Abstract
A 15-day survey of autofluorescence has been conducted upon infection by downy mildew [Plasmopara viticola (Berk. & M.A. Curtis) Berl. & de Toni] of leaves of a susceptible grapevine genotype. Different autofluorescence signals were followed from the cellular to the whole-leaf level by using four types of devices for fluorosensing: a macroscope, a spectrofluorimeter, a portable field optical sensor (the Multiplex 3), and a field fluorescence sensor prototype with 335nm excitation. It was shown for the first time, by the three different techniques and at three different scales, that the stilbene-dependent violet–blue autofluorescence (VBF) had a transitory behaviour, increasing to a maximum 6 days post-inoculation (DPI) and then decreasing to a constant lower level, nevertheless significantly higher than in the control leaf. This behaviour could be sensed from both sides of the leaf. On the abaxial side, VBF could discriminate the presence of infection from 1 DPI, and on the adaxial side from 3 DPI. There was a constant increase in blue-excited green fluorescence starting from 8 DPI, concomitant with a decrease in leaf chlorophyll content sensed by one reflectance and two fluorescence indices available on the Multiplex 3 sensor. These results show that a pre-symptomatic and symptomatic sensing of downy mildew is possible by autofluorescence-based sensors, and this is potentially applicable in the field.
Key words: Disease diagnostics, optical proximal sensors, phenolic compounds, phytoalexin fluorescence, Plasmopara viticola, Vitis vinifera.
Introduction
Early detection of biotic and abiotic stresses is a major issue in precision agriculture. Accordingly, the development of tools for proximal sensing to monitor plant health has gained in importance (Sankaran et al., 2010). Plant health survey and disease detection can be classed in two categories: indirect ground-based methods (reflectance and fluorescence spectroscopy and imaging, and gaseous metabolite profiling techniques) and direct laboratory-based detection methods (serological or molecular). The latter allows highly reliable assessment, but has a delayed response. Laboratory methods are time-consuming, labour intensive, and very dependent on sampling. Indirect methods based on optical properties are rapid, non-invasive, with immediate response, and above all they can be implemented on vehicles for mapping. Unfortunately optical methods are often less specific. In viticulture, downy mildew, caused by the oomycete Plasmopara viticola (Berk. & M.A. Curtis) Berl. & de Toni, is one of the main diseases, requiring numerous fungicide treatments in order to avoid damage and significant economic losses.
As a protection mechanism, grapevine produces stilbene phytoalexins. The role of stilbenes in the resistance of grapevine to P. viticola is well documented (Jeandet et al., 2002; Chong et al., 2009; Jeandet et al., 2010), but it is still not fully elucidated. Stilbenes show a bright UV-induced violet–blue fluorescence (VBF) (Hillis and Ishikura, 1968) that can be measured in vivo on grapevine leaves (Langcake and McCarthy, 1979; Dai et al., 1995a, b ). Indeed, under UV illumination, grapevine leaves are known to emit three types of autofluorescence: (i) the red fluorescence of chlorophyll (ChlF); (ii) the blue-green fluorescence (BGF) assigned to hydroxycinnamic acids (Cerovic et al., 1999; Pfündel et al., 2006); and (iii) a VBF of induced stilbenes in inoculated grapevine leaves (Poutaraud et al., 2007; Bellow et al., 2012). In a previous study (Bellow et al., 2012), confocal spectral microscopy was used for in vivo localization at the cellular level of stilbene fluorescence induced by P. viticola in grapevine leaves. Compartmentation differed between cultivars (resistant versus susceptible). Moreover, it was shown that due to microenvironment effects, compartmentation should strongly affect the fluorescent yield of stilbenes.
Autofluorescence can therefore be used as a non-destructive indicator of the presence of infection. On the one hand, fluorescence imaging is a particularly useful technique to assess autofluorescence changes due to stilbene accumulation in grapevine leaves at both macroscopic (Poutaraud et al., 2007) and microscopic scales (Poutaraud et al., 2007; Bellow et al., 2012). On the other hand, laboratory spectrofluorimetry allows an accurate and quantitative assessment of the intrinsic spectral signature of stilbenes that has been correlated to the stilbene content of leaves analysed by HPLC (Poutaraud et al., 2007). However, there is no report yet on the behaviour of stilbene VBF during the full course of P. viticola infections until the late visible symptoms (oily spots).
The aims of this study were 2-fold: (i) to characterize on a daily basis the kinetics of autofluorescence in plant-attached grapevine leaves responding to P. viticola infection during at least 2 weeks following their inoculation until characteristic visible symptoms are established; and (ii) to analyse different fluorescence indices as the basis for downy mildew diagnosis in the field. To reach these goals, the monitoring of BGF, VBF, ChlF, and blue-excited green fluorescence (GF) at three spatial scales was performed simultaneously: imaging by fluorescence macroscopy (submillimetric), spectrofluorimetry (millimetric), and proximal sensing (whole-leaf scale) with two sensors, the Multiplex 3 sensor (Ben Ghozlen et al., 2010) and a proximal fluorescence sensor prototype with short-wave UV sources.
Materials and methods
Plants of Vitis vinifera cv. Cabernet Sauvignon (a genotype susceptible to P. viticola) were grown from cuttings in Colmar (France) at 22±3 °C, 13/11h light/dark in the greenhouse. The study was started when plants attained the stage of ~15 leaves. The plants were settled outdoors next to the greenhouse in a place that was never in the shade in Colmar (latitude 48°05N, longitude 07°20E) in August 2010 for 15 d. This outdoor regimen guaranteed that the plants which were initially grown in the greenhouse protected from biotic or abiotic stresses had a flavonol content equivalent to vineyard leaves at the moment of inoculation (Kolb et al., 2001). As the constitutive flavonol have been shown to participate in the resistance of grapevine leaves to P. viticola (Agati et al., 2008), the outdoor treatment was fundamental for the results to be applicable to vineyard-grown leaves. Plasmopara viticola was obtained from naturally infected plants in Colmar. Sporangia were periodically grown in order to prepare inoculum. Sporangia were diluted in distilled water, counted, and then adjusted to a concentration of ~5×104 sporangia ml–1. The fifth fully expanded leaf counted from the apex, still attached to the plant, was inoculated. Three protocols of inoculation were used depending on the experiment. For protocols 1 and 2, the shoot was positioned on a horizontal surface and the leaf laid flat with the abaxial side upwards. For fluorescence macroscopy, protocol 1, five drops of 12 µl of inoculum suspension and five drops of distilled water (control) were applied on the abaxial side of each leaf. For spectrofluorimetry, protocol 2, six drops of 200 µl of inoculum suspension and three drops of distilled water were applied on the abaxial side of each leaf. For proximal sensing, protocol 3, nine fifth leaves of different plants were each totally immersed in a test tube containing 60ml of inoculum suspension. After 5h of incubation, leaves were cleaned with distilled water and superficially dried. Plants were then transported (by land transport, in humidified portable mini-greenhouses) from Colmar to Gif-sur-Yvette (France) for fluorescence macroscopy, and to Orsay (France) for spectrofluorimetry and proximal sensing. During the 15 d of experiments, except when being measured, plants were maintained either in their humidified portable mini-greenhouses inside a growth chamber (22 °C, 13/11h light/dark) or directly in the greenhouse for proximal sensing. The success of infection was attested to by the presence of sporulation on leaves kept in a humid environment, or by oily spots (without sporulation) on other leaves. All measurements were done with leaves attached to the plant.
