Abstract
Phosphorus (P) nutrition is always a key issue regarding plants responses to elevated CO2. Yet it is unclear of how elevated CO2 affects P uptake under different nitrogen (N) forms. This study investigated the influence of elevated CO2 (800 µl l–1) on P uptake and utilization by Arabidopsis grown in pH-buffered phosphate (P)-deficient (0.5 µM) hydroponic culture supplying with 2mM nitrate (NO3 −) or ammonium (NH4 +). After 7 d treatment, elevated CO2 enhanced the biomass production of both NO3 −- and NH4 +-fed plants but decreased the P amount absorbed per weight of roots and the P concentration in the shoots of plants supplied with NH4 +. In comparison, elevated CO2 increased the amount of P absorbed per weight of roots, as well as the P concentration in plants and alleviated P deficiency-induced symptoms of plants supplied with NO3 −. Elevated CO2 also increased the root/shoot ratio, total root surface area, and acid phosphatase activity, and enhanced the expression of genes or transcriptional factors involving in P uptake, allocation and remobilization in P deficient plants. Furthermore, elevated CO2 increased the nitric oxide (NO) level in roots of NO3 −-fed plants but decreased it in NH4 +-fed plants. NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) inhibited plant P acquisition by roots under elevated CO2. Considering all of these findings, this study concluded that a combination of elevated CO2 and NO3 − nutrition can induce a set of plant adaptive strategies to improve P status from P-deficient soluble sources and that NO may be a signalling molecule that controls these processes.
Key words: ammonium, anthocyanin, atnos1, elevated CO2, nitrate, nitrate reductase, nitric oxide, nitric oxide synthase, nr, phosphorus acquisition, phosphate deficiency
Introduction
Concentrations of atmospheric CO2 have risen from about 270 µl l–1 in pre-industrial times to over 380 µl l–1 at present, and are predicted to reach between 530 and 970 µl l–1 by the end of this century (IPCC, 2007). It has been demonstrated that increasing atmospheric CO2 concentration has a profound impact on the growth and development of plants. Many studies have investigated plant responses to elevated CO2 on ecosystem, community, population, physiological, and molecular scales (Gibeaut et al., 2001; Teng et al., 2006; Jin et al., 2009; Niu et al., 2011a ). However, relatively little information exists on the effects of elevated CO2 on nutrition acquisition and metabolism. Previous studies have shown that elevated CO2 generally stimulates the rates of carboxylation but suppresses the rate of oxygenation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in C3 plants, thereby increasing plant growth and crop yield (Stitt et al., 1991; Drake et al., 1997). However, sustaining these increased rates requires increased nutrient uptake. Consequently, the role of nutrition availability is a key unresolved issue regarding plant responses to elevated CO2.
Phosphorus is an essential element for plant growth and development. It plays important roles in energy transfer, signal transduction, photosynthesis, enzyme activation/inactivation, membrane synthesis and stability, and respiration, and is a structural component of nucleic acids and phospholipids (Abel et al., 2002; Vance et al., 2003; Chiou and Lin, 2011; Péret et al., 2011). However, the low solubility and high sorption capacity of phosphate in soils make it relatively unavailable to plant roots. In soil solution, concentrations of available P (mainly H2PO4 − and HPO4 2−) are generally less than 10 µM, which is well below the critical level needed for the optimal performance of crops (Schachtman et al., 1998; Batjes, 1997). The problem of P deficiency is mitigated by the application of concentrated fertilizers that provide soluble P to plants. This practice, however, is inherently inefficient due to chemical immobilization and surface runoff of P (Abel et al., 2002). Hence, low P availability is one of the major growth-limiting factors for plants in many natural and agricultural ecosystems (Barber et al., 1963; Raghothama, 1999).
When soil phosphorus availability is low, phosphorus uptake is increased by elevated CO2; however, higher foliar phosphorus concentrations are also required to realize the maximum growth potential at elevated CO2 (Conroy et al., 1990). Although the absolute increase in growth is greatest at high nutrient availability, relatively large gains in productivity can also be achieved at lower nutrient availability (Conroy et al., 1992). Notably, P deficiency reduces the assimilation of NO3 − into the proteins, which might cause negative feedback on NO3 − influx and/or stimulate NO3 − efflux (Schjørring, 1986). Therefore, it seems that, under conditions of nutrition deficiency and elevated CO2, plants sense changes in nutrition availability and trigger a set of plant adaptive responses to increase nutrition uptake and recycling. Numerous studies indicate that plants enhance P acquisition and mobilization to cope with low P availability by inducing a series of biochemical, physiological, and molecular adaptation strategies (Raghothama, 1999; Mukatira et al., 2001; Abel et al., 2002; Rubio et al., 2009; Ramaekers et al., 2010; Péret et al., 2011; Jin et al., 2012; Veneklaas et al., 2012). However, it is unclear how plants sense and respond to P deficiency under conditions of elevated CO2.
Nitrogen (N) has been the centre of attention not only because it is the primary limiting nutrition in most temperate ecosystems, but also because the form of N supply can modify the response of plant growth to nutrition acquisition and elevated CO2. It has been reported that N forms (NO3 − versus NH4 +) can lead to contrasting responses of plant growth and metabolism to elevated CO2. For example, elevated CO2 increased NO3 − uptake and nitrate reductase activity when tobacco was fed with NO3 − but increased NH4 + uptake and inhibited nitrate reductase activity with an NH4 + supply (Matt et al., 2001). Likewise, under elevated CO2, NH4 +-fed wheat showed greater increases in leaf area and smaller decreases in shoot protein concentration than NO3 −-fed plants (Bloom et al., 2002, 2010). Pharmacological experiments and studies of mutants with low activity of NIA (encoding nitrate reductase) showed that nitric oxide (NO) production was induced by NO3 − and elevated CO2 (Gojon et al., 1998), but was inhibited by ammonium, glutamine, or related downstream products of nitrate assimilation (Gojon et al., 1998). Recently, Carlisle et al. (2012) reported that both CO2 concentration and N form strongly affected biomass production in hydroponically grown wheat, as well as nutrient concentrations in above- and below-ground tissues. Consequently, these differences between NO3 − and NH4 + nutrition led us to investigate how N form regulates the responses of P homeostasis of plants grown under elevated CO2. Therefore, the aim of this study was to investigate the interactive effects of N form and elevated CO2 on plant P uptake and utilization in a P-deficient medium. Arabidopsis was used as the test plant.
Materials and methods
Plant material and growth conditions
Seeds of Arabidopsis thaliana wild-type ecotype and mutants nr, AT1G77760 and AT1G37130 locus (Wang et al., 2004) and atnos1, AT3G47450 locus (Guo et al., 2003), were surface-sterilized, and germinated on Petri plates containing 0.8% agar medium supplemented with one-fifth-strength nutrition solution (Niu et al., 2011a ). After 7 d, seedlings of uniform size were transferred to 1.5ml Eppendorf tubes whose bottoms were cut about 0.7cm from the bottom to allow root growth into the nutrition solution. The tubes were inserted in holes of plastic boxes containing 200ml of a half-strength nutrition solution, and plants were grown for 14 d. Then, each seedling was transferred to each pot containing 300ml of nutrition solution. The composition (µM) of the nutrition solution was as follows: 1500 KNO3, 500 MgSO4, 1000 CaCl2, 500 NaH2PO4, 250 (NH4)2SO4, 10 H3BO3, 0.5 MnSO4, 0.5 ZnSO4, 0.1 CuSO4, 0.1 (NH4)6Mo7O24, and 25 Fe-EDTA (Hoagland and Arnon, 1938). The nutrition solution was renewed every 3 d and its pH was adjusted daily to 6.0 using 1M NaOH. All plants were grown in controlled-environment growth chambers (Conviron E7/2, Winnipeg, Manitoba, Canada) with a humidity of 80%, a daily cycle of 22 °C/10h day and 20 °C/14h night. The daytime light intensity was 120 µmol photons m−2 s−1. At least eight independent replicates were used for each treatment.
