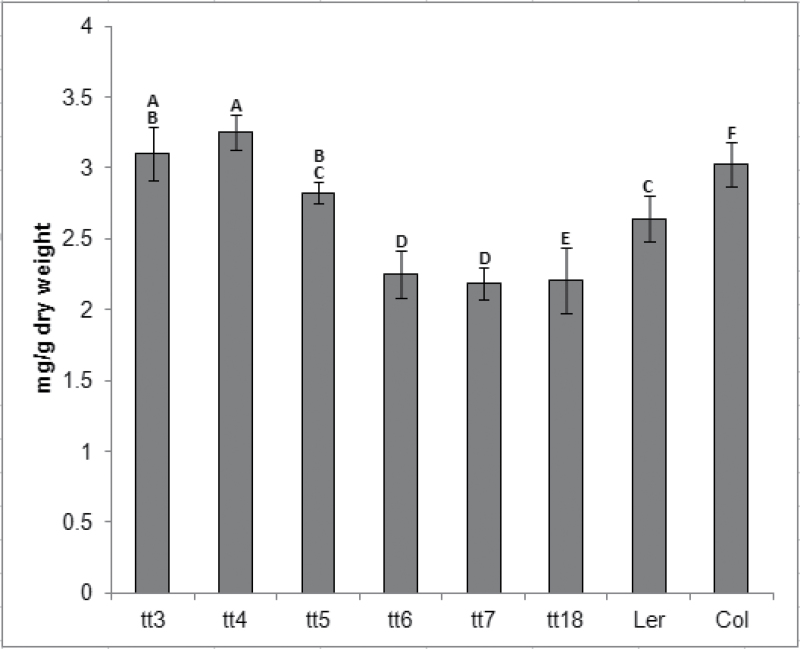
The bleaching phenotype observed in anthocyanin production mutants under various stress conditions could be indicative of accelerated chlorophyll breakdown due to either earlier senescence or photodamage. Anthocyanin and flavonoids have been shown to protect plants from high light damage by reflecting and/or absorbing excess amounts of solar and UV radiation (Feild et al., 2001; Havaux and Kloppstech, 2001). Protective properties of anthocyanin and upstream flavonoids were tested by measuring the chlorophyll levels of whole plants under high light conditions. It was found that the chlorophyll levels vary between different tt lines. Although mutants such as tt3, tt4, and tt5 contained more chlorophyll per unit of dry weight compared with wild-type plants at most time points sampled, others such as tt6 and tt7 had lower chlorophyll content levels (Fig. 2). In agreement with previous studies, there was no correlation between the presence of anthocyanin and chlorophyll content (Gould et al., 2000). In addition, the data suggest that there is no correlation between a specific mutation in the anthocyanin production pathway and the chlorophyll catabolism rate throughout the growth and senescence of tt lines under high light treatment.
Fig. 2.
Chlorophyll a/b content in rosette leaves of anthocyanin production mutants and wild-type controls. The measurements were taken at peak chlorophyll levels around the time of floral transition. All data are the mean ±SD. Bars with the same letters are not statistically different according to the one-way ANOVA LSD test (P < 0.05, n=5).
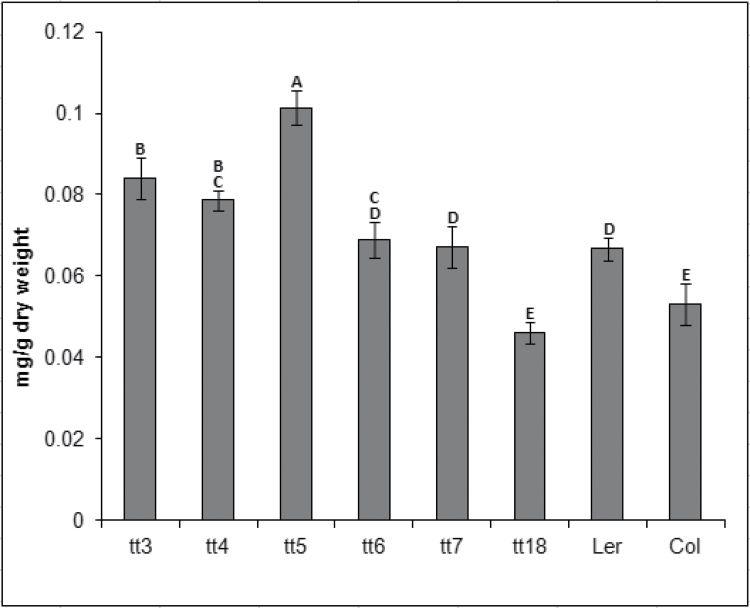
Carotenoid accumulation in plants grown under high light treatment was examined to determine whether this compound helps plants deal with excess irradiance. Carotenoid levels were highest in tt3, tt4, and tt5 lines, whereas tt6, tt7, and tt18 plants produced less or similar amounts of light-protecting flavonoids compared with the wild type (Fig. 3). The tt5 line was the most adversely affected by high light treatment, as indicated by the greatest reduction in biomass accumulation and the highest carotenoid content compared with other tt and wild-type plants.