Fluorescence imaging
Images were acquired using a macroscope (AZ100 multizoom, Nikon, Champigny-sur-Marne, France) equipped with a 130W metal halide lamp white source (Intensilight, Nikon) and a high-resolution colour camera (Ds-Ri, Nikon). The UV-suppression filter of this source was removed. The images of UV-excited visible autofluorescence were recorded using a custom-made filter block from AHF (Tübingen, Germany) with an excitation bandpass filter 340/26 (FF01 Brightline, Semrock, Rochester, NY, USA), a dichroic filter Q380LP (Chroma Technology Corp., Bellows Falls, VT, USA), and a long-pass 371nm emission filter (LP02-364RS, Semrock). The images of blue-excited green autofluorescence were recorded using a GFP-B filter set (excitation band pass filter 472/30, dichroic filter 495nm, and emission bandpass filter 520/35, Nikon). A ×2 objective (NA 0.2, working distanced 45mm, AZ-Plan Fluor, Nikon) was used, and 24-bit RGB colour images were acquired with a 1284×1024 pixel resolution. Imaged leaves, still attached to the plant, were carefully flattened (abaxial side facing the objective) on the glass sample holder (adaxial side lightly moistened for adhesion). The flatness of the imaged area was necessary for a good-quality acquisition. Sporangiophores were washed from the sporulating leaves to avoid their contribution to VBF. Image acquisition was performed using the NIS-Elements software (Nikon). Image analysis, including composition, was performed using the software ImageJ (http://rsbweb.nih.gov/ij/). The presented data are representative results of several experiments.
Spectrofluorimetry
Excitation and emission fluorescence spectra were acquired with a spectrofluorimeter (Cary Eclipse, Varian, Les Ulis, France) using a configuration adapted to attached leaves based on a double-arm optical fibre bundle (C Technologies, Cedar Knolls, NJ, USA) made of 147 randomized fibres. The two arms of the bundle were coupled to the excitation and emission part of the spectrofluorimeter via a fibre-optic coupler accessory provided by Varian (part no. FA-VAR00-AP15). The common part of the fibre bundle was maintained at a fixed distance (5mm) from the samples by a proprietary clip. Under these conditions, every day from 1 DPI to 15 DPI at about the same time, the spectra of the same marked circular regions (diameter 5.5mm) of the abaxial side of each leaf were recorded. Excitation spectra were corrected with a calibrated photodiode (S1337-1010BQ, Hamamatsu, Massy, France), and emission spectra were corrected using a standard lamp with a known spectrum (LI-COR 1800–02, LI-COR, Lincoln, NE, USA) as described in detail previously (Louis et al., 2006). In addition, fluorescence was expressed in quinine sulphate equivalent units (QSEU) (Cerovic et al., 1999): 1000 QSEU correspond to the fluorescence of 1 µM quinine sulphate dihydrate in 0.105M perchloric acid for 1cm light path square cells or, in general, the fluorescence of 1 nmol cm–2 of this standard excited at 347.5nm and emitting at 450nm under the identical conditions used to acquire the sample fluorescence spectrum.
Proximal sensing
Multiplex® 3 (FORCE-A, Orsay, France) is a hand-held, multiparametric fluorescence sensor based on light-emitting diode excitation and filtered photodiode detection that is designed to work in the field under daylight conditions (for detailed description and specifications, see Ben Ghozlen et al., 2010). In the present investigation, the Multiplex 3 (with a 6cm diameter measuring area) was used for daily measurements of the abaxial and adaxial sides of infected (protocol 3) leaves (fifth from the apex) and control leaves (sixth from the apex).
Several Multiplex 3 indices were followed: (i) SFR_R, a chlorophyll fluorescence emission ratio linked to the leaf chlorophyll content; (ii) R-590, the leaf reflectance at 590nm, named YF_G in the Multiplex 3, that also reflects changes in leaf chlorophyll content, increasing with chlorophyll content decrease; (iii) FER_RG, a chlorophyll fluorescence red-to-green (635/516nm) excitation ratio originally designed for fruit anthocyanin content, but which is here inversely correlated to the chlorophyll content of leaves devoid of anthocyanins; (iv) FLAV, a chlorophyll fluorescence red-to-UV (635/375nm) excitation ratio that is a measure of epidermal flavonols; and (v) YF_UV, the 375nm excited yellow autofluorescence (590nm, FWHM 10nm) of the leaves.
The prototype of a new proximal sensor, the Mx-330 (FORCE-A, Orsay, France), was used to measure daily the in vivo VBF of the abaxial and adaxial sides of infected (protocol 3) leaves (fifth from the apex) and control leaves (sixth from the apex), in parallel with the Multiplex 3 measurements (the same region of the leaf). The Mx-330 sensor was based on the Multiplex 3 design (mechanical structure and electronics) but specifically adapted to measure in vivo the stilbene VBF on grapevine leaves (335nm excitation–400nm emission). The sensor illuminates a 6cm diameter surface at a 4cm distance from the source and detectors. The leaves were flattened as much as possible during the measurements.