After plants were further grown in pots for 14 d, treatments commenced. Plants were grown in chambers with a CO2 concentration of either 350±50 (ambient) or 800±50 µl l–1 (elevated CO2). Meanwhile, N and P treatments were initiated. One set of plants were fully supplied with 2mM NO3 − as KNO3, while the other was fully supplied with 2mM NH4 + in each CO2 treatment. For the treatment of NH4 + as the sole N source, 1mM (NH4)2SO4 and 1mM K2SO4 were added. The low-P treatment had 0.5 µM NaH2PO4. The solution pH was buffered with 2mM HEPES at pH 6.8 to minimize pH change, and was adjusted daily to 6.8 using 1M NaOH. The treatment solutions were renewed every 2 d.
The NO-related treatments on wild-type plants were also initiated as follows: (i) ambient treatment: plants were grown continuously under ambient CO2; (ii) elevated CO2 treatment: plants were transferred to a chamber with a CO2 concentration of 800±50 µl l–1; (iii) ambient+sodium nitroprusside (SNP) treatment: plants were transferred to the nutrition solution containing 100 µM SNP under ambient CO2; and (iv) elevated CO2+2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) treatment: plants were transferred to the nutrition solution containing 100 µM cPTIO under elevated CO2.
Analysis of total P in plant tissue
After 7 d treatment with ambient or elevated CO2, plants from various treatments were harvested, washed thoroughly with deionized water, divided into shoots and roots, and dried in an oven at 75 °C for 1 d. The samples were then weighed, digested in sulfuric acid/hydrogen peroxide, and analysed for total P concentration using the vanadium-molybdenum blue photometric method. The ‘P absorbed per unit weight of roots’ was calculated as the total amount of P per plant/root dry weight.
Chlorophyll concentration in leaves
After 7 d treatment, a portable chlorophyll meter (SPAD-502, Minolta, Japan) was used to measure chlorophyll content (Jin et al., 2009). Three leaves were selected randomly from each treatment and two SPAD readings were recorded for each leaf, avoiding the main veins during measurement.
Anthocyanins
Total anthocyanins in leaves were extracted and determined as described by Hodges et al. (1999) with minor modifications. Approximately 0.1g of fresh leaves was homogenized in 3ml of methanol-1% HCl. Samples were centrifuged and 1ml of the supernatant appropriately diluted in methanol-1% HCl was used for analysis. The difference in absorbance at 536 and 600nm was used to calculate total anthocyanin content. Results were expressed as cyanidin 3-glucoside equivalents (mmol g−1 of fresh weight) using an extinction coefficient of 0.449mol l−1 at 536nm (Fiorani et al., 2005).
Root morphology
After 7 d treatment, the root parameters of individual plants were measured using a root analysis instrument (WinRHIZO, Regent, Canada). The number and length of root hairs in each segment were measured using light microscopy with differential interference contrast optics. Micrographs were recorded on a CCD camera (Nikon Eclipse E600, Melville, NY, USA).
Activity of acid phosphatases (APases)
The activity of APases in shoots and roots was determined according to the method of McLachlan et al. (1987) with minor modifications. After 7 d in low-P treatment, the seedlings were washed thoroughly with deionized water, divided into shoots and roots, weighed, and then chilled immediately on ice. About 0.1–0.2g of fresh samples was frozen with liquid nitrogen and homogenized in a cold mortar, and the powder was macerated with 5ml of extraction buffer (0.2M glacial acetic acid-sodium acetate buffer, pH 5.8). The extract was centrifuged at 27 000g at 4 °C for 10min, and the supernatant was used for an assay of APase activity. APase activity was determined by measuring enzyme activity in extraction buffer. Briefly, the APase reaction mixture contained 0.05ml of enzyme extract, 0.45ml of buffer solution and 4.5ml of 5M p-nitrophenyl phosphate. All APase reaction mixtures were incubated at 30 °C for 30min in the dark. The reaction mixtures were stopped by the addition of 2ml of 2M NaOH. After this, the concentration of p-nitrophenol in the APase reaction mixtures was determined in a spectrophotometer at 405nm. The APase activity was expressed as mol substrate hydrolysed g–1 of fresh weight min–1.
In situ measurement of NO in the root
NO was imaged using diaminofluorescein-FM diacetate (DAF-FM DA) and epifluorescence microscopy. DAF-FM DA has been used successfully to detect NO production in both plants and animals. Roots were loaded with 5 µM DAF-FM DA in 20mM HEPES-NaOH buffer (pH 7.4) for 30min, washed three times in fresh buffer and observed under a microscope (Nikon Eclipse E600, Nikon; excitation 488nm, emission 495–575nm). A 100W high-pressure mercury-vapour lamp was used as a light source (HB-10103AF-Hg, Nikon). Fifteen roots of each treatment were measured each time. The signal intensities of green fluorescence in the images of the root tip and young root-hair zone were quantified using Image J.
Determination of nitric oxide synthase (NOS) activity
The activity of NOS was determined as described by Tian et al. (2007) with some modifications. The activity of NOS was determined with an NOS assay kit (Beyotime, Haimen, China). Briefly, 0.1g of roots was frozen in liquid nitrogen and ground to a fine powder. The powder was homogenized in 1ml of extraction buffer containing 100mM HEPES-KOH (pH 7.5), 1mM EDTA, 10% glycerol, 5mM dithiothreitol, 0.1% Triton X-100, 0.5mM phenylmethylsulfonyl, 20 µM flavin adenine dinucleotide, 25 µM leupeptin, 5 µM Na2MoO4, and 1% polyvinylpyrrolidone. The contents were centrifuged at 13 000g for 20min at 4 °C and 0.2ml of clear supernatant was then added to 0.1ml of assay mixture (containing NADPH, L-arginine, NOS assay buffer, and DAF-FM DA) and reacted at 37 °C in the dark for 1h. The NO content was detected under a microscope as described above. The fluorescence intensity was expressed as colour level on a scale ranging from 0 to 255, and the fluorescence intensity was quantified by using Image J. Data are presented as the mean of fluorescence intensity relative to the control treatment. The control treatment was the plant grown in ambient CO2.
Determination of maximum nitrate reductase (NR) activity
The activity of maximum NR was determined as described by Tian et al. (2007) with some modifications. Briefly, a total of 0.2ml of clear supernatant prepared as described above was added into 0.4ml of pre-warmed assay buffer containing 100mM HEPES-KOH (pH 7.5), 5mM KNO3, and 0.25mM NADPH. The mixed solution was reacted at 30 °C for 1h, and the reaction was stopped by adding zinc acetate. The nitrite produced was measured colorimetrically at 540nm by adding 1ml of 1% sulfanilamide in 3M HCl plus 1ml of 0.2% N-(1-naphthyl)ethylenediamine.
