Abstract
In some plants, pollen grains accumulate storage lipids that serve as energy supply during germination. Here, three enzymes involved in early steps of oil body mobilization in the male gametophyte were functionally characterized for the first time. The effect of extracellular sugars on pollen performance and oil body dynamics was also analysed. Olive pollen oil bodies showed phospholipase A, lipase, and lipoxygenase activities on their surface. Enzyme activity levels increased during germination with a maximum after 3h. Removal of extracellular sugars from the germination medium did not affect pollen performance but increased enzyme activity rates and sped up oil body mobilization. Inhibitors seriously hampered pollen germination and pollen tube growth, leading to a characteristic accumulation of oil bodies in the germinative aperture. It can be concluded that storage lipids are sufficient for proper olive pollen germination. A lipase and a lipoxygenase are likely involved in oil body mobilization. Extracellular sugars may modulate their function, while a phospholipase A may promote their access to the storage lipids.
Key words: Lipase, lipoxygenase, oil body, olive, phospholipase A, pollen germination
Introduction
In oleaginous plants, storage neutral lipids serve as energy supply and provide carbon equivalents for periods of active metabolism (Murphy, 2012). Storage lipids can be found in different sporophytic plant organs and tissues such as oilseeds and oleaginous fruits (Huang, 1994; Zienkiewicz et al., 2011a), as well as in cells of both male (Piffanelli et al., 1998) and female (Jiang et al., 2009) germ lines. In plant seeds, neutral lipids are stored in specialized organelles called oil bodies. Despite they have often been regarded as simple storage sites, a plethora of unsuspected functions has been suggested for these organelles, including subcellular lipid trafficking and turnover, and calcium signalling (Murphy, 2012).
Oil bodies consist of a triacylglycerol (TAG) matrix surrounded by a monolayer of phospholipids, in which a set of a few unique proteins are embedded (Huang, 1994). The disruption of this barrier may be required to promote access of degrading enzymes to the storage TAGs. In a pioneer study, Matsui et al. (1999) found that trypsin-assisted in vitro digestion of oil body-associated proteins led to oxygenation of TAGs by the action of a specific lipoxygenase (LOX) enzyme in cucumber cotyledons. More recently, it was reported that a patatin-type phospholipase promotes the LOX-dependent oxygenation of oil body phospholipids in cucumber cotyledons (Rudolph et al., 2011). Thus, phospholipase A (PLA) effectively produces 80-nm holes in the phospholipid monolayer (Noll et al., 2000).
The biochemical machinery and the metabolic pathways involved in conversion of storage TAGs to sugars during seed germination has been extensively studied over the last 50 years (Graham, 2008; Murphy, 2012). It is widely accepted that fatty acids are initially released from TAGs and transported to glyoxysomes, where they are activated to their acyl-CoA esters and degraded via β-oxidation. The SUGAR-DEPENDENT1 (SDP1) gene encodes a bona-fide TAG lipase that contains a patatin-like domain and is involved in TAG breakdown (Eastmond, 2006). An alternative pathway of TAG mobilization, which is dependent of a specific LOX enzyme, may occur in parallel in some oilseeds (Feussner et al., 1994). This LOX enzyme catalyses oxygenation of linoleate moieties (18:2) of oil body-derived TAGs to (9Z,11E,13S)-13-hydroperoxy octadeca-9,11-dienoic acid (13-HPOD) (Gerhardt et al., 2005). 13-HPOD is then released from TAGs, reduced to (9Z,11E,13S)-13-hydroxy octadeca-9,11-dienoic acid (13-HOD) by the peroxygenase activity of caleosin (Hanano et al., 2006), and degraded via the β-oxidation spiral.
The pollen grain is the second most active site, after the seed, in TAG biosynthesis (Piffanelli et al., 1998). The mature olive pollen grain accumulates a high number of oil bodies in the cytoplasm of the vegetative cell (Rodríguez-García et al., 2003; Zienkiewicz et al., 2011b). During pollen germination, these organelles polarize near the germinative aperture and move to the emerging pollen tube (Rodríguez-García et al., 2003). Storage lipids are progressively metabolized during further steps of pollen tube growth (Zienkiewicz et al., 2010). These data suggest that oil bodies might provide the pollen tube with energy for a rapid apical growth. However, despite the importance of storage lipids for the growing of the pollen tube, little is known about the enzyme machinery involved in storage lipid mobilization in the male gamethophyte. Here, this study functionally characterizes for the first time three enzymes, namely lipase, PLA, and LOX, involved in early steps of oil body mobilization in the olive (Olea europaea L.) pollen. It also analyses the effect of extracellular sugars on pollen performance and oil body dynamics during germination and pollen tube growth.
Materials and methods
Plant material
Olive (Olea europaea L. cv. Picual) pollen grains were harvested as previously described (Zienkiewicz et al., 2010). Pollen samples were germinated according to Zienkiewicz et al. (2010) in a liquid culture medium with [(+)Su] or without [(–)Su] 10% (w/v) sucrose. Germinated pollen grains were sampled after 1, 3, 6, and 12h of culture, and the germination rate (%) was calculated as previously described (Rejón et al., 2012).
Oil body morphometry
Oil bodies were isolated from olive mature and germinated pollen [1, 3, and 6h in either (+)Su or (–)Su medium], stained with Nile red, and their number and size (i.e. the largest diameter) were recorded as described by Zienkiewicz et al. (2010).
Protein extraction
Oil body-associated proteins were extracted as described by Zienkiewicz et al. (2010). Mature and germinated pollen [1, 3, and 6h in (+)Su medium] samples were ground in N2 to a very fine powder using a mortar and pestle and resuspended in 1.5ml of 0.05M phosphate buffer (pH 7.0). Total proteins were eluted under continuous stirring at 4 °C for 1h. Protein suspensions were clarified by centrifugation at 13,500 g for 30min at 4 °C and the resulting supernatants were stored at –20 °C until use. The protein concentration was measured using the 2D Quant Kit (Amersham Biosciences, USA) following the manufacturer’s instructions.
In vitro assays of lipase and PLA activity
Proteins were extracted from germinated pollen (1, 3, and 6h) grown in (+)Su and (–)Su medium, as described above. For lipase activity, 50 µl protein extract (~50 µg of protein) were incubated with 10 µl of 25 µg ml–1 resorufin ester (Sigma-Aldrich, USA) for 10min, and the absorbance was read at A550 in an iMark Microplate Reader (Bio-Rad, USA). The PLA activity was assayed at A488 using 10 µl of 1mM BODIPY FL C11-PC (Molecular Probes, USA) as substrate. Control reactions were performed by omitting protein extracts in the reaction mixture.