Statistical analyses
Statistical analyses were performed using the software Statistica 6.1 (StatSoft Inc., Maison-Alfort, France). As normality (Shapiro–Wilk test) or homoscedasticity (Levene test and Brown–Forsythe test) were often violated, the analysis of variance (ANOVA) could not be used. Instead, the significance of the difference between means was assessed by three non-parametric tests: a Mann–Whitney U-test, a Wald–Wolfowitz runs test, and a Kolmogorov–Smirnov test. The three non-parametric tests usually produced the same results. In the event of discordance, the result of the most stringent test was retained and presented.
Results
Daily imaging of autofluorescence of a grapevine leaf infected by P. viticola
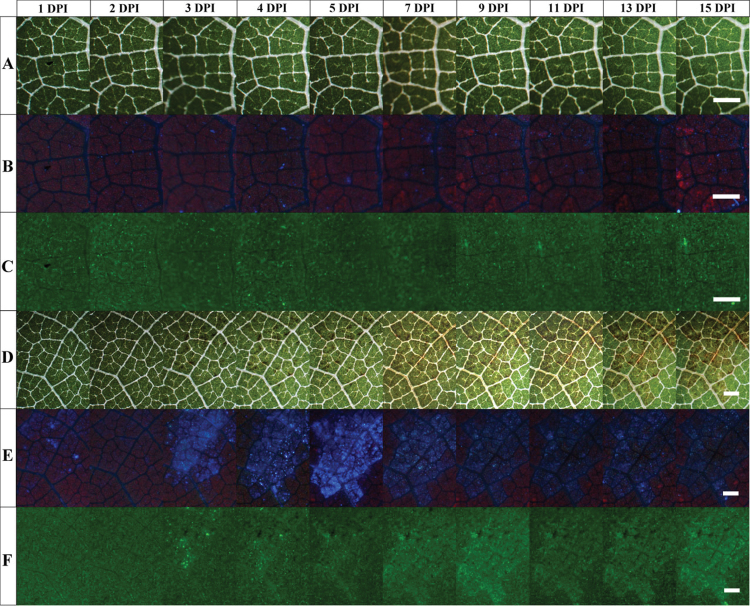
For each treatment (inoculated or control), similar results were obtained in five regions. Images presented in Fig. 1D–F are representative of an inoculated region occupied by one inoculum drop of 12 µl followed during 15 d. The first tiny necrotic spots could be seen on transmission images in the middle of infected areoles at 3 DPI (Fig. 1D). A thickening and an extension of the necrotic spots were observed on the following days. At 7 DPI, a yellowing of the abaxial surface was observed due to a local decrease of the chlorophyll content in leaf tissues (Fig. 1D). This chlorosis of infected areoles increased daily during the remaining course of the survey. At 15 DPI, which corresponds to the end of the survey, the chlorosis appeared clearly limited by veins of infected areoles. The UV-induced autofluorescence images revealed a few spots of VBF at 1 DPI (Fig. 1E). At 2 DPI, these early spots of VBF were no longer visible. At 3 DPI, infected areoles displayed an intense VBF. This signal reached a maximum intensity at 5 DPI and then decreased at 7 DPI and remained at a low but noticeable level for the rest of the survey. From 5 to 15 DPI, the VBF appeared clearly limited to the infected areoles. The chlorosis and the stilbene VBF appeared co-localized. Bright GF spots also appeared at 3 DPI (Fig. 1F). A larger and more diffuse GF was present at 7 DPI in infected tissue, but also restricted to infected areoles. Thereafter, GF covered a region (Fig. 1F) seemingly superimposed upon the chlorotic region (Fig. 1D). From 7 DPI, GF progressively increased until the end of the survey. Images of control regions displayed no changes during the whole survey. Transmission images remained green due to the presence of chlorophyll, with the exception of white veins (Fig. 1A), and no variation was observed on fluorescence images (Fig. 1B, C). The UV-induced visible fluorescence of the abaxial side of control regions appeared purple due to the mix between the red ChlF abundant in the mesophyll and the BGF of constitutive phenolic compounds present in epidermal cell walls (Fig. 1B). The GF displayed no variation in the control region (Fig. 1C).
Fig. 1.
Images of the abaxial side of a V. vinifera cv. Cabernet Sauvignon leaf during P. viticola infection. Numbers of days post-inoculation (DPI) by P. viticola are increasing from left to right. (A–C) Control region (2.28×1.71mm). (D–F) Inoculated region (4.07×3.04mm). A and D are white-light transmission images. B and E are images of UV-excited visible autofluorescence. C and F are images of blue-excited green autofluorescence. Images were taken daily, but not all are shown. Bars, 500 µm. For details, see the Materials and methods.