Quantitative PCR analysis
Total RNA was extracted using RNAiso Plus (Takara, Otsu, Shiga, Japan) from about 70mg of fresh root tissues. At least five plants were used for extraction. All RNA samples were checked for DNA contamination before cDNA synthesis. cDNA was synthesized, and possible residual genomic DNA contamination was verified as described by us previously (Niu et al., 2011a ). The mRNA levels of the genes PHOSPHATE STARVATION RESPONSE 1 (AtPHR1), PHOSPHATE TRANSPORTER 1 (AtPHT1), PHOSPHATE 1 (AtPHO1), PHOSPHATE 2 (AtPHO2), and PURPLE ACID PHOSPHATASE 2 (AtPAP2) were detected using a SYBR Green RT-PCR kit (Takara) with the following pairs of gene-specific primers: AtPHR1, forward: 5’-ACAGCAATAACGGAACGGGCAAG-3’ and reverse: 5’-GCTCTTTCACTACCGCCAAGACTG-3’; AtPHT1, forward: 5’-GCCAAGGTAGACGCAGGATA-3’ and reverse: 5’-AACCTCAG CCTCACCAGAGA-3’; AtPHO1, forward: 5’-TACGCGAGAGAAA ACAACGA-3’ and reverse: 5’-TTCCGGAGAACCAAATTGTC-3’; AtPHO2, forward: 5’- TTTTACACAAGCCACCAAAGC-3’ and reverse: 5’-TCACGAGCATGTCCAACAA-3’; AtPAP2, forward: 5’-CATCAAGTTCCTTTGAGAGC-3’ and reverse: 5’-TTGGACCGG TGTTGTAGGAG-3’.
Real-time PCR was carried out similar to as described by Niu et al. (2011a). A pair of primers for the housekeeping gene UBQ10 (forward: 5’-GGTTCGTACCTTTGTCCAAGCA-3’, reverse: 5’-CCTTCGTTA AACCAAGCTCAGTATC-3’) was used for a control reference gene for the PCR (Gan et al., 2006). Melting-curve analysis and gel electrophoresis of the PCR products were used to confirm the absence of non-specific amplification products. Relative expression levels were calculated by subtracting the threshold cycle (C t) values for UBQ10 from those of the target gene (to give ΔC t) and then calculating 2−ΔCt, where C t was the cycle number at which the fluorescence rose above the set threshold of the quantitative PCR.
Statistical analyses
All statistical analyses were conducted with DPS software (Stirling Technologies Inc., China). Means were compared using Student’s t-test or Fisher’s least significant difference test at P=0.05 in all cases.
Results
Plant growth and P uptake in low-P culture
After 7 d growth in ambient CO2 and P-deficient medium containing NO3 − supplement as the sole N source, Arabidopsis leaves were dark green with a SPAD reading of 33. By comparison, the leaves of plants grown under the same nutrition conditions but with elevated CO2 were a normal green colour (Fig. 1a) with SPAD readings of 26 (see Supplementary Fig. S2a at JXB online). Elevated CO2 decreased the concentration of anthocyanins in leaves of NO3 −-supplied plants (see Supplementary Fig. S3a at JXB online), indicating that elevated CO2 alleviated the P-deficiency-induced symptoms of the NO3 −-supplied plants. However, with a supply of NH4 + as the sole N source, plants grown in the low-P solution had a similar appearance under both ambient and elevated CO2 treatments (Fig. 1a). Elevated CO2 did not change the concentrations of chlorophyll and anthocyanins (Supplementary Figs S2a and S3a). Furthermore, ammonium toxicity was not evident in the NH4 +-fed plants.
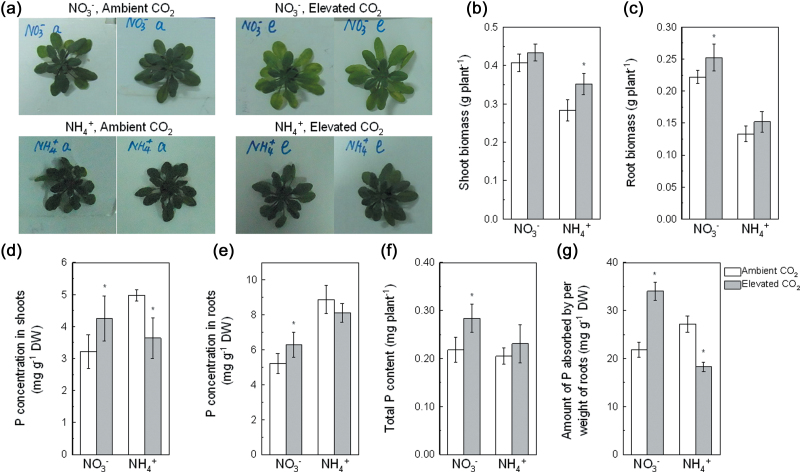
Fig. 1.
Images (a), fresh biomass production of shoots (b) and roots (c), concentration of P in shoots (d) and roots (e), total P content (f), and amount of P absorbed per weight of roots (g) of 5-week-old wild-type Arabidopsis grown for 7 d in P-deficient nutrition solutions with NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1). Data are means ±SD (n=5). Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05). DW, dry weight.
Elevated CO2 enhanced shoot growth exclusively under NH4 + nutrition (Fig. 1b), whereas it enhanced root growth under NO3 – nutrition (Fig. 1c). Elevated CO2 decreased the P concentration in shoots by 27% but did not alter it in the roots of NH4 +-supplied plants. However, elevated CO2 increased the P concentration by 32 and 21%, respectively, in the shoots and roots of NO3 −-fed plants (Fig. 1d, e). Meanwhile, elevated CO2 increased the amount of P absorbed per unit weight of roots of NO3 –-fed plants by 56% but decreased that of NH4 +-fed plants by 33% (Fig. 1g), indicating that NO3 − nutrition facilitates P uptake in roots.
After 7 d growth in P-adequate medium with either N source, the leaves were a normal green colour (data not shown). CO2 treatment did not affect the concentration of anthocyanins in leaves, but NH4 + supply significantly increased SPAD readings (Supplementary Figs S2b and S3b). Elevated CO2 increased biomass production (Supplementary Fig. S1a, b), but decreased the P concentration and the amount of P absorbed per weight of roots, with the decrease being greater in the NO3 − than in the NH4 + treatment (Fig. S1c, d).
Physiological and morphological responses to P deficiency
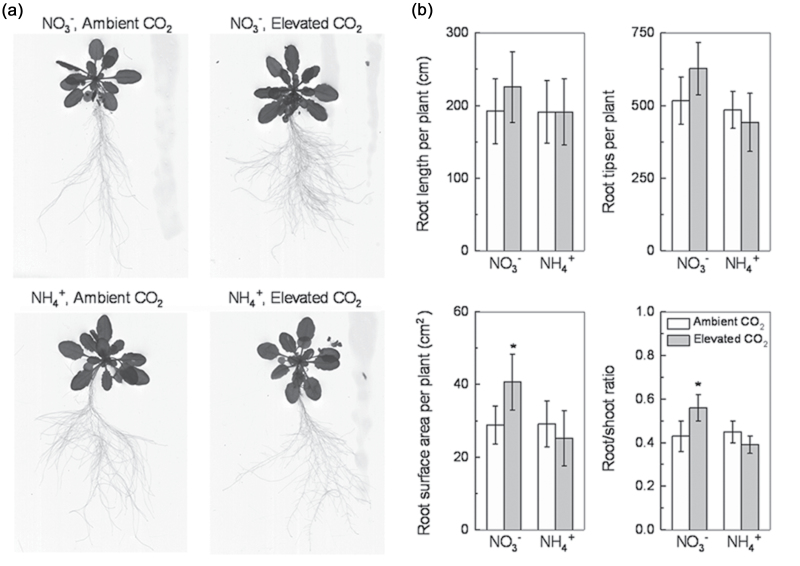
Elevated CO2 greatly enhanced the number of lateral roots in the NO3 −-supplied plants but restrained it in the NH4 +-supplied plants (Fig. 2a). The root/shoot ratio of NO3 −-fed plants was greater and that of NH4 +-fed plants was lower under elevated compared with ambient CO2 (Fig. 2b). Furthermore, compared with ambient CO2, elevated CO2 increased the total root surface area and root/shoot ratio of the NO3 −-supplied plants but not that of the NH4 +-supplied plants (Fig. 2b). Elevated CO2 did not affect the length or density of root hairs of plants grown in either N treatment (data not shown).