Fig. 3.
Carotenoid production was induced in anthocyanin production mutants and wild-type controls by exposure to high light throughout the plant life cycle. All data are the mean ±SD. Bars with the same letters are not statistically different according to the one-way ANOVA LSD test (P < 0.05, n=5).
Chlorophyll catabolism in rosette leaves and inflorescence growth are not influenced by anthocyanin and flavonoid precursors
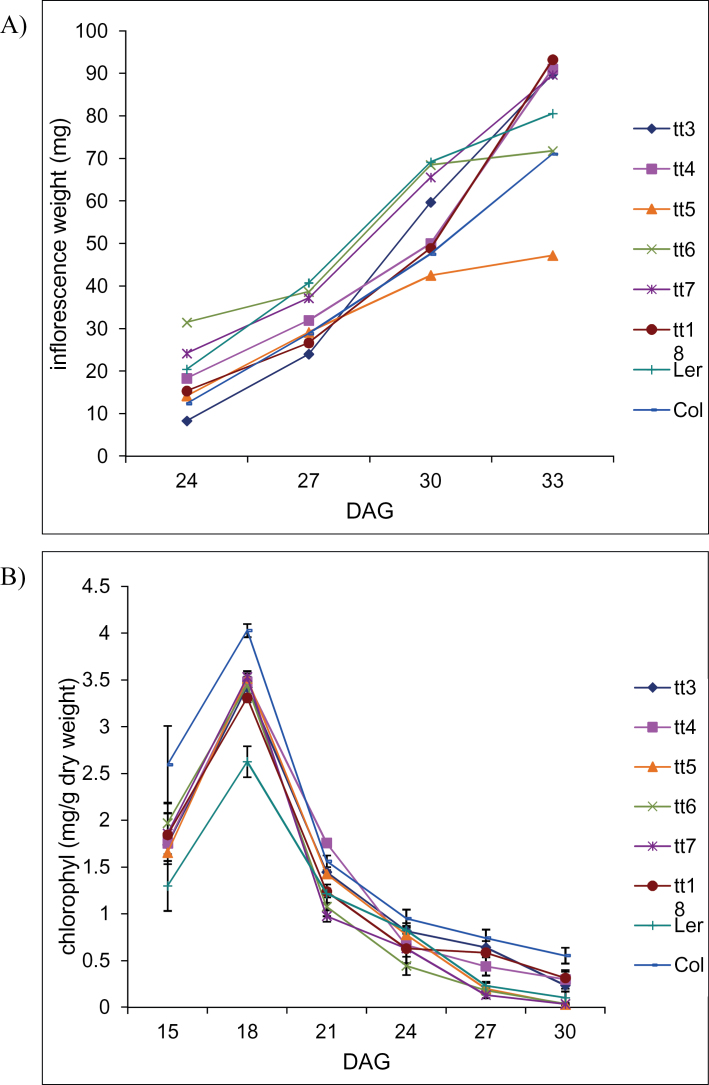
It has been proposed that anthocyanin is able to protect the machinery responsible for remobilization of nutrients from chlorophyll catabolism (Feild et al., 2001). Changes in inflorescence biomass and rosette leaf chlorophyll content were used to assess the ability of plants to remobilize and utilize the recycled material from the rosette leaves to support inflorescence growth. Rosette chlorophyll levels of all anthocyanin production mutants and wild-type control plants decreased gradually throughout the life cycle, as shown in Fig. 4. There were no observable differences in the rate of chlorophyll catabolism between different mutant lines and wild-type control plants (Fig. 4B). All tt lines, with the exception of tt5, were able to accumulate inflorescence biomass steadily throughout their life cycle (Fig. 4A). Therefore, the ability of plants to remobilize nutrients was not affected by the presence of anthocyanin and/or upstream flavonoid precursors.
Fig. 4.
Inflorescence biomass accumulation (A) and rosette chlorophyll a+b levels (B) were measured throughout the plant life cycle to obtain an estimate of its ability to remobilize nutrients from vegetative to reproductive tissues. All data are the mean ±SD.