Daily kinetics of autofluorescence measured by spectrofluorimetry at the abaxial side of grapevine leaf infected by P. viticola
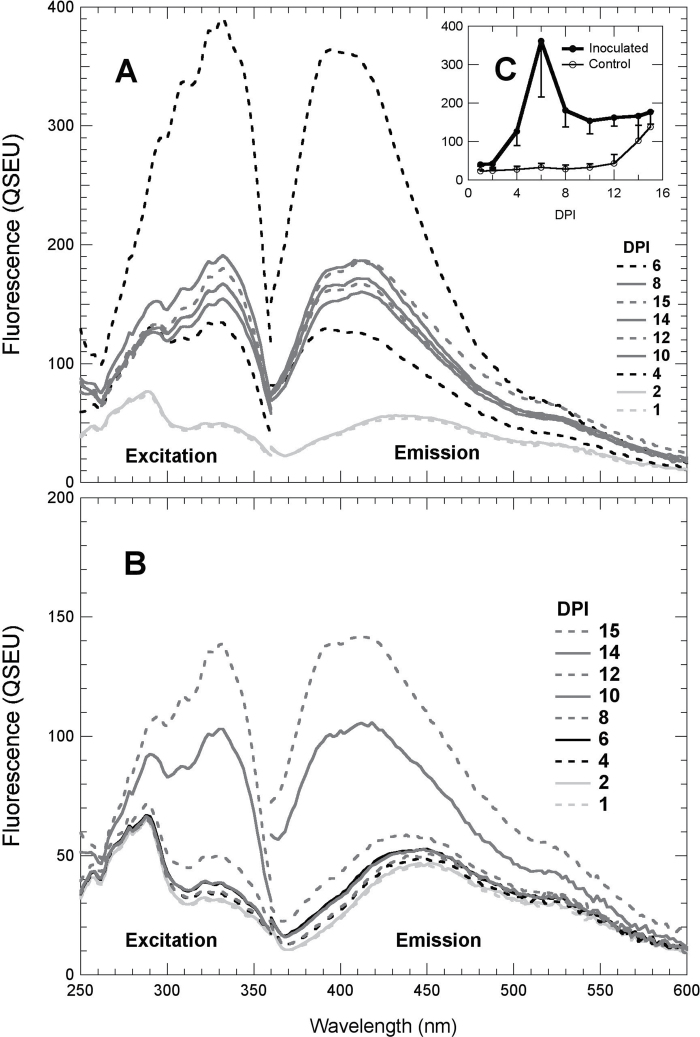
Changes in both BGF excitation and emission spectra associated with the P. viticola infection have been investigated at the abaxial side of a leaf from 1 to 15 DPI (Fig. 2). The spectra were obtained from samples independent of but comparable with those used for images presented in Fig. 1. The results shown are means of measurements on six infected regions and three control regions. From 1 to 12 DPI on control regions (Fig. 2B) and at 1 and 2 DPI on the inoculated regions (Fig. 2A), fluorescence excitation and emission spectra had the usual shape of control grapevine leaves (Cerovic et al., 1999; Poutaraud et al., 2007). From 4 DPI onwards, a second peak emerged at 330nm on fluorescence excitation spectra of inoculated regions due to the accumulation of stilbenes (Fig. 2A). In fluorescence emission spectra excited at 330nm, this resulted in a hypsochromic shift of the maximum emission from 440nm to 400nm (Fig. 2A). These spectral signatures that reflect the accumulation of stilbenes (Poutaraud et al., 2007; Bellow et al., 2012) were observed from 4 to 15 DPI on the inoculated region and the two last DPI, 14 and 15, on control regions (Fig. 2B). It should be noted here that the control regions were situated on the same leaf, close to infected regions. The daily dynamics of the VBF (330nm excitation and 400nm emission) are represented in the inset in Fig. 2C. The increase in VBF on inoculated regions is clear at 4 DPI. The maximum was reached at 6 DPI and then it decreased to reach a plateau from 8 DPI onwards. On control regions, as expected, the VBF value remained low from 1 to 12 DPI. However, at 12 DPI, the autofluorescence started to increase to reach a value close to the plateau of VBF in inoculated regions at 15 DPI.
Fig. 2.
Changes in UV-excited visible autofluorescence spectra of grapevine leaves during P. viticola infection. Daily fluorescence excitation spectra with an emission wavelength at 400nm and emission spectra with the UV-excitation wavelength set at 330nm were acquired from the abaxial side of a Cabernet Sauvignon leaf on circular spots (5.5mm diameter). The presented results are means of spectra acquired on six inoculated spots (A) and on three control spots (B). Note that the y-axis scale in A is double that in B. All spots were exactly the same every day. Standard deviations of the spectra can be visualized from the inset C that displays the daily means and standard deviation of VBF (330nm excitation and 400nm emission).
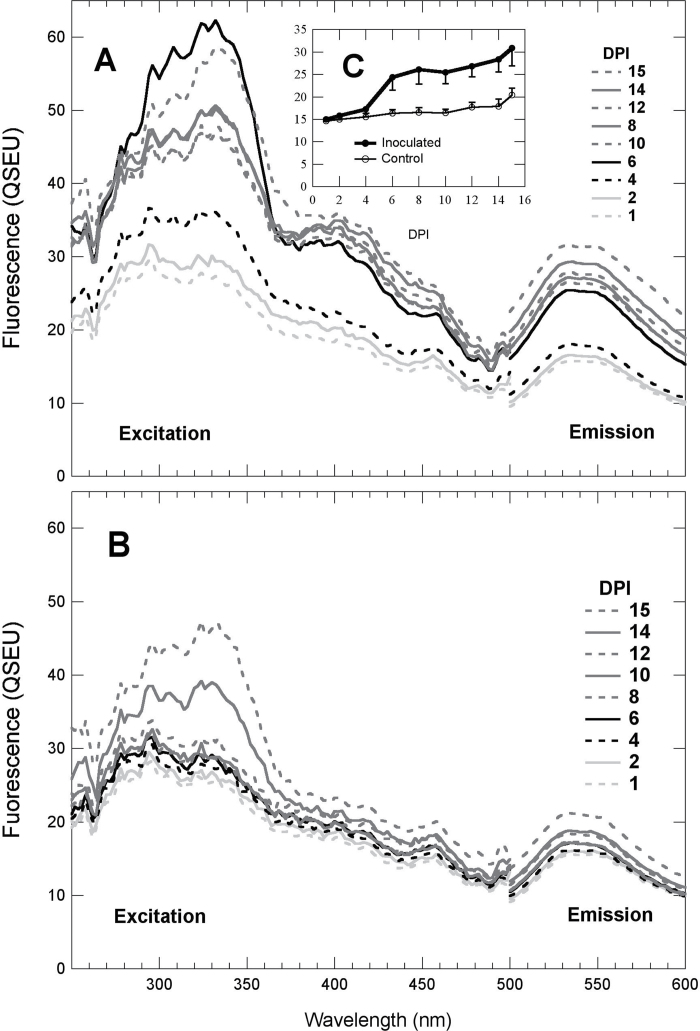
Fluorescence excitation and emission spectra of Fig. 3 were acquired in order to analyse the GF. In inoculated regions (Fig. 3A), on the excitation spectra (emission at 525nm), a small peak appeared at 400nm in addition to the increase at 330nm due to stilbenes. On control regions, fluorescence spectra changed neither in shape nor in amplitude (Fig. 3B) until 14 DPI. Indeed, the GF emission spectra did not change in shape during the whole survey, both in inoculated and in control regions. The daily dynamics of the fluorescence emission at 525nm excited at 430nm are represented in the inset (Fig. 3C). On inoculated regions, the GF, which significantly increased from 4 to 6 DPI, remained higher for infected regions than for control regions until 15 DPI, with a slight increase from 10 to 15 DPI. On control regions, a very small but continuous increase in the GF was observed from 4 to 15 DPI.
Fig. 3.