Fig. 2.
Root morphology (a), and root length, number of root tips, root surface area, and root/shoot ratio (b) of 5-week-old wild-type Arabidopsis grown for 7 d in P-deficient nutrition solutions containing NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1). Data are means ±SD (n=8) of two independent experiments. Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05).
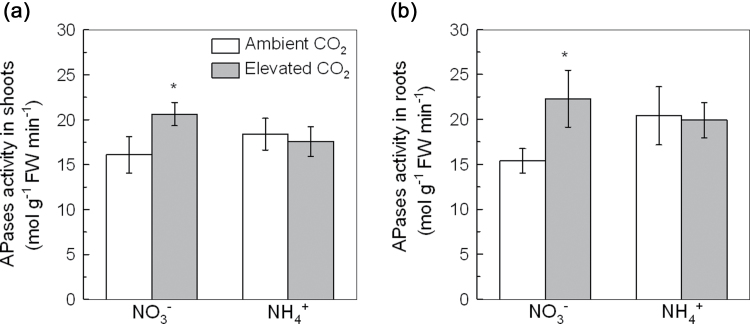
Another general response of plants to P starvation is the enhanced activity of APases. APase activity in both the shoots and roots of NH4 +-supplied plants was similar between the ambient and elevated CO2 treatments (Fig. 3a, b). However, elevated CO2 strongly increased APase activity in the shoots and roots of NO3 −-fed plants (Fig. 3a, b).
Fig. 3.
APase activity in shoots (a) and roots (b) of 5-week-old wild-type Arabidopsis grown for 7 d in P-deficient nutrition solutions containing NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1). Data are means ±SD (n=5). Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05). FW, fresh weight.
Expressions of genes involved in P uptake, translocation, and allocation in P-deficient plants
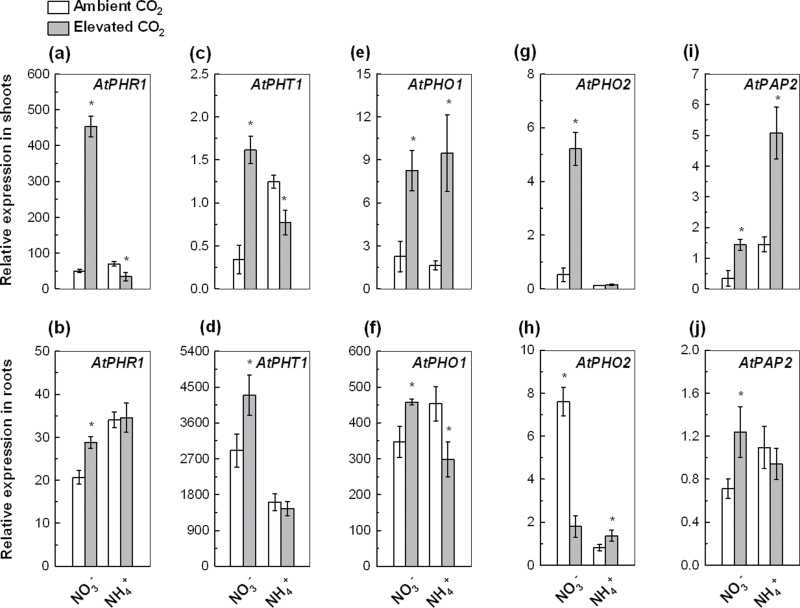
Increased expression of genes involved in P uptake, transport, allocation, and remobilization is a significant adaptive response of plants to facilitate external P acquisition and internal P utilization under P-limited conditions (Rubio et al., 2001; Vance et al., 2003; Chiou and Lin, 2011). Elevated CO2 enhanced the expression of the P-uptake gene AtPHR1 and the P-transport gene AtPHT1; their expression in shoots were increased by 8-fold and 3.7-fold, respectively (Fig. 4a–d). By contrast, elevated CO2 did not change the expression of AtPHR1 and AtPHT1 in the roots of NH4 +-fed plants, and decreased their expression in the shoots (Fig. 4a–d).
Fig. 4.
Relative expression levels of the genes AtPHR1, AtPHT1, AtPHO1, AtPHO2, and AtPAP2 in the shoots and roots of 5-week-old wild-type Arabidopsis grown for 7 d in P-deficient nutrition solutions with NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1). Relative expression levels were calculated and normalized with respect to expression of UBQ10 mRNA. Data are means ±SD (n=5). Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05).
In order to observe the allocation and retranslocation of internal P between shoots and roots, the expression of AtPHO1 and AtPHO2 was analysed. Elevated CO2 increased AtPHO1 expression in the shoots and roots of NO3 −-supplied plants by 2.6-fold and 32%, respectively. In comparison, elevated CO2 increased AtPHO1 expression in the shoots by 4.7-fold but decreased it by 34% in the roots of NH4 +-fed plants (Fig. 4e, f). Elevated CO2 increased the expression of AtPHO2 by 8.8-fold in the shoots but decreased it by 76% in the roots of NO3 −-supplied plants, whereas it did not significantly change its expression in the shoots of NH4 +-fed plants (Fig. 4g, h).
Possible role of NO in P nutrition under P deficiency and elevated CO2
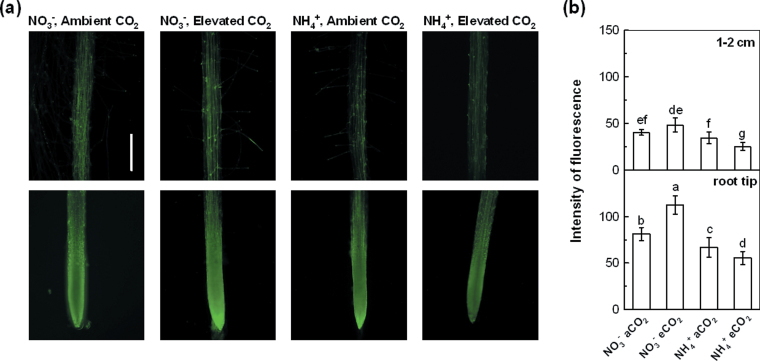
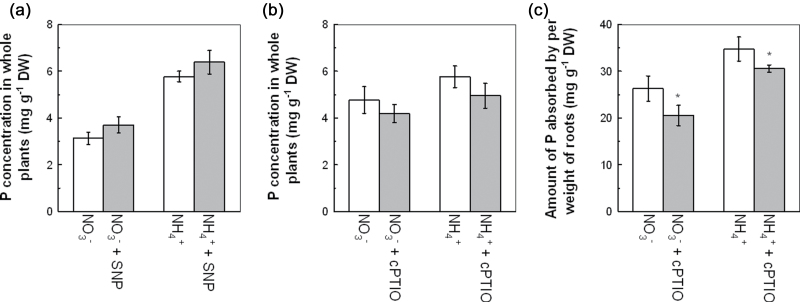
NO was demonstrated recently to be a signal molecule involved in the regulation of root growth during P deficiency (Wang et al., 2010). We observed that elevated CO2 resulted in stronger fluorescence intensity in the root tip and root-hair region in NO3 −-fed plants but decreased it in NH4 +-fed plants, compared with results under ambient CO2 (Fig. 5a). To confirm the observation, the fluorescence of 15 roots from each treatment was measured. As shown in Fig. 5b, elevated CO2 significantly increased the NO level in the roots of NO3 −-supplied plants but decreased it in NH4 +-supplied plants. A correlation was found between endogenous NO level and P concentration in the roots. Whereas the application of SNP (a NO donor) to the plants under ambient CO2 tended to promote the P concentration to levels below those measured under elevated CO2 (Fig. 6a), the addition of the NO scavenger cPTIO under elevated CO2 reduced the amount of P absorbed per unit weight of roots in both NO3 −- and NH4 +-supplied plants and inhibited it to levels significantly below those measured under ambient CO2 (Fig. 6c), suggesting some involvement of NO in modulating P uptake in roots. The treatment effect of total P uptake followed the same pattern (data not shown). These results implied that an appropriate concentration of endogenous NO in roots is critical for the regulation of elevated CO2-induced P uptake in both NO3 − and NH4 + medium.