Comparison of expression of chlorophyll and senescence-related genes in anthocyanin biosynthesis mutants
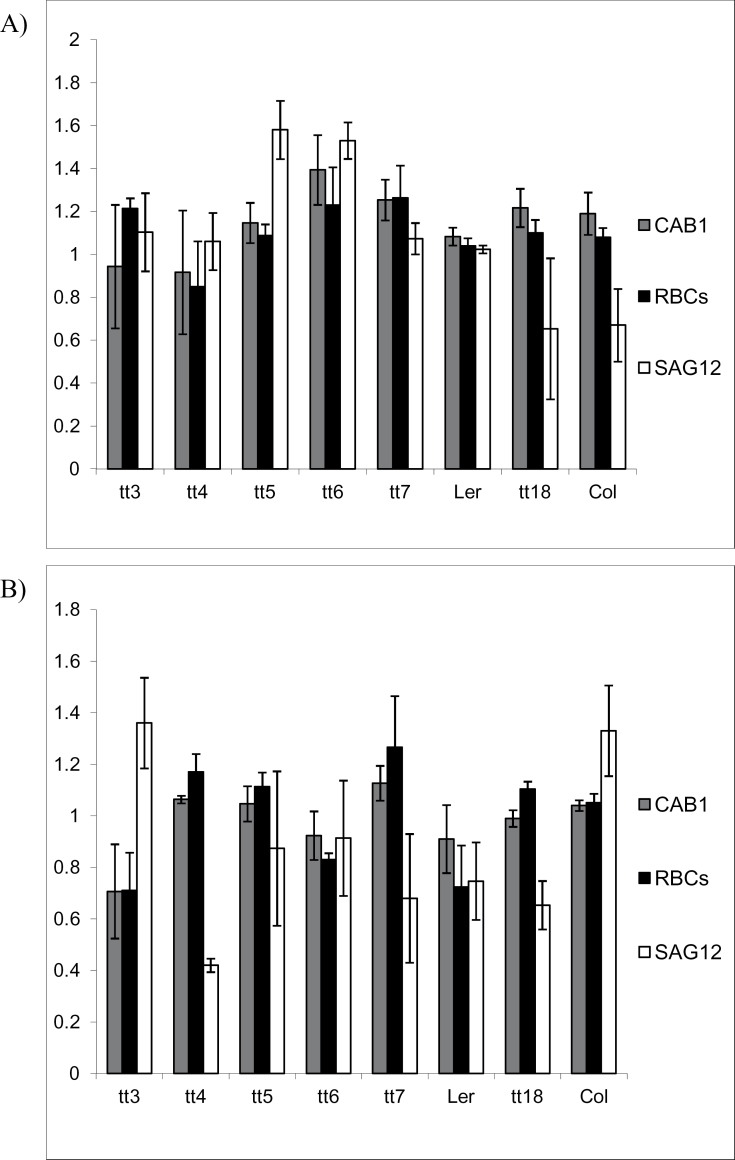
To test whether the early yellowing phenotype first observed under limiting nitrogen conditions is correlated with the absence of anthocyanin leading to earlier senescence, the expression level of SAG12 was assessed by qRT-PCR. SAG12 is a well known molecular marker for senescence (Noh and Amasino, 1999). At 14 DAG, tt3, tt5, and tt6 showed a small increase of <1.4-fold in relative SAG12 expression levels compared with wild-type plants (Fig. 5A). At 24 DAG, increases in expression of SAG12 were observed in tt3, tt4, tt5, and tt6 lines compared with the Landsberg wild-type control. The changes in SAG12 expression in tt3 and tt4 lines were larger compared with other tt lines; however, they are not nearly as high a change as compared with experiments where senescence is occurring (see, for example, Fisher-Kilbienski et al., 2010) and thus are not likely to be physiologically significant. The relative amounts of SAG12 transcript in tt6 and tt7 increased 1.5-fold compared with that of wild-type plants (Fig. 5B); however, it is unlikely that these small differences are physiologically significant.
Fig. 5.
Comparison of expression levels of senescence-associated gene 12, small subunit of Rubisco, and chlorophyll a/b-binding protein in transparent testa mutants with those in Landsberg and Columbia wild-type controls using qRT-PCR. The plants were grown under limiting nitrogen conditions to induce production of genes associated with the plant’s response to the treatment. The first set of samples was taken at 14 DAG when plants did not display any signs of stress (A). The sampling was repeated at 24 DAG when wild-type controls have accumulated visible amounts of anthocyanin and the mutants showed a pronounced yellowing phenotype (B). All data are the mean ±SD.
Reduction in expression of RbcS and CAB1 genes is associated with reduced photosynthetic activity as plants senesce (Smart, 1994; Weaver et al., 1998). In addition, these genes serve as markers for the normal limiting nitrogen response (Peng et al., 2007). In order to provide additional support for the chlorophyll content observation, expression levels of CAB1 and RbcS were measured by qRT-PCR. Levels of CAB1 and RbcS transcript in almost all of the anthocyanin production mutants were nearly equal to those of wild-type controls at both time points (Fig. 5). Therefore, the limiting nitrogen response of plants at both molecular genetic and physiological levels was not affected by the presence of anthocyanin and/or a varying flavonoid profile.