Western blot analysis
Proteins from mature and germinated pollen [3h in (+)Su medium] were electroblotted as previously described (Zienkiewicz et al., 2010). Immunodetection of LOX was performed by incubation with a polyclonal anti-Glycine max (soybean) LOX antibody (Agrisera, Sweden), diluted 1:1000 in TBS buffer (pH 7.2) containing 1% (w/v) bovine serum albumin (BSA) overnight at 4 °C, followed by a DyLight 488-conjugated anti-rabbit IgG secondary antibody (Agrisera), diluted 1:2000 in TBS for 2h. The signal was detected in a Pharos FX imager (Bio-Rad).
In-gel assays of lipase and LOX activities
Lipase activity was assayed in gel using β-naphthyl palmitate as substrate, as previously described (Rejón et al., 2012). LOX activity was measured according to Heinish et al. (1996), with minor modifications. Briefly, the gel was incubated for 30min in a solution containing either α-linolenic acid or a mixture of α-linolenic acid and 10mM sodium cyanide. Subsequently, the gel was stained with 100ml of a solution containing 0.5g of N,N-dimethyl-p-phenylenediamine, 4.5ml methanol, and 0.5ml acetic acid. Control reactions were performed by excluding the substrate.
Lipase and LOX inhibition assays
Pollen samples were germinated in either (+)Su or (–)Su medium supplemented with the corresponding inhibitor at a final concentration of 2mM. Pollen was sampled after 3h of culture and the germination rate (%) was calculated as described above. Ebelactone B (dissolved in ethanol/H2O, 1:1) and ferulic acid (prepared in ethanol) were purchased from Sigma-Aldrich. Controls were carried without inhibitors.
To perform in-gel inhibition assays, protein extracts from germinated pollen [3h in (+)Su medium] were prepared as above but the extraction buffer contained 2mM ferulic acid. After SDS-PAGE, LOX activity was detected as described above. Reproducibility of results was confirmed in three independent experiments.
LOX immunoprecipitation and mass spectrometry analysis
Proteins were extracted from oil bodies as described above. LOX immunoprecipitation was carried out using the anti-soybean LOX antibody, as described by Zienkiewicz et al. (2010). After electrophoresis, the CBB-stained LOX band was sliced and subjected to MS/MS analysis. The identification of olive pollen LOX was carried out at the Laboratory of Proteomics (CSIC/UAB), a member of ProteoRed network (www.proteored.org). Samples were analysed using a linear LTQ ion trap equipped with a microESI ion source (ThermoFisher, USA). MS/MS spectra were analysed using the PEAKS Studio v5.1 software (Bioinformatics Solutions, Canada). Sequence tags with a confidence higher than 70% were sent for protein identification against Uniprot database (taxonomy: 3193 embryophyta, release 15.15) using the Basic Local Alignment Search Tool (BLAST). Only the sequence tags with more than 80% of coincidence with the identified protein were considered. The identifications were manually validated to ensure the quality of the spectral data.
In situ localization of lipase and PLA activity
Oil bodies were isolated from germinated pollen [3h in (+)Su medium] and incubated with either an aqueous solution of 25 µg ml–1 resorufin ester (lipase substrate) or an ethanolic solution of 1mM BODIPY FL C11–PC 9 (PLA substrate). In situ localization of lipase and PLA activity was also carried out in intact pollen tubes. Samples were incubated in either 25 µg resorufin ester ml–1 for 30min or 1mM BODIPY FL C11-PC for 10min, and examined with a C1 confocal laser scanning microscope (Nikon, Japan) using a He-Ne (549nm) and an argon (488nm) laser, respectively.
Light microscopy immunolocalization of LOX
LOX was immunolocalized on intact pollen tubes and isolated oil bodies [3h in (+)Su medium]. Oil body samples were processed as described by Zienkiewicz et al. (2010) and incubated with the anti-soybean LOX antibody (diluted 1:30 in PBS buffer, pH 7.2, containing 1% BSA) for 3h, followed by incubation with an anti-rabbit IgG DyLight 488-conjugated secondary antibody (diluted 1:200 in PBS), for 1h. Germinated pollen samples were processed as above but the primary antibody was diluted 1:50 and incubation was carried out overnight at 4 °C. All the samples were re-suspended in an anti-fading solution of Citifluor (Sigma-Aldrich) and examined in a C1 confocal microscope using an argon (488nm) laser. Ten µl of 0.1mg Nile red ml–1 were added to the germinated pollen samples before observation. Z-series images were collected using the EZ-C1 Gold version 2.10 software (Nikon). Negative controls were prepared by excluding the primary antibody.
Transmission electron microscopy immunolocalization of LOX
Germinated pollen [3h in (+)Su medium] samples were processed for transmission electron microscopy as previously described (Zienkiewicz et al., 2010). Sections were incubated with an anti-soybean LOX antibody (diluted 1:50 in PBS buffer, pH 7.2, containing 0.5% BSA), overnight at 4 °C, followed by an anti-rabbit IgG 15-nm gold-conjugated secondary antibody (British Biocell International, UK), diluted 1:100 in PBS, for 1h. After washing, sections were stained with 5% (w/v) uranyl acetate for 30min. Samples were observed in a JEM-1011 transmission electron microscope (JEOL, Japan) operating at 80kV. Control reactions were carried out by omitting the primary antibody.
Statistical analysis
Standard analysis of variance (t-test) was used to assess the significance of the means at P < 0.01 level using the SSPS Statistics version 19 software (IBM, USA).