Fig. 5.
NO production of 5-week-old wild-type Arabidopsis grown for 7 d in P-deficient nutrition solutions containing NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1). (a) Photographs of NO production shown as green fluorescence in the root tips and root-hair region of representative roots of plants supplied with NO3 − and exposed to ambient or elevated CO2, and with NH4 + exposed to ambient or elevated CO2. Bar, 500 µm. (b) Effects of N form and CO2 treatment on NO production expressed as relative fluorescence. NO production was visualized with DAF-FM DA dye. Data are means ±SD (n=15). Means followed by the same letter within a root segment are not significantly different at P <0.05.
Fig. 6.
Effect of SNP under ambient CO2 (350±50 µl l–1) (a) and cPTIO under elevated CO2 (800±50 µl l–1) (b) on P concentration per plant, and the effect of cPTIO under elevated CO2 (800±50 µl l–1) (c) on the amount of P absorbed per weight of roots of 5-week-old wild-type Arabidopsis treated with P-deficient nutrition solutions containing NO3 − or NH4 +. Data are means ±SD (n=5). Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05).
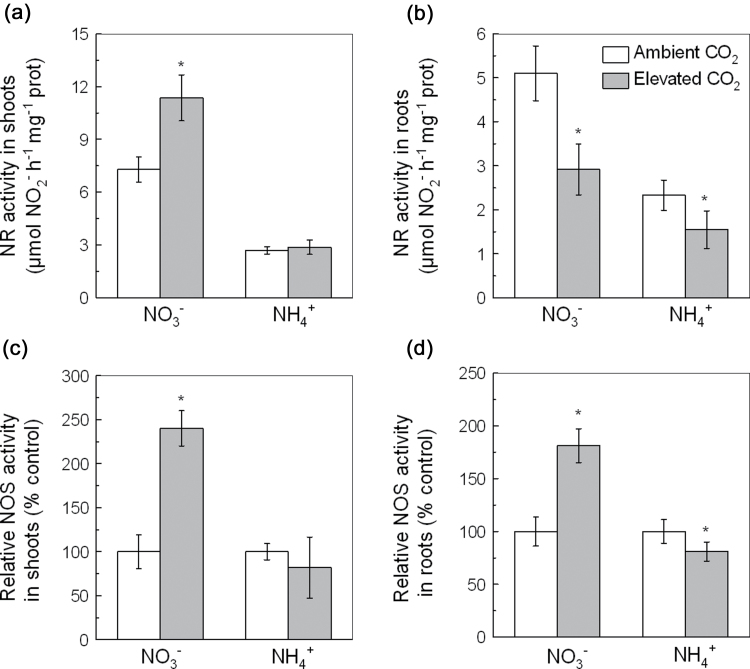
To confirm the above findings further, the effects of elevated CO2 on the activity of NR and NOS were studied (Fig. 7). In the NO3 − treatment, elevated CO2 increased NR activity in the shoots but decreased it in the roots (Fig. 7a, b). Elevated CO2 also increased NOS activity in both the shoots and roots of the NO3 −-fed plants (Fig. 7c, d). In the NH4 + treatment, elevated CO2 did not affect the NOS and NR activity in shoots but inhibited their activity in roots (Fig. 7).
Fig. 7.
NR activity in shoots (a) and roots (b) and NOS activity in shoots (c) and roots (d) of 5-week-old wild-type Arabidopsis treated with P-deficient nutrition solutions containing NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1) for 7 d. Data are means ±SD (n=5 for NR; n=10 for NOS). Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05).
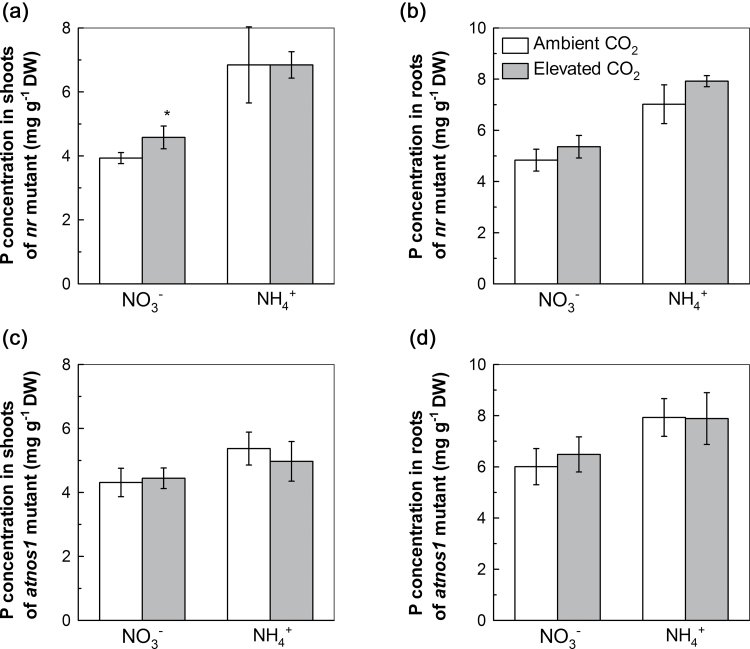
To further verify the role of NO in mediating elevated CO2-induced uptake of P during P deficiency, mutants nr (an NR-reduced mutant) and atnos1 (an NOS-reduced mutant) were used to observe their capacity of P uptake compared with wild-type Arabidopsis. Overall, the P concentrations of mutants nr and atnos1 grown in both NO3 −- and NH4 +-supplied media were similar under ambient and elevated CO2 (Fig. 8a, b). Specially, elevated CO2 increased the P concentration in shoots of the nr mutant supplied with NO3 −. These results demonstrated that NO is the signal involved in the regulation of elevated CO2-induced uptake of P during P deficiency.
Fig. 8.
P concentration in shoots (a, c) and roots (b, d) of Arabidopsis NR-reduced mutant nr and nitric oxide synthase-reduced mutant atnos1 treated with P-deficient nutrition solutions containing NO3 − or NH4 + exposed to ambient CO2 (350±50 µl l–1) or elevated CO2 (800±50 µl l–1) for 7 d. Data are means ±SD (n=5). Asterisks indicate that the mean values are significantly different between the ambient and elevated CO2 treatments (P <0.05).
Discussion
Previous studies have indicated that CO2-dependent stimulation of growth is likely to enhance the requirements of plants for macronutrients such as N and P (Baxter et al., 1994; Kogawara et al., 2006; Lagomarsino et al., 2008). This study demonstrated that a combination of elevated CO2 and nitrate induced a set of morphological, physiological, and molecular responses that enabled the plants to better access and utilize P and to alleviate P-deficiency symptoms.
Elevated CO2 improves P nutrition under a nitrate supply
The promotion of plant growth under elevated CO2 would be associated with increased requirements for mineral nutrition to optimize the nutrition balance in plants. This was the case in a 6-year Free Air CO2 Enrichment (FACE) experiment in which photosynthetic stimulation by elevated CO2 was largely regulated by N and P availability in the soil (Lagomarsino et al., 2008). In the present study, elevated CO2 enhanced biomass production in both NO3 −- and NH4 +-fed Arabidopsis plants during P deficiency (Fig. 1). However, elevated CO2 preferentially stimulated specific P (pool) concentration as well as total P concentration and reduced the accumulation of anthocyanins in NO3 −-fed plants. This result is consistent with the findings of Carlisle et al. (2012) that NH4 +-supplied plants tended to have decreased nutrient concentrations with increasing CO2 concentration, while NO3 −-supplied plants varied widely across CO2 treatments. The present study concluded that NO3 − nutrition facilitates the P uptake of roots under elevated CO2.