Green autofluorescence (GF) emission spectra and associated excitation spectra of grapevine leaves during P. viticola infection. Daily fluorescence excitation spectra with an emission at 525nm and emission spectra with the blue-excitation wavelength set at 430nm were obtained as described in Fig. 2. (A) Mean spectra of inoculated spots, (B) mean spectra of control spots, (C) daily means and standard deviation of GF (430nm excitation and 525nm emission).
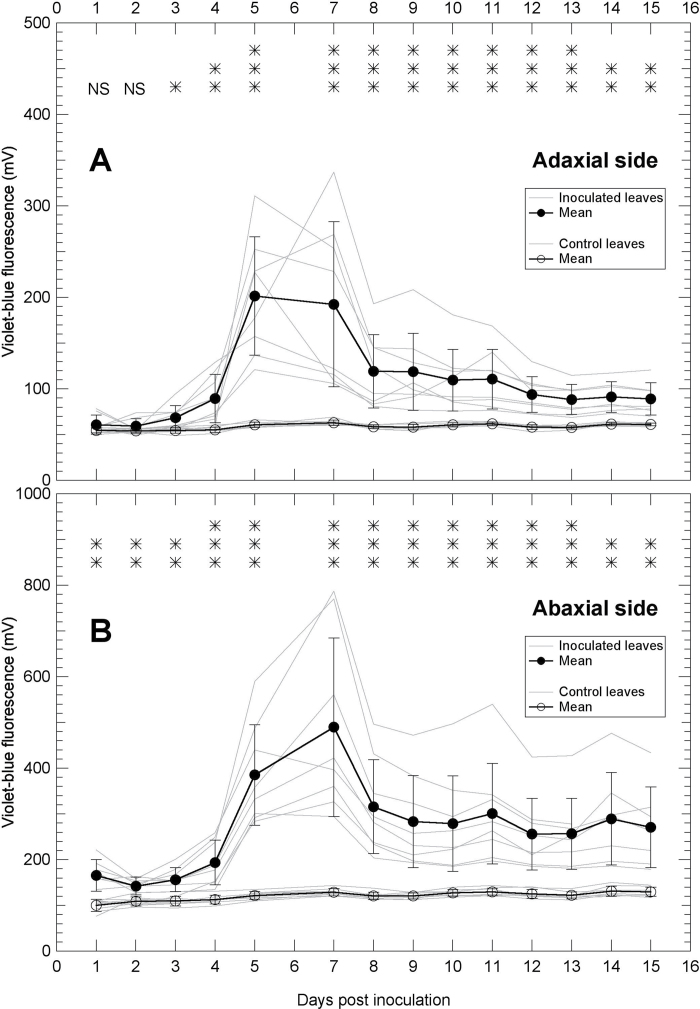
Proximal sensing with the Mx-330 of the daily kinetics of violet–blue fluorescence in leaves infected by P. viticola
Mx-330 is a new prototype sensor optimized for the detection of stilbene VBF conceived for outdoor field studies. In order to profit from the large surface sensed by the Mx-330, the entire leaves were inoculated. A significant difference in VBF was measured already at 1 DPI at the abaxial side of infected leaves (Fig. 4B). At the adaxial side, the difference in stilbene VBF between inoculated and control leaves started to be significant at 3 DPI (Fig. 4A). The signal increased on both sides to reach a maximum intensity at ~6–7 DPI. A decrease of the signal was observed from 7 to 8 DPI and then it tended to stabilize until the end of the survey at a significantly larger value than the control. The signal measured at the abaxial side of infected leaves was about double that of the adaxial side. Apart from this difference of fluorescence level, the same dynamics of stilbene VBF were measured on both sides of the leaf.
Fig. 4.
Changes in violet–blue autofluorescence of grapevine leaves during P. viticola infection followed by the Mx-330 sensor. A large surface (6cm diameter) of both the adaxial (A) and the abaxial (B) side of Cabernet Sauvignon leaves was sensed from the first to the 15th day after inoculation. Note that the y-axis scale in B is double that in A. Grey lines are daily kinetics of individual leaves and bold lines are means with standard deviations of eight inoculated leaves and eight control leaves. The significance of the difference between control and inoculated leaves for each day is indicated on the top of the graphs: NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Proximal sensing with the Multiplex 3 of the daily kinetics of optical parameters in leaves infected by P. viticola
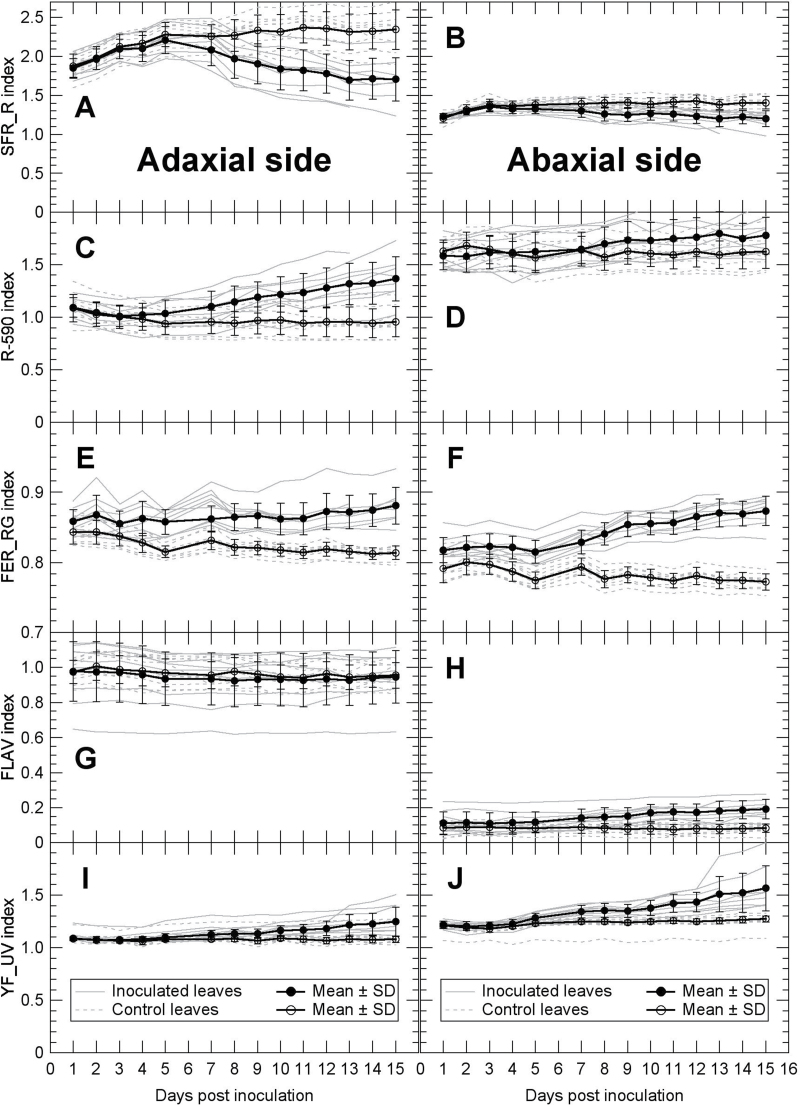
The Multiplex 3 is a field-portable proximal sensor that can gather information on the changes in leaf chlorophyll content, flavonol content, and yellow fluorescence. The sensing of whole leaves was done on the same leaves analysed with the Mx-330. Globally, at the adaxial leaf side, the SFR_R index, reflecting the leaf chlorophyll content (Tremblay et al., 2012), had values about double those of the abaxial side (Fig. 5A, B). A slight increase in SFR_R, corresponding to an accumulation of chlorophyll by the still developing leaves, was observed from 1 to 5 DPI, both in inoculated and in control leaves, especially at the adaxial side. As expected, the SFR_R index remained constant in control leaves throughout the rest of the survey, while a significant decrease was observed on both abaxial and adaxial sides of inoculated leaves (Fig. 5A, B). Two other Multiplex 3 indices, the reflectance at 590nm, R-590 (Fig. 5C, D), and the red-to-green fluorescence excitation ratio, FER_RG (Fig. 5E, F), reflected the same changes in chlorophyll. The latter index is sensitive to chlorophyll changes in the absence of anthocyanins. The observed loss of chlorophyll confirmed by three Multiplex 3 indices corroborated the visual observations (data not shown) and the chlorosis reported in Fig. 1D.
Fig. 5.
Changes in Multiplex 3 indices during P. viticola infection of grapevine leaves. Both adaxial (A, C, E, G, I) and abaxial (B, D, F, H, J) side of leaves were measured on the same leaves shown in Fig. 4. Grey lines are individual leaves and bold lines are means with standard deviations of nine inoculated leaves and nine control leaves. For the description of different Multiplex 3 indices, see the Materials and methods.