Results
Olive pollen oil bodies’ behaviour in sucrose-supplemented vs. sucrose-free culture medium
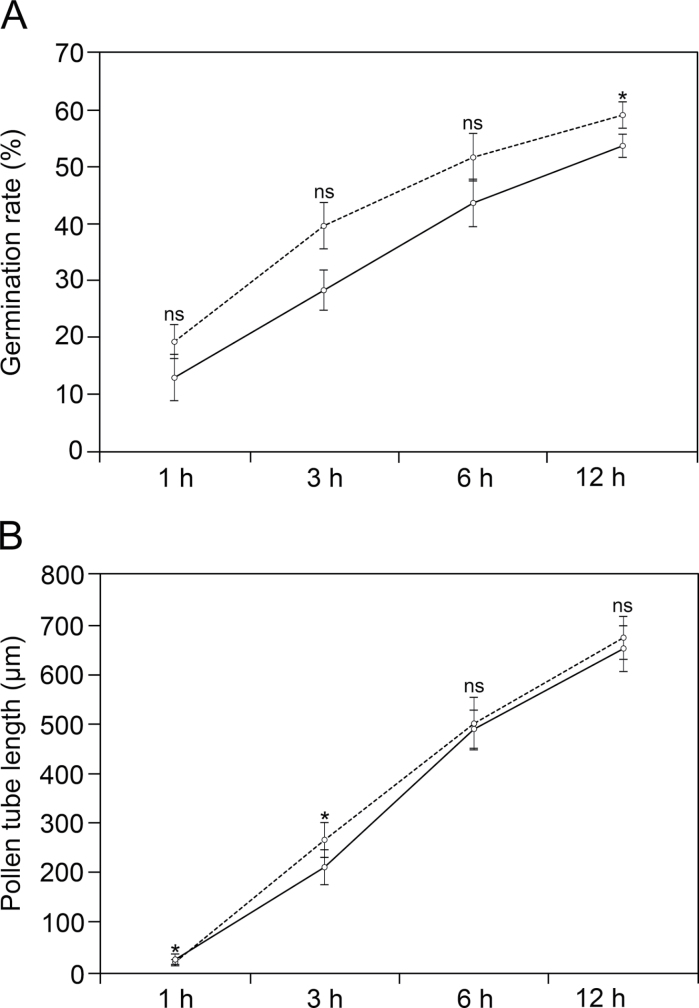
This study first compared germination and pollen tube growth rates in (+)Su vs. (–)Su cultured pollen. Germination rates were slightly lower in (–)Su medium (Fig. 1A and Supplementary Fig. S1, available at JXB online). Furthermore, there were slight differences in the average pollen tube length between (+)Su and (–)Su germination media at the early stages of culture (Fig. 1B).
Fig. 1.
Comparison of olive pollen germination rate (A) and pollen tube growth rate (B) in culture medium with sucrose (dashed line) and without sucrose (solid line). Values are mean ± SD calculated from 1000 (A) and 500 (B) pollen grains. Asterisks correspond to significant difference (P ≤ 0.01); ns, no significant difference.
Nile red staining allowed visualization of oil bodies inside pollen as fluorescent round-shaped structures (Supplementary Fig. S2). After 1h of culture, a single pollen grain contained about 1000 oil bodies (Fig. 2A). The average diameter of these oil bodies was similar in (–)Su and (+)Su medium (Supplementary Table S1). Subsequently, the number of oil bodies progressively diminished in both culture variants but this process occurred more rapidly in pollen grains germinated in (–)Su medium. After 3h of culture, oil bodies doubled their number and size in pollen tubes grown in (–)Su medium (Fig. 2B and Supplementary Fig. S2). The average number and size of oil bodies gradually decreased in both culture variants. However, lipid mobilization occurred more rapidly in pollen tubes growing in (–)Su medium and oil bodies were noticeably larger (Fig. 2B and Supplementary Table S1).
Fig. 2.
Oil body dynamics in olive pollen grains (A) and pollen tubes (B) at different times of germination in culture medium with sucrose (dashed line) and without sucrose (solid line). Values are mean ± SD from Z-series images corresponding to 45 germinated pollen grains from three independent experiments (N = 45). Asterisks correspond to significant difference (P ≤ 0.01); ns, no significant difference.
Detection of oil body-associated enzymatic activities in the olive pollen during germination
The oil body-associated protein profile is displayed in Fig. 3A. A single lipolytic band of 44.6kDa was associated with oil bodies in the mature pollen, whereas a unique 43.1-kDa lipase was detected in the germinated pollen (Fig. 3B). A protein of about 99kDa with LOX activity was also detected in oil bodies of germinated pollen (Fig. 3C, D). The specificity of the anti-LOX antibody was confirmed by immunoprecipitation and MS/MS analyses (Supplementary Fig. S3 and Table S2).
Fig. 3.
(A) Coomassie-stained polyacrylamide gel showing mature (MP) and germinated (GP) pollen oil body-associated protein profiles. (B) In-gel assay of lipase activity (arrows). (C) Immunodetection of lipoxygenase (arrow). (D) In-gel assay of lipoxygenase activity (arrows) in experiments with (+Cy) or without (–Cy) cyanide.
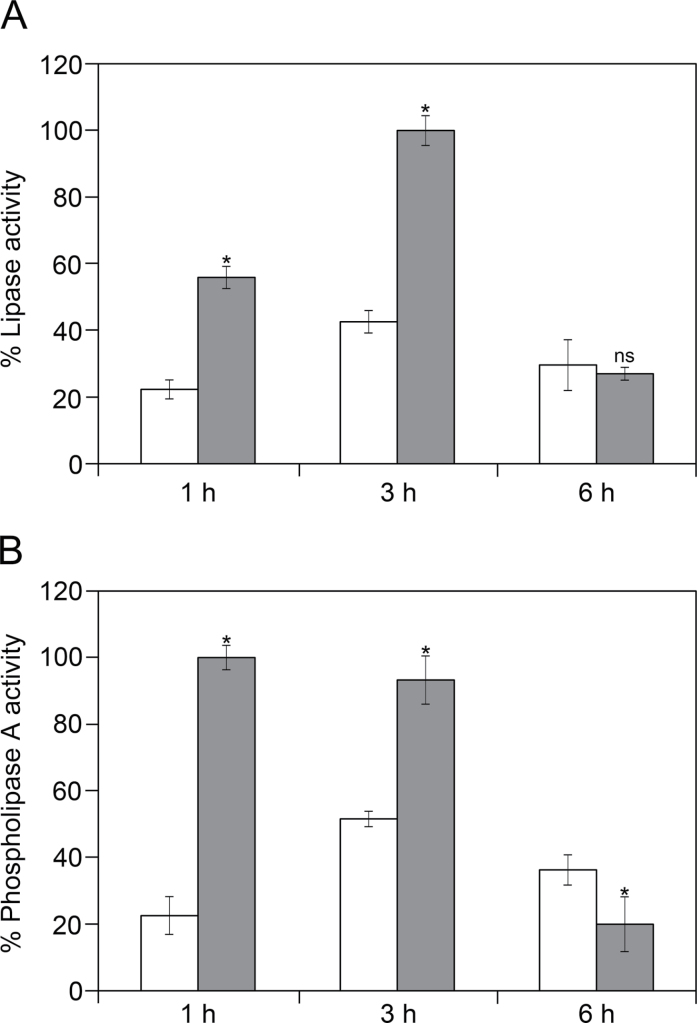
Lipase activity values reached a peak after 3h in both culture variants (Fig. 4A), but they were 2-fold higher in (–)Su samples. PLA activity profiles were similar to those of lipase (Fig. 4B) and PLA activity levels were also significantly higher in (–)Su samples. After 6h of culture, the intensity of both lipase and PLA activities significantly decreased.