To test whether the improved P uptake occurred only in NO3 −-fed P-deficient plants under elevated CO2 conditions, we further examined the influence of elevated CO2 on P nutrition of P-adequate Arabidopsis supplied with different N forms. Interestingly, elevated CO2 only increased the shoot biomass, but decreased specific P uptake with the decrease being greater in the NO3 − than the NH4 + treatment (Fig. S1). Numerous studies have elucidated that plants adapt to P deficiency through modifications of root architecture, carbon metabolism and membrane structure; exudation of carboxylates, protons and enzymes; and enhanced expressions of genes (Raghothama, 1999; Vance et al., 2003; Shenoy and Kalagudi, 2005; Shulaev et al., 2008; Wang et al., 2010). Therefore, the increase in P concentration in NO3 −-fed Arabidopsis plants by elevated CO2 cannot be attributed to increased photosynthesis alone but also to the improved P nutrition of the plants through morphological, physiological, and molecular responses to P deficiency.
This study showed that NO3 −-fed plants had more lateral roots but a shorter taproot under elevated compared with ambient CO2, whereas NH4 +-fed plants had longer roots with fewer lateral roots under elevated CO2 (Fig. 2). In the field, particularly with no-till systems, P concentration is great in topsoil and declines substantially with depth (Lynch and Brown, 2001; Ao et al., 2010). The reduced primary root and increased lateral roots, resulting in a shallower root system, is a positive adaptive response to low-P availability by reducing inter-root competition within the same plant (Miller et al., 2003; Lynch, 2007). In addition, the greater root/shoot ratios seen in NO3 −- compared with NH4 +-supplied plants during P deficiency under high CO2 concentrations (Fig. 2) also favour P acquisition. Nevertheless, favourable root architecture may only play a minor role in increasing P uptake of plants under the combined conditions of P limitation and elevated CO2 in the hydroponic culture supplied with H2PO4 −.
Another general response of plants to P starvation is the enhanced activity of APases (Richardson et al., 2009; Tran et al., 2010). In bean plants, a high activity of APases contributes to the increased P-utilization efficiency through remobilization of P from old leaves to young tissues (Kouas et al., 2009). Similarly, the physiological function of root APases has also been demonstrated by Xiao et al. (2006) and Wang et al. (2009) where transgenic plants overexpressing APase genes exhibited greater P acquisition from an organic P resource than wild-type plants. Our results showed that elevated CO2 increased APase activity in both the shoots and roots of Arabidopsis plants supplied with NO3 −, which is consistent with results reported in the literature (Lagomarsino et al., 2008). The enhanced APase activity, especially in the root of NO3 −-fed plants, under elevated CO2 conditions (Fig. 3) would be expected to facilitate hydrolysis of organic P and thus plant P uptake in the field.
Biochemical and molecular studies have identified a number of genes that are involved in plant alteration in response to P starvation. PHR1 is a key transcriptional activator in controlling P uptake, allocation, and anthyocyanin accumulation (Rubio et al., 2001; Nilsson et al., 2007). The enhanced expression of AtPHR1 under elevated CO2 could activate numerous P-deficiency-response genes, which in turn change molecular, cellular, and physiological processes to cope with P deficiency. P assimilation is initiated by the transport of P into the roots and the number of P transporters present in the roots reflects the complexity and significance of the process. In Arabidopsis, the reduced P uptake activity is caused by a defect in targeting the PHT1 transporter to the plasma membrane (González et al., 2005; Jain et al., 2007). Our present study showed that elevated CO2 enhanced the expression of AtPHT1 genes in the shoots and roots of NO3 −-fed plants with expression being much greater in the roots (Fig. 4), indicting that P transport was stimulated by elevated CO2 in the NO3 − medium.
Partitioning and allocation of internal P is also crucial for maintenance of P homeostasis within plants. AtPHO1 was first identified in Arabidopsis as a gene playing an important role in P transport from the roots to the shoots (Poirier et al., 1991), while AtPHO2 is responsible for P translocation from the shoots to the roots. It was reported that a pho1 mutant plant accumulated adequate P in the roots but less P in the shoots (Poirier et al., 1991; Hamburger et al., 2002; Stefanovic et al., 2007). The downregulated expression of AtPHO1 in the roots and the similar expression of AtPHO2 in the shoots of NH4 +-supplied plants in the present study (Fig. 4) probably form a negative-feedback loop against internal P recycling between shoots and roots. In contrast, the PHO2 gene, which regulates P uptake, allocation, and remobilization, is a target gene of miR399s pho2, which displays P toxicity with excessive P accumulation in the shoots of Arabidopsis (Aung et al., 2006). Accordingly, the high expression of AtPHO1 in the roots and AtPHO2 in the shoots of the NO3 −-supplied plants under elevated CO2 (Fig. 4) indicated that internal P recycling between shoots and roots was stimulated by elevated CO2 during the onset of P starvation. Furthermore, the enhanced AtPAP2 expression in shoots under elevated CO2 (Fig. 4) indicated that elevated CO2 could excite intracellular P remobilization during P deficiency.
A possible role of NO
It has been shown that P deficiency significantly increases the endogenous NO concentration in roots of white lupin (Wang et al., 2010). In this study, we observed that elevated CO2 increased the NO level in roots of NO3 −-supplied plants, while the NO scavenger cPTIO inhibited specific P uptake by the root. In contrast, the NH4 + supply was associated with a rapid decrease in NO production under elevated CO2, while the NO scavenger cPTIO inhibited specific P uptake (Figs 5 and 6). These results are supported by recent observations that NO3 − but not NH4 + increased the NO level in the roots of tomato (Solanum lycopersicum) in both cadmium-free and cadmium-supplemented growth solutions (Luo et al., 2012). These results suggest that NO is partly responsible for NO3 −-facilitated P accumulation in plants.
It is unclear how elevated CO2 changes the NO levels in roots under P deficiency. It is recognized that NR and NOS are two potential enzymatic sources of NO in plants (Neill et al., 2008). Although NOS genes have not yet been identified in plants (Neill et al., 2008), several studies support the presence of NOS activity in plants (Corpas et al., 2006; Valderrama et al., 2007). As shown in Fig. 7, NR activity in the shoots of NO3 −-fed plants was greater under elevated compared with ambient CO2, while NR activity in the roots was lower under elevated CO2, irrespective of N form. These results are in accordance with the findings of other studies (Buchanan et al., 2000; Poonnachit and Darnell, 2004). Nitrate supply has been shown to enhance NR activity (Shaner and Boyer, 1976), whereas NH4 + is an inhibitor of NR (Jin et al., 2011). On the one hand, increasing NO3 − availability should enhance the NR-dependent NO production. Accordingly, a higher NO level in the roots of the NO3 −-fed plants and a lower NO level in the roots of NH4 +-fed plants under elevated CO2 is partly due to the change in NR activity. On the other hand, elevated CO2 enhanced the root uptake capacity for NO3 −, but not for NH4 +, in field-grown loblolly pine saplings (Bassirirad et al., 1996). Therefore, it is reasonable to propose that stimulated NO3 − uptake under elevated CO2 may facilitate NO production in roots, form a positive feedback loop, and upregulate P uptake.