Discussion
This study was designed to gain understanding of the roles that anthocyanin pigment and/or its upstream flavonoid precursors play in the response of plants to challenging environmental conditions at specific developmental time points when plants are particularly susceptible to such treatments. Mild chronic growth conditions of limiting nitrogen and moderately high light stress conditions were chosen to ensure that the plants were able to complete their life cycles and produce seeds.
Anthocyanin pigment has been implicated both directly and indirectly in the ability of plants to tolerate chronic unfavourable conditions. For example, mutants in the anthocyanin production pathway were found to be hypersensitive to UV-B (Li et al., 1993; Philpott et al., 2004). In a previous study, a few different tt lines were analysed for final yield and whether they simply had lower vigour as opposed to having lower tolerance for adverse growth conditions such as drought, cold, and UV-B (von Wettberg et al., 2010). While mutations in the anthocyanin production pathway were correlated with decreased final yield, even under optimal growth conditions, the authors did not report pathway-related trends. A photoprotective role for anthocyanins may be one of the alternative mechanisms that plants can employ to deal with high light conditions (Hughes and Smith, 2007). It is important to note that upstream flavonoid precursors must be considered since these molecules could play a role in overall growth and development of a plant. From an evolutionary standpoint, the prevalence of anthocyanin production pathways among flowering plants suggests that compounds produced by this pathway must be advantageous for plant growth and survival (Rausher, 2006). For example, red leaf colour could have evolved as a defensive response to pest colonization as noted by Hamilton and Brown in their co-evolution hypothesis (Hamilton and Brown, 2001).
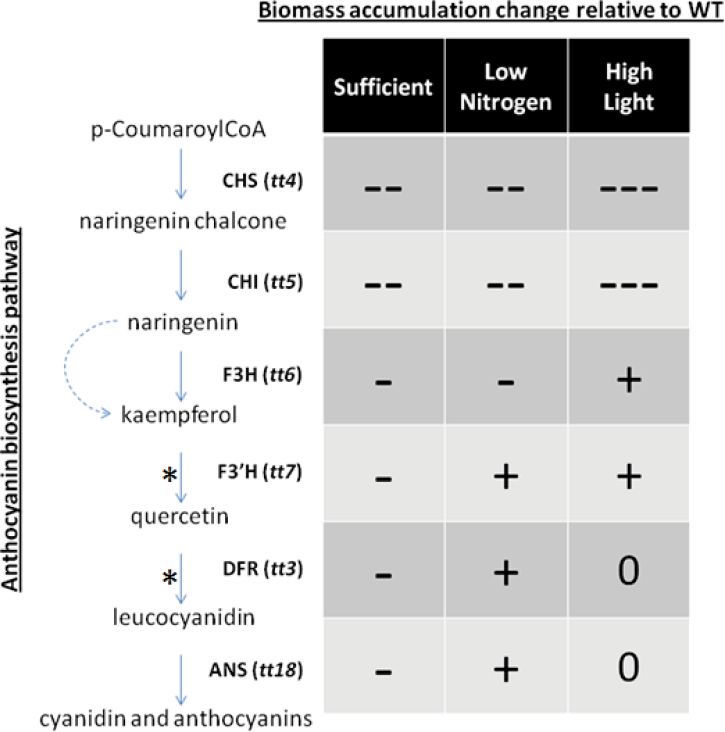
The importance of anthocyanin production is evident even under non-stress growth conditions where, on average, all anthocyanin production mutants performed more poorly compared with wild-type controls (Fig. 6). The knockout lines at various steps in the anthocyanin production pathway were examined to investigate whether changes in the growth rate were due to lack of anthocyanin or overaccumulation of flavonoid intermediates. Mutants defective in early steps in the pathway (tt4 and tt5) that do not produce most of the flavonoid intermediates had a significantly negative effect on biomass accumulation at 24 DAG (Fig. 1). This suggests that downstream flavonoids could be playing a role in normal plant growth and development. While mutants defective in later stages of the pathway (tt6, tt7, tt3, and tt18) performed better than early mutants by comparison, they consistently had between 11% and 34% lower biomass accumulation than wild-type plants at this time point depending on the mutant line tested. Therefore, inability to produce flavonoids has a negative effect on plant growth and/or development.
Fig. 6.
A simplified diagram of the anthocyanin production pathway is shown on the left. Conversions that are highlighted (*) require additional enzymatic steps not shown in the figure. The biosynthesis genes and their respective transparent testa (tt) mutant annotations are indicated at each enzymatic step: chalcone synthase (Santelia et al., 2008), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3’-hydroxylase (F3’H), dihydroflavonol 4-reductase (DFR), and anthocyanidin synthase (Hermans et al., 2006). The effect of the mutations on biomass accumulation is presented in the table on the right. A decrease (-, - -, - - -), increase (+), and no change (0) in biomass accumulation relative to the wild-type control are shown under each condition tested.