Fig. 4.
In vitro assays of lipase activity (A) and phospholipase A activity (B) at different time intervals of olive pollen germination in culture medium with sucrose (white bars) and without sucrose (grey bars). Values are mean ± SD relative percentages referred to the highest optical density value for five independent experiments with three replicas each (N = 15) performed in each case. Asterisks correspond to significant difference (P ≤ 0.01); ns, no significant difference.
In-gel assays of enzyme activity revealed up to 12 putative lipases in germinated pollen in both (–)Su and (+)Su medium, with two prominent activity bands of 38.8 and 51.6kDa (Fig. 5A). Lipase activities showed significantly higher intensities in (–)Su samples (Fig. 5A). Early steps of pollen germination were also accompanied by LOX activity, with bands of about 63, 90, and 99kDa (Fig. 5B). Average levels of LOX activity were also higher in (–)Su samples (Fig. 5B).
Fig. 5.
(A) In-gel detection of lipase activity in 3-h germinated pollen in culture medium with sucrose [(+)Su and without sucrose [(–)Su]. Major activity bands are indicated with arrows. The chart at the bottom display the average densitometric data corresponding to the lipolytic bands detected on three gels. (B) In-gel detection of lipoxygenase activity in 3-h germinated pollen in (+)Su and (–)Su culture media. Major activity bands are indicated with arrows. The chart at the bottom displays the average densitometric data corresponding to the lipoxygenase bands detected on three gels. Asterisks correspond to significant difference (P ≤ 0.01).
Functional analysis of oil body-associated enzymes using specific inhibitors
When ebelactone B and ferulic acid inhibitors were added to the germination medium, they significantly hampered the capacity of pollen to germinate in the two culture variants assayed (Supplementary Fig. S4). This effect was accompanied by a characteristic accumulation of oil bodies in the germinative swollen aperture (Supplementary Fig. S5). Moreover, in-gel inhibition assays showed that LOX activities corresponding to 90- and 99-kDa bands were fully inhibited by the action of ferulic acid, while the 63-kDa band remained unaffected (Fig. 6).
Fig. 6.
In-gel inhibition assay of lipoxygenase activity in olive pollen after 3h of in vitro germination. In experiments with 2mM ferulic acid (+FA), lipoxygenase activities corresponding to 90 and 99kDa bands (arrows) were inhibited. In control experiments (–FA), the lipoxygenase activity profile was similar with (+Cy) and without (–Cy) cyanide. M, protein markers.
Cellular localization of lipase and PLA activities in the olive pollen
Purified pollen oil bodies were visible as spherical structures that stained positively with Nile red (Supplementary Fig. S6). Isolated oil bodies did not show lipase activity at pollen maturity (Supplementary Fig. S7A). After 3h of germination, oil bodies displayed a strong lipase activity on their surface (Supplementary Fig. S7B). This study could also detect the existence of PLA activity on the oil bodies boundaries in both mature and germinated pollen samples (Supplementary Fig. S7C, D).
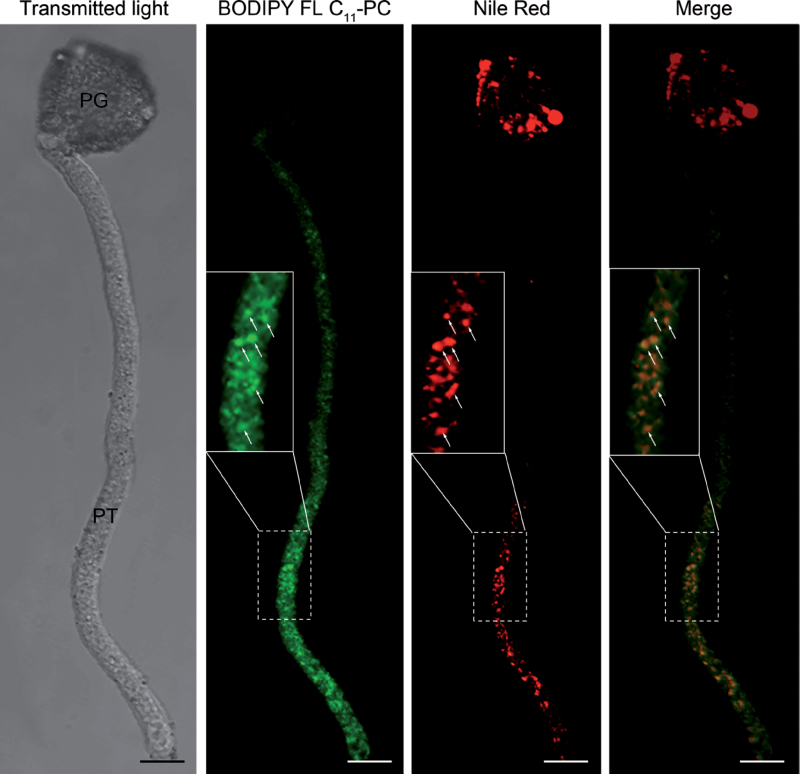
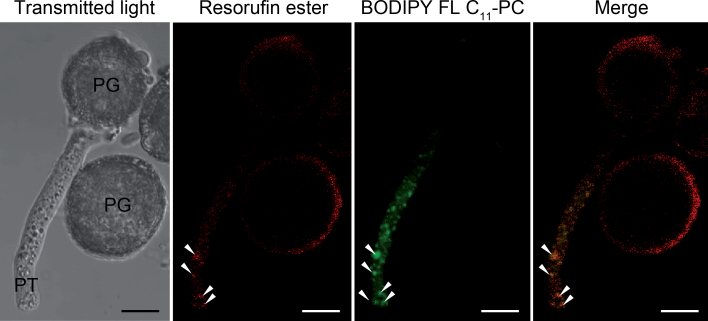
The lipase activity was detected around the pollen wall and the vicinity of the pollen aperture from which the pollen tube emerged (Supplementary Fig. S8). In the pollen tube, the fluorescent signal was mainly located along the cytoplasm. The cytoplasmic labelling was visualized as red fluorescent spots (Supplementary Fig. S8, inset). The PLA activity was exclusively located in the pollen tube cytoplasm as green fluorescent spots (Fig. 7) and co-localized with oil bodies (Fig. 7, inset). Simultaneous fluorescent labelling with resorufin ester and BODIPY FL C11-PC showed that lipase and PLA activities also co-localize in the pollen tube cytoplasm (Fig. 8). Negative controls did not show any fluorescence (Supplementary Fig. S9).