The FLAV index was ~5 times larger on the adaxial side than on the abaxial leaf side (Fig. 5G, H). This reflects the difference in epidermal flavonol contents between adaxial and abaxial side of leaves, a well-known characteristic of dicot plants (Cerovic et al., 2002). While the FLAV index remained constant through the entire study on the adaxial side, an increase could be seen on the abaxial side of inoculated leaves. An increase of the YF_UV index was also recorded for inoculated leaves, especially at the abaxial leaf side. Although not optimized for the present study, this narrow-band index of yellow fluorescence excited by a 375nm UV light is related to the GF presented in Figs 1C, F, and 3.
Discussion
Fluorescence measured by spectroscopy or imaging has two advantages: it is very sensitive and can be applied at different scales, from microscopy to remote sensing. Imaging allows assessment of the spatial distribution of signals, while spectroscopy can assess the variation of signals with larger sensitivity and from a distance. Works of Poutaraud et al. (2007) and Bellow et al. (2012) have shown that in vivo VBF is a good indicator of the presence of stilbene phytoalexins in grapevine leaves induced upon infection by P. viticola. In addition, changes in variable ChlF were shown to be a good indicator of downy mildew due to its impairment of photosynthesis (Csefalvay et al., 2009).
Spatiotemporal characteristics of the autofluorescence of grapevine leaves during infection by P. viticola
Images acquired in this study showed that the stilbene VBF was strictly limited to the vein-delimited areoles inoculated by P. viticola. Outside this region, no extensions of visible symptoms of chlorosis or necrosis were observed. Apparently, the development of P. viticola hyphae is generally stopped by veins in leaf tissues (Unger et al., 2007). Therefore, the results confirm that visible symptoms and fluorescence induced by the P. viticola infection are both limited to the primary infection site areoles. Sporulation appeared at 4 DPI for the leaves kept in a water-saturated atmosphere in portable mini-greenhouses; this confirmed the success of the infection (not shown). Sporangiophores were washed away daily so they appear neither on transmission (Fig. 1D) nor on fluorescence images (Fig. 1E, F), nor did they influence the fluorescence spectra (Figs 2, 3) (cf. Supplementary Fig. S1 available at JXB online). For proximal sensing, plants were kept in the dry atmosphere of a greenhouse and there was no sporulation, hence also no influence of the sporangiophores on the measurements.
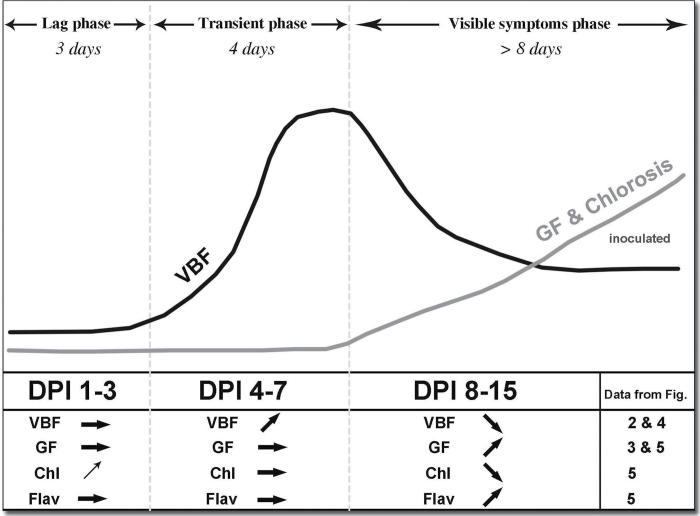
The results of this study set the detection threshold of stilbene VBF at 3 DPI at the adaxial leaf side of a susceptible grapevine leaf. On the abaxial side, inoculated leaves had significantly larger VBF than control leaves from day 1 after inoculation. Therefore, the stilbene VBF seems a promising asymptomatic signal to detect infections. These 15 d of the survey revealed the transient nature of the stilbene VBF summarized in Fig. 6, with three characteristic phases: a lag phase, a transient phase characterized by an increase in VBF to a maximum around 6 DPI, followed by a decrease and a stabilization of VBF at a level significantly above the control in the third phase. This last phase was concomitant with the appearance of visible symptoms of chlorosis. The same phases were observed with fluorescence imaging on 0.2mm2, measured with the spectrofluorimeter on 20mm2, and assessed with the new proximal sensor Mx-330 on 2000mm2 on both the abaxial and the adaxial side of the whole infected leaves. Blue-excited GF increased continuously during the third phase. Sporulation appeared at the beginning of the transient phase (4 DPI). The transitory nature of VBF raises the question of the fate of stilbenes during the infection. The dynamics of stilbene VBF suggest that a large proportion of phytoalexins detected in infected grapevine leaves are either degraded or metabolized into molecules with a significantly lower fluorescence yield soon after the transient phase. Laccases are fungal enzymes that have the capability to metabolize stilbene phytoalexins produced by the hosts, as seen in the case of Botrytis cinerea infections (Pezet et al., 1991; Sbaghi et al., 1996). Laccase activity is assumed to detoxify trans-resveratrol by oxidative polymerization and subsequently to facilitate the infection (Jeandet et al., 2002). Although laccases have not been found in P. viticola, a multicopper oxidase was identified recently in this oomycete that could have the same role (Mestre et al., 2012).