Anthocyanin accumulation has been observed under nitrogen limitation conditions (Peng et al., 2007). A link between nitrogen deficiency and photoinhibition has been reported (Henley et al., 1991), with anthocyanins proposed to have a role in protection against photoinhibition. Interestingly, the reduction in biomass accumulation by the same tt lines was also observed under non-stress growth conditions. In addition, several anthocyanin production mutants used in this study (tt7, tt3, and tt18) accumulated more biomass compared with their respective wild-type controls under nitrogen limitation condition at 24 DAG, while tt6 grew slightly more slowly than the wild type and accumulated less biomass (Figs 1, 6). None of these mutants produce a detectable level of anthocyanins, suggesting that reduced anthocyanin production does not result in a reduced growth rate under this moderate stress condition. Therefore, it was hypothesized that a mutation at or upstream of the flavonoid 3’-hydroxylase step in the anthocyanin biosynthesis pathway, which results in the absence of kaempferol and quercetin flavonoids, had a negative effect on plant growth under non-stress and limiting nitrogen conditions. However, plants with mutations downstream of these steps that still do not produce anthocyanins, while growing more poorly under ideal conditions, grow at least as well as the wild type under the limiting nitrogen condition tested.
Plants up-regulate anthocyanin biosynthesis in response to light stimulus (Christie and Jenkins, 1996). This role of anthocyanin has been studied in various species at high light intensities (Hoch et al., 2003; Karageorgou and Manetas, 2006). Previous work has shown that anthocyanin acts as a protective agent preventing high levels of solar radiation from damaging photosynthetic machinery. Here, where a more moderate high light condition was chosen so that Arabidopsis was able to complete its life cycle, growth of Landsberg wild-type plants was decreased by ~30% while Columbia growth was lower by only 7%. It was found that the growth of tt4 and tt5 mutants was affected most negatively by high light treatment compared with other tt lines; that is, the upstream mutants appeared to be more sensitive to high light by displaying a more pronounced decrease in growth rate. Plants carrying these mutations accumulated significantly less biomass compared with other mutant lines and the wild-type control plants. Interestingly, as with the limiting nitrogen results, some mutant lines (tt6, tt7, and tt3) were able to accumulate more biomass than the wild-type control at 24 DAG. Consistent with the present results from non-stress and limiting nitrogen conditions, any mutation downstream of the chalcone isomerase (tt5) step performed significantly better at 24 DAG.
A clear difference in the growth rates of mutant lines in the first two committed steps of the anthocyanin production pathway compared with the subsequent steps was observed, as shown in Fig. 6. Whether under high light, limiting nitrogen, or non-stress conditions, upstream mutants tt4 and tt5 performed much more poorly compared with downstream mutants and wild-type control plants. The absence of anthocyanin production alone does not explain the drastic reduction in growth of the tt4 and tt5 plants given that other tt lines do not show such a decrease. The mutants in the early steps of the anthocyanin biosynthesis pathway are unable to synthesize most of the flavonoids, including kaempferol and quercetin. Kaempferol has been shown to accumulate to higher levels than other flavonoids under optimal growth conditions in Arabidopsis (Veit and Pauli, 1999). Plants are also known to accumulate quercetin, which is just downstream of kaempferol in the pathway, in response to nitrogen depletion (Olsen et al., 2008). Additionally, the ratio of kaempferol to quercetin changes in response to various environmental conditions (Lovdal et al., 2010). These studies imply that flavonoid intermediates can have additional unknown functions in normal plant growth and response to environmental stresses. It is important to note that the mutant line lacking the flavanone 3-hydroxylase step (tt6) performed significantly better compared with tt4 and tt5 under non-stress and high light conditions. This mutation is leaky and these plants contain both kaempferol and some quercetin (Peer et al., 2001). As such, the tt6 plants were able to grow on a par with tt7 and tt18 lines. Therefore, the absence of kaempferol and possibly quercetin leads to a dramatic decrease in biomass accumulation which is amplified under high light conditions.
Under limiting nitrogen conditions, the anthocyanin biosynthesis mutants were also found to display leaf yellowing during senescence. SAG12 expression confirmed the senescence rates in all of the lines tested. Based on these results, it was concluded that both anthocyanin production mutants and wild-type controls senesced at similar rates during the life cycle. Therefore, the leaf yellowing that was observed in the present experiments was an artefact of the absence of anthocyanin masking the regular process of chlorophyll degradation, as opposed to accelerated senescence as was originally suspected. It should also be noted that, despite differences in biomass accumulation, the tt lines and wild-type controls were at approximately the same developmental stages, as indicated by similar levels of SAG12 expression at 24 DAG.