Fig. 7.
Co-localization of phospholipase A activity (BODIPY FL C11 -PC) with oil bodies (Nile red) in olive pollen tubes (arrows) after 3h of culture. Insets show the magnified areas marked with the dashed squares. PG, pollen grain; PT, pollen tube. Bars, 10 µm.
Fig. 8.
Co-localization of lipase (resorufin ester) and phospholipase A (BODIPY FL C11-PC) activities in olive pollen tubes (arrowheads) after 2h of culture. PG, pollen grain; PT, pollen tube. Bars, 10 µm.
Immunolocalization of LOX enzyme in the olive pollen
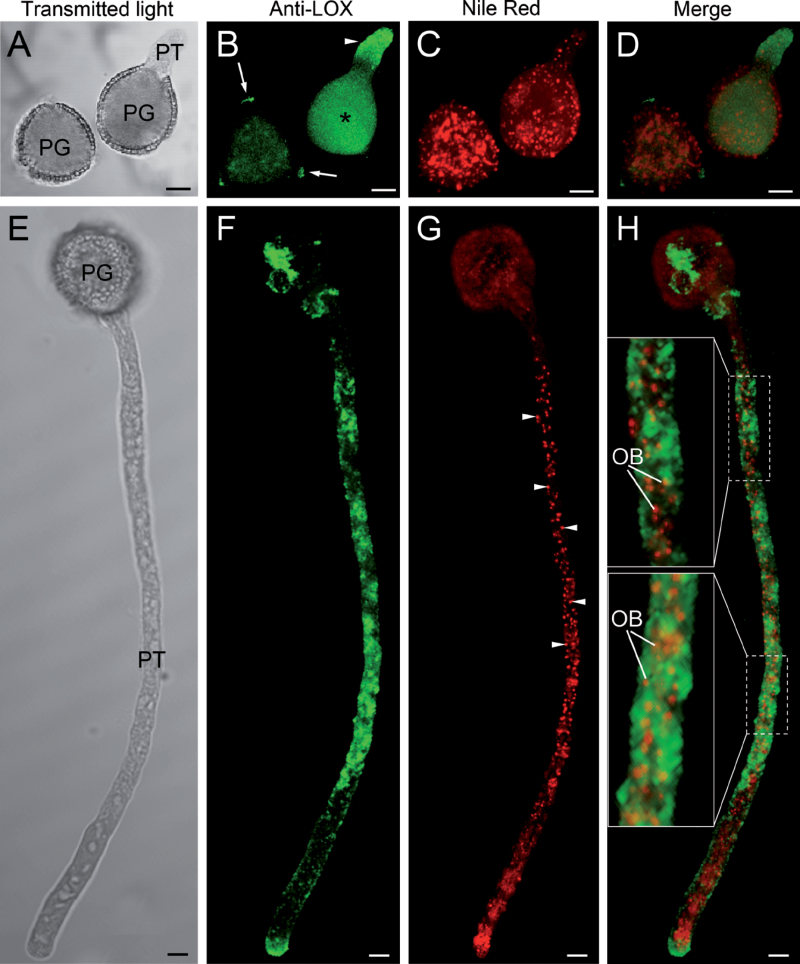
Fluorescent immunolabelling of isolated oil bodies confirmed the presence of a LOX enzyme on the oil body surface (Supplementary Fig. S7E, F). In the incipient pollen tube, green fluorescence was found along the cytoplasm (Fig. 9A–D). After 3h of culture, intense labelling, forming fluorescent spots and patches, was detected along the pollen tube cytoplasm except for the subapical region (Fig. 9E–H). Simultaneous labelling with Nile red showed some co-localization of LOX with oil bodies (Fig. 9H, insets). Green fluorescence was also visible at the pollen tube tip, where no oil bodies were detected. Negative controls did not show any fluorescence (Supplementary Fig. S9).
Fig. 9.
Co-localization of lipoxygenase (anti-LOX antibody) with oil bodies (Nile red). (A–D) Optical section by confocal laser scanning microscopy of olive pollen cultured in vitro for 1h. Fluorescent labelling of LOX was mainly located in the cytoplasm of the pollen grain (B, asterisk) and the pollen tube (B, arrowhead). In the non-germinated pollen grains, fluorescence was lower and located in the cytoplasm and the three apertures (B, arrows). (E–H) Optical section by confocal laser scanning microscopy of olive pollen cultured in vitro for 3h. Fluorescent labelling of LOX was heterogeneously distributed along the pollen tube cytoplasm except for the subapical region. The pollen tube contained numerous oil bodies (G, arrowheads) that co-localized with LOX (H, insets). OB, oil body; PG, pollen grain; PT, pollen tube. Bars, 10 µm.