Elevated CO2 also increased the NOS activity of Arabidopsis plants supplied with NO3 − but decreased it in NH4 +-fed plants (Fig. 7). It is well established that elevated CO2 represses NIA in NH4 +-fed plants and increases NH4 + uptake and assimilation, and the synthesis of glutamine (Gojon et al., 1998; Stitt and Krapp, 1999). Thus, increased NH4 + uptake and assimilation under elevated CO2 may restrain the activity of NR, and possibly of NOS. Furthermore, in mutants with low NIA activity, NO production was suppressed by NH4 + (Gojon et al., 1998; Stitt and Krapp, 1999), which supports our findings.
In Arabidopsis, two mutants defective in NO production displayed altered phenotypes. The nr mutant had a deletion of the major NR gene, NIA2, and an insertion in the NIA1 NR gene (Wang et al., 2004), and the atnos1 mutant has a mutation in an NOS structural gene NOS1 (Guo et al., 2003). In this study, the P concentrations of both mutants, nr and nos, were similar under elevated and ambient CO2 (Fig. 8), suggesting that regulation of P nutrition under elevated CO2 is NO-signalling dependent. However, relative to the ambient CO2 condition, elevated CO2 tended to increase the P concentration in both the shoots and roots of NO3 −-fed plants. These results are consistent with a major or general role of NO in the regulation of plant development and metabolism. The results also support our hypothesis that elevated CO2 increases P uptake in NO3 −-fed plants but suppresses it in NH4 +-fed plants through the effect on the activity of NR and/or NOS, leading to changes in NO production and physiology. However, the details about how NO exerts its effects on P nutrition remain unclear. The studies by Neill et al. (2003) and Niu et al. (2011b) on interrelations between NO, Ca2+, cyclic ADP ribose, cyclic GMP, salicylic acid, reactive oxygen species, hydrogen peroxide signalling, and protein kinases have provided an important focal point for future research.
Overall, although previous reports have provided other evidence concerning NH4 + nutrition facilitating P uptake in the roots of different plant species. Here, using wild-type Arabidopsis plants, we demonstrated that NO3 − nutrition facilitates the P uptake in roots from P-deficient soluble sources under elevated CO2 by upregulating plant adaptive strategies. The increase in NO production may be a signalling pathway controlling the above process. To our knowledge, this finding uncovers a mechanism that has not been described previously and provides a new insight into N forms regulating P nutrition under elevated CO2, an important part of climate change in the 21st century. This study also helps to determine whether the carbon costs of P efficiency under elevated CO2 should be prioritized in crop improvement programmes.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Effect of elevated CO2 and N form on shoot and root fresh biomass production, P concentration in whole plants, and amount of P absorbed per weight of roots.
Supplementary Fig. S2. Effect of elevated CO2 and N form on chlorophyll content (SPAD readings) of wild-type Arabidopsis grown in P-deficient (0.5 µM) (a) and P-adequate (0.5mM) (b) nutrition solutions containing NO3 − or NH4 +.
Supplementary Fig. S3. Effect of elevated CO2 and N form on anthocyanin content of wild-type Arabidopsis grown in P-deficient (0.5 µM) (a) and P-adequate (0.5mM) (b) nutrition solutions containing NO3 − or NH4 +.
Acknowledgements
We thank the anonymous reviewers for their constructive comments. This work was financially supported by the National Key Project on Science and Technology of China (2012BAC17B02), the State Key Development Program for Basic Research of China (973 Program, no. 2009CB119003), and the Project of Transformation Fund for Agricultural Scientific and Technological Achievements of China (2010GB23600669). C.T. was supported by the Australian Research Council through the Linkage Projects funding scheme.
Glossary
Abbreviations:
- APase
acid phosphatase
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-FM DA
diaminofluorescein-FM diacetate
- N
nitrogen
- NO
nitric oxide
- NOS
nitric oxide synthase
- NR
nitrate reductase
- P
phosphorus
- SNP
sodium nitroprusside
- AtPHR1
PHOSPHATE STARVATION RESPONSE 1
- AtPHT1
PHOSPHATE TRANSPORTER 1
- AtPHO1
PHOSPHATE 1
- AtPHO2
PHOSPHATE 2. AtPAP2, PURPLE ACID PHOSPHATASE 2
References
- Abel S, Ticconi CA, Delatorre CA. 2002. Phosphate sensing in higher plants. Physiologia Plantarum. 115, 1–8 [DOI] [PubMed] [Google Scholar]
- Ao JH, Fu JB, Tian J, Yan XL, Liao H. 2010. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean.. Functional Plant Biology. 37, 304–312 [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. 2006. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a MicroRNA399 target gene. Plant Physiology. 141, 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SA, Walker JM, Vasey EH. 1963. Mechanisms for movement of plant nutrients from soil and fertilizer to plant root. Journal of Agricultural and Food Chemistry. 11, 204–207 [Google Scholar]
- Bassirirad H, Thomas RB, Reynolds JF, Strain BR. 1996. Differential responses of root uptake kinetics of NH4 + and NO3 − to enriched atmospheric CO2 concentration in field-grown loblolly pine. Plant, Cell and Environment. 19, 367–371 [Google Scholar]
- Batjes NH. 1997. A world data set for derived soil properties by FAO-UNESCO soil unit for global modelling. Soil Use and Management. 13, 9–16 [Google Scholar]
- Baxter R, Gantley M, Ashenden TW, Farrar JF. 1994. Effects of elevated carbon dioxide on three grass species from montane pasture. Nutrient uptake, allocation and efficiency of use. Journal of Experimental Botany. 45, 1267–1278 [Google Scholar]
- Bloom AJ, Burger M, Asensio JSR, Cousins AB. 2010. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis . Science. 328, 899–903 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Smart DR, Nguyen DT, Searles PS. 2002. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proceedings of the National Academy of Sciences U S A. 99, 1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. 2000. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; [Google Scholar]
- Carlisle E, Myers S, Raboy V, Bloom A. 2012. The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Frontiers in Plant Science. 3, 195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI. 2011. Signaling network in sensing phosphate availability in plants. Annual Review of Plant Biology. 62, 185–206 [DOI] [PubMed] [Google Scholar]
- Conroy JP, Milham PJ, Barlow EWR. 1992. Effect of nitrogen and phosphorus availability on the growth response of Eucalyptus grandis to high CO2 . Plant, Cell and Environment. 15, 843–847 [Google Scholar]
- Conroy JP, Milham PJ, Reed ML, Barlow EWR. 1990. Increases in phosphorus requirements for CO2-enriched pine species. Plant Physiology. 92, 977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA. 2006. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta. 224, 246–254 [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzàlez-Meler MA, Long SP. 1997. More efficient plants: a consequence of rising atmospheric CO2?. Annual Review of Plant Physiology and Plant Molecular Biology. 48, 609–639 [DOI] [PubMed] [Google Scholar]
- Fiorani F, Umbach AL, Siedow JN. 2005. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiology. 139, 1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan YB, Kumimoto R, Liu C, Ratcliffe O, Yu H, Broun P. 2006. GLABROUS INFLORESCENCE STEMS modulates the regulation by gibberellins of epidermal differentiation and shoot maturation in Arabidopsis . The Plant Cell. 18, 1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Cramer GR, Seemann JR. 2001. Growth, cell walls, and UDP-Glc dehydrogenase activity of Arabidopsis thaliana grown in elevated carbon dioxide. Journal of Plant Physiology. 158, 569–576 [Google Scholar]
- Gojon A, Dapoigny L, Lejay L, Tillard P, Rufty TW. 1998. Effects of genetic modification of nitrate reductase expression on 15NO3 − uptake and reduction in Nicotiana plants. Plant, Cell and Environment. 21, 43–53 [Google Scholar]