The ability of plants to remobilize nutrients from rosette leaves to support inflorescence growth and seed development is essential for survival (Hortensteiner and Feller, 2002). An increase in inflorescence biomass and a decrease in rosette chlorophyll levels indicates that tt lines are not hindered in this process compared with wild-type control plants under high light treatment. No correlation was observed between absence of anthocyanin or upstream flavonoid precursors and adverse effects on growth and development of reproductive organs in anthocyanin biosynthesis mutants. Therefore, products of the anthocyanin biosynthesis pathway do not seem to play a role in the protection of the nutrient remobilization machinery under the treatments used in this study.
Carotenoid accumulation is another defensive strategy used by plants to protect against high levels of solar radiation (Koka and Song, 1978). Therefore, in the absence of protection provided by anthocyanin and some precursor flavonoids, and depending on the tt line examined, the carotenoid levels were expected to increase as a result of more photooxidative damage. It is reasonable to assume that tt5 mutant plants are affected the most by high light treatment as indicated by low biomass accumulation and high carotenoid content. Interestingly, tt4 mutants were not found to have higher carotenoid levels despite being negatively affected by the high light growing conditions.
In this study, mutants defective at various steps in the anthocyanin biosynthesis pathway were used to examine the response of plants to mildly adverse environmental conditions. The absence of anthocyanin pigment production did not have a significant negative effect on plant growth on its own. The inability of plants to produce flavonoid precursors was found to be correlated with negative effects on growth rate under all conditions. In contrast, mutations later in the flavonoid pathway that still do not produce anthocyanins led to a smaller decrease in biomass under more ideal conditions. There was no noticeable effect of these mutations on the senescence process or any effect on nutrient remobilization. Interestingly some mutant lines exhibited a better growth rate than wild-type plants under chronic mild nitrogen and light stress, suggesting that flavonoid precursor levels could be manipulated in crop plants to enhance plant growth under stress conditions.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Early yellowing phenotype of anthocyanin production mutants under challenging environmental conditions differs from wild-type plants.
Acknowledgements
The authors thank Paul Kerrigan for his help with sample collection, biomass, chlorophyll, and carotenoid measurements. Michael Mucci and Tannis Slimmon provided expert plant care. Arabidopsis seeds were obtained from the Arabidopsis Biological Resource Center at Ohio State University. Research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant and the Ontario Research Fund (ORF).
References
- Ahmad M, Lin CT, Cashmore AR. 1995. Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. The Plant Journal. 8, 653–658 [DOI] [PubMed] [Google Scholar]
- Bhatla SC, Pant RC. 1977. Isolation and characterization of anthocyanin pigment from phosphorus-deficient maize plants. Current Science. 46, 700–702 [Google Scholar]
- Bloor SJ. 2001. Overview of methods for analysis and identification of flavonoids. Methods in Enzymology. 335, 3–14 [DOI] [PubMed] [Google Scholar]
- Chalker-Scott L. 1999. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology. 70, 1–9 [Google Scholar]
- Christie JM, Jenkins GI. 1996. Distinct UV-B and UV-A blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. The Plant Cell. 8, 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BH. 1976. Carotenoids. In: Goodwin TW, ed. Chemistry and biochemistry of plant pigments. London: Academic Press; 38–165 [Google Scholar]
- Davies CS. 2000. Strategy differences of two potato species in response to nitrogen starvation. Do plants have a genetic switch for nitrogen signalling?. Plant, Cell and Environment. 23, 759–765 [Google Scholar]
- Davies KM, Schwinn KE. 2003. Transcriptional regulation of secondary metabolism. Functional Plant Biology. 30, 913–925 [DOI] [PubMed] [Google Scholar]
- Diaz C, Purdy S, Christ A, Morot-Gaudry J-F, Wingler A, Masclaux-Daubresse C. 2005. Characterization of markers to determine the extent and variability of leaf senescence in arabidopsis. a metabolic profiling approach. Plant Physiology. 138, 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz C, Saliba-Colombani V, Loudet O, Belluomo P, Moreau L, Daniel-Vedele F, Morot-Gaudry J-F, Masclaux-Daubresse C. 2006. Leaf yellowing and anthocyanin accumulation are two genetically independent strategies in response to nitrogen limitation in Arabidopsis thaliana. Plant and Cell Physiology. 47, 74–83 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Steele CL. 1999. Flavonoids and isoflavonoids—a gold mine for metabolic engineering. Trends in Plant Science. 4, 394–400 [DOI] [PubMed] [Google Scholar]