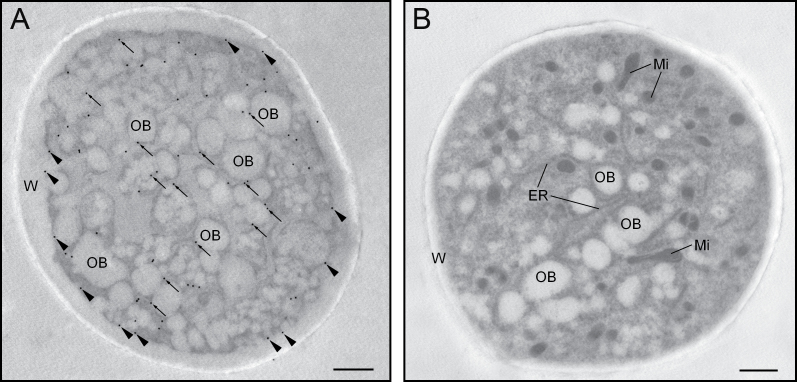
At ultrastructural level, the olive pollen LOX was mainly localized attached to the surface of oil bodies that appeared randomly distributed along the pollen tube cytoplasm (Fig. 10A). Moreover, a significant labelling was found attached to small cytoplasmic vesicles that fused with the plasma membrane, which was also decorated with gold particles (Fig. 10A). Negative controls showed no labelling (Fig. 10B).
Fig. 10.
Ultrastructural localization of lipoxygenase in the olive pollen tube. (A) Transversal section of a pollen tube after 3h of germination. Gold particles were located on the oil bodies’ boundaries (arrows) and in the surrounding cytoplasm. Some labelling was also present at the plasma membrane (arrowheads). (B) Negative control showing no gold labelling. ER, endoplasmic reticulum; Mi, mitochondria; OB, oil body; W, pollen tube wall. Bars, 1 µm.
Discussion
This work first addressed the question of whether olive pollen lipidic reserves are sufficient to promote the autonomous growth of the pollen tube. Sugar removal from the culture medium did not significantly influence pollen tube growth rate, suggesting that storage TAGs are sufficient as carbon supply for proper early pollen tube growth. Mobilization of seed storage lipids in Arabidopsis thaliana seedlings was significantly retarded in the presence of exogenous glucose (To et al., 2002). This effect seems to be correlated with changes on expression of genes encoding lipid mobilization-related enzymes. Thus, lipase and catalase activities were significantly enhanced in yellow lupine seeds germinated in sugar-free medium (Borek et al., 2006; Borek and Nuc, 2011). Similarly, the present study observed that oil body mobilization during olive pollen germination occurred more rapidly when the culture medium was not supplemented with sucrose. In parallel, under sugar-deprivation conditions, lipase, PLA, and LOX activities significantly increased in the olive pollen tube, confirming that the catabolism of storage lipids is more intense in the absence of an extracellular carbon supply.
The olive stigma exudate contains pectins and free sugars that are likely products of starch hydrolysis in papillae prior to pollination and may serve as pollen tube cell wall precursors (Suárez et al., 2012). However, these extracellular sugars might also modulate the expression of oil body-associated enzymes during pollen tube growth on the stigma, as shown in vitro. Olive pollen tubes grow in vitro at rates of about 1 µm min–1 (this paper), and storage lipids are mostly degraded within 12h of culture (Zienkiewicz et al., 2010). Interestingly, oil bodies present in pollen and some arbuscular mycorrhizal fungi show a strong resemblance in terms of size and distribution and movement within pollen and germ tubes (Bago et al., 2002). Pollen oil bodies are entirely mobilized in the olive stigma (unpublished data), and pollen tube growth is much faster than in vitro (Suárez et al., 2012). In the style and ovary, the passage of the pollen tubes across the transmitting track is accompanied by the disappearance of starch (Suárez et al. 2012). All these data suggest that the olive pollen tube growth in the stigma is mainly fuelled by its own reserves, but is markedly heterotrophic in the style and ovary.
Seed oil bodies are stabilized by proteins (e.g. oleosins) docked in the phospholipids that surrounds the TAG matrix (Huang, 1994). Therefore, a first step in oil body mobilization should be the disruption of this barrier in order to promote access of lipases to the stored TAGs (Noll et al., 2000; Gupta and Bhatla, 2007). A recent paper found evidence that the mobilization of storage lipids by LOX in cucumber cotyledons is promoted by a patatin-type phospholipase (Rudolph et al., 2011). Here, the present study has detected for the first time the presence of PLA activity associated to the surface of oil bodies. Moreover, a good temporal correlation was observed between PLA and lipase activities during germination. These data suggest that the action of the oil body-associated lipase might be promoted by the co-localizing PLA enzyme. Interestingly, the mature pollen grain also showed PLA activity suggesting that this enzyme may be active and stably associated to oil bodies at this developmental stage. This is in good agreement with the idea that phospholipids breakdown is a prerequisite for TAG mobilization.
The pollen coat lipids play a key role in pollen hydration on dry stigmas (Wolters-Arts et al., 1998). Lipases are present in the extracellular pollen coat that is deposited on the pollen wall surface at the final steps of pollen maturation (Mayfield et al., 2001; Shakya and Bhatla 2010). Recently, it has been demonstrated that the extracellular lipase EXL4 is required for efficient hydration of Arabidopsis pollen (Updegraff et al., 2009). Similarly, the olive pollen wall surface showed a strong lipase activity. Whether such enzyme activity has a function in olive pollen hydration is still a matter for future investigations.
The lipase activity was also tightly associated to the surface of olive pollen oil bodies. As far as is known, this is the first study reporting the intracellular localization of lipases in the pollen grain. It is widely accepted that storage lipid breakdown during oilseed germination is initiated by the activation of TAG lipases, which hydrolyse TAGs and release glycerol and fatty acids (Graham, 2008). In a previous study, Rejón et al. (2012) examined the lipolytic capacity of the mature olive pollen and identified up to 12 putative lipases. Here, the present study has identified a unique lipolytic band associated to olive pollen oil bodies. Interestingly, the lipolytic activity was only detectable on oil bodies in germinated pollen grains. In the light of the biochemical and microscopy findings reported here, this lipase enzyme might be inhibited in vivo at the mature pollen stage, being further activated during germination. Indeed, some studies carried out in seeds suggested that lipase activity could be inhibited by acyl-CoAs (plus CoA) (Hills et al., 1989). In a previous work, it was demonstrated that olive pollen lipase activity is effectively inhibited both in vitro and in gel by ebelactone B (Rejón et al., 2012). Here, the present study found that the addition of ebelactone B to the culture medium resulted in strong reduction of germination capacity of olive pollen, likely by hampering storage lipid mobilization. The size of the olive pollen oil body-associated lipase is similar to LeLID1 (45kDa) and AtLIP1 (42kDa) seed TAG lipases from tomato and Arabidopsis, respectively. The gene SDP1 encodes a 95-kDa patatin domain TAG lipase that is involved in storage lipid mobilization in germinating Arabidopsis seeds (Eastmond 2006). LeLID1 and AtLIP1, however, do not possess the patatin domain and they are not directly involved in oil body breakdown (Matsui et al., 2004; El-Kouhen et al., 2005). At present, there is a lack of conclusive evidence that a TAG lipase is involved in storage lipid mobilization in olive pollen. Further analysis will be necessary to determine if the pollen oil body-associated lipase is a bona-fide TAG lipase.