Fig. 6.
Schematic summary of optical indicators of downy mildew infection. The initial increase in chlorophyll (Chl) seen during the lag phase is due to the maturation of the leaf. It was identical on control and on inoculated leaves. DPI, days post-infection. The optical indicator increased (↑), decreased (↓), or remained constant (→).
The origin of the GF has yet to be determined. However, GF seems both temporally and spatially correlated with the loss of chlorophyll, and therefore the visual symptoms of chlorosis. The decrease in chlorophyll content in infected leaves was sensed by Multiplex 3 indices of chlorophyll content (SFR_R, R-590 and FER_RG) on both the adaxial and the abaxial side of leaves. Dicot leaves, such as in V. vinifera, have palisade (on the adaxial side) and spongy (on the abaxial side) parenchyma with very different histological and optical properties and chlorophyll content. Palisade parenchyma had higher chlorophyll content than spongy parenchyma, shown by the SFR difference in Fig. 5A versus B, and the reflectance index (R-590) in Fig. 5C versus D (opposite response to SFR) for both inoculated and control leaves. The chlorophyll content of a leaf will be the sum of the index measured on each of the two sides. The highly diffusive properties of the abaxial leaf side precluded the detection of significant differences between inoculated and control leaves for R-590. The green-to-red fluorescence excitation ratio (FER_RG) has only recently been linked to leaf chlorophyll content when anthocyanins are absent (Z.G. Cerovic, unpublished). It will have higher values for lower chlorophyll contents (opposite response to SFR) due to lower green light absorption. The local loss of chlorophyll, seen by fluorescence indices in the third phase of the infection, will decrease the reabsorption of pre-existing green-fluorescing compounds and lead to an increase in GF (Cerovic et al., 1999). This is supported by the absence of changes in the shape of GF spectra during the infection.
Towards new non-destructive indices to detect downy mildew
The similar results obtained by the three approaches used in this study, albeit a four order difference in sensed area size, prove the universality and robustness of the stilbene VBF and GF as indicators of P. viticola infection. In addition to the use of VBF in microscopy to study P. viticola–grapevine interactions (Bellow et al., 2012), stilbene fluorescence seems suited for the development of an accurate tool for field detection. The spectra obtained allow the optimal wavelengths (330nm excitation, 400nm emission) most specific for stilbene fluorescence in grapevine leaves to be defined. Although the stilbene signal measured at the adaxial side of the leaves was half that measured at the abaxial side, the Mx-330 index allowed a promising discrimination as early as 3 DPI. However, the inoculation performed on entire leaves reached all parts of the leaves. Natural P. viticola droplet infections rarely cover the whole leaf surface. So a lower stilbene-dependent signal can be expected in the field. Still, the specificity and advantage of fluorescence over reflectance is its very high sensitivity. Up to a limit (concentration quenching), fluorescence will be the same from a given amount of fluorophore distributed uniformly over an area or aggregated on small spots. Further experiments in the field on spontaneous infections are required, and these are now feasible thanks to the availability of the Mx-330.
VBF appears as an early indicator of downy mildew, while GF is specific to the late stage of the infection, temporally correlated with visible symptoms of chlorosis. Stilbenes are phytoalexins of grapevine, induced by either biotic or abiotic stresses (Jeandet et al., 2002) and not specific to a particular disease. Using stilbene VBF to probe downy mildew in the field might not be specific enough because other factors could lead to the production of stilbenes. Therefore, additional signatures of the infection could be advantageous to discriminate specifically P. viticola infections. In the present study, it was found that GF of grapevine leaves was both spatially and temporally correlated with downy mildew late symptoms of chlorosis. Even if this signal does not provide an early diagnosis, it provides evidence of the presence of downy mildew. The comparison of four independent sensing techniques—imaging, specrofluorimetry, UV-excited blue fluorescence sensing (Mx-330), and multiwavelength proximal sensing (Multiplex 3)—has allowed a robust description of the three characteristic phases of autofluorescence of grapevine leaves during the infection by P. viticola (Fig. 6). Variable ChlF (Csefalvay et al., 2009) needs imaging and is not available for measurement with mobile platforms. This seems not to be the case with simple autofluorescence signals or ratios such as VBF, VBF/GF, SFR, and FER_RG. Furthermore, the availability of several signals could help to discriminate downy mildew among the biotic and abiotic stresses.
This study was performed on attached leaves in order to maintain them under physiological conditions. However, plants were grown in a controlled environment compared with field-grown plants that are potentially facing multiple stresses. So the next step would be to perform a similar kinetic investigation involving Multiplex 3 and Mx-330 in the field during spontaneous infections. Although demonstrated here only for the V. vinifera–P. viticola pathosystem, the approach presented herein can be extended to other pathosystems involving fluorescent phytoalexins found in other species, such as coumarins in sunflower or isoflavonoids in soybean (Grayer and Harborne, 1994).
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Images of sporulating leaves before and after removal of sporangiophores.