- Faragher JD. 1983. Temperature regulation of anthocyanin accumulation in apple skin. Journal of Experimental Botany. 34, 1291–1298 [Google Scholar]
- Feild TS, Lee DW, Holbrook NM. 2001. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology. 127, 566–574 [PMC free article] [PubMed] [Google Scholar]
- Fischer-Kilbienski I, Miao Y, Roitsch T, Zschiesche W, Humbeck K, Krupinska K. 2010. Nuclear targeted AtS40 modulates senescence associated gene expression in Arabidopsis thaliana during natural development and in darkness. Plant Molecular Biology. 73, 379–390 [DOI] [PubMed] [Google Scholar]
- Gould KS, Markham KR, Smith RH, Goris JJ. 2000. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. Journal of Experimental Botany. 51, 1107–1115 [DOI] [PubMed] [Google Scholar]
- Hamilton WD, Brown SP. 2001. Autumn tree colours as a handicap signal. Proceedings of the Royal Society B: Biological Sciences. 268, 1489–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. 2000. Advances in flavonoid research since 1992. Phytochemistry. 55, 481–504 [DOI] [PubMed] [Google Scholar]
- Harvaux M, Kloppstech K. 2001. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta. 213, 953–966 [DOI] [PubMed] [Google Scholar]
- Henley WJ, Levavasseur G, Franklin LA, Osmond CB, Ramus J. 1991. Photoacclimation and photoinhibition in Ulva rotundata as influenced by nitrogen availability. Planta. 184, 235–243 [DOI] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. 2006. How do plants respond to nutrient shortage by biomass allocation?. Trends in Plant Science. 11, 610–617 [DOI] [PubMed] [Google Scholar]
- Hipskind J, Wood K, Nicholson RL. 1996. Localized stimulation of anthocyanin accumulation and delineation of pathogen ingress in maize genetically resistant to Bipolaris maydis race O. Physiological and Molecular Plant Pathology. 49, 247–256 [Google Scholar]
- Hoch WA, Singsaas EL, McCown BH. 2003. Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiology. 133, 1296–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortensteiner S, Feller U. 2002. Nitrogen metabolism and remobilization during senescence. Journal of Experimental Botany. 53, 927–937 [DOI] [PubMed] [Google Scholar]
- Hortensteiner S, Krautler B. 2010. Chlorophyll breakdown in higher plants. Biochimica et Biophysica Acta. 1807, 977–988 [DOI] [PubMed] [Google Scholar]
- Hughes NM, Burkey KO, Cavender-Bares J, Smith WK. 2011. Xanthophyll cycle pigment and antioxidant profiles of winter-red (anthocyanic) and winter-green (acyanic) angiosperm evergreen species. Journal of Experimental Botany. 63, 1895–1905 [DOI] [PubMed] [Google Scholar]
- Hughes NM, Neufeld HS, Burkey KO. 2005. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytologist. 168, 575–587 [DOI] [PubMed] [Google Scholar]
- Hughes NM, Reinhardt K, Feild TS, Gerardi AR, Smith WK. 2010. Association between winter anthocyanin production and drought stress in angiosperm evergreen species. Journal of Experimental Botany. 61, 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes NM, Smith WK. 2007. Seasonal photosynthesis and anthocyanin production in 10 broadleaf evergreen species. Functional Plant Biology. 34, 1072–1079 [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. 1988. Naturally-occurring auxin transport regulators. Science. 241, 346–349 [DOI] [PubMed] [Google Scholar]
- Karageorgou P, Manetas Y. 2006. The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiology. 26, 613–621 [DOI] [PubMed] [Google Scholar]
- Koka P, Song PS. 1978. Protection of chlorophyll alpha by carotenoid from photodynamic decomposition. Photochemistry and Photobiology. 28, 509–515 [Google Scholar]
- Kolesnikov PA, Zore SV. 1957. Anthocyanin formation in wheat shoots, induced by visible and invisible ultraviolet light. Doklady Akademii Nauk SSSR. 112, 1079–1081 [Google Scholar]
- Landry LG, Chapple CCS, Last RL. 1995. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiology. 109, 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng P, Itamura H, Yamamura H, Deng XM. 2000. Anthocyanin accumulation in apple and peach shoots during cold acclimation. Scientia Horticulturae. 83, 43–50 [Google Scholar]
- Li JY, Oulee TM, Raba R, Amundson RG, Last RL. 1993. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. The Plant Cell. 5, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Woo HR, Nam HG. 2003. Molecular genetics of leaf senescence in Arabidopsis. Trends in Plant Science. 8, 272–278 [DOI] [PubMed] [Google Scholar]
- Lovdal T, Olsen KM, Slimestad R, Verheul M, Lillo C. 2010. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry. 71, 605–613 [DOI] [PubMed] [Google Scholar]
- Ma YY, Guo XL, Liu BH, Liu ZH, Shao HB. 2011. The changes of organelle ultrastructure and Ca(2+) homeostasis in maize mesophyll cells during the process of drought-induced leaf senescence. Electronic Journal of Biotechnology. 14, 10 [Google Scholar]