Fatty acids released from TAGs may be further degraded either via the β-oxidation in glyoxysomes (Graham, 2008), or by an alternative pathway, which is dependent on a LOX enzyme (Feussner et al., 1994). The present study detected for the first time the existence of LOX activity in germinating olive pollen. The anti-LOX antibody used in this work recognized a unique 99-kDa protein band on Western blots. However, the LOX activity profile revealed the existence of a second LOX isoenzyme with an estimated molecular weight of ~90kDa. The size of both olive pollen LOXs are in the molecular weight range reported for other plant LOXs (Andreou and Feussner, 2009). The present study also detected a smaller protein band of ~63kDa, which showed enhanced LOX activity. Interestingly, limited proteolysis of soybean LOX1 generated a ~60-kDa fragment with enhanced activity and membrane-binding ability (Maccarrone et al., 2001). Therefore, it is plausible that the 63-kDa LOX found in the olive pollen comes from one of the other two larger LOXs, although this point needs to be experimentally verified.
The olive pollen 99-kDa LOX was mainly localized on the oil body surface, as previously reported for other LOX enzymes in seeds and cotyledons (Rodríguez-Rosales et al., 1998; Rudolph et al., 2011; Yadav and Bhatla, 2011), which suggests that it is a type 1-LOX. The present study could not detect either the protein or its activity in oil bodies from mature pollen grains. This fact suggests that the 99-kDa LOX enzyme might be recruited by oil bodies just after pollen hydration. Olive pollen LOX in-gel activity was effectively inhibited by ferulic acid, as previously demonstrated for other plant LOXs (Redrejo-Rodríguez et al., 2004; Szymanowska et al., 2009). The present study found that the capacity of olive pollen to germinate was seriously hampered when ferulic acid was added to the culture medium. This effect was characteristically accompanied by a strong accumulation of oil bodies at the germinative aperture. However, there is no conclusive experimental evidence that ferulic acid only inhibits LOX activity, so these results should be taken cautiously. Further functional analysis will be necessary to confirm that pollen performance is affected when LOX enzyme is blocked. All together, these data support the idea that a LOX-mediated pathway of storage lipid mobilization, which is of major importance for germination, exists in the olive pollen grain.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. In vitro germination rates of olive pollen in sucrose-supplemented and sucrose-free medium.
Supplementary Fig. S2. Nile red staining of oil bodies in olive pollen germinated in sucrose-supplemented and sucrose-free medium.
Supplementary Fig. S3. Immunoprecipitation of olive pollen lipoxygenase.
Supplementary Fig. S4. Effect of lipase and lipoxygenase inhibitors on olive pollen germination.
Supplementary Fig. S5. Inhibitory effects of ferulic acid and ebelactone B on pollen germination.
Supplementary Fig. S6. Nile red staining of olive pollen oil bodies.
Supplementary Fig. S7. Detection of lipase, phospholipase A, and lipoxygenase on the oil body surface.
Supplementary Fig. S8. Immunolocalization of lipase activity in olive pollen during germination.
Supplementary Fig. S9. Negative controls for lipase, phospholipase A, and lipoxygenase detection.
Supplementary Table S1. Average size of olive pollen oil bodies during in vitro germination.
Supplementary Table S2. Identification of olive pollen lipoxygenase.
Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation (ERDF-cofinanced project AGL2008-00517) and the Junta de Andalucía (ERDF-cofinanced project P2010-CVI5767). A. Zienkiewicz thanks the CSIC for providing JAEdoc grant funding. We also thank Ms. C. Martínez-Sierra for her expert technical assistance and the CIFA ‘Venta del Llano’ (Mengíbar, Spain) for kindly providing us with the pollen.
References
- Andreou A, Feussner I. 2009. Lipoxygenases. Structure and reaction mechanism. Phytochemistry. 70, 1504–1510 [DOI] [PubMed] [Google Scholar]
- Bago A, Zipfel W, Williams RM, Jun J, Arreola R, Lammers PJ, Pfeffer PE, Shachar-Hill Y. 2002. Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiology. 128, 108–124 [PMC free article] [PubMed] [Google Scholar]
- Borek S, Nuc K. 2011. Sucrose controls storage lipid breakdown on gene expression level in germinating yellow lupine ( Lupinus luteus L.) seeds. Journal of Plant Physiology. 168, 1795–1803 [DOI] [PubMed] [Google Scholar]
- Borek S, Ratajczak W, Ratajczak L. 2006. Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Science. 170, 441–452 [Google Scholar]
- Eastmond PJ. 2006. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. The Plant Cell. 18, 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kouhen K, Blangy S, Ortiz E, Gardies AM, Ferte N, Arondel V. 2005. Identification and characterization of a triacylglycerol lipase in Arabidopsis homologous to mammalian acid lipases. FEBS Letters. 579, 6067–6073 [DOI] [PubMed] [Google Scholar]
- Feussner I, Wasternack C, Kindl H, Kühn H. 1994. Lipoxygenase-catalyzed oxygenation of storage lipids is implicated in lipid mobilization during germination. Proceedings of the National Academy of Sciences, USA. 92, 11849–11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B, Fischer K, Balkenhohl TJ, Pohnert G, Kühn H, Wasternack C, Feussner I. 2005. Lipoxygenase-mediated metabolism of storage lipids in germinating sunflower cotyledons and β-oxidation of (9Z,11E,13S)-13-hydroxy-octadeca-9,11-dienoic acid by the cotyledonary glyoxysomes. Planta. 220, 919–930 [DOI] [PubMed] [Google Scholar]
- Graham IA. 2008. Seed storage oil mobilization. Annual Reviews of Plant Biology. 59, 115–142 [DOI] [PubMed] [Google Scholar]
- Gupta A, Bhatla SC. 2007. Preferential phospholipase A2 activity on the oil bodies in cotyledons during seed germination in Helianthus annuus L. cv. Morden . Plant Science. 172, 535–543 [Google Scholar]