- González E, Solano R, Rubio V, Leyva A, Paz-Ares J. 2005. PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidospis . The Plant Cell. 17, 3500–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM. 2003. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 302, 100–103 [DOI] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, Petétot JMC, Somerville C, Poirier Y. 2002. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. The Plant Cell. 14, 889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. 1938. The water-culture method for growing plants without soil. Circ. 347 Berkeley, CA: University of California, College of Agriculture; [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 207, 604–611 [DOI] [PubMed] [Google Scholar]
- IPCC 2007. Climate change 2007: the fourth IPCC assessment report. Valencia, Spain: Intergovernmental Panel on Climate Change; [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. 2007. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiology. 144, 232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. 2009. Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiology. 150, 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Shamsi IR, Luo BF, Lin XY. 2011. NO synthase-generated NO acts downstream of auxin in regulating Fe-deficiency induced root branching that enhances Fe-deficiency tolerance in tomato plants. Journal of Experimental Botany. 62, 3875–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Tang CX, Armstrong R, Sale P. 2012. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient Vertisol. Plant and Soil. 358, 91–104 [Google Scholar]
- Kogawara S, Norisada M, Tange T, Yagi H, Kojima K. 2006. Elevated atmospheric CO2 concentration alters the effect of phosphate supply on growth of Japanese red pine (Pinus densiflora) seedlings. Tree Physiology. 26, 25–33 [DOI] [PubMed] [Google Scholar]
- Kouas S, Debez A, Slatni T, Labidi N, Drevon JJ, Abdelly C. 2009. Root proliferation, proton efflux, and acid phosphatase activity in common bean (Phaseolus vulgaris) under phosphorus shortage. Journal of Plant Biology. 52, 395–402 [Google Scholar]
- Lagomarsino A, Moscatelli MC, Hoosbeek MR, De Angelis P, Grego S. 2008. Assessment of soil nitrogen and phosphorous availability under elevated CO2 and N-fertilization in a short rotation poplar plantation. Plant and Soil. 308, 131–147 [Google Scholar]
- Luo BF, Du ST, Lu KX, Liu WJ, Lin XY, Jin CW. 2012. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. Journal of Experimental Botany. 63, 3127–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany. 55, 493–512 [Google Scholar]
- Lynch JP, Brown KM. 2001. Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 237, 225–237 [Google Scholar]
- Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M. 2001. Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant, Cell and Environment. 24, 1119–1137 [Google Scholar]
- McLachlan KD, Elliott DE, De Marco DG, Garran JH. 1987. Leaf acid-phosphatase isozymes in the diagnosis of phosphorus status in field-grown wheat. Australian Journal of Agricultural Research. 38, 1–13 [Google Scholar]
- Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP. 2003. Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Functional Plant Biology. 30, 973–985 [DOI] [PubMed] [Google Scholar]
- Mukatira UT, Liu CM, Varadarajan DK, Raghothama KG. 2001. Negative regulation of phosphate starvation-induced genes. Plant Physiology. 127, 1854–1862 [PMC free article] [PubMed] [Google Scholar]
- Neill S, Bright J, Desikan R, Hancock J, Harrison J, Wilson I. 2008. Nitric oxide evolution and perception. Journal of Experimental Botany. 59, 25–35 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. 2003. Nitric oxide signalling in plants. New Phytologist. 159, 11–35 [DOI] [PubMed] [Google Scholar]
- Nilsson L, Müller R, Nielsen TH. 2007. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana . Plant, Cell and Environment. 30, 1499–1512 [DOI] [PubMed] [Google Scholar]
- Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS. 2011. a Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2 . Plant, Cell and Environment. 34, 1304–1317 [DOI] [PubMed] [Google Scholar]
- Niu YF, Jin GL, Chai RS, Wang H, Zhang YS. 2011. b Responses of root hair development to elevated CO2 . Plant Signaling and Behavior. 6, 1414–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. 2011. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science. 16, 442–450 [DOI] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J. 1991. A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiology. 97, 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonnachit U, Darnell R. 2004. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Annals of Botany. 93, 399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. 1999. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology. 50, 665–693 [DOI] [PubMed] [Google Scholar]
- Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J. 2010. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Research. 117, 169–176 [Google Scholar]
- Richardson AE, Hocking PJ, Simpson RJ, George TS. 2009. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Science. 60, 124–143 [Google Scholar]
- Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J. 2009. Plant hormones and nutrient signaling. Plant Molecular Biology. 69, 361–373 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J. 2001. A conserved MYB transcription factor involved in phosphate starvation signalling both in vascular plants and in unicellular algae. Genes & Development. 15, 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. 1998. Phosphorus uptake by plants: from soil to cell. Plant Physiology. 116, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjørring JK. 1986. Nitrate and ammonium absorption by plants growing at a sufficient or insufficient level of phosphorus in nutrient solutions. Plant and Soil. 91, 313–318 [Google Scholar]
- Shaner DL, Boyer JS. 1976. Nitrate reductase activity in maize (Zea mays L.) leaves. I. Regulation by nitrate flux. Plant Physiology. 58, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy VV, Kalagudi GM. 2005. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnology Advances. 23, 501–513 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Cortes D, Miller G, Mittler R. 2008. Metabolomics for plant stress response. Physiologia Plantarum. 132, 199–208 [DOI] [PubMed] [Google Scholar]
- Stefanovic A, Ribot C, Rouached H, Wang Y, Chong J, Belbahri L, Delessert S, Poirier Y. 2007. Members of the PHO1 gene family show limited functional redundancy in phosphate transfer to the shoot, and are regulated by phosphate deficiency via distinct pathways. Plant Journal. 50, 982–994 [DOI] [PubMed] [Google Scholar]
- Stitt M, Krapp A. 1999. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell and Environment. 22, 583–621 [Google Scholar]
- Stitt M, Quick WP, Schurr U, Scheibe R, Schulze ED, Rodermel SR, Bogorad L. 1991. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with ‘antisense’ rbcS. Planta. 183, 542–554 [DOI] [PubMed] [Google Scholar]
- Teng NJ, Wang J, Chen T, Wu XQ, Wang YH, Lin JX. 2006. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana . New Phytologist. 172, 92–103 [DOI] [PubMed] [Google Scholar]
- Tian QY, Sun DH, Zhao MG, Zhang WH. 2007. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos . New Phytologist. 174, 322–331 [DOI] [PubMed] [Google Scholar]
- Tran HT, Hurley BA, Plaxton WC. 2010. Feeding hungry plants: the role of purple acid phosphatases in phosphate nutrition. Plant Science. 179, 14–27 [Google Scholar]
- Valderrama R, Corpas FJ, Carreras A, Fernandez-Ocana A, Chaki M, Luque F, Gómez-Rodríguez MV, Colmenero-Varea P, del Río LA, Barroso JB. 2007. Nitrosative stress in plants. FEBS Letters. 581, 453–461 [DOI] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 157, 423–447 [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, et al. 2012. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytologist. 195, 306–320 [DOI] [PubMed] [Google Scholar]
- Wang BL, Tang XY, Cheng LY, Zhang AZ, Zhang WH, Zhang FS, Liu JQ, Cao Y. 2010. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytologist. 187, 1112–1123 [DOI] [PubMed] [Google Scholar]
- Wang RC, Tischner R, Gutiérrez RA, Hoffman M, Xing XJ, Chen MS, Coruzzi G, Crawford NM. 2004. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiology. 136, 2512–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y, Tian J, Lim BL, Yan X, Liao H. 2009. Overexpressing AtPAP15 enhances phosphorus efficiency in soybean. Plant Physiology. 151, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Zhang JH, Harrison MJ, Wang ZY. 2006. Ectopic expression of a phytase gene from Medicago truncatula barrel medic enhances phosphorus absorption in plants. Journal of Integrative Biology. 48, 35–43 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.