- Mackinney G. 1941. Absorption of light by chlorophyll solutions. Journal of Biological Chemistry. 140, 315–322 [Google Scholar]
- Martinez V, Del Amor FM, Marcelis LFM. 2005. Growth and physiological response of tomato plants to different periods of nitrogen starvation and recovery. Journal of Horticultural Science and Biotechnology. 80, 147–153 [Google Scholar]
- Mulligan JT, Long SR. 1989. A family of activator genes regulates expression of Rhizobium meliloti nodulation genes. Genetics. 122, 7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. 1999. Regulation of developmental senescence is conserved between Arabidopsis and Brassica napus. Plant Molecular Biology. 41, 195–206 [DOI] [PubMed] [Google Scholar]
- Oh SA, Park JH, Lee GI, Paek KH, Park SK, Nam HG. 1997. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. The Plant Journal. 12, 527–535 [DOI] [PubMed] [Google Scholar]
- Olsen KM, Lea US, Slimestad R, Verheul M, Lillo C. 2008. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. Journal of Plant Physiology. 165, 1491–1499 [DOI] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS. 2001. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiology. 126, 536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng MS, Bi YM, Zhu T, Rothstein SJ. 2007. Genome-wide analysis of Arabidopsis responsive transcriptome to nitrogen limitation and its regulation by the ubiquitin ligase gene NLA. Plant Molecular Biology. 65, 775–797 [DOI] [PubMed] [Google Scholar]
- Peng MS, Hudson D, Schofield A, Tsao R, Yang R, Gu HL, Bi YM, Rothstein SJ. 2008. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. Journal of Experimental Botany. 59, 2933–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott M, Gould KS, Lim C, Ferguson LR. 2004. In situ and in vitro antioxidant activity of sweetpotato anthocyanins. Journal of Agricultural and Food Chemistry. 52, 1511–1513 [DOI] [PubMed] [Google Scholar]
- Rausher M. 2006. The evolution of flavonoids and their genes. In: Grotewold E, ed. The science of flavonoids. New York: Springer; 175–211 [Google Scholar]
- Saito N, Harborne JB. 1992. Correlations between anthocyanin type, pollinator and flower color in the Labiatae . Phytochemistry. 31, 3009–3015 [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, et al. 2008. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. Journal of Biological Chemistry. 283, 31218–31226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg P, Murakami P, Turner M, Heitz H, Hawley G. 2008. Association of red coloration with senescence of sugar maple leaves in autumn. Trees–Structure and Function. 22, 573–578 [Google Scholar]
- Schoeneweiss DF, Grunwald C. 1979. Anthocyanin accumulation in stems of Cornus stolonifera in response to infection by Botryosphaeria dothidea . Phytopathology. 69, 542–542 [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM. 1995. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. The Plant Journal. 8, 659–671 [DOI] [PubMed] [Google Scholar]
- Smart CM. 1994. Tansley Review No. 64. Gene expression during leaf senescence. New Phytologist. 126, 419–448 [DOI] [PubMed] [Google Scholar]
- Stapleton AE, Walbot V. 1994. Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiology. 105, 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Takeda K, Ohnishi T. 1991. Light-induced anthocyanin reduces the extent of damage to DNA in UV-irradiated Centaurea cyanus cells in culture. Plant and Cell Physiology. 32, 541–547 [Google Scholar]
- Thomas H, Huang L, Young M, Ougham H. 2009. Evolution of plant senescence. BMC Evolutionary Biology. 9, 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Pauli GF. 1999. Major flavonoids from Arabidopsis thaliana leaves. Journal of Natural Products. 62, 1301–1303 [DOI] [PubMed] [Google Scholar]
- von Wettberg EJ, Stanton ML, Whittall JB. 2010. How anthocyanin mutants respond to stress: the need to distinguish between stress tolerance and maximal vigour. Evolutionary Ecology Research. 12, 457–476 [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM. 1998. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Molecular Biology. 37, 455–469 [DOI] [PubMed] [Google Scholar]
- Williams CA, Grayer RJ. 2004. Anthocyanins and other flavonoids. Natural Product Reports. 21, 539–573 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. 2001. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology. 126, 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerback R, Dressler K, Hess D. 1989. Flavonoid compounds from pollen and stigma of Petunia hybrida—inducers of the VIR region of the Agrobacterium tumefaciens Ti plasmid. Plant Science. 62, 83–91 [Google Scholar]
- Zhang AH, Lu QT, Yin Y, Ding SH, Wen XG, Lu CM. 2010. Comparative proteomic analysis provides new insights into the regulation of carbon metabolism during leaf senescence of rice grown under field conditions. Journal of Plant Physiology. 167, 1380–1389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.