- Hanano A, Burcklen M, Flenet M, Ivancich A, Louwagie M, Garin J, Blee E. 2006. Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. Journal of Biological Chemistry. 281, 33140–33151 [DOI] [PubMed] [Google Scholar]
- Heinish O, Kowalski E, Ludwig H, Tauscher B. 1996. Staining for soybean lipoxygenase activity in electrophoretic gels. European Journal of Lipid Science and Technology. 98, 183–184 [Google Scholar]
- Hills MJ, Murphy DJ, Beevers H. 1989. Inhibition of neutral lipase from castor bean lipid bodies by coenzyme A (CoA) and oleoyl CoA. Plant Physiology. 89, 1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AHC. 1994. Structure of plant seed oil bodies. Current Opinion in Structural Biology. 4, 493–498 [Google Scholar]
- Jiang PL, Chen JC, Chiu ST, Tzen JT. 2009. Stable oil bodies sheltered by a unique caleosin in cycad megagametophytes. Plant Physiology and Biochemistry. 47, 1009–1016 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Salucci ML, van Zadelhoff G, Malatesta F, Veldink G, Vliegenthart JFG, Finazzi-Agrò A. 2001. Tryptic digestion of soybean lipoxygenase-1 generates a 60kDa fragment with improved activity and membrane binding ability. Biochemistry. 40, 6819–6827 [DOI] [PubMed] [Google Scholar]
- Matsui K, Fukutomi S, Ishii M, Kajiwara T. 2004. A tomato lipase homologous to DAD1 (LeLID1) is induced in postgerminative growing stage and encodes a triacylglycerol lipase. FEBS Letters. 569, 195–200 [DOI] [PubMed] [Google Scholar]
- Matsui K, Hijiya K, Tabuchi Y, Kajiwara T. 1999. Cucumber cotyledon lipoxygenase during postgerminative growth. Its expression and action on lipid bodies. Plant Physiology. 119, 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield JA, Fiebig A, Johnstone S, Preuss D. 2001. Gene families from Arabidopsis thaliana pollen coat proteome. Science. 292, 2482–2485 [DOI] [PubMed] [Google Scholar]
- Murphy DJ. 2012. The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma. 249, 541–585 [DOI] [PubMed] [Google Scholar]
- Noll F, May C, Kindl H. 2000. Phospholipid monolayer of plant lipid bodies attacked by phospholipase A2 shows 80nm holes analyzed by atomic force microscopy. Biophysical Chemistry. 86, 29–35 [DOI] [PubMed] [Google Scholar]
- Piffanelli P, Ross JHE, Murphy DJ. 1998. Biogenesis and function of the lipidic structures of pollen grains. Sexual Plant Reproduction. 11, 65–80 [Google Scholar]
- Redrejo-Rodríguez M, Tejeda Cano A, Pinto C, Macías P. 2004. Lipoxygenase inhibition by flavonoids: semi-empirical study of the structure activity relation. Journal of Molecular Structure. 674, 121–124 [Google Scholar]
- Rejón JD, Zienkiewicz A, Rodríguez-García MI, Castro AJ. 2012. Profiling and functional classification of esterases in olive (Olea europaea) pollen during germination. Annals of Botany. 110, 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-García MI, M’rani-Alaoui M, Fernández MC. 2003. Behavior of storage lipids during development and germination of olive (Olea europaea L) pollen. Protoplasma. 221, 237–244 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rosales MP, Kerbeb L, Ferrol N, Donaire JP. 1998. Lipoxygenase activity and lipid composition of cotyledons and oil bodies of two sunflower hybrids. Plant Physiology and Biochemistry. 36, 285–291 [Google Scholar]
- Rudolph M, Schlereth A, Körner M, Feussner K, Berndt E, Melzer M, Hornung E, Feussner I. 2011. The lipoxygenase-dependent oxygenation of lipid body membranes is promoted by a patatin-type phospholipase in cucumber cotyledons. Journal of Experimental Botany. 62, 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R, Bhatla SCh. 2010. A comparative analysis of the distribution and composition of lipidic constituents and associated enzymes in pollen and stigma of sunflower. Sexual Plant Reproduction. 23, 163–172 [DOI] [PubMed] [Google Scholar]
- Suárez C, Castro AJ, Rapoport HF, Rodríguez-García MI. 2012. Morphological, histological and ultrastructural changes associated with olive pistil pre- to post-anthesis events. Sexual Plant Reproduction. 25, 133–146 [DOI] [PubMed] [Google Scholar]
- Szymanowska U, Jakubczyk A, Baraniak B, Kur A. 2009. Characterisation of lipoxygenase from pea seeds (Pisum sativum var. Telephone L.). Food Chemistry. 116, 906–910 [Google Scholar]
- To JPC, Reiter WD, Gibson SI. 2002. Mobilization of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biology. 2, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff EP, Zhao F, Preuss D. 2009. The extracellular lipase EXL4 is required for efficient hydration of Arabidopsis pollen. Sexual Plant Reproduction. 22, 197–204 [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C. 1998. Lipids are required for directional pollen tube growth. Nature. 392, 818–821 [DOI] [PubMed] [Google Scholar]
- Yadav MK, Bhatla Sch. 2011. Localization of lipoxygenase activity on the oil bodies and in protoplasts using a novel fluorescence imaging method. Plant Physiology and Biochemistry. 49, 230–234 [DOI] [PubMed] [Google Scholar]
- . Zienkiewicz A, Jiménez-López JC, Zienkiewicz K, Alché JD, Rodríguez-García MI. 2011a. Development of the cotyledon cells during olive (Olea europaea L.) in vitro seed germination and seedling growth. Protoplasma. 248, 1751–756 [DOI] [PubMed] [Google Scholar]
- Zienkiewicz K, Castro AJ, Alché JD, Zienkiewicz A, Suarez C, Rodríguez-García MI. 2010. Identification and localization of a caleosin in olive (Olea europaea L.) pollen during in vitro germination. Journal of Experimental Botany. 61, 1537–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienkiewicz K, Zienkiewicz A, Rodríguez-García MI, Castro AJ. 2011b. Characterization of a caleosin expressed during olive (Olea europaea L.) pollen ontogeny. BMC Plant Biology. 11, 122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.