Acknowledgements
This work was supported by FORCE-A (Orsay) in a joint project with Centre National de la Recherche Scientifique (CNRS), Institut National de la Recherche Agronomique (INRA), and Université Paris-Sud 11 (UPS) (grant nos CNRS 065103, INRA 28000039, and UPS N73800) and by IFR87 ‘La plante et son environnement’ local grant. Fluorescence macroscopy was done on the Imagif Platform with the support of Marie-Noëlle Soler (IFR87). We are grateful to Marie Annick Dorne and Pascale Coste (INRA, Colmar) for growing the grapevine plants and for the inoculation, and to Sabine Merdinoglu and Didier Merdinoglu (INRA, Colmar) for their support.
References
- Agati G, Cerovic ZG, Dalla Marta A, Di Stefano V, Pinelli P, Traversi ML, Orlandini S. 2008. Optically-assessed preformed flavonoids and susceptibility of grapevine to Plasmopara viticola under different light regimes. Functional Plant Biology. 35, 77–84 [DOI] [PubMed] [Google Scholar]
- Bellow S, Latouche G, Brown SC, Poutaraud A, Cerovic ZG. 2012. In vivo localization at the cellular level of stilbene fluorescence induced by Plasmopara viticola in grapevine leaves. Journal of Experimental Botany. 63, 3697–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ghozlen N, Cerovic ZG, Germain C, Toutain S, Latouche G. 2010. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors. 10, 10040–10068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, Moya I. 2002. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant, Cell and Environment. 25, 1663–1676 [Google Scholar]
- Cerovic ZG, Samson G, Morales F, Tremblay N, Moya I. 1999. Ultraviolet-induced fluorescence for plant monitoring: present state and prospects. Agronomie. 19, 543–578 [Google Scholar]
- Chong J, Poutaraud A, Hugueney P. 2009. Metabolism and roles of stilbenes in plants. Plant Science. 177, 143–155 [Google Scholar]
- Csefalvay L, Di Gaspero G, Matous K, Bellin D, Ruperti B, Olejnickova J. 2009. Pre-symptomatic detection of Plasmopara viticola infection in grapevine leaves using chlorophyll fluorescence imaging. European Journal of Plant Pathology. 125, 291–302 [Google Scholar]
- Dai GH, Andary C, Mondolot-Cosson L, Boubals D. 1995. a Histochemical responses of leaves of in-vitro plantlets of Vitis spp to infection with Plasmopara viticola . Phytopathology. 85, 149–154 [Google Scholar]
- Dai GH, Andary C, Mondolot-Cosson L, Boubals D. 1995. b Histochemical studies on the interaction between three species of grapevine, Vitis vinifera. V. rupestris V. rotundifolia, and the downy mildew fungus. Physiological and Molecular Plant Pathology. 46, 177–188 [Google Scholar]
- Grayer RJ, Harborne JB. 1994. A survey of antifungal compounds from higher-plants, 1982–1993. Phytochemistry. 37, 19–42 [DOI] [PubMed] [Google Scholar]
- Hillis WE, Ishikura N. 1968. Chromatographic and spectral properties of stilbene derivatives. Journal of Chromatography. 32, 323–336 [DOI] [PubMed] [Google Scholar]
- Jeandet P, Delaunois B, Conreux A, Donnez D, Nuzzo V, Cordelier S, Clement C, Courot E. 2010. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. Biofactors. 36, 331–341 [DOI] [PubMed] [Google Scholar]
- Jeandet P, Douillet-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M. 2002. Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. Journal of Agricultural and Food Chemistry. 50, 2731–2741 [DOI] [PubMed] [Google Scholar]
- Kolb C, Käser MA, Kopecky J, Zotz G, Riederer M, Pfündel EE. 2001. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiology. 127, 863–875 [PMC free article] [PubMed] [Google Scholar]
- Langcake P, McCarthy WV. 1979. Relationship of resveratrol production to infection of grapevine leaves by Botrytis cinerea . Vitis. 18, 244–253 [Google Scholar]
- Louis J, Cerovic ZG, Moya I. 2006. Quantitative study of fluorescence excitation and emission spectra of bean leaves. Journal of Photochemistry and Photobiology B: Biology. 85, 65–71 [DOI] [PubMed] [Google Scholar]
- Mestre P, Pirona M-C, Merdinoglu D. 2012. Identification of effector genes from the phytopathogenic Oomycete Plasmopara viticola through the analysis of gene expression in germinated zoospores. Fungal Biology. 116, 825–835 [DOI] [PubMed] [Google Scholar]
- Pezet R, Pont V, Hoangvan K. 1991. Evidence for oxidative detoxication of pterostilbene and resveratrol by a laccase-like stilbene oxidase produced by Botrytis cinerea . Physiological and Molecular Plant Pathology. 39, 441–450 [Google Scholar]
- Pfündel EE, Agati G, Cerovic ZG. 2006. Optical properties of plant surfaces. In: Reiderer M, Müller C, eds. Biology of the plant cuticle. Vol. 23, Oxford: Blackwell Publishing; 216–249 [Google Scholar]
- Poutaraud A, Latouche G, Martins S, Meyer S, Merdinoglu D, Cerovic ZG. 2007. Fast and local assessment of stilbene content in grapevine leaf by in vivo fluorometry. Journal of Agricultural and Food Chemistry. 55, 4913–4920 [DOI] [PubMed] [Google Scholar]
- Sankaran S, Mishra A, Ehsani R, Davis C. 2010. A review of advanced techniques for detecting plant diseases. Computers and Electronics in Agriculture. 72, 1–13 [Google Scholar]
- Sbaghi M, Jeandet P, Bessis R, Leroux P. 1996. Degradation of stilbene-type phytoalexins in relation to the pathogenicity of Botrytis cinerea to grapevines. Plant Pathology. 45, 139–144 [Google Scholar]
- Tremblay N, Cerovic ZG, Wang Z. 2012. Sensing crop nitrogen status with fluorescence indicators. A review. Agronomy for Sustainable Development. 32, 451–464 [Google Scholar]
- Unger S, Bueche C, Boso S, Kassemeyer HH. 2007. The course of colonization of two different Vitis genotypes by Plasmopara viticola indicates compatible and incompatible host–pathogen interactions. Phytopathology. 97, 780–786 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.